Abstract
In overdose the analgesic/antipyretic acetaminophen (APAP) is hepatotoxic. Toxicity is mediated by initial hepatic metabolism to N-acetyl-p-benzoquinone imine (NAPQI). After low doses NAPQI is efficiently detoxified by GSH. However, in overdose GSH is depleted, NAPQI covalently binds to proteins as APAP adducts, and oxygen/nitrogen stress occurs. Toxicity is believed to occur by mitochondrial dysfunction. Manganese superoxide dismutase (MnSOD) inactivation by protein nitration has been reported to occur during other oxidant stress-mediated diseases. MnSOD is a critical mitochondrial antioxidant enzyme that prevents peroxynitrite formation within the mitochondria. To examine the role of MnSOD in APAP toxicity, mice were treated with 300 mg/kg APAP. GSH was significantly reduced by 65% at 0.5 h and remained reduced from 1 to 4 h. Serum alanine aminotransferase did not significantly increase until 4 h and was 2290 IU/liter at 6 h. MnSOD activity was significantly reduced by 50% at 1 and 2 h. At 1 h, GSH was significantly depleted by 62 and 80% at nontoxic doses of 50 and 100 mg/kg, respectively. No further GSH depletion occurred with hepatotoxic doses of 200 and 300 mg/kg APAP. A dose response decrease in MnSOD activity was observed for APAP at 100, 200, and 300 mg/kg. Immunoprecipitation of MnSOD from livers of APAP-treated mice followed by Western blot analysis revealed nitrated MnSOD. APAP-MnSOD adducts were not detected. Treatment of recombinant MnSOD with NAPQI did not produce APAP protein adducts. The data indicate that MnSOD inactivation by nitration is an early event in APAP-induced hepatic toxicity.
Introduction
Acetaminophen (APAP; N-acetyl-p-aminophenol), a commonly used analgesic/antipyretic drug, is the most prevalent cause of drug-induced acute liver failure in the United States (Lee et al., 2008). In overdose it produces a centrilobular hepatic necrosis. Toxicity is believed to occur by initial hepatic metabolism by cytochrome P450 enzymes to the highly reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI). After a therapeutic dose NAPQI is efficiently detoxified by GSH. When taken in overdose NAPQI leads to depletion of hepatic GSH levels (Mitchell et al., 1973) and covalent binding to hepatic cellular proteins to form 3-(cystein-S-yl)-acetaminophen (APAP-Cys) adducts (Cohen et al., 1997). Covalent binding has been shown to correlate with development of necrosis (Jollow et al., 1973; Roberts et al., 1991). Subsequent events important in the toxicity are poorly understood (Jaeschke and Bajt, 2006; Hinson et al., 2010).
We have been investigating the hypothesis that GSH depletion and covalent binding are initial events in APAP toxicity and these events lead to a subsequent oxygen/nitrogen stress. Previously, we reported the presence of 3-nitrotyrosine in livers of APAP-treated mice and their occurrence coincided in the centrilobular hepatocytes with APAP-protein adducts and developing necrosis (Hinson et al., 1998). It was postulated that the reactive nitrogen species peroxynitrite, formed by a rapid reaction of superoxide with nitric oxide, was important in toxicity. Peroxynitrite is both a nitrating agent and an oxidizing agent and is detoxified by GSH (Sies et al., 1997), which is depleted in APAP toxicity (Mitchell et al., 1973).
In work using freshly isolated mouse hepatocytes we presented evidence that reactive nitrogen species was formed during mitochondrial permeability transition (MPT) and this was critical to toxicity (Reid et al., 2005; Burke et al., 2010). This mechanism has also been shown to occur in cultured mouse hepatocytes (Kon et al., 2004). MPT is an abrupt increase in the permeability of the inner mitochondrial membrane to ions and small-molecular-weight solutes. It is promoted by oxidative stress and calcium and leads to a large increase in oxidative stress. MPT occurs with the loss of the ability of the mitochondria to produce ATP (Byrne et al., 1999; Zorov et al., 2000), a lethal event for the cell. In our previous studies using freshly isolated hepatocytes, we showed that APAP toxicity occurs in two phases (Reid et al., 2005) as reported previously (Boobis et al., 1986; Grewal and Racz, 1993). The initial phase (0–2 h) occurs with GSH depletion and covalent binding followed by a toxic phase (3–5 h). We found that the toxic phase occurred with MPT and nitration of protein. Toxicity and nitration could be blocked by a MPT inhibitor (cyclosporine A), an antioxidant (N-acetylcysteine), a mitochondrial nitric-oxide synthase inhibitor (7-nitroindazole), and high concentrations of a peroxynitrite scavenger (acetaminophen). We postulated that MPT occurred with formation of reactive nitrogen species, leading to toxicity (Burke et al., 2010).
