Abstract
Adenylyl cyclases (AC) are important regulators of airway smooth muscle function, because β-adrenergic receptor (AR) agonists stimulate AC activity and increase airway diameter. We assessed expression of AC isoforms in human bronchial smooth muscle cells (hBSMC). Reverse transcriptase-polymerase chain reaction and immunoblot analyses detected expression of AC2, AC4, and AC6. Forskolin-stimulated AC activity in membranes from hBSMC displayed Ca2+-inhibited and Gβγ-stimulated AC activity, consistent with expression of AC6, AC2, and AC4. Isoproterenol-stimulated AC activity was inhibited by Ca2+ but unaltered by Gβγ, whereas butaprost-stimulated AC activity was stimulated by Gβγ but unaffected by Ca2+ addition. Using sucrose density centrifugation to isolate lipid raft fractions, we found that only AC6 localized in lipid raft fractions, whereas AC2 and AC4 localized in nonraft fractions. Immunoisolation of caveolae using caveolin-1 antibodies yielded Ca2+-inhibited AC activity (consistent with AC6 expression), whereas the nonprecipitated material displayed Gβγ-stimulated AC activity (consistent with expression of AC2 and/or AC4). Overexpression of AC6 enhanced cAMP production in response to isoproterenol and beraprost but did not increase responses to prostaglandin E2 or butaprost. β2AR, but not prostanoid EP2 or EP4 receptors, colocalized with AC5/6 in lipid raft fractions. Thus, particular G protein-coupled receptors couple to discreet AC isoforms based, in part, on their colocalization in membrane microdomains. These different cAMP signaling compartments in airway smooth muscle cells are responsive to different hormones and neurotransmitters and can be regulated by different coincident signals such as Ca2+ and Gβγ.
Introduction
Smooth muscle tone is influenced by extracellular hormones and neurotransmitters, many of which activate G protein-coupled receptors (GPCRs) that modulate the activity of effector enzymes and the level of intracellular second messengers. Intracellular calcium and cAMP, key second messengers of GPCRs, exert opposite effects on smooth muscle contraction, with Ca2+ causing contraction and cAMP inducing relaxation (Torphy et al., 1982; Billington and Penn, 2003). β-Adrenergic receptor (AR) agonists, which stimulate cAMP production via activation of Gs and adenylyl cyclase (AC) activity, induce relaxation of smooth muscle (Kume et al., 1994; Kotlikoff and Kamm, 1996). However, several investigators have found that βAR agonists induce relaxation of airway smooth muscle via both cAMP-dependent and -independent mechanisms, possibly indicating other roles for cAMP signaling (Torphy, 1994; Ostrom and Ehlert, 1998; Spicuzza et al., 2001). In addition, other hormones can regulate cAMP production in smooth muscle via receptors coupled to Gs. These include prostaglandin E2 (PGE2) and prostacyclin (Madison et al., 1989; Tamaoki et al., 1993). cAMP acts through protein kinase A (PKA) to initiate rapid effects such as regulation of ion channels and cellular metabolism and more delayed effects such as changes in gene expression, growth, and proliferation (Billington et al., 1999; Scott et al., 1999). The exchange protein activated by cAMP, Epac, can also mediate a subset of effects of cAMP in many cells (Grandoch et al., 2010). Airway smooth muscle cells also express GPCRs coupled to Gi and the inhibition of cAMP production, including M2 muscarinin acetylcholine receptors, lysophosphatidic acid receptors, and endothelin-1 receptors (Ehlert et al., 1997; Hirshman and Emala, 1999).
Nine different transmembrane AC isoforms exist, each with different amino acid sequences, tissue and chromosomal distribution, and regulation (Hurley, 1999; Hanoune and Defer, 2001). Differences in regulation include stimulation or inhibition by Gβγ, Ca2+, and various protein kinases. AC5 and AC6 represent a subfamily of ACs related in structure and regulation. These isoforms are inhibited by PKA, Ca2+, nitric oxide, Gi, and Gβγ (McVey et al., 1999; Hill et al., 2000; Hanoune and Defer, 2001). In contrast, AC3 can be either stimulated by Ca2+/calmodulin or specifically inhibited by calmodulin kinase II, whereas AC2 is activated by Gβγ (Wei et al., 1996; Hanoune and Defer, 2001). Although these unique properties of AC isoforms have been recognized for some time (on the basis of reconstituted enzymatic assays), the effects these features have on cell physiology are poorly understood (Sadana and Dessauer, 2009). One possible reason for this is the fact that most cells express at least three or four different AC isoforms, implying a high degree of duplicity (Ostrom and Insel, 2004). In addition, all AC isoforms are activated by the same G protein, and there are no good isoform-specific drugs, making it difficult to activate select AC isoforms.
It is becoming a commonly accepted notion that various proteins involved in GPCR signal transduction are enriched and spatially organized within plasma membrane microdomains (Neubig, 1994; Steinberg and Brunton, 2001; Ostrom, 2002). Numerous studies have focused on caveolae and lipid rafts as membrane microdomains where receptors, G proteins, effector molecules, and even second messengers are concentrated (Shaul and Anderson, 1998; Davare et al., 2001; Ostrom and Insel, 2004). Caveolae are cholesterol- and sphingolipid-enriched portions of the membrane that form distinct flask-like invaginations when the protein caveolin is expressed (Anderson, 1998). Lipid rafts are microdomains of the plasma membrane that are biochemically similar to caveolae but morphologically indistinguishable from the rest of the plasma membrane (Hooper, 1999; Simons and Toomre, 2000; Galbiati et al., 2001). We have previously reported that compartmentation of GPCR signaling may be a means by which cells target specific hormonal signals to distinct cellular responses despite using similar signaling pathways (Ostrom et al., 2001; Liu et al., 2008). One means by which this may occur is through the distinct localization of AC isoforms in either lipid raft or nonraft domains.
Our goal was to define the AC isoforms expressed in hBSMC, a convenient cell model of human airway smooth muscle. We also sought to characterize the localization of these proteins in membrane microdomains and define the regulation of their activity by key receptors. We found that hBSMC express primarily AC2, AC4, and AC6. AC6 localizes in caveolin-rich lipid raft fractions where it is stimulated by β2AR and prostacyclin receptors and inhibited by Ca2+, whereas AC2 and AC4 expression is excluded from these domains and is regulated by prostanoid EP2 receptors and stimulated by Gβγ.
