Abstract
Several antidepressant drugs have previously been reported to increase neurogenesis in the dentate gyrus of the hippocampus in laboratory animals. We found no effect of the selective serotonin reuptake inhibitor fluoxetine or the corticotropin-releasing factor receptor 1 antagonist R121919 [3-[6-(dimethylamino)-4-methylpyridin-3-yl]-2,5-dimethyl-N,N-dipropyl-1H-pyrazolo[1,5-a]pyrimidin-8-ium-7-amine] on the rate of cell proliferation or hippocampal brain-derived neurotrophic factor (BDNF) mRNA expression in either adult or adolescent rats after long-term administration. In adults, the mood stabilizer lithium was found to significantly increase cell proliferation; the atypical antipsychotic paliperidone did not affect proliferation, either alone or when combined with lithium. Fourteen-day survival of neuronally fated cells showed a significant interaction effect of lithium and paliperidone but no effect of either drug alone. BDNF mRNA expression was significantly decreased by lithium in the CA1/2 cell fields and increased by paliperidone in the CA1/2, CA3, and dentate gyrus. These results raise questions concerning the hypothesis that all antidepressants increase neurogenesis under nonstressed conditions. They also confirm and extend previous reports of lithium-induced increases in cell proliferation but not survival.
Introduction
Monoaminergic-acting antidepressants, as a class, have been hypothesized to up-regulate several processes involved in hippocampal neurogenesis, including neural progenitor cell (NPC) proliferation, short- and long-term survival of neuroblasts and immature neurons, expression of neurotrophic factors, and growth and branching of neuronal processes. NPC proliferation rate and immature neuron survival rate have been up-regulated by 14 or more days of treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine, but not by shorter regimens (Malberg et al., 2000; Kodama et al., 2004; Huang and Herbert, 2006; Marcussen et al., 2008), although this effect has not been observed universally (Cowen et al., 2008; David et al., 2009). Experiments involving the SSRI citalopram/escitalopram have been less successful, with negative results outnumbering positive ones, with regard to both proliferation and survival (Jaako-Movits et al., 2006; Jayatissa et al., 2006). The tricyclic antidepressant imipramine, although less frequently used in studies of neurogenesis, has been shown to positively have an impact on both proliferation and survival (Keilhoff et al., 2006; Surget et al., 2008).
In addition, CRF1 receptor antagonists, which have recently been explored clinically as alternatives to conventional monoaminergic antidepressants (reviewed in Holsboer and Ising, 2008), have also been hypothesized to up-regulate hippocampal neurogenesis. One CRF1 receptor antagonist, SSR125543, has been reported by two groups to block stress-induced deficits in cell proliferation, although it did not affect cell proliferation under nonstressed conditions (Alonso et al., 2004; Surget et al., 2008). The CRF1 antagonist used in experiments here, R121919 [3-[6-(dimethylamino)-4-methylpyridin-3-yl]-2,5-dimethyl-N,N-dipropyl-1H-pyrazolo[1,5-a]pyrimidin-8-ium-7-amine; also known as NBI 30775; PubChem compound ID 44400634; http://www.ncbi.nlm.nih.gov/sites/entrez?db = pccompound&term = r121919], has been shown to attenuate both behavioral and endocrine responses to stress in rats (Gutman et al., 2003; Rivier et al., 2003). There are no reports of the effects of R121919 on neurogenesis.
A series of experiments involving the mood stabilizer lithium augmented with the atypical antipsychotic paliperidone (9-hydroxyrisperidone) are included in this report. The rationale for performing these experiments arises from the common clinical practice of using antipsychotic augmentation in the treatment of refractory bipolar and unipolar depression, a regimen that has been shown to be effective in controlled studies (Ghaemi and Goodwin, 1999; Rothschild, 2003). Moreover, both atypical antipsychotics and lithium are effective in augmenting the effects of antidepressants in nonresponders (Simon and Nemeroff, 2005; Rapaport et al., 2006; Keitner et al., 2009; Nelson and Papakostas, 2009), and similarly, atypical antipsychotic drugs augment the therapeutic effects of lithium (Bowden, 2005). The mechanism(s) underlying these augmenting effects of atypical antipsychotics remain uncertain, although electrophysiological studies have found several atypical antipsychotic drugs to increase firing rates of noradrenergic neurons in the locus coeruleus in opposition to the decrease in firing caused by SSRIs (Seager et al., 2005; Dremencov et al., 2007a,b). If neurogenesis plays a crucial role in the therapeutic effects of antidepressants and mood stabilizers, it is plausible that the effects of atypical antipsychotics in augmenting these drugs may also be mediated by a change in neurogenesis.
