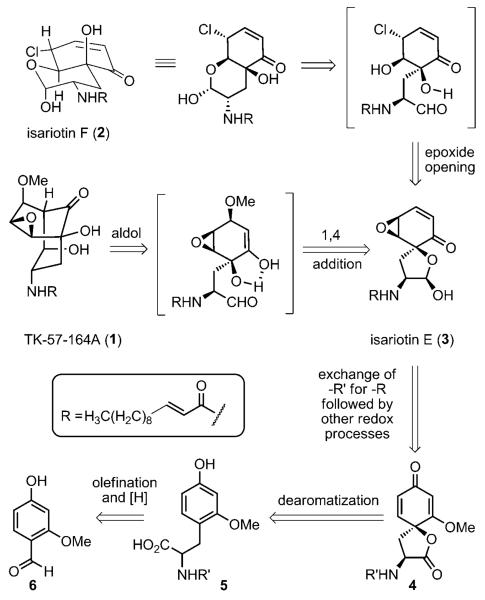
Cyclohexenone monoepoxides enjoy widespread attention because of their useful properties and structural complexity.[1] In a search for bioactive secondary metabolites of entomopathic fungi-afflicting insects, Bunyapaiboonsri et al. reported isolating two novel compounds, isariotin E (3) and F (2) along with the known bicycle TK-57-164A (1), a compound previously described as an agricultural bactericide.[2, 3] Isariotin F (2) exhibited activity against the malaria parasite Plasmodium falciparum K1, three cancer cell lines (KB, BC, NCI-H187), and nonmalignant (Vero) cells (IC50 = 5.1, 15.8, 2.4, 1.6, and 2.9 μm, respectively). Furthermore Bunyapaiboonsri et al. speculated that isariotin E (3) might serve as the biosynthetic progenitor of 1 and 2 (Scheme 1). However, the paucity of compound 3 thwarted investigation of its speculated biosynthetic pathway as well as biological evaluation.
Scheme 1.
Metabolites from Isaria tenuipes BCC 12625 and our synthetic plan.
Natural products 1–3 contain a diverse array of congested and reactive functionality. Seven contiguous stereocenters are embedded within the [3.3.1]-bicyclic framework of 1; expressed functionality includes both secondary and tertiary alcohols, an epoxide, a methyl ether, a hydrophobic amide, and a bridging carbonyl group. A trans-decalin-like system possessing an ethereal ring union describes the skeleton of 2. The periphery features five stereogenic centers; a stereo-defined hemiketal derived from a secondary alcohol, an allylic chloride/enone moiety, and the same tertiary alcohol and amide functionalities found in 1. Similarly, five stereo-centers comprise the backbone of the spirocycle 3. It includes the same tertiary alcohol, epoxide, and amide functionalities found in 1, but also a highly electrophilic enone and a hemiketal consisting of a tertiary alcohol. Given these structural attributes coupled to the putative biosynthesis and biological activity of these compounds, we felt that all three natural products warranted our attention. Herein, we detail our strategy for the total synthesis of isariotin E (3), isariotin F (2), and TK-57-164A (1).
Benefiting from Bunyapaiboonsri's insight,[2] we suspected that the [3.3.1]-bicyclic nonane core found in compound 1 might arise via the intermediacy of an enol formed by the diastereoselective conjugate addition of methoxide to compound 3 (Scheme 1). An observation of a similar epoxide directed conjugate addition reported by Ogita and co-workers for scyphostatin supported this notion.[4] However, the outcome of this reaction was far from assured given the report from Nishiyama and co-workers on an indiscriminate Michael/aldol cascade affording gymnastatin F.[5] The ethereal trans-decalin skeleton of 2 was imagined to be derived from a chloride triggered epoxide-opening event with 3, which would generate a free secondary alcohol which could serve as the new center for hemiketalization to afford the bicycle 2. We speculated that the core of the spirocycle 3 could arise from a related dissymmetric 2,5-cyclohexadienone, 4, provided that the necessary series of chemoselective and diastereoselective reactions could be developed to successfully transform its conjoined enone and vinylogous ester functional groups. The dissymmetric spirolactone 4 was envisioned to serve as a key platform, whose fixed geometry might enable smooth exchange of amine functionality. It could arise from an ipso dearomatization of the unnatural amino acid 5, a resorcinol surrogate of tyrosine, which we suspected could be procured through a Horner–Wadsworth–Emmons olefination with the commercially available benzaldehyde 6, and subsequent reduction. With regards to the selection of the starting amine protecting group (R′), our decision to capitalize upon the amino acid construction method of Schmidt et al.,[6] and the customary questions of functional group compatibility constrained our choices to carbamates.