Materials and Methods
Materials and Cell Culture.
The primary antibody for FLAG (monoclonal) was obtained from Sigma-Aldrich. Primary antibodies for caveolin-1 (monoclonal), caveolin-2 (monoclonal), and caveolin-3 (monoclonal) were obtained from BD Biosciences (San Jose, CA). Primary antibodies for AC2 (polyclonal), AC3 (polyclonal), AC4 (polyclonal), AC5/6 (polyclonal), AC7 (polyclonal), β2-adrenergic receptor (polyclonal), EP2 receptor (polyclonal), and EP4 receptor (polyclonal) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. Beraprost and butaprost were obtained from Cayman Chemical (Ann Arbor, MI). Purified bovine Gβγ protein was purchased from EMD Biosciences (San Diego, CA). All other chemicals and reagents were obtained from Sigma-Aldrich.
hBSMC were obtained from LonzaBio (Basel, Switzerland) and were maintained in smooth muscle basal medium supplemented with the SmGM-2 bullet kit (5% fetal bovine serum, 0.1% insulin, 0.1% human epidermal growth factor, 0.2% human fibroblast growth factor-B, and gentamicin sulfate/amphotericin B; LonzaBio) and kept in a 37°C incubator with 5% CO2. Cells were used between passages 4 and 12 for all experiments. In some studies, cells were incubated with approximately 100 viral particles per cell of recombinant adenoviruses expressing either the lacZ cDNA (control) or the murine AC6 cDNA. Cells were incubated with adenovirus for 24 h and then were washed and equilibrated in growth medium for 24 h before assays were performed. Preliminary studies using lacZ adenovirus and β-galactosidase staining indicated that the efficiency of the adenoviral gene transfer was greater than 90% (data not shown).
RT-PCR.
RT-PCR for AC isoforms was performed using the primer pairs described in Table 1. Total RNA was extracted from WI-38 cells grown to 80 to 90% confluence on 10-cm plates using TRIzol reagent (Invitrogen, Carlsbad, CA) and an RNeasy RNA isolation kit (QIAGEN, Valencia, CA). A DNase reaction was performed to eliminate DNA contaminants, and the RNA was reverse transcribed using Superscript II (Invitrogen) and poly(dT) primer. PCR with each primer pair were performed on cDNA, genomic DNA (positive control), and minus RT (negative control) templates. The primers were designed on the basis of GenBank sequences and are listed in Table 1. The thermal profile for all real-time PCR was 50°C for 2 min and 95°C for 10 min, followed by 35 cycles of 95°C for 10 s and 60 to 62°C for 1 min. PCR products were analyzed by agarose gel electrophoresis and visualized under UV light with ethidium bromide.
TABLE 1.
AC isoform primer pairs used for RT-PCR
Some sequences were adapted from Xu et al. (2001).
| Isoform | Forward | Reverse |
|---|---|---|
| AC1 | ACTACGAGGTAGAACCGGGT | ACAGGACAGTGCGAATCTGAA |
| AC2 | CTGCCAAAAACGTCTGTCCT | CTACCCAGAACAAGCAGTGT |
| AC3 | GACCCCTGGCTAATGACAAA | AGCGTCGCATCTCATAGACA |
| AC4 | CTGGACAGGATGCACAACAG | GAAGGCGAGTGCAATCTCAG |
| AC5 | ATTCACAGCGGGCGAGTA | GGTTCATCTTGGCGATCA |
| AC6 | CAAGGAGCAGCACATTGAGA | AGAGCTGGCCAAGAGACTCA |
| AC7 | GCTATGTGCTGGTGTTCGAC | TAGTAGCAGTCGCCGAGGAT |
| AC8 | CGGGATTTGGAACGCCTCTA | CCTCCGAGTCTCCAGGAAAG |
| AC9 | GAGATGGTGAACATGAGAGTCG | CCTTCTCCTGCAAGATCTCACAC |
Measurement of cAMP Accumulation.
hBSMC were washed three times with serum- and NaHCO3-free Dulbecco's modified Eagle's medium supplemented with 20 mM HEPES, pH 7.4, and equilibrated for 30 min. An assay for cAMP accumulation was performed by incubation with drugs of interest and 0.2 mM isobutylmethylxanthine, a phosphodiesterase inhibitor, for 10 min. To terminate reactions, assay medium was aspirated, and 250 μl of ice-cold trichloroacetic acid (7.5% w/v) was immediately added to each well. cAMP content in trichloroacetic acid extracts was determined by EIA (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Production of cAMP was normalized to the amount of protein per sample as determined using a dye-binding protein assay (Bio-Rad Laboratories, Hercules, CA).
Measurement of AC Activity.
AC and nitric-oxide synthase activities were measured in membranes prepared from neonatal rat ventricular myocytes. Cells were rinsed twice in ice-cold phosphate-buffered saline (PBS) and then were scraped into hypotonic homogenizing buffer (30 mM Na-HEPES, 5 mM MgCl2, 1 mM EGTA, and 2 mM DTT, pH 7.5) and homogenized with 20 strokes of a Dounce homogenizer. The homogenate was centrifuged at 300g for 5 min at 4°C; the supernatant was then transferred to a centrifuge tube and centrifuged at 5000g for 10 min. The pellet was suspended in buffer (30 mM Na-HEPES, 5 mM MgCl2, and 2 mM DTT, pH 7.5) to attain approximately 1 mg/ml total protein concentration before being added to tubes containing drug and AC assay buffer (30 mM Na-HEPES, 100 mM NaCl, 1 mM EGTA, 10 mM MgCl2, 1 mM isobutylmethylxanthine, 1 mM ATP, 10 mM phosphocreatine, 5 μM GTP, 60 U/ml creatine phosphokinase, and 0.1% bovine serum albumin, pH 7.5.). AC activity assays were terminated after 15 min by boiling and cAMP content was determined by EIA (GE Healthcare).