In laboratory animals, long-term lithium treatment has been shown to increase both cell proliferation and survival (Chen et al., 2000; Son et al., 2003; Silva et al., 2008). The atypical antipsychotic drug olanzapine has been reported to increase the rate of cell proliferation in the dentate gyrus with an effect size similar to fluoxetine, although combination olanzapine–fluoxetine treatment did not increase the rate of neurogenesis above that observed with fluoxetine alone (Kodama et al., 2004). Other atypical antipsychotic drugs reported to increase hippocampal neurogenesis include clozapine, quetiapine, and risperidone (Wakade et al., 2002; Halim et al., 2004; Luo et al., 2005). Furthermore, after chronic restraint stress, quetiapine and venlafaxine exhibit additive effects on neurogenesis (Xu et al., 2006). However, numerous studies have reported no effects of atypical antipsychotics on hippocampal neurogenesis (Wakade et al., 2002; Green et al., 2006).
These experiments were undertaken to test the hypothesis that all medications with utility in treating depression, with distinct mechanisms of action, share the common feature of augmenting neurogenesis and/or neuronal survival.
Materials and Methods
Animals.
Adult male Sprague-Dawley rats, approximately 8 weeks old in the adult experiments or 3 weeks old in the adolescent experiment, were obtained 1 week before initiation of the experimental protocols (Charles River Laboratories, Wilmington, MA). Animals were maintained in standard laboratory conditions: pair-housed in polycarbonate cages (30 × 20 × 20 cm) with corn-cob bedding on a 12/12-light cycle, with ad libitum access to food and water. All procedures used were approved by the Institutional Animal Care and Use Committee of Emory University and are in compliance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drug Administration.
Fluoxetine (11 mg/kg as fluoxetine HCl) or R121919 (30 mg/kg, donated by Neurocrine Biosciences, San Diego, CA) were administered as oral gavage in water once daily for 3 weeks, with precise dose matched to the body weight of each animal within the last 3 days. Control animals were administered the corresponding volume of water. These doses were chosen because they are both behaviorally active and mimic therapeutic serum concentrations (fluoxetine) or provide robust CRF1 receptor occupancy (R121919) (Gutman et al., 2003).
Paliperidone (0.75 mg/kg per day, supplied by Janssen Pharmaceutica, New Brunswick, NJ) or vehicle (0.3% tartaric acid) were delivered by subcutaneous osmotic minipumps (Alzet model 2ML4; Durect Corporation, Cupertino, CA). Although risperidone has a longer history of use as an atypical antipsychotic, paliperidone represents approximately 90% of the active drug after systemic risperidone administration. Furthermore, the solubility characteristics of paliperidone were superior to risperidone for use in long-term dosing studies (M. J. Owens, unpublished observations). After recovering from implantation surgery, animals were administered chow containing 1.2 g/kg lithium carbonate for 1 week, followed by chow containing 2.4 g/kg lithium carbonate or control chow (Bio-Serv, Frenchtown, NJ). This regimen continued for 3 weeks total for the cell proliferation experiment and 4 weeks total for the cell survival experiment. Animals receiving lithium chow were provided with 0.9% NaCl in water to minimize hyperuria. These doses of lithium were chosen to mimic therapeutic serum concentrations.
Serum drug analysis was performed by the laboratory of Dr. James C. Ritchie (Core and Toxicology Laboratories, Emory University Hospital) for fluoxetine and lithium and by the laboratory of Drs. Jennifer Donovan and Lindsay DeVane (Medical University of South Carolina, Charleston, SC) for paliperidone. Serum concentrations of R121919 were not measured because of its short half-life and unknown identity of possible active metabolites, but dosing was based upon our previous experience (Gutman et al., 2003).
Bromodeoxyuridine Administration and Sacrifice.
Bromodeoxyuridine [(BrdU) 200 mg/kg in 0.9% saline; Sigma-Aldrich, St. Louis, MO] was administered via a single intraperitoneal injection according to the timelines given for each experiment. For assessment of cell proliferation rate, BrdU injection was given on day 21 or 22 of drug administration, and animals were killed 24 (in the fluoxetine and R121919 experiments) or 2 h (in the lithium and paliperidone experiment) later. For assessment of newborn cell survival in the lithium and paliperidone experiment, BrdU injection was given on day 14 or 15 of drug administration, and animals were killed 14 days later. At the time of death, animals were deeply anesthetized with pentobarbital and transcardially perfused with ice-cold 0.1 M phosphate-buffered saline followed by 4% paraformaldehyde. At the time of cardiac puncture, blood was collected for serum drug analysis. The brains were removed, postfixed overnight, and equilibrated in a 30% sucrose solution. Sections of 25 μm through the entire hippocampus (bregma −2.4 to −6.2 mm) were cut on a cryostat and mounted on slides for immunocytochemistry and in situ hybridization procedures.
