Abstract
P2X receptors (P2XRs) are ion channels gated by synaptically released ATP. The P2X4 is the most abundant P2XR subtype expressed in the central nervous system and to date is the most ethanol-sensitive. In addition, genomic findings suggest that P2X4Rs may play a role in alcohol intake/preference. However, little is known regarding how ethanol causes the inhibition of ATP-gated currents in P2X4Rs. We begin to address this issue by investigating the effects of ethanol in wild-type and mutant D331A and M336A P2X4Rs expressed in human embryonic kidney (HEK) 293 cells using whole-cell patch-clamp methods. The results suggest that residues D331 and M336 play a role in P2X4R gating and ethanol inhibits channel functioning via a mechanism different from that in other P2XRs. Key findings from the study include: 1) ethanol inhibits ATP-gated currents in a rapid manner; 2) ethanol inhibition of ATP-gated currents does not depend on voltage and ATP concentration; 3) residues 331 and 336 slow P2X4 current deactivation and regulate the inhibitory effects of ethanol; and 4) ethanol effects are similar in HEK293 cells transfected with P2X4Rs and cultured rat hippocampal neurons transduced with P2X4Rs using a recombinant lentiviral system. Overall, these findings provide key information regarding the mechanism of ethanol action on ATP-gated currents in P2X4Rs and provide new insights into the biophysical properties of P2X4Rs.
Introduction
P2X receptors (P2XRs) are a superfamily of cation-permeable ligand-gated ion channels activated by synaptically released extracellular ATP. P2XRs are ubiquitous in mammalian organisms and are expressed in virtually all types of tissues (Khakh, 2001; Rubio and Soto, 2001; North, 2002). ATP induces nonselective cationic currents through homomeric and heteromeric channels formed by seven subunits (P2X1-P2X7) (Torres et al., 1999; North, 2002). P2X subunits consist of two transmembrane (TM) domains connected by a large extracellular domain (ectodomain) (North, 2002; Burnstock, 2008). Crystallographic investigations have confirmed that P2XRs have a trimeric structure with a large rigid ectodomain and an α-helical segment (TM2) lining the pore (Kawate et al., 2009).
P2XRs are becoming a focus of investigation in ethanol studies because of building evidence suggesting that P2XRs are important mediators of ethanol-induced effects (Li et al., 2000; Davies et al., 2006; Asatryan et al., 2008; Tabakoff et al., 2009). ATP-gated currents are inhibited by ethanol at intoxicating and anesthetic concentrations when measured in P2X2Rs and P2X4Rs expressed in Xenopus oocytes (Xiong et al., 2000; Davies et al., 2002, 2005), whereas ATP-gated currents are potentiated by ethanol in P2X3Rs (Davies et al., 2005). Moreover, preliminary investigations found that ethanol inhibits ATP-gated currents of P2X7Rs expressed in oocytes.
The P2X4 subtype is the most abundant P2XR subtype expressed in the central nervous system (Buell et al., 1996; Soto et al., 1996). Moreover, P2X4Rs are the most ethanol-sensitive P2XRs identified to date; in the oocyte expression system ethanol inhibits P2X4R currents starting at concentrations below 10 mM (Xiong et al., 1999, 2000, 2005; Davies et al., 2002, 2005; Popova et al., 2010). In addition, findings from a genomic investigation suggest that P2X4Rs may play a role in alcohol intake and preference in rats. Specifically, whole brain expression of the p2rx4 gene in naive animals was inversely related to innate 24-h alcohol preference, and P2X4R mRNA expression was decreased in alcohol-preferring rats (Tabakoff et al., 2009). In support of this notion, we have shown that null mutant P2X4 male mice drink significantly more alcohol than their wild-type (WT) littermates (D. Davies, unpublished preliminary findings).
Investigations are beginning to shed light on potential sites of ethanol action in P2X4Rs. Previous findings suggested that the ectodomain and the TM interfaces in P2X4Rs contained targets for ethanol action (Xiong et al., 2005; Asatryan et al., 2008; Yi et al., 2009). Additional support for this hypothesis comes from a report that mutations at positions 331 or 336 in the ectodomain-TM2 region of P2X4Rs significantly reduced or eliminated the modulatory effects of ethanol (Popova et al., 2010).
Less is known regarding the mechanism of ethanol action on P2X4Rs. To date, the majority of electrophysiological investigations exploring the sites and mechanisms of ethanol action on P2XRs have used two-electrode voltage clamp on Xenopus oocytes (Weight et al., 1999; Li et al., 2000; Davies et al., 2002, 2005; Popova et al., 2010). However, there are some limitations to the use of oocytes for the study of drug effects on ion channels. The large size of the oocyte and the relatively slow perfusion rates typically used in these studies make it difficult to carry out detailed biophysical characterization of ligand-gated currents.
Whole-cell patch-clamp electrophysiology provides a higher level of resolution and insights into the biophysical properties of the receptor and the mechanism of drug-receptor interaction. However, at this time, few studies have used this approach to investigate the effects of ethanol on P2XRs. The work that is available has focused on mouse hippocampal and bullfrog DRG neurons (Li et al., 1998, 2000). These early neuronal studies found that ATP-gated currents were inhibited by ethanol in a concentration-dependent manner and that ethanol affected the rate of deactivation of the currents. Moreover, the authors of the latter study suggested that ethanol acted by altering the affinity of ATP. Overall, the findings from these initial patch-clamp studies were consistent with findings reported in oocytes (Weight et al., 1999; Li et al., 2000; Davies et al., 2002, 2005; Popova et al., 2010).