In other studies, AC activity was measured in caveolin-1 immunoprecipitates. Cells were scraped and homogenized in a modified lysis buffer with 1% Triton X-100 (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 2 mM DTT, and 1% Triton X-100 plus mammalian protease inhibitor cocktail). Homogenate was precleared with 50 μl of protein A-agarose for 30 min at 4°C and then was incubated with caveolin-1 monoclonal antibody (BD Pharmingen, San Diego, CA) for 1 h. Antibody complexes were then precipitated after incubation with protein A-agarose and resuspended in membrane buffer. The assay was conducted by adding 30-μl sample (membranes or immunoprecipitate) to tubes containing assay buffer (30 mM Na-HEPES, 100 mM NaCl, 1 mM EGTA, 10 mM MgCl2, 1 mM isobutylmethylxanthine, 1 mM ATP, 10 mM phosphocreatine, 5 μM GTP, 60 U/ml creatine phosphokinase, and 0.1% bovine serum albumin, pH 7.5.) and drugs of interest. The mixture was incubated for 15 min at 30°C, and reactions were stopped by boiling for 5 min. The cAMP content of each tube was assayed by EIA (GE Healthcare). The total protein concentration was determined using a dye-binding protein assay (Bio-Rad Laboratories).
Nondetergent Isolation of Lipid Raft and Nonraft Membranes.
Cells were fractionated using a detergent-free method. Ten-centimeter plates at 70 to 80% confluence were washed twice in ice-cold PBS and scraped into a total of 1.5 ml of 500 mM sodium carbonate, pH 11. Cells were homogenized with 20 strokes in a tissue grinder followed by three 20-s bursts with an ultrasonic cell disruptor. A full 1-min rest period was included between each ultrasonic burst. Then, 1 ml of homogenate was brought to 45% sucrose by addition of an equal volume of 90% sucrose in MBS (25 mM MES and 150 mM NaCl, pH 6.5) and loaded in an ultracentrifuge tube. A discontinuous sucrose gradient was layered on top of the sample by placing 2 ml of 35% sucrose prepared in MBS with 250 mM sodium carbonate and then 1 ml of 5% sucrose (also in MBS-Na2CO3). The gradient was centrifuged at 46,000 rpm on a SW55Ti rotor (Beckman Coulter, Fullerton, CA) for 16 to 18 h at 4°C. The faint light-scattering band was collected from the 5 to 35% sucrose interface (lipid raft fractions), and the bottom of the gradient (45% sucrose) was collected as nonraft material. Raft and nonraft fractions, along with whole-cell lysate, were then analyzed by SDS-PAGE (loading equal proportions of each fraction) and immunoblotting. For AC isoform detection, samples were deglycosylated and concentrated before SDS-PAGE. Eighty to 100 μg of protein was incubated with peptide-N-glycosidase F in 60 mM NaCl, 1.25 mM EDTA, 143 mM β-mercaptoethanol, 5 mM sodium phosphate, 15 mM Tris-Cl (pH 7.5), 0.1% SDS, and 0.5% Nonidet P-40 for 2 h at 37°C. Reactions were terminated with 0.33 volumes of 4× SDS-PAGE sample buffer.
Immunoblot Analysis.
Whole-cell lysates were obtained by scraping cells in modified radioimmunoprecipitation assay lysis buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 1% Igepal CA-630 plus mammalian protease inhibitor cocktail (Sigma Aldrich)] and homogenizing by sonication. Whole-cell lysates or cell fractions were separated on 10% SDS-polyacrylamide gels by electrophoresis before being transferred to polyvinylidene difluoride membranes (Millipore Corporation, Billerica, MA) by electroblotting. Membranes were blocked in 20 mM PBS with 3% nonfat dry milk and incubated with primary antibody for 2 to 12 h at 4°C with constant rocking. Bound primary antibodies were visualized using an appropriate secondary antibody with conjugated horseradish peroxidase (Santa Cruz Biotechnology, Inc.) and ECL reagent (Pierce Chemical, Rockford, IL). Some primary antibodies recognized multiple nonspecific protein species, in which case whole blot images are shown. When just specific bands were evident, only the appropriately sized immunoreactive bands (based on the expected molecular weight of the protein of interest) are shown.
Data Analysis and Statistics.
Data are presented as the mean ± S.E.M. and in some cases as representative images of at least three separate experiments. Statistical comparisons (t tests and one-way analysis of variance) were performed and graphics were generated using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
Results
Because AC isoforms possess different regulatory properties and can be compartmentalized in specific signaling microdomains, we sought to define the AC isoforms expressed in hBSMC. We used RT-PCR analysis with isoform-specific primers to detect mRNA for each of the nine mammalian AC isoforms. We detected mRNA for AC2, AC4, and AC6 from hBSMC (Fig. 1). Each of these primer pairs amplified appropriately sized DNA sequences but did not yield PCR products when RNA (samples not treated with reverse transcriptase) was used as the template (data not shown). Because most primer pairs produced some nonspecific bands, we used two different template concentrations to confirm that only primers for AC2, AC4, and AC6 yielded specific product. Primers for other AC isoforms yielded products that were not of the expected size or sequence.
Fig. 1.
Total RNA from hBSMC was treated with DNase and then reverse-transcribed using poly-T priming, and 20 or 50 ng of template was used for PCRs with AC isoform-specific primer pairs (Table 1). PCR was run for 35 cycles with an annealing temp of 63°C. The image is representative of three experiments. Arrows indicate the size of the expected PCR product for each primer pair.
We also conducted immunoblot analyses to detect expression of AC isoforms and to determine the localization of these isoforms in plasma membrane microdomains. We fractionated hBSMC using a nondetergent method to isolate caveolin-rich lipid raft fractions and performed immunoblot analyses with AC isoform-specific antibodies. hBSMC express caveolin-1 and caveolin-2 but not caveolin-3 in buoyant lipid raft fractions and whole-cell lysates (Fig. 2). Only trace immunoreactivity for caveolins could be detected in nonraft fractions. AC isoform-specific antibodies detected multiple nonspecific bands, but only bands of the expected molecular weights were considered positive. We detected immunoreactivity for AC2, AC4, and AC5/6 (using an antibody unable to distinguish between AC5 and AC6) in hBSMC (Fig. 2). AC2 and AC4 immunoreactivity was detected only in nonraft fractions, whereas immunoreactivity for AC5/6 was detected only in lipid raft fractions. We could not detect immunoreactivity for any of the other AC isoforms.
Fig. 2.