Immunocytochemistry.
Immunocytochemical staining was performed on every 12th section throughout the hippocampus. For BrdU staining, antigen retrieval was performed by incubation in 50% formamide/SSC at 65°C, followed by 2 M HCl at 37°C, and then neutralization in 0.1 M boric acid, pH 8.5. For Ki-67 staining, antigen retrieval was performed by incubation in 10 mM citrate buffer at 90°C. After quenching in 2% hydrogen peroxide, sections were blocked in 3% normal horse serum (Vector Laboratories, Burlingame, CA) and incubated overnight in 1:100 mouse monoclonal anti-BrdU antibody (BD Biosciences, Franklin Lakes, NJ) or 1:100 mouse monoclonal anti-Ki-67 antibody (Vector Laboratories). The next day, they were treated with 1:200 horse anti-mouse rat-adsorbed secondary antibody and then treated with a Vectastain ABC kit and visualized with diaminobenzidine substrate (all obtained from Vector Laboratories).
Stained sections were examined at 40× magnification under a light microscope. Labeled cells were counted if they were within the subgranular zone of the dentate gyrus or within one cell width from its edge. A total number of positively labeled cells was acquired for each animal and multiplied by 12 to approximate the number of labeled cells throughout the entire dentate gyrus. In addition, cell counts were divided into dorsal and ventral parts of the dentate gyrus. This procedure follows established guidelines for unbiased rare event stereology (Mouton, 2002). All cell counting was performed by the same individual under treatment-blind conditions.
A Student's t test (for each group of the fluoxetine/R121919 experiments) or two-way ANOVA (for the lithium/paliperidone experiment) was performed on each of the resulting measures, with total dentate gyrus and dorsal and ventral regions considered separately. The criterion for significance was p < 0.05. Furthermore, when BrdU and Ki-67 cell counts were available for the same animal cohorts, these individual points, ungrouped by treatment, were subjected to correlations analysis (Pearson's r). Because both numbers represent the same population of proliferating cells, these numbers should correlate strongly.
In Situ Hybridization.
BDNF mRNA was quantified using in situ hybridization with an 35S-labeled riboprobe. As in the immunocytological protocol above, every 12th section was treated and analyzed. BDNF riboprobe was constructed from cDNA inserts, including the full coding regions plus some flanking sequence, ligated into a pR1112-8 plasmid. Radiolabeled antisense cRNA was synthesized by incorporating [35S]CTP into the probe. The transcription reaction was performed using an Ambion MAXIscript kit with T3 RNA polymerase (Applied Biosystems, Foster City, CA), according to the instructions provided. After transcription and removal of the cDNA template with DNase, the cRNA probe was recovered through gel filtration using a G-50 Sephadex Quick Spin column (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
The slides underwent 10 min of acetylation (0.5% acetic anhydride in 0.1 M triethanolamine, pH 8.0), two rinses in 2× SSC, and dehydration through a graded ethanol series. The sections were then air-dried for at least 1 h before hybridization. The brain sections were hybridized overnight at 60°C with 1 to 2 × 106 cpm of 35S-labeled cRNA probe diluted into hybridization buffer [50% formamide, 10% dextran sulfate, 0.3 M NaCl, 1× Denhardt's solution, 10 mM Tris, 1 mM EDTA, 2 mg/ml yeast tRNA, 10 mM dithiothreitol (DTT)] in humidified Nunc trays.
The next day, slides were allowed to cool to room temperature before being washed four times in SSC. The sections were then treated with 250 μg/ml RNase A for 30 min at 37°C. Afterward, the slides underwent a series of SSC washes (supplemented with 1 mM DTT), with salt concentrations decreasing from 2 to 0.5 times followed by a 60-min high stringency wash, with 0.1× SSC + 1 mM DTT at 60°C, and then dehydration through a graded ethanol series. The slides were air-dried and then apposed to Kodak Biomax MR film (Carestream Health, Rochester, NY) until signal reached the desired intensity.
Images on film were digitized with a CCD-72 camera and image analysis system (Dage-MTI, Michigan City, IN). Semiquantitative analysis was performed using AIS software (Imaging Research, Inc., St. Catherines, ON, Canada). Messenger RNA expression levels were calculated by subtracting the neutral background density from the specific signal. Sections were matched for rostrocaudal level and analyzed for values representing the regions CA1/2, CA3, and dentate gyrus, which were then averaged between sections to produce a single value for each region for each animal.
A Student's t test (for each group of the fluoxetine/R121919 experiment) or two-way ANOVA (for the lithium/paliperidone experiment) was performed on each of the resulting measures, with total dentate gyrus and dorsal and ventral regions considered separately. The criterion for significance was p < 0.05.