In models of nephrotoxicity associated with renal transplantation it has been shown that the activity of the mitochondrial enzyme manganese superoxide dismutase (MnSOD) is dramatically decreased by a mechanism involving tyrosine nitration and oxidation of the enzyme (MacMillan-Crow et al., 1996; Macmillan-Crow and Cruthirds, 2001). MnSOD is a critical enzyme in mitochondria that is important in detoxification of superoxide. MnSOD found in the mitochondrial matrix detoxifies superoxide radical (O2−), forming hydrogen peroxide (H2O2) and molecular oxygen (O2). MnSOD thus limits the reaction of superoxide with nitric oxide to form the reactive nitrogen species peroxynitrite. Because the hepatotoxicity of APAP occurs with increased nitration of proteins and mitochondrial dysfunction, and MnSOD heterozygote mice have been reported to be more sensitive to APAP toxicity compared with wild-type mice (Fujimoto et al., 2009), we examined hepatic MnSOD activity and possible nitration in APAP-induced hepatotoxicity.
Materials and Methods
Reagents.
Acetaminophen (4-acetamidophenol, paracetamol), protein G Sepharose, and xanthine oxidase were obtained from Sigma-Aldrich. (St. Louis, MO). Coomassie Plus Protein Assay Reagent and SuperSignal chemiluminescent substrate reagent were obtained from Pierce Chemical (Rockford, IL). Triethylammonium bicarbonate buffer and tris(2-carboxyethyl) phosphine hydrochloride were purchased from Pierce Biochemical (Milwaukee, WI). Peroxidase-labeled goat anti-rabbit IgG and goat anti-mouse IgG antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and Thermo Fisher Scientific (Rockford, IL), respectively. Protease inhibitor cocktail was obtained from Roche Diagnostics (Mannheim, Germany). Hybond-ECL nitrocellulose membranes for Western blotting were obtained from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Anti-3-nitrotyrosine and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Millipore Bioscience Research Reagents (Temecula, CA). MnSOD antibodies were obtained from Assay Designs (Ann Arbor, MI). Zeba TM Desalt spin columns were purchased from Thermo Fisher Scientific. All chemicals were the highest grade commercially available. NAPQI was synthesized and purified using a method described by Dahlin et al. (1984). The development and utilization of the anti-APAP antibody has been described previously (Roberts et al., 1991; Michael et al., 2001).
Animals.
Six-week-old male B6C3F1 mice (mean weight, 24.4 g) were obtained from Harlan (Indianapolis, IN). Mice were acclimatized for 1 week before the planned experiments. Mice were fed ad libitum and housed in individual cages on a 12-h light/dark cycle. All animal experimentation and protocols were approved by the institutional animal care and use committee. Animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. On the day before experiments, mice were fasted overnight. In the APAP studies, control mice received only saline, and treated mice received acetaminophen in 0.4 ml of saline (intraperitoneally). In a dose-response experiment, mice were administered APAP (50, 100, 200, and 300 mg/kg) and sacrificed at 1 h after APAP treatment (n = 3 per experimental group). In a time-course experiment, mice were administered APAP (300 mg/kg) and sacrificed at 0.5, 1, 2, 4, and 6 h after APAP treatment (n = 3 per time point) with 6 h as the time of maximal toxicity. At the designated time, mice were anesthetized in a carbon dioxide chamber; blood was withdrawn by cardiac puncture, and the animals were subsequently euthanized under carbon dioxide followed by cervical dislocation. Livers were removed and weighed, and 200 mg of liver was preserved in GSH homogenization buffer, while the remaining tissue was snap-frozen in liquid nitrogen and stored at −80°C for later analysis.
Liver Toxicity Analysis.
Toxicity was determined by the quantitation of alanine aminotransferase (ALT) in the blood, which occurs as a result of liver damage. Blood was allowed to coagulate at room temperature for at least 1 h and centrifuged, and the serum was removed. ALT measurements were determined using a Liquid ALT (SGPT) reagent set kit (Pointe Scientific Inc., Lincoln Park, MI) according to the manufacturer's protocol. Spectral changes were followed using Roche Cobas Mira Classic Chemistry Analyzer (Roche Molecular Systems, Inc., Branchburg, NJ).
Hepatic Assays.