Immunoblot analysis for AC isoforms expressed in lipid raft (raft), nonraft, or whole-cell lysate (WCL) fractions from hBSMC. Cells were fractionated using a nondetergent method and separated by sucrose density centrifugation (see Materials and Methods). Gradients were collected in 0.5-ml fractions and analyzed for appropriate separation of marker proteins (data not shown). Buoyant fractions containing caveolin were pooled and loaded into a single lane (raft fraction). Nonbuoyant “heavy” fractions were also pooled and loaded into a single lane (nonraft fraction). Samples were separated by SDS-PAGE and immunoblotted with the indicated primary antibody. Blots are labeled at the approximate molecular weight of the expected immunoreactive band. Images shown are representative of three to five experiments.
RT-PCR and immunoblot analyses did not yield definitive information about the predominant AC isoforms expressed. Thus, we measured AC activity in membranes and probed for characteristic regulatory properties to determine the predominant AC isoform(s) in hBSMC. Membrane AC activity was stimulated above basal levels by addition of either forskolin (direct activator of AC, 10 μM), isoproterenol (βAR agonist, 1 μM), beraprost [prostacyclin receptor (IPR) agonist, 1 μM] or butaprost (EP2 receptor agonist, 1 μM) (Fig. 3). Addition of 1 μM calcium caused no significant change in basal, forskolin-stimulated, beraprost-stimulated, or butaprost-stimulated AC activity but did inhibit isoproterenol-stimulated activity. These data imply that β-adrenergic receptors couple to a calcium-inhibitable AC isoform. In contrast, addition of 200 nM purified Gβγ protein increased enzymatic activity in the presence of either forskolin or butaprost but had no significant effect on other conditions. Thus, total membrane AC activity (forskolin) and EP2 receptor responses are sensitive to Gβγ. These data are consistent with expression of both a calcium-inhibitable AC isoform (AC6) and a Gβγ-stimulated isoform (AC2 and AC4), with the latter type being the predominant isoform.
Fig. 3.
AC activity in hBSMC membranes is regulated by several GPCR agonists and influenced by Ca2+ and Gβγ. Membranes were prepared from hBSMC as described (Materials and Methods) and then incubated with forskolin (Fsk), isoproterenol (Iso), beraprost (Bera), or butaprost (Buta) in buffer only (vehicle, □), buffer plus 1 μM free Ca2+ (■), or buffer plus 200 nM purified Gβγ ( ). Data are presented as the mean ± S.E.M. of n = 6. *, p < 0.05; **, p < 0.01 by paired t test compared with basal condition (no drug); #, p < 0.05 by paired t test compared with vehicle (□).
). Data are presented as the mean ± S.E.M. of n = 6. *, p < 0.05; **, p < 0.01 by paired t test compared with basal condition (no drug); #, p < 0.05 by paired t test compared with vehicle (□).
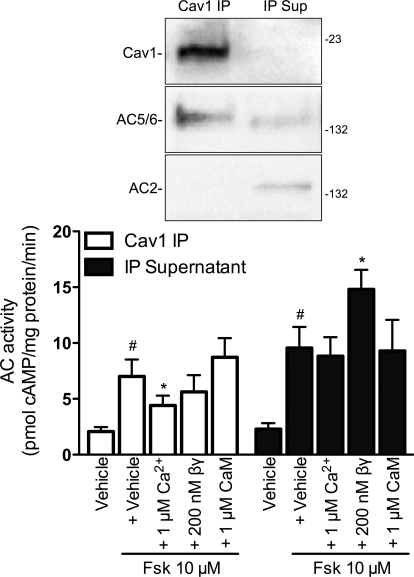
The receptor-selective effects of calcium and Gβγ on AC activity and the localized expression of AC isoforms led us to hypothesize that lipid raft AC expression would display regulatory properties distinct from that in nonraft fractions. Therefore, we immunoprecipitated (IP) caveolin-1 from hBSMC using a modified protocol that maintains the integrity of the lipid raft domain and supports AC enzymatic activity (Ostrom et al., 2001; Liu et al., 2008). This method is essentially an immunoisolation of caveolae. As we have observed in other cells, these immunoprecipitates contain AC5/6 immunoreactivity (Fig. 4, top) (Ostrom et al., 2001, 2004; Liu et al., 2008). We detected low levels of AC2 immunoreactivity in supernatants from the immunoprecipitates but not in the IP samples. We could not detect AC4 immunoreactivity in either fraction (data not shown). We measured AC activity in both IP and supernatant samples and found that both contained forskolin-stimulated AC activity (Fig. 4, bottom). The addition of 1 μM calcium inhibited forskolin-stimulated AC activity in the caveolin-1 IP samples but had no effect in the IP supernatant. Inclusion of 200 nM purified Gβγ had no effect on AC activity in the caveolin-1 IP samples but stimulated activity in the IP supernatant. Because calcium-stimulable AC isoforms (AC1, AC3, and AC8) require calmodulin, we included 1 μM calmodulin plus calcium in some conditions. We saw no calcium-calmodulin stimulation of AC activity in either basal (data not shown) or forskolin-stimulated (Fig. 4) conditions. These data are consistent with the idea that hBSMC express the calcium-inhibitable AC6 in lipid rafts and caveolae and Gβγ-sensitive isoforms (AC2 and AC4) in nonraft membrane microdomains.
Fig. 4.
Adenylyl cyclase activity in immunoisolated caveolae or isolation supernatants. Caveolae were immunoisolated from hBSMC by immunoprecipitating caveolin-1 in reduced detergent conditions (see Materials and Methods). Caveolin-1 (Cav) IP samples or IP supernatants (IP Sup) were separated by SDS-PAGE and probed for caveolin-1, AC5/6, and AC2 immunoreactivity (top). Forskolin (Fsk)-stimulated AC activity was measured in caveolin-1 IP samples (□) or IP supernatants (■) incubated with either vehicle, 1 μM free Ca2+, 200 nM purified Gβγ (βγ), or 1 μM Ca2+ plus 1 μM calmodulin (CaM). Data are presented as the mean ± S.E.M. of n = 5. #, p < 0.05 by paired t test compared with vehicle alone; *, p < 0.05 by paired t test compared with 10 μM forskolin alone.