Results
Fluoxetine and R121919.
In a pilot study in adult animals, chronic daily dosing of the chosen fluoxetine dosage produced reliable serum concentrations in a narrow range of 600 to 700 ng/ml (combined fluoxetine plus its active metabolite norfluoxetine) at 18 h past dose time. This represents approximately the 80th to 85th percentile for patients treated with fluoxetine at our site (Z. N. Stowe and J. C. Ritchie, unpublished observations). The timing of daily dosing for the experiment resulted in animals that were past drug nadir at the time of sacrifice, thus serum concentrations were not measured. In rats, the half-life of fluoxetine is approximately 2 h, and the half-life of norfluoxetine, which possesses identical pharmacology (Owens et al., 1997), is approximately 30 h (M. J. Owens, unpublished data). The dose-serum concentration relationship for fluoxetine + norfluoxetine has been shown experimentally to be equivalent in adult and adolescent rats (G. Neigh, personal communication).
In adult animals, no effect of either fluoxetine or R121919 on cell proliferation (as measured by 24-h BrdU incorporation) was seen in either the total, dorsal, or ventral dentate gyrus (Table 1). No effect of either drug on BDNF mRNA expression was observed in any hippocampal area (Table 2).
TABLE 1.
Chronic treatment with fluoxetine or R121919 did not affect cell proliferation rate (measured using 24-h BrdU incorporation or endogenous Ki-67 expression) in the dentate gyrus of adult or adolescent rats
Data are presented as number of labeled cells within the entire dentate gyrus and its dorsal and ventral parts ± S.E.M. n = 8–12 for all groups.
| Total |
Dorsal |
Ventral |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Fluoxetine | R121919 | Vehicle | Fluoxetine | R121919 | Vehicle | Fluoxetine | R121919 | |
| Adult (BrdU) | 1967 ± 174 | 1983 ± 173 | 1812 ± 118 | 1295 ± 146 | 1185 ± 131 | 1140 ± 114 | 668 ± 77 | 778 ± 73 | 664 ± 66 |
| Adolescent (BrdU) experiment 1 | 4691 ± 319 | 4659 ± 479 | 5320 ± 655 | 2853 ± 261 | 2538 ± 248 | 3231 ± 447 | 1743 ± 125 | 1879 ± 240 | 1976 ± 221 |
| Adolescent (BrdU) experiment 2 | 6230 ± 332 | 5828 ± 366 | 5072 ± 359 | 3224 ± 107 | 3182 ± 301 | 2918 ± 254 | 2625 ± 184 | 2352 ± 123 | 1994 ± 151 |
| Adolescent (Ki-67) experiment 1 | 14710 ± 807 | 15931 ± 736 | 16817 ± 903 | 8883 ± 492 | 9323 ± 575 | 10679 ± 681 | 5596 ± 416 | 6239 ± 500 | 5964 ± 349 |
| Adolescent (Ki-67) experiment 2 | 16726 ± 732 | 17353 ± 1070 | 16485 ± 1222 | 9849 ± 722 | 9824 ± 650 | 9126 ± 882 | 6352 ± 340 | 6724 ± 550 | 6759 ± 442 |
TABLE 2.
Chronic treatment with fluoxetine or R121919 did not affect expression of BDNF mRNA measured by in situ hybridization in adult or adolescent rats
Data are presented as relative optical density normalized to vehicle levels for each area ± S.E.M. n = 8–12 for all groups.
| CA1/2 |
CA3 |
Dentate Gyrus |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Fluoxetine | R121919 | Vehicle | Fluoxetine | R121919 | Vehicle | Fluoxetine | R121919 | |
| Adult | 1 ± 0.043 | 0.962 ± 0.034 | 0.888 ± 0.027 | 1 ± 0.049 | 0.897 ± 0.041 | 0.863 ± 0.036 | 1 ± 0.063 | 0.940 ± 0.078 | 0.840 ± 0.050 |
| Adolescent | 1 ± 0.027 | 1.103 ± 0.040 | 1.004 ± 0.037 | 1 ± 0.021 | 1.1101 ± 0.041 | 0.997 ± 0.035 | 1 ± 0.030 | 1.073 ± 0.036 | 0.975 ± 0.050 |
In adolescent animals, no effect of either fluoxetine or R121919 on cell proliferation (as measured by 24-h BrdU incorporation or Ki-67 expression) was noted in either the total, dorsal, or ventral dentate gyrus (Table 1). Correlation analysis of Ki-67 versus BrdU cell counts revealed a strong linear correlation (r = 0.5461; p < 0.0001), with a slope of 0.2669 ± 0.05423. No effect of either drug on BDNF mRNA expression was seen in any hippocampal area (Table 2).