MnSOD activity in liver homogenates was measured by a cytochrome c reduction method using 1 mM potassium cyanide to inactivate copper-zinc SOD (SOD1) and extracellular SOD (SOD3) as described previously (McCord and Fridovich, 1969; MacMillan-Crow et al., 1996). GSH content in liver tissue was determined using a modification of the Elman procedure (Mitchell et al., 1973).
Protein Extraction.
Liver extracts were prepared by homogenizing 0.1 g of tissue in 1 ml of 1× PBS, pH 7.4, containing a protease inhibitor cocktail and 1 mM phenyl methanesulfonyl fluoride using a Tissue-Tearor homogenizer (BioSpec Products, Inc., Bartlesville, OK). These extracts were sonicated for two 5-s bursts in continuous mode at 30% power using a Sonic dismembrator (Thermo Fisher Scientific) and then centrifuged at 10,000 rpm for 5 min to remove tissue debris. Protein concentration was determined using the Bradford assay (Pierce Chemical Co.).
Immunoprecipitation.
Solubilized proteins (1 mg/sample) were precleared (1 h, 4°C) with 25 μl of Protein G Sepharose. The precleared supernatant was incubated with 10 μg of anti-MnSOD antibody overnight at 4°C. Immune complexes were precipitated (90 min) with 50 μl of Protein G Sepharose, washed twice with PBS, resuspended in sample loading buffer, boiled, and immediately separated using SDS-PAGE followed by Western blot analysis (MacMillan-Crow and Thompson, 1999; MacMillan-Crow et al., 2001).
Western Blot Analysis.
Western blot analyses were performed using the liver homogenates of each combined treatment group (100 μg) by SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes as described previously (Michael et al., 2001). Membranes were blocked 30 min for MnSOD and GAPDH and overnight for APAP and 3-nitrotyrosine in blocking buffer (5% milk in TBS, 0.1% Tween 20). The blocked immunoblots were incubated with anti-MnSOD (1:1000), anti-GAPDH (1:1000), and anti-3-nitrotyrosine (1:500) for 120 min and with anti-APAP (1:1000) for 90 min. Membranes for MnSOD and APAP were next incubated with peroxidase-labeled goat anti-rabbit IgG, and 3-nitrotyrosine and GAPDH were next incubated with peroxidase-labeled goat anti-mouse IgG for 60 min. Membranes were visualized using SuperSignal chemiluminescent substrate reagent, and the relative amount of protein in the blots was determined by densitometry analysis using a Fluorchem 8900 imaging densitometer (Alpha Innotech, San Leandro, CA).
Reaction of Proteins with NAPQI and Analysis for APAP Covalent Binding to Proteins.
In preparation for covalent modification by NAPQI, proteins [bovine serum albumin (BSA), ovalbumin (OA), and MnSOD] were diluted in triethylammonium bicarbonate buffer buffer (200 mM, pH 8) and then reduced for 10 min at room temperature with tris(2-carboxyethyl) phosphine hydrochloride (final concentration 12–20 mM) (Pierce Biochemical). Subsequently, NAPQI in dioxane was added to the reduced protein at a molar ratio of 6 to 10 times the protein molar concentration [final concentration of dioxane <1.2% (v/v)] and incubated for 5 min at room temperature with gentle mixing. Small molecules were removed from the reaction mixtures by gel filtration on centrifugal desalting columns equilibrated with PBS, pH 7.2 (exclusion volume >6000 Da). The excluded, putatively modified proteins were analyzed for the presence of APAP-protein adducts after enzymatic digestion using high-pressure liquid chromatography with electrochemical detection (HPLC-EC) of APAP-Cys as described previously (Muldrew et al., 2002; James et al., 2009).
Statistical Analysis.
All animal experiments used three animals per treatment group. The animal experiments were repeated twice. For statistical analyses Western blots of liver homogenates from each liver (100 μg) were performed and the density of the blots was determined as described above. Numerical data are reported as mean ± S.E. The data were analyzed using one-way analysis of variance followed by the Tukey Honestly Significant Difference post hoc test. Statistical significance was defined as experimental being p ≤ 0.05 from control. PASW Statistics Student Version 18 (SPSS Inc., Chicago, IL) was used for statistical analyses.
Results
Effect of APAP on Hepatic MnSOD Activity.