We next wondered how altering the endogenous AC isoform expression would change signaling. We measured cAMP production in hBSMC that had been incubated with recombinant adenoviruses expressing either lacZ (control) or AC6 genes. Whereas basal cAMP accumulation did not differ between control cells and cells overexpressing AC6, AC6 overexpression enhanced responses to forskolin (10 μM) or isoproterenol (1 μM) 3- to 4-fold compared with that in control cells (Fig. 5A). cAMP accumulation stimulated by beraprost (1 μM) was enhanced almost 2-fold by AC6 overexpression. In contrast, responses to other agonists that increase cAMP in hBSMC, including PGE2 (10 μM) and butaprost (1 μM), were unchanged in cells overexpressing AC6. We examined the cAMP response across a range of concentrations for each of these receptor-selective agonists. AC6 overexpression increased the Emax of isoproterenol and beraprost approximately 3-fold without significantly altering the potency of these responses (Fig. 5, B and C). Neither the potency nor the efficacy of butaprost-stimulated cAMP production was altered by AC6 overexpression (Fig. 5D). Thus, AC6 overexpression in hBSMC selectively enhanced cAMP generation by βAR and IPR but not by other Gs-coupled receptors.
Fig. 5.
Overexpression of AC6 selectively enhances βAR-mediated cAMP responses. cAMP accumulation was measured in hBSMC incubated with recombinant adenoviruses expressing either lacZ (□) or AC6 (■) for 24 h. A, responses to forskolin (Fsk), isoproterenol (Iso), PGE2, beraprost (Bera), butaprost (Buta), CGRP, or pituitary adenylate cyclase activating peptide (PACAP) are shown as the mean ± S.E.M. of n = 6. Responses were also measured to increasing concentrations of isoproterenol (B), beraprost (C), or butaprost (D). Data are expressed as the mean ± S.E.M. of n = 3. *, p < 0.05 by paired t test compared with lacZ.
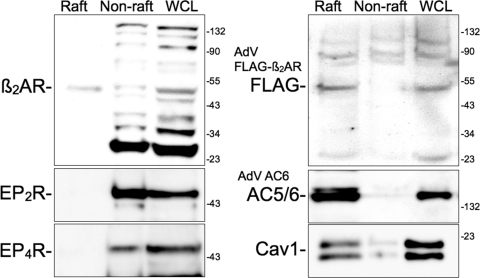
In other cell types, AC6 overexpression selectively enhances βAR- and IPR-mediated cAMP accumulation because of the compartmentation of AC6 with these receptors in lipid rafts (Liu et al., 2008). We tested the hypothesis that lipid raft microdomains may be one site for the βAR-AC6 colocalization in hBSMC. We isolated lipid raft fractions from hBSMC using the same method as described for Fig. 2. Caveolin-1 was enriched in the buoyant, lipid raft fractions as was virtually all of the AC5/6 immunoreactivity when AC6 was overexpressed (Fig. 6). We detected endogenously expressed β2AR in both lipid raft fractions and nonraft fractions. Multiple β2AR immunoreactive bands were detected in nonraft and whole-cell lysate fractions, making interpretation difficult. Therefore, we overexpressed an epitope-tagged β2AR using a recombinant adenovirus. Immunoblotting for the FLAG epitope showed that the bulk of β2AR are localized in lipid raft fractions. In contrast, immunodetection of native prostanoid EP2 and EP4 receptors occurred only in noncaveolin-rich, nonraft fractions. Taken together, these results imply that β2AR, IPR, and AC6 colocalize in lipid rafts and that this microdomain excludes prostanoid E receptors and the other principle AC isoforms of hBSMC (AC2 and AC4).
Fig. 6.
Immunoblot analysis for GPCRs and AC6 expressed in lipid raft and nonraft fractions from hBSMC. Cells were fractionated using a nondetergent method and separated by sucrose density centrifugation (see Materials and Methods). Buoyant fractions (Raft) and nonbuoyant “heavy” fractions (Non-raft) were pooled and loaded along with whole-cell lysates (WCL) into individual lanes and separated by SDS-PAGE. Blots are labeled with the primary antibody at the approximate molecular weight of the expected immunoreactive band. In some studies, hBSMC were incubated for 24 h with recombinant adenoviruses expressing either FLAG-β2AR or AC6. Images shown are representative of three to four experiments. Cav1, caveolin-1.
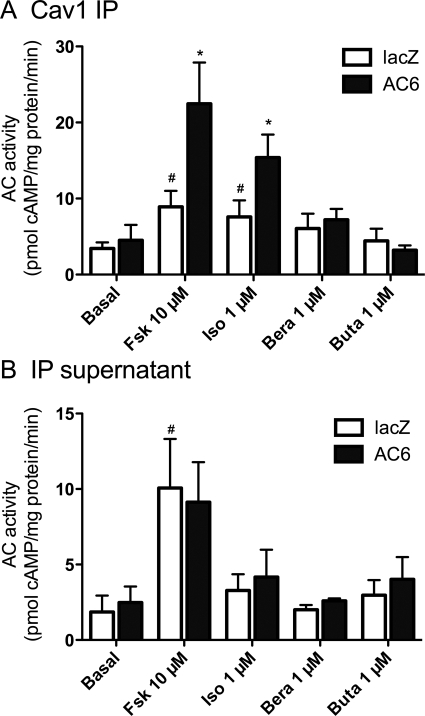
We used AC activity assays in caveolin-1 IP samples as an alternative approach to confirm the selective localization and coupling of GPCRs and AC isoforms observed above. In these studies, we immunoprecipitated caveolin-1 from lacZ (control) cells or cells overexpressing AC6 and measured AC activity in response to receptor-selective agonists. Caveolin-1 IP samples contained forskolin- and isoproterenol-stimulated AC activity but no significant stimulation by beraprost or butaprost (Fig. 7A). Caveolin-1 IP samples from AC6-overexpressing cells possessed 1.5- to 2-fold higher AC activity when stimulated with forskolin or isoproterenol (compared with control cells). However, no significant increase in AC activity could be detected in response to beraprost and butaprost. We also examined AC activity in the IP supernatants. In these fractions, forskolin increased AC activity approximately 4-fold in both lacZ and AC6 cells (Fig. 7B). However, we could not observe increased AC activity in supernatants stimulated with isoproterenol, butaprost, or beraprost. Thus, IP supernatants contain significant AC activity, of which none appears to be from AC6, and do not retain any GPCR-stimulable activity. Taken together, these data confirm that AC6 overexpression localizes to lipid rafts and/or caveolae where it can be selectively activated by β2AR.