Lithium and Paliperidone.
Animals with serum lithium concentrations under 0.40 mM were excluded (2 of 48 total animals); serum concentrations of lithium in remaining animals averaged 0.62 mM in the cell proliferation experiment and 0.67 mM in the cell survival experiment. These concentrations are within the clinical therapeutic range. Animals with serum paliperidone concentrations under 20 ng/ml were excluded (4 of 48 total animals); serum concentrations of paliperidone in remaining animals averaged 66 ng/ml in the proliferation experiment. In the cell survival experiment, serum concentrations of paliperidone varied greatly between those animals receiving paliperidone only and those also receiving lithium: paliperidone only animals averaged 74 ng/ml, whereas Li + Pal animals averaged only 31.4 ng/ml. Because the lithium groups in the survival experiment exhibited greater urination than did the lithium groups in the proliferation experiment (we hypothesize that this was the result of the longer duration of lithium treatment), we suspect that this affected the rate of paliperidone excretion in these animals (Mannens et al., 1993). Mean peak to trough ratio of paliperidone (Invega) in humans is 1.7 according to manufacturer's instructions (Janssen Pharmaceutica), with the trough 75th percentile of serum concentrations being 52 ng/ml (middle 50th percentile 20–52 ng/ml) (Nazirizadeh et al., 2010).
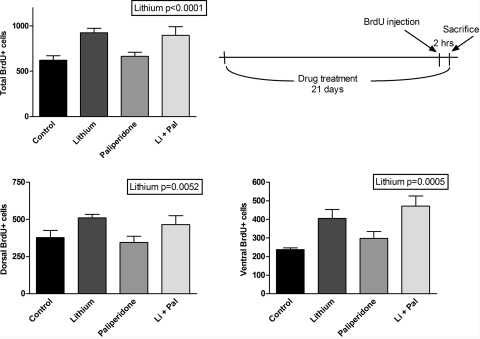
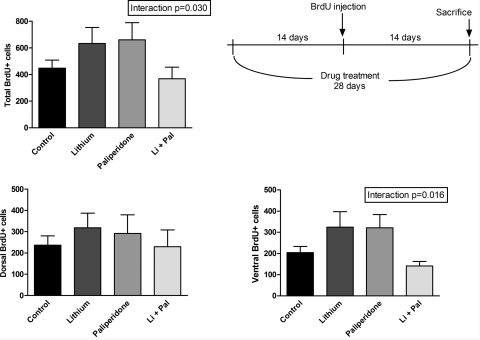
A significant effect of lithium was observed on cell proliferation (as measured by 24-h BrdU incorporation) in all areas (p < 0.0001 for total; p = 0.0052 for dorsal; p = 0.0005 for ventral); no effect of paliperidone was observed (Fig. 1). Fourteen-day cell survival (as measured by 14-day BrdU incorporation) showed a significant interaction effect of lithium plus paliperidone in the total and ventral measurements, such that the combination decreases proliferation relative to either drug alone (p = 0.0295 and p = 0.0156, respectively). There was a trend for increased cell number in the lithium and paliperidone alone groups, which decreased to a level similar to control in the lithium/paliperidone combination group (Fig. 2).
Fig. 1.
Long-term treatment with lithium but not paliperidone significantly increased cell proliferation rate (measured using 2-h BrdU incorporation) in the dentate gyrus of adult rats. Two-way ANOVA revealed significant effect of lithium in all areas (p < 0.0001 for total; p = 0.0052 for dorsal; p = 0.0005 for ventral). n = 12 for lithium group; n = 8 for each other group. Data are presented as mean ± S.E.M. Li + Pal, lithium and paliperidone.
Fig. 2.
Long-term treatment with lithium and/or paliperidone produced an interaction effect but no significant effect of either drug on BrdU cell counts, indicating 14-day cell survival rate in the dentate gyrus. Two-way ANOVA revealed significant interaction effect for total and ventral areas (p = 0.030 and p = 0.016, respectively). n = 12 for control and lithium groups; n = 8 for paliperidone and paliperidone + lithium groups. Data are presented as mean ± S.E.M. Li + Pal, lithium and paliperidone.
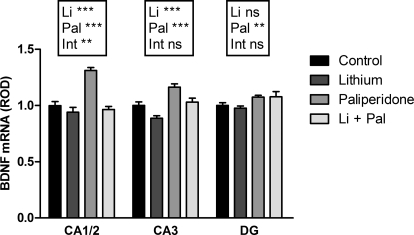
BDNF mRNA expression was negatively affected by lithium in the CA1/2 and CA3 (p < 0.0001 and p = 0.0003, respectively), positively affected by paliperidone in the CA1/2, CA3, and dentate gyrus (p = 0.0002, p < 0.0001, p = 0.006), and showed a drug interaction effect in the CA1/2 (p = 0.001) (Fig. 3). The BDNF data were taken from the cell proliferation experiment in which serum paliperidone concentrations were similar between the paliperidone only and the lithium + paliperidone groups.