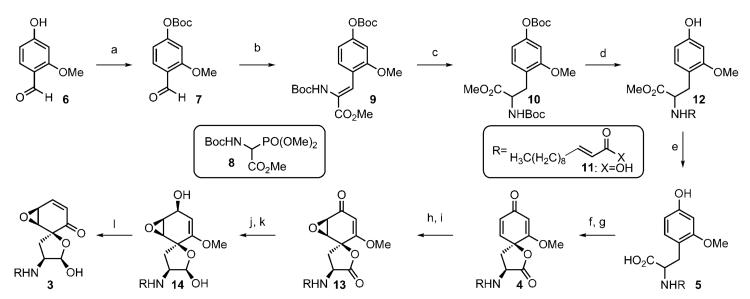
Our route therefore began with O-Boc protection of the commercially available phenol 6 (Scheme 2). We found that in the presence of 1,1,3,3-tetramethyl guanidine (TMG), the differentially protected benzaldehyde 7 can be elaborated with N-Boc-protected phosphonate 8,[7] prepared using the protocol of Schmidt et al., to afford the vinyl carbamate 9 (78 % yield, E/Z 1:9, > 10 g scale). For our purposes, both of these olefinic isomers proceeded to compound 10 upon hydrogenation (Pd/C, H2), and therefore improvement of this ratio necessitated no further attention. Global removal of -NBoc and -OBoc residues in compound 10 (5:1 CH2Cl2/ TFA) afforded the corresponding ammonium salt, which subsequently underwent selective amide bond formation with the acid 11 to produce the amide 12 (82 % yield). Additional hydrolysis of the methyl ester 12 with lithium hydroxide afforded compound 5 (88 % yield). With the desired monoprotected resorcinol tyrosine analogue in hand, we were poised to test the oxidative dearomatization of the dissymmetric resorcinol.[8,9] We hoped that the methoxy and amine residues would emerge being positioned on opposite sides of the conjoined spirocyclic molecule in a diastereoselective manner, reminiscent of a reaction developed by Wardrop et al. for the production of spiroamides from nitrenes.[10] To our delight, sequential exposure of the acid 5 to hypervalent iodine and DBN provided the spirocyclic lactone 4 as the major diastereomer (61 % overall yield, > 12:1 d.r.); this outcome was confirmed by nOe examination of the chromatographically separable isomers.[11]
Scheme 2.
Synthesis of isariotin E (3). Reagents and conditions: a) Boc2O (1.5 equiv), NEt3 (3.0 equiv), DMAP (0.1 equiv), CH2Cl2, RT, 12 h, > 95 %; b) 7 (1.3 equiv), TMG (1.5 equiv), THF, RT, 18 h, 78 %; c) Pd/C (0.04 equiv), H2, EtOAc/MeOH (1:1), RT, 12 h, > 95 %; d) TFA/CH2Cl2 (1:5), RT, 24 h, concentrate; then 13 (1.3 equiv), EDCI (1.3 equiv), NEt3 (5.0 equiv), DMF, RT, 16 h, 82 %; e) LiOH·H2O (3.0 equiv), THF/MeOH/H2O (1:1:1), RT, 20 h, 88 %; f) PhI(OAc)2 (1.5 equiv), TFA (2.3 equiv), CH2Cl2, 0°C→RT, 3 h; g) DBN (0.5 equiv), CH2Cl2, RT, 2 h, 61 % over two steps, > 12:1 d.r.; h) pyrrolidine (1.5 equiv), CH2Cl2, RT, 14 h, concentrate; then tBuOOH (10 equiv), DBN (10 equiv), CH2Cl2, RT, 6 h, 62%; i) AcOH (5 equiv), CH2Cl2, reflux, 8 h, 92 %; j) NaBH4 (3.0 equiv), THF/H2O (10:1), 0°C, 30 min; k) Dibal-H (4.5 equiv), CH2Cl2, −78°C, 10 min, 56 % over 2 steps; l) Cl3CCO2H (11 equiv), CH2Cl2/H2O (10:1), RT, 6 h, 74 %. Boc = tert-butoxycarbonyl, DMAP = 4-dimethylaminopyridine, TMG = 1,1,3,3,-tetramethylguanidine, THF = tetrahydrofuran, TFA = trifluoroacetic acid, EDCI = 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, DMF = N,N-dimethylformamide, DBN = 1,5-diazabicyclo[4.3.0]non-5-ene, Dibal-H = diisobutylaluminum hydride.
To capitalize upon the directing effect of the tertiary alcohol,[12] we next exposed the dienone 4 to pyrrolidine. Without purification, the corresponding enone-amide participated in sequential nucleophilic epoxidation (tert-butyl hydroperoxide, DBN) and afforded a syn-epoxide (62 % yield, single diastereomer, see the Supporting Information). Relactonization proceeded upon the addition of acetic acid and furnished the spirolactone 13 (92 % yield).