Fig. 7.
Adenylyl cyclase activity in immunoisolated caveolae or isolation supernatants from hBSMC overexpressing AC6. Caveolae were immunoisolated from hBSMC by immunoprecipitating caveolin-1 (Cav1) in reduced detergent conditions (see Materials and Methods). Cav1 IP samples (A) or IP supernatants (B) were collected from hBSMC incubated for 24 h with recombinant adenoviruses expressing either lacZ (□) or AC6 (■), then incubated with either vehicle (basal), forskolin (Fsk), isoproterenol (Iso), beraprost (Bera), or butaprost (Buta). Data are presented as the mean ± S.E.M. of n = 3. #, p < 0.05 by paired t test compared with basal; *, p < 0.05 by paired t test compared with lacZ.
Discussion
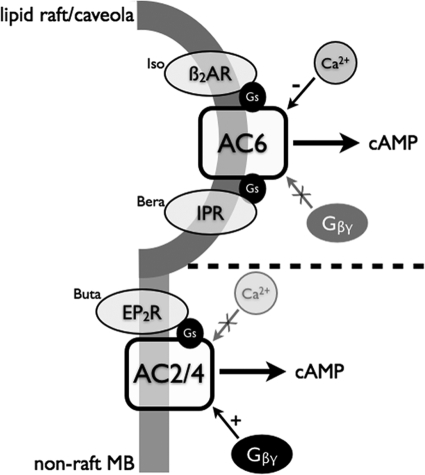
The objective of these studies was to characterize the AC isoforms expressed in hBSMC and to define their localization and coupling to specific receptors. We used several approaches to define the AC isoforms expressed, including RT-PCR, immunoblot analysis, and characterization of the isoform-specific regulatory properties of AC activity in hBSMC. Our data indicate that hBSMC express three isoforms of AC: AC2, AC4, and AC6. AC2 and AC4, both isoforms stimulated by Gβγ, appear similar in that they are expressed in nonlipid raft membrane fractions where they colocalize with and couple to EP2 receptors. The specific coupling of EP2 receptors with AC2/4 seems to impart a Gβγ-stimulable but Ca2+-insensitive profile of cAMP signaling stimulated by PGE2. In contrast, AC6 is expressed in lipid raft domains of hBSMC where this protein is colocalized with at least a portion of the cell's complement of β2AR. The lipid raft-localized AC activity is Ca2+-sensitive but not altered by Gβγ, imparting a Ca2+ sensitivity to the βAR agonist-stimulated cAMP signaling. These data are consistent with compartmentalized cAMP signaling that is imparted by distinct AC isoform localization and coupling to colocalized receptors. Airway smooth muscle may interpret catecholamine (via βAR) and PGE2 (via EP2 receptors) signals differently because of this compartmentalized cAMP accumulation and differential regulation by coincident signals (Ca2+ and Gβγ). More work is needed to determine whether the cellular responses to these hormones differ, but it is clear that both βAR agonists and PGE2 have both common and divergent effects in smooth muscle (Madison et al., 1989; Yan et al., 2010). Figure 8 is a schematic diagram that summarizes the localization and signaling mechanisms we propose on the basis of our findings.
Fig. 8.
Schematic diagram of GPCR/AC localization and signaling in hBSMC. β2AR and IPR signal via AC6 primarily in lipid raft microdomains. Ca2+ appears to inhibit these signals, whereas Gβγ has no effect. In nonraft membranes, prostanoid EP2 receptors localize with AC2 and AC4 and signal via a Ca2+-insensitive cyclase that is Gβγ stimulated. The dashed line represents a presumed barrier between the two illustrated cAMP compartments.
One approach we used was to immunoprecipitate caveolin-1 in reduced detergent conditions that maintain lipid raft integrity. Under these conditions, immunoprecipitation of caveolin-1 pulls down the entire caveolar microdomain with lipids, cholesterol, and associated proteins (Ostrom et al., 2001; Liu et al., 2008). These samples are essentially immunoisolated caveolae and have an advantage over other lipid raft/caveolar isolation methods in that most enzymatic activity persists through the isolation. We have used this approach in other cell types to measure AC activity regulated by different receptors (Liu et al., 2008). In the present studies, we used this method to also determine the AC isoform-specific regulation of cAMP production in these membrane microdomains. Our data are consistent with the idea that AC2 and AC4 reside outside the lipid raft/caveolar microdomain where they couple primarily to EP2 receptors, but that AC6 (endogenous and overexpressed) resides in caveolae where it can be regulated by β2AR and prostacyclin receptors.
We used an overexpression approach to measure the isoform-specific effects of AC. Overexpression of AC6 enhanced signaling by βAR or prostacyclin receptor activation but did not affect responses to EP2 receptors. This observation was consistent across both whole-cell cAMP accumulation studies and assays of AC activity in caveolin-1 IP samples and supernatants. The fact that β2AR (both native and overexpressed) were observed in the same lipid raft fractions as the overexpressed AC6 but that EP2 and EP4 receptors were excluded from these domains seemingly corroborates these findings. Prostacyclin receptors have been shown to localize in lipid raft domains in other cell types (Liu et al., 2008). Although we were unable to detect these receptors using available antibodies, the fact that beraprost-stimulated cAMP production was enhanced by AC6 overexpression implies that prostacyclin receptors are also lipid raft-localized in hBSMC.
At first glance, the AC isoforms expressed by hBSMC that we report appear different from those previously reported in studies of airway smooth muscle and primary cultured cells isolated from human samples (Xu et al., 2001). Xu et al. (2001) detected mRNA for AC isoforms 1, 3, 4, 5, 6, 7, and 9. They performed immunoblot analysis on whole-cell lysates and detected expression of just AC5/6 (using the same antibody used here, which is unable to distinguish between these two isoforms). To determine the predominant AC isoform, they examined cAMP production in intact cells for regulation by protein kinase C (PKC) and PKA. These authors concluded that the predominant AC isoform was probably AC5, because cAMP production was inhibited by PKA activation but stimulated by treatment with phorbol ester (an activator of PKC). Given that Xu et al. (2001) could not detect AC2 or AC4 by immunoblot, their conclusions were logical because AC6 activity is inhibited by PKC. However, the AC activity profile they observed could also be explained by expression of both AC2/4 with AC6, consistent with our present findings. The fact that Xu et al. (2001) did not enrich their protein samples via lipid raft fractionation, as in the present studies, makes it possible that they missed the expression of AC2 and AC4 at the protein level. Thus, our data may not be inconsistent with those reported by Xu et al. (2001), even though the sources of the cells differ between these studies.