Fig. 3.
Long-term treatment with lithium and/or paliperidone produced opposing effects on expression of BDNF mRNA. Two-way ANOVA revealed significant effects of lithium, paliperidone, and interaction in CA1/2 (**, p ≤ 0.001, ***, p < 0.001); significant effects of lithium and paliperidone in CA3 (p = 0.0003, p < 0.0001); and significant effect of paliperidone in the dentate gyrus. n = 12 for lithium group; n = 8 for each other group. ROD, relative optical density; ns, not significant. Data are presented as mean ± S.E.M. Li + Pal, lithium and paliperidone.
Discussion
As noted earlier, these experiments were undertaken to test the hypothesis that all medications with efficacy in treating depression, even when possessing different initial mechanisms of action, share the common feature of augmenting neurogenesis and/or neuronal survival. The failure of chronic fluoxetine treatment to increase cell proliferation rate was quite surprising. Because the extant literature reported this consequence of long-term fluoxetine treatment to be a reliable function, this SSRI treatment group was originally included as a positive control in our experiments. However, after lack of success in producing the expected result, communications between our group and researchers from other laboratories revealed that many had experienced similar difficulties in reproducing this effect. Most of these negative results have not been published, with notable exceptions involving fluoxetine in two studies and citalopram in a third (Jaako-Movits et al., 2006; Cowen et al., 2008; David et al., 2009; it is important to note that each of these studies also used behaviorally active antidepressant doses).
The fact that fluoxetine did not produce changes in cell proliferation, despite the use of a dose that was reported to be behaviorally active effects and at clinically therapeutically relevant concentrations, suggests that it is not a simple problem with drug administration or a fluke experiment that is responsible for this deviation from the prevailing dogma of antidepressant effects on neurogenesis. Rather, it is possible that some finer point related to the stress levels or previous experiences of the animals involved determines their responsiveness to antidepressant effects. These factors might include housing conditions, ambient noise level, personnel activity, conditions related to shipping or breeding colony, or any number of small details that are impossible to entirely control even at the hands of researchers skilled in minimizing laboratory stress conditions. Early life stress, which might have occurred under the domain of the breeding facility, has been shown to greatly reduce responsivity of adult neurogenesis to both stress and antidepressant treatment (Mirescu et al., 2004; Navailles et al., 2008). Some studies testing the same drugs under both stressed and nonstressed conditions report that an antidepressant regimen, which does not alter neurogenesis under nonstressed conditions, will fully or partially block the decrease caused by stress (Jaako-Movits et al., 2006). This raises the possibility that successful experiments may require a certain level of stress and that expertise in minimizing laboratory stress conditions might actually be a hindrance in replicating these experiments. Whatever the key factor(s) might be between laboratories that are responsible for these discordant results, alteration of neurogenesis is clearly not a reliable effect of behaviorally active doses of antidepressants.
The failure of the CRF1 antagonist R121919 to produce an effect, while confounded by the same issues discussed for the antidepressants above, is not surprising. Of the two reports of the effects of CRF1 antagonists on cell proliferation under nonstressed conditions, both were in agreement with these results in finding no change (Alonso et al., 2004; Surget et al., 2008). Moreover, the behavioral antidepressant effects of a CRF1 antagonist have been observed, even when hippocampal neurogenesis was ablated by x-irradiation or methylazoxymethanol; this suggests that neurogenic actions of these drugs are not necessary for their antidepressant effects (Surget et al., 2008; Bessa et al., 2009). Other recent evidence also supports the hypothesis that neurogenesis may not in itself be necessary for antidepressant action and may simply occur in association with other features of neuroplasticity that are closely tied to antidepressant effects (Bessa et al., 2009).
In the lithium and paliperidone experiments, the primary finding was a robust effect of chronic lithium treatment on NPC proliferation rate; in contrast, paliperidone had no appreciable effect either alone or as an augmentation to lithium treatment. It is noteworthy that, when the newborn cells were followed to 2 weeks postgeneration with continuing drug exposure, the lithium effect was lost. This suggests that, although lithium increases proliferation rate, there is a corresponding increase in rate of cell death of this newborn cell population, which results in no net gain in new cell number at 2 weeks of age. One report that long-term lithium treatment increases apoptosis as well as cell proliferation in the dentate gyrus supports this interpretation (Silva et al., 2008). For adult-generated granule cells, the first few weeks of life have been established as crucial in establishing connections to reach functional maturity and enable survival (Dayer et al., 2003). Therefore, it may be beneficial to have a larger pool of immature neurons from which the most successfully integrated may be selected, although the resulting number of mature neurons is unchanged.