To determine the potential role of MnSOD in APAP toxicity, mice were treated with a toxic dose of APAP (300 mg/kg i.p.). Control mice received saline only. Mice were sacrificed at 0.5, 1, 2, 4, and 6 h after APAP treatment. Consistent with previous observations, ALT levels were significantly increased at 4 and 6 h (Fig. 1A). Hepatic GSH levels were significantly depleted by 0.5 h and remained significantly depleted until 4 h. By 6 h, GSH levels had recovered to levels comparable with that of saline-treated animals (Fig. 1B). The effect of APAP toxicity on hepatic MnSOD was examined by measuring MnSOD activity in liver homogenates. The MnSOD activity decreased significantly (55% reduction) by 1 h and remained significantly decreased at 2 h. By 4 h MnSOD activity had recovered to levels comparable with saline-treated animals, and by 6 h levels were significantly increased over that observed in control animals (Fig. 1C). To determine whether this change in MnSOD activity was caused by a change in protein levels, MnSOD expression was analyzed by Western blot analysis using APAP-treated liver homogenates. A single band of 24 kDa was detected in the lanes of control and APAP-treated mice. The same blot was stripped and reprobed with an anti-GAPDH antibody to ensure equal loading of protein between samples. Densitometric analysis data showed no significant changes in immunochemically detectable MnSOD levels (Fig. 1D).
Fig. 1.
Time course for effect of APAP on serum ALT, hepatic GSH, hepatic MnSOD activity, and hepatic MnSOD protein. Mice (n = 3) were treated with a 300 mg/kg dose of APAP and sacrificed at the indicated times, and serum and liver were collected. The 0 time is the saline-treated control mice. A, ALT levels in serum (hepatotoxicity). B, GSH levels in liver. C, MnSOD activity in liver. D, MnSOD protein levels in liver. Western blot analyses were performed on each homogenate using anti-MnSOD. GAPDH was used as a loading control. The data are presented as mean ± S.E. of the relative intensity. One representative gel is shown above the density values. *, significant difference from saline, p ≤ 0.05.
To further understand the effect of APAP on MnSOD activity, a dose-response study was performed. Mice were injected with APAP (50, 100, 200, and 300 mg/kg i.p.) and sacrificed at 1 h after APAP treatment. Because the 1-h time point is before significant toxicity (Fig. 1), serum ALT remained unchanged throughout the dose response (Fig. 2A). Hepatic GSH levels were significantly reduced at all APAP doses (Fig. 2B). MnSOD activity decreased by 28% after 50 mg/kg APAP and significantly decreased further to 32, 55, and 57% after doses of 100, 200, and 300 mg/kg, respectively (Fig. 2C). MnSOD expression was analyzed by Western blot analysis using APAP-treated dose-dependent liver homogenates. A single band of 24 kDa was detected in the lanes of control and APAP-treated mice. The same blot was stripped and reprobed with an anti-GAPDH antibody to ensure equal loading of protein between samples. Densitometric analysis indicated that MnSOD protein was gradually decreased at doses of 50, 100, 200, and 300 mg/kg (Fig. 2D). Because APAP (100 mg/kg) significantly decreased MnSOD activity at 1 h and this dose has not been shown to be hepatotoxic (Pumford et al., 1989) another experiment was performed. APAP (100 mg/kg) was administered to mice, which were sacrificed at 1 and 6 h. At 1 h, MnSOD activity was significantly decreased by 33%. At 6 h, ALT levels in APAP-treated mice (19.6 ± 2 IU/liter) were not significantly different from saline-treated controls (28 ± 10 IU/liter). Therefore, the ALT data suggest that 100 mg/kg APAP was a nontoxic dose.
Fig. 2.
Dose response for effect of APAP on serum ALT, hepatic GSH, hepatic MnSOD activity, and hepatic MnSOD protein at 1 h. Mice (n = 3) were treated with 0, 50, 100, 200, and 300 mg/kg APAP and sacrificed after 1 h. A, ALT levels in serum (hepatotoxicity). B, GSH levels in liver. C, MnSOD activity in liver. D, MnSOD protein levels in liver. Western blot analyses were performed on each homogenate using anti-MnSOD. GAPDH was used as a loading control. Data are presented as mean ± S.E. of the relative intensity. One representative gel is shown above the density values. *, significant difference from saline, p ≤ 0.05.
Examination of MnSOD for APAP Protein Adducts and/or 3-Nitrotyrosine.
Two possible mechanisms were envisioned that could account for the decrease in MnSOD activity after APAP treatment. One possible mechanism was reaction of NAPQI, the reactive metabolite of APAP, with cysteine groups or other nucleophilic groups on MnSOD causing loss of activity. MnSOD has been reported previously to contain two cysteine groups (DiSilvestre et al., 1995). The second mechanism was nitration of tyrosine of MnSOD by reactive nitrogen species, leading to its inactivation. Tyrosine nitration leading to inactivation of MnSOD has been shown to occur in other diseases (MacMillan-Crow et al., 1996; Xu et al., 2006; Bayir et al., 2007).