The characterization of AC isoforms in the present report depends heavily on immunological approaches, which can present significant pitfalls. Foremost, the commercially available antibodies for AC isoforms are varied in their usefulness and their degree of nonspecificity. In our experience, the antibodies for AC2, AC3, AC4, and AC5/6 are relatively reliable (we have rarely detected expression of AC7 in the cells we have analyzed). Use of these antibodies to characterize AC isoforms in cultured cells depends on concentrating cell lysates to maximize detection and then fractionating cells to separate lipid raft and nonraft microdomains, which splits many of the nonspecific bands into different fractions and facilitates identification. We mitigate the shortcomings of using these antibodies by also analyzing mRNA expression and the enzymatic activity characteristic of each AC isoform (Figs. 1, 3, and 4). However, other pharmacological approaches for defining AC isoform expression are available and are particularly applicable for characterizing AC isoforms in tissues, whereas using these antibodies is more problematic. AC isoforms can be defined by rank order of sensitivity to various nucleotide and non-nucleotide inhibitors or by activation by isoform-selective derivatives of forskolin (Gille et al., 2004; Pinto et al., 2008; Göttle et al., 2009). Whereas no single approach can be used to reliably characterize the AC isoforms expressed in a given system, a combination of molecular and pharmacological tools can be deployed to achieve this goal.
It will be particularly important to understand the AC isoform expression and localization in airway smooth muscle from patients with asthma or chronic obstructive pulmonary disease. Furthermore, it is not known whether the human population is heterogeneous with respect to AC isoform expression in airway smooth muscle. Therefore, more work needs to be done before one can generalize about the AC isoforms expressed across patient populations. Our data define AC isoform expression in a convenient cell culture model of airway smooth muscle but also highlight how the cellular response to hormones and neurotransmitters such as catecholamines and prostaglandins can be largely influenced by the AC isoforms expressed by the cells. If development, age, or disease induces changes in AC expression, the cAMP signaling by airway smooth muscle could be altered significantly. In addition, if patients differ in their AC isoform expression profile they might display divergent responses to drugs such as βAR agonists, phosphodiesterase inhibitors, or cyclooxygenase inhibitors. Our data may also provide an impetus for ongoing work to develop AC isoform-specific inhibitors or activators, because such drugs could allow alteration of signaling by a subset of the cell's receptors (Onda et al., 2001; Mou et al., 2006). Such agents may have two advantages over traditional receptor-targeting drugs: 1) they may be more specific because receptors initiate other signaling cascades in addition to the canonical second messengers, and 2) they may have greater efficacy because AC expression is the stoichiometrically limiting component in the pathway (Daaka et al., 1998; Ostrom et al., 2000).
In conclusion, we detected expression of three AC isoforms, AC2, AC4, and AC6, in human airway smooth muscle cells in culture. This pattern of expression probably does not differ from that of primary cultures from human airways (Xu et al., 2001), indicating that this cultured cell model is appropriate for examining GPCR signaling and regulation that is relevant to humans. Different hormone receptors appear to couple to distinct AC isoforms, based largely on their colocalization in membrane microdomains, and their regulation of cAMP signaling is sensitive to different factors that affect the activity of these specific AC isoforms. More work is required to understand how (and whether) cAMP signaling in these different microdomains leads to unique patterns of cellular regulation. Given that cAMP signaling is known to be compartmentalized by expression of phosphodiesterases and A kinase anchoring proteins (Michel and Scott, 2002; Dessauer, 2009), we predict that such signaling microdomains are relevant to airway physiology and disease.
Acknowledgments
We thank Joseph Kaminsky and Fengying Li for their technical assistance with these studies.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL079166].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.177923.
ABBREVIATIONS:
- GPCR
- G protein coupled-receptor
- AR
- adrenergic receptor(s)
- AC
- adenylyl cyclase
- PGE2
- prostaglandin E2
- PKA
- protein kinase A
- hBSMC
- human bronchial smooth muscle cell(s)
- RT
- reverse transcriptase
- PCR
- polymerase chain reaction
- EIA
- enzyme immunoassay
- PBS
- phosphate-buffered saline
- DTT
- dithiothreitol
- PAGE
- polyacrylamide gel electrophoresis
- IPR
- prostacyclin receptor(s)
- IP
- immunoprecipitated
- PKC
- protein kinase C.
Authorship Contributions
Participated in research design: Bogard, Xu, and Ostrom.
Conducted experiments: Bogard, Xu, and Ostrom.
Performed data analysis: Bogard, Xu, and Ostrom.
Wrote or contributed to the writing of the manuscript: Bogard and Ostrom.
Other: Ostrom acquired funding for the research.