It should be noted that animals on a lithium diet stabilized at a body weight averaging 10 to 15% less than animals on a standard chow diet, although they maintained similar standards of general health. This was presumably due to the tendency of animals being presented with ad libitum chow to eat slightly less (approximately 6%) of the lithium-containing chow, which was nutritionally equivalent to the control chow (data from pilot animals, not shown). This is common and expected for the dosages used (H. M. Johnson & Johnson, personal communication). Thus, there is the small possibility that the effect of lithium on proliferation rate observed here is not truly a drug effect but is related to the decreased food intake and/or corresponding decreased growth of body mass. There is some evidence for increased neurogenesis under calorie-restricted conditions, but only when total caloric intake is reduced, much more than the 6% seen here (Kwon et al., 2008).
The failure of paliperidone to increase either proliferation or survival of new hippocampal neurons was somewhat surprising, considering reported efficacy of atypical antipsychotic treatment in bipolar disorder and as augmenting agents in refractory depression. This suggests that treatment of bipolar disorder and/or refractory depression with atypical antipsychotic drugs probably involves crucial pathways distinct from neurogenesis. The paliperidone-induced increase of BDNF mRNA in the CA1/2 and CA3 of the hippocampus seen here may be one contributing factor. BDNF mRNA expression levels often, but do not always, correlate with the rate of neurogenesis in the hippocampus, and BDNF/TrkB signaling is theorized to play a positive role in general neuroplasticity (Schmidt and Duman, 2007). Here, the disconnect between cell proliferation rate and BDNF expression is glaring, because BDNF expression is increased only in the case of paliperidone treatment alone and normalizes with the addition of lithium.
Acknowledgments
We thank the laboratories of Drs. James C. Ritchie (Emory University, Atlanta, GA) and Jennifer Donovan and Lindsay DeVane (Medical University of South Carolina, Charleston, SC) for the performance of serum drug analyses. We also thank Dr. Ronald Duman (Yale University, New Haven, CT) for thoughtful contributions to the design and interpretation of these studies.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant 5R01-MH042088]; National Institutes of Health National Institute on Drug Abuse [Grant 5T32-DA015040-05]; and Janssen Pharmaceutica.
Parts of the work presented in this study have previously been presented: Hanson ND (2010) Effects of stress and psychoactive drugs on adult hippocampal neurogenesis in the rat, doctoral dissertation, Emory University, Atlanta, GA; Hanson ND, Nemeroff CB, and Owens MJ (2008) Effects of a CRF1 antagonist on cell proliferation in the dentate gyrus (Abstract). Soc Neurosci Abstr 34:559.6; and Hanson ND, Brotherton TE, Nemeroff CB, and Owens MJ (2007) Paliperidone does not augment effects of lithium on cell proliferation in the dentate gyrus (Abstract). Soc Neurosci Abstr 33:267.5.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175372.
ABBREVIATIONS:
- NPC
- neural progenitor cell
- BDNF
- brain-derived neurotrophic factor
- BrdU
- bromodeoxyuridine
- R121919
- 3-[6-(dimethylamino)-4-methylpyridin-3-yl]-2,5-dimethyl-N,N-dipropyl-1H-pyrazolo[1,5-a]pyrimidin-8-ium-7-amine
- CRF
- corticotropin-releasing factor
- SSRI
- selective serotonin reuptake inhibitor
- ANOVA
- analysis of variance
- SSC
- saline-sodium citrate buffer
- DTT
- dithiothreitol.
Authorship Contributions
Participated in research design: Hanson, Nemeroff, and Owens.
Conducted experiments: Hanson.
Performed data analysis: Hanson.
Wrote or contributed to the writing of the manuscript: Hanson, Nemeroff, and Owens.