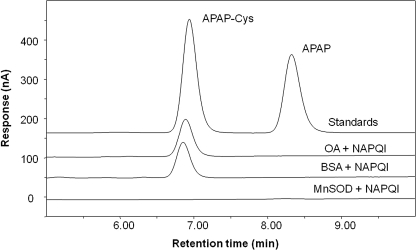
To determine whether MnSOD could be adducted by NAPQI, the recombinant protein was treated with synthetic NAPQI. In control incubations, BSA and OA were likewise treated with NAPQI. Before treatment with NAPQI the proteins were pretreated with tris(2-carboxyethyl) phosphine hydrochloride to reduce cysteine residues to the thiolate form. After treatment with NAPQI, the proteins were gel filtered, hydrolyzed by protease, and analyzed for APAP-Cys by HPLC-EC (Muldrew et al., 2002; James et al., 2009). As shown in Fig. 3, APAP-Cys was not observed in MnSOD treated with NAPQI. However, treatment of BSA and OA with synthetic NAPQI yielded a HPLC-EC peak with both proteins corresponding to authentic APAP-Cys (Fig. 3). These data suggest that MnSOD is not readily derivitized by NAPQI.
Fig. 3.
HPLC-EC analysis of NAPQI-treated MnSOD, BSA, and OVA for APAP-Cys. BSA, OA, and recombinant MnSOD were reacted with synthetic NAPQI. The resultant proteins were gel-filtered, treated with protease, and analyzed by HPLC/EC. HPLC reference standards were APAP-Cys (6.85 min, 20 pmol on column) and APAP (8.33 min, 13.2 pmol on column). Detection signal is current in nA at a potential of 180 mV versus a palladium reference electrode.
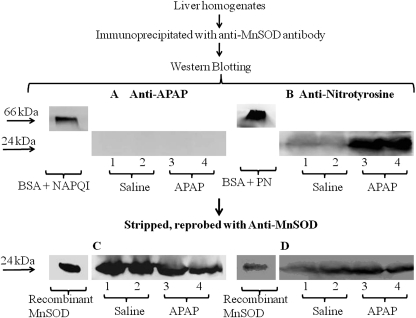
To confirm that MnSOD was not derivitized by NAPQI to form APAP protein adducts and to examine the possibility that the protein was nitrated, a MnSOD immunoprecipitation experiment was performed. In this experiment mice received APAP (300 mg/kg) or saline and were sacrificed at 1 h. Liver homogenates were subjected to immunoprecipitation with anti-MnSOD antibody. The immunoprecipitated protein was analyzed by SDS-PAGE and subsequently blotted and stained with polyclonal anti-APAP antibody. NAPQI-treated BSA was used as a positive control. A single band of 66 kDa was found in the control lane consistent with APAP covalently bound to BSA; however, no evidence was found for APAP covalently bound to MnSOD (Fig. 4A). The same blot was stripped and reprobed with monoclonal anti-MnSOD antibody to ensure immunoprecipitation occurred. Recombinant MnSOD was used as a positive control. A 24-kDa band was detected in control, saline, and APAP-treated samples (Fig. 4C). This result is in agreement with the HPLC/EC data suggesting that NAPQI does not covalently bind with MnSOD. Furthermore, to determine whether MnSOD was nitrated after APAP administration, liver homogenates were subjected to immunoprecipitation with anti-MnSOD antibody, analyzed by Western blot, and stained with monoclonal anti-3-nitrotyrosine antibody. Peroxynitrite-treated BSA was used as a positive control. As shown in Fig. 4B, 3-nitrotyrosine was detected in the immunoprecipitated MnSOD from the liver homogenates of the APAP-treated mice. To ensure immunoprecipitation occurred, the same blot was stripped and reprobed with monoclonal anti-MnSOD antibody (Fig. 4D). These data indicate that MnSOD was nitrated in the livers of APAP-treated mice.
Fig. 4.
Western blot of immunoprecipitated MnSOD for APAP covalent binding and tyrosine nitration. Liver homogenates were immunoprecipitated with anti-MnSOD antibody and resolved in 10% SDS-PAGE followed by Western blot analysis. Lanes 1 and 2 are from saline-treated mouse livers. Lanes 3 and 4 are from APAP-treated (300 mg/kg) mouse liver at 1 h. A and B, Western blot analyses were performed using anti-APAP antibody (A) and anti-3-nitrotyrosine antibody (B). C and D, subsequently, the blots were stripped and reprobed with anti-MnSOD antibody. BSA treated with NAPQI (A), peroxynitrite (B) at 66 kDa, and recombinant MnSOD at 24 kDa (C and D) were used as positive controls. One representative experiment is shown.