Having successfully established four of the stereocenters, we imagined that stereoselective reduction of the carbonyl residues and rearrangement should complete the synthesis of isariotin E (3) from the vinylogous ester 13. The necessary diastereocontrol required to prevent an undesired Payne rearrangement required a two-step reduction protocol. The vinylogous ester 13 undergoes reduction with sodium borohydride to afford the corresponding syn-epoxy alcohol, which underwent additional reduction as a crude reaction mixture using Dibal-H to arrive at a hemiketal; a compound that we have tentatively assigned as the lactol 14 (56 % overall yield, one diastereomer). Treatment of this allylic alcohol functionality with trichloroacetic acid (1:10 H2O/CH2Cl2) resulted in isariotin E (3) in 74 % yield. The NMR data for the synthetic compound 3 matched those of isariotin E in every respect, concluding the first total synthesis for 3.
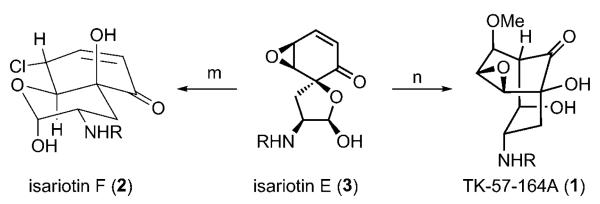
With ample quantities of the hemiketal 3 in hand, we were positioned to test the putative skeletal rearrangements (Scheme 3). Initially, we suspected that both isariotin F (2) and TK-57-164A (1) were artifacts from the isolation of 3. However, we were unable to cause isariotin E (3) to convert into either 1 or 2 using prior isolation conditions (CaCl2, MeOH, 2 days). We were therefore extremely gratified to find that exposure of isariotin E (3) to a freshly prepared solution of NaOMe in MeOH provided the formidable [3.3.1]-bicyclic framework of TK-57-164A (1) in nearly quantitative yield and perfect diastereoselective control (> 95 % yield, single diastereomer). Alternatively, we found that treatment of isariotin E (3) with cerium(III) chloride heptahydrate in acetonitirile for 16 hours led to what we believe, from preliminary 1H NMR analysis, to be the chloride 2 along with an aldehyde, and epimeric or isomeric lactols. However, upon standing and solidfying over four days, this complex mixture smoothly converged into isariotin F (2) in good yield (86 % from 3). NMR data for synthetic 1 and 2 matched those of natural 1 and 2 in every respect.
Scheme 3.
Confirmation of the skeletal rearrangements. Reagents and conditions: m) CeCl3·7 H2O (20 equiv), MeCN, RT, 16 h, 86 %; n) NaOMe (10 equiv), MeOH, RT, 1 h, > 95 %.
In summary, we successfully traversed the gauntlet of reactive functional groups presented by these molecules to complete the first total synthesis of isariotin E (3, 12 steps, 7.8 % overall yield), isariotin F (2, 13 steps, 6.7 % overall yield), and TK-57-164A (1, 13 steps, 7.7 % overall yield). The chemistry developed for manipulating dissymmetric cyclohexadienones offers new potential for synthesizing other family members and analogues in a fairly rapid manner, possibly resulting in new compounds with greater selectivity and enhanced potency. The reported syntheses prove efficient because of their protecting group and redox economy, a natural consequence of following biological pathways.[13] Furthermore, the unusual conditions required for the last two transformations suggest that compounds 1 and 2 may indeed be natural products supporting Bunyapaiboonsri s original biosynthetic postulate. These three total syntheses also substantiate our confidence in the unique synthetic opportunities available to disymmetric cyclohexadienones displaying conjoined enone and vinylogous ester components and their potential for complex molecule synthesis.[14] We speculate that similar synthetic potential is not achievable for cyclohexadienone derivatives derived from o-tyrosine because of a propensity for dimerization.
Footnotes
T.R.R.P. is grateful for financial support from National Institute of General Medical Sciences (64831-06) for this work and related studies on scyphostatin. We thank Taridorn Buajarern for providing 1H and 13C NMR spectral data for 1, 2, and 3 in [D6]acetone for comparative purposes. We appreciate Dr. Arkady Krasovskiy for his insightful ideas regarding epoxide opening with lanthanide halides. This project has also benefited from earlier studies of R. W. Van De Water and C. Hoarau within our group.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200904716.