References
- Anderson RG. (1998) The caveolae membrane system. Annu Rev Biochem 67:199–225 [DOI] [PubMed] [Google Scholar]
- Billington CK, Hall IP, Mundell SJ, Parent JL, Panettieri RA, Jr, Benovic JL, Penn RB. (1999) Inflammatory and contractile agents sensitize specific adenylyl cyclase isoforms in human airway smooth muscle. Am J Respir Cell Mol Biol 21:597–606 [DOI] [PubMed] [Google Scholar]
- Billington CK, Penn RB. (2003) Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 4:2. [PMC free article] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. (1998) Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem 273:685–688 [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. (2001) A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293:98–101 [DOI] [PubMed] [Google Scholar]
- Dessauer CW. (2009) Adenylyl cyclase—A-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76:935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ, Ostrom RS, Sawyer GW. (1997) Subtypes of the muscarinic receptor in smooth muscle. Life Sci 61:1729–1740 [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. (2001) Emerging themes in lipid rafts and caveolae. Cell 106:403–411 [DOI] [PubMed] [Google Scholar]
- Gille A, Lushington GH, Mou TC, Doughty MB, Johnson RA, Seifert R. (2004) Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. J Biol Chem 279:19955–19969 [DOI] [PubMed] [Google Scholar]
- Göttle M, Geduhn J, König B, Gille A, Höcherl K, Seifert R. (2009) Characterization of mouse heart adenylyl cyclase. J Pharmacol Exp Ther 329:1156–1165 [DOI] [PubMed] [Google Scholar]
- Grandoch M, Roscioni SS, Schmidt M. (2010) The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol 159:265–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N. (2001) Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41:145–174 [DOI] [PubMed] [Google Scholar]
- Hill J, Howlett A, Klein C. (2000) Nitric oxide selectively inhibits adenylyl cyclase isoforms 5 and 6. Cell Signal 12:233–237 [DOI] [PubMed] [Google Scholar]
- Hirshman CA, Emala CW. (1999) Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol 277:L653–L661 [DOI] [PubMed] [Google Scholar]
- Hooper NM. (1999) Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review). Mol Membr Biol 16:145–156 [DOI] [PubMed] [Google Scholar]
- Hurley JH. (1999) Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem 274:7599–7602 [DOI] [PubMed] [Google Scholar]
- Kotlikoff MI, Kamm KE. (1996) Molecular mechanisms of β-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol 58:115–141 [DOI] [PubMed] [Google Scholar]
- Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. (1994) β-Adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest 93:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Thangavel M, Sun SQ, Kaminsky J, Mahautmr P, Stitham J, Hwa J, Ostrom RS. (2008) Adenylyl cyclase type 6 overexpression selectively enhances β-adrenergic and prostacyclin receptor-mediated inhibition of cardiac fibroblast function because of colocalization in lipid rafts. Naunyn Schmiedebergs Arch Pharmacol 377:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JM, Jones CA, Sankary RM, Brown JK. (1989) Differential effects of prostaglandin E2 on contractions of airway smooth muscle. J Appl Physiol 66:1397–1407 [DOI] [PubMed] [Google Scholar]
- McVey M, Hill J, Howlett A, Klein C. (1999) Adenylyl cyclase, a coincidence detector for nitric oxide. J Biol Chem 274:18887–18892 [DOI] [PubMed] [Google Scholar]
- Michel JJ, Scott JD. (2002) AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol 42:235–257 [DOI] [PubMed] [Google Scholar]
- Mou TC, Gille A, Suryanarayana S, Richter M, Seifert R, Sprang SR. (2006) Broad specificity of mammalian adenylyl cyclase for interaction with 2′,3′-substituted purine- and pyrimidine nucleotide inhibitors. Mol Pharmacol 70:878–886 [DOI] [PubMed] [Google Scholar]
- Neubig RR. (1994) Membrane organization in G-protein mechanisms. FASEB J 8:939–946 [DOI] [PubMed] [Google Scholar]
- Onda T, Hashimoto Y, Nagai M, Kuramochi H, Saito S, Yamazaki H, Toya Y, Sakai I, Homcy CJ, Nishikawa K, et al. (2001) Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J Biol Chem 276:47785–47793 [DOI] [PubMed] [Google Scholar]
- Ostrom RS. (2002) New determinants of receptor-effector coupling: trafficking and compartmentation in membrane microdomains. Mol Pharmacol 61:473–476 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Bundey RA, Insel PA. (2004) Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J Biol Chem 279:19846–19853 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Ehlert FJ. (1998) M2 muscarinic receptors inhibit forskolin- but not isoproterenol-mediated relaxation in bovine tracheal smooth muscle. J Pharmacol Exp Ther 286:234–242 [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. (2001) Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 276:42063–42069 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. (2004) The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol 143:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom RS, Post SR, Insel PA. (2000) Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving Gs. J Pharmacol Exp Ther 294:407–412 [PubMed] [Google Scholar]
- Pinto C, Papa D, Hübner M, Mou TC, Lushington GH, Seifert R. (2008) Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. J Pharmacol Exp Ther 325:27–36 [DOI] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals 17:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MG, Swan C, Jobson TM, Rees S, Hall IP. (1999) Effects of a range of β2 adrenoceptor agonists on changes in intracellular cyclic AMP and on cyclic AMP driven gene expression in cultured human airway smooth muscle cells. Br J Pharmacol 128:721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul PW, Anderson RG. (1998) Role of plasmalemmal caveolae in signal transduction. Am J Physiol 275:L843–L851 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1:31–39 [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Belvisi MG, Birrell MA, Barnes PJ, Hele DJ, Giembycz MA. (2001) Evidence that the anti-spasmogenic effect of the β-adrenoceptor agonist, isoprenaline, on guinea-pig trachealis is not mediated by cyclic AMP-dependent protein kinase. Br J Pharmacol 133:1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF, Brunton LL. (2001) Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41:751–773 [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Chiyotani A, Takeyama K, Yamauchi F, Tagaya E, Konno K. (1993) Relaxation and inhibition of contractile response to electrical field stimulation by Beraprost sodium in canine airway smooth muscle. Prostaglandins 45:363–373 [DOI] [PubMed] [Google Scholar]
- Torphy TJ. (1994) β-Adrenoceptors, cAMP and airway smooth muscle relaxation: challenges to the dogma. Trends Pharmacol Sci 15:370–374 [DOI] [PubMed] [Google Scholar]
- Torphy TJ, Freese WB, Rinard GA, Brunton LL, Mayer SE. (1982) Cyclic nucleotide-dependent protein kinases in airway smooth muscle. J Biol Chem 257:11609–11616 [PubMed] [Google Scholar]
- Wei J, Wayman G, Storm DR. (1996) Phosphorylation and inhibition of type III adenylyl cyclase by calmodulin-dependent protein kinase II in vivo. J Biol Chem 271:24231–24235 [DOI] [PubMed] [Google Scholar]
- Xu D, Isaacs C, Hall IP, Emala CW. (2001) Human airway smooth muscle expresses 7 isoforms of adenylyl cyclase: a dominant role for isoform V. Am J Physiol Lung Cell Mol Physiol 281:L832–L843 [DOI] [PubMed] [Google Scholar]
- Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. (2011) Anti-mitogenic effects of β-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J 25:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]