APAP Time Course and Dose Response for Nitration of MnSOD.
The time course and dose response for nitration of tyrosine in proteins in the APAP-treated mouse livers were determined. Western blots (Figs. 1D and 2D) were stripped and reprobed with polyclonal anti-3-nitrotyrosine antibody. As shown in Fig. 5, a nitrated protein with a molecular mass the same as MnSOD (24 kDa) was detected in livers of APAP-treated mice. Densitometric analysis showed that the relative amount of 3-nitrotyrosine in this protein significantly increased at 2, 4, and 6 h after a toxic dose of APAP (300 mg/kg) (Fig. 5A). The dose response for the increase of 3-nitrotyrosine in this protein showed a significant increase at 100, 200, and 300 mg/kg by 1 h after APAP (Fig. 5B). These data are consistent with nitration of MnSOD after doses of APAP (100, 200, and 300 mg/kg).
Fig. 5.
APAP time course and dose response for nitration of MnSOD. A, the samples from the time course (Fig. 1D) were stripped and reprobed with anti-3-nitrotyrosine antibody. B, the samples from the dose response (Fig. 2D) were stripped and reprobed with anti-3-nitrotyrosine antibody. The data are presented as mean ± S.E. of the relative intensity. One representative gel is shown. *, significant difference from saline, p ≤ 0.05.
Discussion
MnSOD is a critical enzyme found in the mitochondrial matrix. It catalyzes the dismutation of superoxide, forming hydrogen peroxide and molecular oxygen (McCord and Fridovich, 1988; Macmillan-Crow and Cruthirds, 2001). By scavenging superoxide its activity limits the reaction of superoxide with nitric oxide to form the reactive nitrogen species peroxynitrite. Peroxynitrite is both an oxidizing agent and a nitrating agent. It can nitrate tyrosine residues forming 3-nitrotyrosine (Beckman, 1996). It is detoxified by GSH (Sies et al., 1997), which is depleted in APAP toxicity (Figs. 1B and 2B). The data presented here show that the activity of hepatic MnSOD is dramatically reduced at 1 and 2 h after a hepatotoxic dose of APAP administered to mice (Fig. 1C).
The mechanism of the decrease of MnSOD activity was examined. Because a significant decrease in MnSOD activity was observed by 1 h (Figs. 1 and 2), the decrease was not a result of loss of the enzyme into the blood caused by hepatocyte lysis. Hepatocyte lysis was not observed until 4 and 6 h (Fig. 1A). In addition, there was no evidence that NAPQI covalently bound to the enzyme because APAP-protein adducts were not detected either upon addition of NAPQI to MnSOD (Fig. 3) or in livers of APAP-treated mice (Fig. 4A). However, loss of MnSOD activity occurred with the formation of nitrated enzyme (Fig. 4B). Western blot analysis for 3-nitrotyrosine in the 24-kDa protein suggested significant nitration of MnSOD by 1 h at doses of 100, 200, and 300 mg/kg. These data suggest that the loss of activity of MnSOD was a result of nitration and that nitration occurs at a dose of APAP that is not hepatotoxic (100 mg/kg).
We previously examined APAP toxicity in freshly isolated hepatocytes. Toxicity was shown to occur after GSH depletion and covalent binding and to occur by MPT with increased oxidative stress. MPT is known to be initiated by oxidative stress and leads to a large increase in oxidative stress. It occurs with loss of the ability of the mitochondria to produce ATP, a lethal event for the cell. However, in earlier work the nature of the oxidative stress was not determined (Reid et al., 2005). In more recent work we showed that MPT occurred with increased nitration of proteins. Inhibitors of MPT inhibited both toxicity and nitration. The finding that a neuronal nitric-oxide synthase inhibitor, 7-nitroindazole, but not two inducible nitric-oxide synthase inhibitors, inhibited both toxicity and nitration suggested involvement of a mitochondrial nitric-oxide synthase as the source of nitric oxide (Burke et al., 2010). Thus, the source of both the nitric oxide and the superoxide may be from mitochondria and explain the previous finding that the nitration of proteins in APAP hepatic toxicity occurs exclusively in mitochondria (Cover et al., 2005). The finding here that MnSOD is inhibited supports the hypothesis that APAP toxicity is by mitochondrial dysfunction promoted by reactive nitrogen species.