References
- 1.Marco-Contelles J, Molina MT, Anjum S. Chem. Rev. 2004;104:2857–2900. doi: 10.1021/cr980013j. For more recent examples, see: Haritakun R, Srikitikulchai P, Khoyaiklang P, Isaka M. J. Nat. Prod. 2007;70:1478–1480. doi: 10.1021/np070291q. Amagata T, Minoura K, Numata A. J. Nat. Prod. 2006;69:1384–1388. doi: 10.1021/np0600189. Amagata T, Tanaka M, Yamada T, Minoura K, Numata A. J. Nat. Prod. 2008;71:340–345. doi: 10.1021/np070529a. For total syntheses of a related epoxide known as scyphostatin, see: Inoue M, Yokota W, Murugesh MG, Izuhara T, Katoh T. Angew. Chem. 2004;116:4303–4305. doi: 10.1002/anie.200454192. Angew. Chem. Int. Ed. 2004;43:4207–4209. doi: 10.1002/anie.200454192. Fujioka H, Sawama Y, Kotoku N, Ohnaka T, Okitsu T, Murata N, Kubo O, Li R, Kita Y. Chem. Eur. J. 2007;13:10225–10238. doi: 10.1002/chem.200700871. Takagi R, Miyanaga W, Tojo K, Tsuyumine S, Ohkata K. J. Org. Chem. 2007;72:4117–4125. doi: 10.1021/jo070337k.
- 2.Bunyapaiboonsri T, Yoiprommarat S, Intereya K, Rachtawee P, Hywel-Jones NL, Isaka M. J. Nat. Prod. 2009;72:756–759. doi: 10.1021/np800702c. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa A, Nishikawa N, Takahashi S, Yamamoto K. WO 2004-074269A1. 2004 [Google Scholar]
- 4.Tanaka M, Nara F, Suzuki-Konagai K, Hosoya T, Ogita T. J. Am. Chem. Soc. 1997;119:7871–7872. [Google Scholar]
- 5.Murayama K, Tanabe T, Ishikawa Y, Nakamura K, Nishiyama S. Tetrahedron Lett. 2009;50:3191–3194. [Google Scholar]
- 6.a) Schmidt U, Beuttler T, Lieberknecht A, Griesser H. Tetrahedron Lett. 1983;24:3573–3576. [Google Scholar]; b) Schmidt U, Lieberknecht A, Schanbacher U, Beuttler T, Wild J. Angew. Chem. 1982;94:797–798. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1982;21:770–777. [Google Scholar]
- 7.Commercially available, but for large scale preparation, see: Zoller U, Ben-Ishai D. Tetrahedron. 1975;31:863–866. Schmidt U, Lieberknecht A, Wild J. Synthesis. 1984:53–60.
- 8.a) Wenderski TA, Huang S, Pettus TRR. J. Org. Chem. 2009;74:4104–4109. doi: 10.1021/jo900401k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mejorado L, Pettus TRR. J. Am. Chem. Soc. 2006;128:15625–15631. doi: 10.1021/ja062987w. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Hoarau C, Pettus TRR. Org. Lett. 2006;8:2843–2846. doi: 10.1021/ol061000s. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pettus LH, Van De Water RW, Pettus TRR. Org. Lett. 2001;3:905–908. doi: 10.1021/ol0155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.While superficially related to prior tyrosine dearomatizations that yield symmetric spirocyclic cyclohexadienone lactones demonstrated by P. Wipf, we are aware of only one other prior diastereoselective dearomatization yielding a dissymmetric spirocyclic cyclohexadienone lactone, see: Plourde G, Spaetzel R, Kwasnitza JS, Scully TW. Molecules. 2007;12:2215–2222. doi: 10.3390/12092215.
- 10.a) Wardrop DJ, Burge MS, Zhang W, Ortiz JA. Tetrahedron Lett. 2003;44:2587–2591. [Google Scholar]; b) Wardrop DJ, Burge MS. J. Org. Chem. 2005;70:10271–10284. doi: 10.1021/jo051252r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The initial dearomatization affords a separable 1.35:1.00 ratio of diastereomers which epimerizes to 12:1 upon addition of DBN. A full account of dearomatization and epimerization studies will be reported in due course.
- 12.For a nice review of symmetric dienone reactivity see: Wipf P, Jung J-K. Chem. Rev. 1999;99:1469–1480. doi: 10.1021/cr9803838. and references cited therein. For examples of hydroxy-directed syn-epoxidation under anhydrous conditions, see: Adam W, Kiliç H, Saha-Möller CR. Synlett. 2002:510–512. Zilbeyaz K, Sahin E, Kiliç H. Tetrahedron: Asymmetry. 2007;18:791–796.
- 13.Burns NL, Baran PS, Hoffmann RW. Angew. Chem. 2009;121:2896–2910. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 14.Magdziak D, Meek SJ, Pettus TRR. Chem. Rev. 2004;104:1383–1429. doi: 10.1021/cr0306900. [DOI] [PubMed] [Google Scholar]