References
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. (2004) Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 9:278–286, 224 [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773, 739 [DOI] [PubMed] [Google Scholar]
- Bowden CL. (2005) Atypical antipsychotic augmentation of mood stabilizer therapy in bipolar disorder. J Clin Psychiatry 66 (Suppl 3):12–19 Review [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. (2000) Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75:1729–1734 [DOI] [PubMed] [Google Scholar]
- Cowen DS, Takase LF, Fornal CA, Jacobs BL. (2008) Age-dependent decline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res 1228:14–19 [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, et al. (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. (2003) Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol 460:563–572 [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. (2007a) Distinct electrophysiological effects of paliperidone and risperidone on the firing activity of rat serotonin and norepinephrine neurons. Psychopharmacology (Berl) 194:63–72 [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. (2007b) Noradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brain. Biol Psychiatry 61:671–678 [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Goodwin FK. (1999) Use of atypical antipsychotic agents in bipolar and schizoaffective disorders: review of the empirical literature. J Clin Psychopharmacol 19:354–361 [DOI] [PubMed] [Google Scholar]
- Green W, Patil P, Marsden CA, Bennett GW, Wigmore PM. (2006) Treatment with olanzapine increases cell proliferation in the subventricular zone and prefrontal cortex. Brain Res 1070:242–245 [DOI] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. (2003) The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther 304:874–880 [DOI] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. (2004) Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology 29:1063–1069 [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. (2008) Central CRH system in depression and anxiety–evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol 583:350–357 [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. (2006) Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry 59:619–624 [DOI] [PubMed] [Google Scholar]
- Jaako-Movits K, Zharkovsky T, Pedersen M, Zharkovsky A. (2006) Decreased hippocampal neurogenesis following olfactory bulbectomy is reversed by repeated citalopram administration. Cell Mol Neurobiol 26:1559–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. (2006) Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology 31:2395–2404 [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Bernstein HG, Wolf G. (2006) Cell proliferation is influenced by bulbectomy and normalized by imipramine treatment in a region-specific manner. Neuropsychopharmacology 31:1165–1176 [DOI] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, Ninan PT, Solomon DA, Nemeroff CB, Keller MB. (2009) A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res 43:205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS. (2004) Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 56:570–580 [DOI] [PubMed] [Google Scholar]
- Kwon YS, Jeong SW, Kim DW, Choi ES, Son BK. (2008) Effects of the ketogenic diet on neurogenesis after kainic acid-induced seizures in mice. Epilepsy Res 78:186–194 [DOI] [PubMed] [Google Scholar]
- Luo C, Xu H, Li XM. (2005) Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res 1063:32–39 [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannens G, Huang ML, Meuldermans W, Hendrickx J, Woestenborghs R, Heykants J. (1993) Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos 21:1134–1141 [PubMed] [Google Scholar]
- Marcussen AB, Flagstad P, Kristjansen PE, Johansen FF, Englund U. (2008) Increase in neurogenesis and behavioural benefit after chronic fluoxetine treatment in Wistar rats. Acta Neurol Scand 117:94–100 [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. (2004) Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 7:841–846 [DOI] [PubMed] [Google Scholar]
- Mouton P. (2002) Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Johns Hopkins, Baltimore, MD [Google Scholar]
- Navailles S, Hof PR, Schmauss C. (2008) Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol 509:372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazirizadeh Y, Vogel F, Bader W, Haen E, Pfuhlmann B, Gründer G, Paulzen M, Schwarz M, Zernig G, Hiemke C. (2010) Serum concentrations of paliperidone versus risperidone and clinical effects. Eur J Clin Pharmacol 66:797–803 [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166:980–991 [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. (1997) Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 283:1305–1322 [PubMed] [Google Scholar]
- Rapaport MH, Gharabawi GM, Canuso CM, Mahmoud RA, Keller MB, Bossie CA, Turkoz I, Lasser RA, Loescher A, Bouhours P, et al. (2006) Effects of risperidone augmentation in patients with treatment-resistant depression: Results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 31:2505–2513 [DOI] [PubMed] [Google Scholar]
- Rivier CL, Grigoriadis DE, Rivier JE. (2003) Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology 144:2396–2403 [DOI] [PubMed] [Google Scholar]
- Rothschild AJ. (2003) Challenges in the treatment of depression with psychotic features. Biol Psychiatry 53:680–690 [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. (2007) The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 18:391–418 [DOI] [PubMed] [Google Scholar]
- Seager MA, Barth VN, Phebus LA, Rasmussen K. (2005) Chronic coadministration of olanzapine and fluoxetine activates locus coeruleus neurons in rats: implications for bipolar disorder. Psychopharmacology (Berl) 181:126–133 [DOI] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leão P, Almeida OF, Sousa N. (2008) Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience 152:656–669 [DOI] [PubMed] [Google Scholar]
- Simon JS, Nemeroff CB. (2005) Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 66:1216–1220 [DOI] [PubMed] [Google Scholar]
- Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK, Lee SH. (2003) Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem 85:872–881 [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. (2008) Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64:293–301 [DOI] [PubMed] [Google Scholar]
- Wakade CG, Mahadik SP, Waller JL, Chiu FC. (2002) Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res 69:72–79 [DOI] [PubMed] [Google Scholar]
- Xu H, Chen Z, He J, Haimanot S, Li X, Dyck L, Li XM. (2006) Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 16:551–559 [DOI] [PubMed] [Google Scholar]