An interesting finding was the large increase in hepatic MnSOD activity (Fig. 1C) observed at 6 h after treatment with APAP. The increase seemed to occur without a statistically significant alteration in detectable protein levels. Whereas the decrease in the activity of MnSOD at 1 and 2 h is believed to be a result of enzyme nitration (Fig. 4A), there was a doubling of activity by 6 h. Likewise, Mladenović et al. (2009) reported that MnSOD activity increased at 6 h after a toxic dose of APAP. This increase may be an effect on the antioxidant response element. Increased oxidative stress and NAPQI are believed to increase nuclear factor erythroid 2-related factor 2 translocation into the nucleus in APAP toxicity, and the antioxidant response element leads to the increased synthesis of a large number of antioxidant enzymes including MnSOD and enzymes controlling GSH synthesis (Goldring et al., 2004; Okawa et al., 2006; Reisman et al., 2009). The difference between the large increase in activity without a significant increase in protein levels is not clear, but may be related to either alterations in the immunoaffinity of the enzyme after oxidation/nitration and/or precipitation of the protein after oxidation/nitration. Thus, the Western blot analysis of the supernatant of the sonicated homogenate may not accurately measure the relative amount of MnSOD once the protein is oxidized/nitrated. This conclusion is supported by the finding that there was less immunodetectable MnSOD at 1 h at doses of 100, 200, and 300 mg/kg APAP (Fig. 2D) because these losses occurred without lysis of hepatocytes (Fig. 2A).
An examination of a dose response for loss of MnSOD activity at 1 h indicated that there was a significant loss of activity at APAP doses of 100, 200, and 300 mg/kg (Fig. 2C). Whereas 200 and 300 mg/kg APAP are known to be hepatotoxic doses, previously a dose of 100 mg/kg was not hepatotoxic (Pumford et al., 1989). A repeat of this experiment showed that there was not a significant increase of ALT after 100 mg/kg APAP at 6 h, a time of maximal ALT levels. However, we report herein that there was a significant decrease in MnSOD activity at this dose (Fig. 2C) and this was accompanied by a significant increase in nitration at 1 h (Fig. 5B). Thus, the data suggest that formation of reactive nitrogen species with loss of MnSOD activity is an early event in APAP toxicity and occurs at nonhepatotoxic doses.
In conclusion, the data in this article show that MnSOD activity is decreased in APAP toxicity. The decrease in activity is attributed to nitration by reactive nitrogen species. The decrease in activity is postulated to augment the developing toxicity by shunting superoxide in mitochondria to react with nitric oxide, leading to increased levels of reactive nitrogen species with nitration and oxidation (Fig. 6). The mechanism of how reactive nitrogen produces the toxicity is unclear, but there is a significant amount of literature that toxicity occurs with alteration in mitochondrial activity (Burcham and Harman, 1991; Donnelly et al., 1994; Myers et al., 1995).
Fig. 6.
Schematic representation of how reactive nitrogen can alter oxidant stress in mitochondria.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK079008, R01-DK059872, R01-DK75936]. D.W.R. and L.P.J. received partial support from an Arkansas Biosciences Institute grant.
This work was previously presented in part: Agarwal R, MacMillan-Crow LA, Rafferty T, Saba H, and Hinson JA (2010) Acetaminophen-induced alterations in hepatic mitochondrial manganese superoxide dismutase (MnSOD; SOD2) activity in mice. Experimental Biology Meeting; 2010 Apr 23–28; Anaheim, CA. American Society for Pharmacology and Experimental Therapeutics, Bethesda, MD.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.176321.
ABBREVIATIONS:
- APAP
- acetaminophen
- APAP-Cys
- 3-(cystein-S-yl)-APAP
- ALT
- alanine aminotransferase
- BSA
- bovine serum albumin
- HPLC-EC
- high-pressure liquid chromatography with electrochemical detection
- SOD
- superoxide dismutase
- MnSOD
- manganese SOD
- MPT
- mitochondrial permeability transition
- NAPQI
- N-acetyl-p-benzoquinone imine
- OA
- ovalbumin
- PAGE
- polyacrylamide gel electrophoresis
- PBS
- phosphate-buffered saline
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
Authorship Contributions
Participated in research design: Agarwal, MacMillan-Crow, and Hinson.
Conducted experiments: Agarwal, MacMillan-Crow, Saba, Rafferty, Roberts, and Hinson.
Contributed new reagents or analytic tools: Fifer.
Performed data analysis: Agarwal, Saba, and Hinson.
Wrote or contributed to the writing of the manuscript: Agarwal, MacMillan-Crow, James, and Hinson.
References
- Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. (2007) Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem 101:168–181 [DOI] [PubMed] [Google Scholar]
- Beckman JS. (1996) Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9:836–844 [DOI] [PubMed] [Google Scholar]
- Boobis AR, Tee LB, Hampden CE, Davies DS. (1986) Freshly isolated hepatocytes as a model for studying the toxicity of paracetamol. Food Chem Toxicol 24:731–736 [DOI] [PubMed] [Google Scholar]
- Burcham PC, Harman AW. (1991) Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem 266:5049–5054 [PubMed] [Google Scholar]
- Burke AS, MacMillan-Crow LA, Hinson JA. (2010) Reactive nitrogen species in acetaminophen-induced mitochondrial damage and toxicity in mouse hepatocytes. Chem Res Toxicol 23:1286–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AM, Lemasters JJ, Nieminen AL. (1999) Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology 29:1523–1531 [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. (1997) Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol 143:1–12 [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. (2005) Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–887 [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. (1984) N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA 81:1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSilvestre D, Kleeberger SR, Johns J, Levitt RC. (1995) Structure and DNA sequence of the mouse MnSOD gene. Mamm Genome 6:281–284 [DOI] [PubMed] [Google Scholar]
- Donnelly PJ, Walker RM, Racz WJ. (1994) Inhibition of mitochondrial respiration in vivo is an early event in acetaminophen-induced hepatotoxicity. Arch Toxicol 68:110–118 [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. (2009) Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol 37:193–200 [DOI] [PubMed] [Google Scholar]
- Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, McMahon M, Hayes JD, Itoh K, Yamamoto M, et al. (2004) Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39:1267–1276 [DOI] [PubMed] [Google Scholar]
- Grewal KK, Racz WJ. (1993) Intracellular calcium disruption as a secondary event in acetaminophen-induced hepatotoxicity. Can J Physiol Pharmacol 71:26–33 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. (1998) Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol 11:604–607 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89:31–41 [DOI] [PubMed] [Google Scholar]
- James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. (2009) Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos 37:1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. (1973) Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther 187:195–202 [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–1179 [DOI] [PubMed] [Google Scholar]
- Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. (2008) Acute liver failure: summary of a workshop. Hepatology 47:1401–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA 93:11853–11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. (2001) Invited review: manganese superoxide dismutase in disease. Free Radic Res 34:325–336 [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. (2001) Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med 31:1603–1608 [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Thompson JA. (1999) Immunoprecipitation of nitrotyrosine-containing proteins. Methods Enzymol 301:135–145 [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055 [PubMed] [Google Scholar]
- McCord JM, Fridovich I. (1988) Superoxide dismutase: the first twenty years (1968–1988). Free Radic Biol Med 5:363–369 [DOI] [PubMed] [Google Scholar]
- Michael SL, Mayeux PR, Bucci TJ, Warbritton AR, Irwin LK, Pumford NR, Hinson JA. (2001) Acetaminophen-induced hepatotoxicity in mice lacking inducible nitric oxide synthase activity. Nitric Oxide 5:432–441 [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217 [PubMed] [Google Scholar]
- Mladenović D, Radosavljević T, Ninković M, Vucević D, Jesić-Vukićević R, Todorović V. (2009) Liver antioxidant capacity in the early phase of acute paracetamol-induced liver injury in mice. Food Chem Toxicol 47:866–870 [DOI] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. (2002) Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos 30:446–451 [DOI] [PubMed] [Google Scholar]
- Myers TG, Dietz EC, Anderson NL, Khairallah EA, Cohen SD, Nelson SD. (1995) A comparative study of mouse liver proteins arylated by reactive metabolites of acetaminophen and its nonhepatotoxic regioisomer, 3′-hydroxyacetanilide. Chem Res Toxicol 8:403–413 [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. (2006) Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339:79–88 [DOI] [PubMed] [Google Scholar]
- Pumford NR, Hinson JA, Potter DW, Rowland KL, Benson RW, Roberts DW. (1989) Immunochemical quantitation of 3-(cystein-S-yl)acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther 248:190–196 [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. (2005) Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 312:509–516 [DOI] [PubMed] [Google Scholar]
- Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD. (2009) Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci 109:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. (1991) Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol 138:359–371 [PMC free article] [PubMed] [Google Scholar]
- Sies H, Sharov VS, Klotz LO, Briviba K. (1997) Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J Biol Chem 272:27812–27817 [DOI] [PubMed] [Google Scholar]
- Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, et al. (2006) The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes 55:110–119 [PubMed] [Google Scholar]
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192:1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]