The current study represents the first comprehensive investigation that focuses on the mechanism of ethanol action on P2X4Rs expressed in a mammalian cell system using whole-cell patch-clamp technique combined with a mutagenesis approach. We found that ethanol inhibits channel gating by affecting the functioning of the receptor around residues D331 and M336 that are thought to play a role in P2X4R gating. To gain insight into the physiological consequences stemming from the ethanol effects on P2XRs, we overexpressed P2X4R in cultured rat hippocampal neurons using a recombinant lentiviral system. Investigations on these neurons revealed a similar degree and pattern of ethanol inhibition compared with those of transfected HEK293 cells. Overall, the results provide key insights into the biophysical properties of P2X4Rs and new knowledge regarding the mechanism of ethanol-receptor interaction.
Materials and Methods
Site-Directed Mutagenesis.
The cDNA of rat P2X4R (GenBank accession no. X87763) was subcloned into pcDNA3 vector (Invitrogen, Carlsbad, CA). Mutagenesis was performed to introduce single point mutations using the QuikChange IIXL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The sequences of mutant constructs were verified using automated DNA sequencing (USC/Norris DNA Core Facility, University of Southern California).
HEK293 Cell Culture.
HEK293 cells, obtained from the American Type Culture Collection (Manassas, VA), were grown in Dulbecco's modified Eagle's medium/F12 media (Invitrogen) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT) and penicillin/streptomycin in O2/CO2 (95:5%) atmosphere. Cells were seeded in six-well plates 1 day before transfections that were performed using 0.2 to 8 μg of DNA and 5 μl of Lipofectamine 2000 reagent in OPTIMEM (Invitrogen). Four to six hours after transfection, cells were trypsinized and plated in 35-mm single dishes in growth medium. Patch-clamp recordings were performed after 24 to 48 h.
Isolation of Hippocampal Neurons.
Timed-pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN) were sacrificed by CO2 inhalation (derived from a tank source). After euthanasia, embryonic day 18 (E18) rat pups were removed from the uterus and decapitated. Fetal hippocampi were dissected out, incubated in cold Hanks' buffer solution (Invitrogen), and then subjected to enzymatic digestion in 0.02% trypsin solution at 37°C for 5 min. Digested tissue was rinsed three times with Hanks' buffer solution and then dissociated with mechanical force by triturating through a series of different sizes of polished Pasteur pipettes. Cells were plated onto poly-d-lysine-coated culture dishes containing neurobasal media supplemented with B-27, primocin, and 25 μM glutamate. Media changes occurred every 3 to 4 days using supplemented neurobasal media without glutamate. The neurons were cultured for at least 5 days to allow for maturation before lentiviral infection.
Lentivirus Production and Transduction of Neurons.
cDNA encoding rat WT P2X4R was subcloned into the pLVX-AcGFP-N1 lentiviral expression vector (Clontech, Mountain View, CA) that results in a C terminus-tagged P2X4-GFP fusion protein. The lentiviral vector construct was verified by enzymatic digestion and gel electrophoresis and DNA sequencing (USC/Norris DNA Core Facility). This construct was then mixed with Lenti-X HT proprietary packaging mix (Clontech) and used with Lentiphos HT transfection system (Clontech) to transfect 293T cells (50–60% confluence) for production of vesicular stomatitis virus G pseudotyped, replication-incompetent lentivirus. Viral titers were determined using HT-1080 fibroblastoma cells and through reverse transcription-polymerase chain reaction of viral RNAs. Neurons were infected at days in vitro 5 to 7 with 106 infectious units/ml and after 48 h, transduction confirmation of protein expression was visualized by GFP fluorescence.
Patch-Clamp Recordings.
HEK293 cells and neurons were voltage-clamped at −50 mV at room temperature, and ATP-induced currents were acquired using an Axopatch 200B amplifier, Digidata 1320 interface, and pClamp 9.0 software (Molecular Devices, Sunnyvale, CA). Data were digitized at 5 kHz and filtered at 1 kHz. Composition of the external solution was 135 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose with pH of 7.4 adjusted with NaOH. Osmolarity was adjusted to 315 mmol/kg with sucrose. Patch electrodes (2–6 MΩ were filled with 140 mM KCl, 2 mM MgCl2, 2.5 mM EGTA, 2 mM TEA-Cl, 4 mM K2ATP, and 10 mM HEPES, pH 7.25 with KOH and osmolarity of 310 mmol/kg. Agonist and drug containing solutions were prepared freshly on the day of an experiment. To avoid strong run-down in P2X4R currents, ATP was applied for the shortest period of time sufficient to reach the peak in most experiments. Time between applications was 15 to 120 s depending on cell recovery from desensitization. In experiments with ethanol preapplication, cells were pretreated with ethanol for at least 30 s between ATP applications. Fast drug applications were performed through a three-barrel flow pipe using a Warner SB-77B Fast Perfusion apparatus and VC-6 Valve Controller (Warner Instruments, Hamden, CT). Solution exchange rate was tested by using solutions with different ionic strength (open tip) or different K+ concentration (whole cell). Speed of solution change in open tip mode was in the 2- to 10-ms range. In the whole-cell mode for lifted-up cells it was approximately 10 ms, whereas for attached cells it was in the range of 50 to 100 ms. Neurons were tested 9 to 14 days after isolation.
Data Analysis and Statistics.
Data were analyzed with pClamp 9.0 (Molecular Devices) and Prism (GraphPad Software Inc., San Diego, CA) software. Drug effects were expressed as normalized percentage of control peak current responses obtained with agonist alone. Agonist control responses were measured before and after each drug application to take into account possible shifts in the control currents as the recordings proceed. Each experiment was carried out with three or more cells from at least two different batches. To generate concentration-response curves, the currents evoked by different ATP concentrations were normalized to peak amplitudes evoked by 100 μM ATP. Concentration-response data were fit to Hill equation I/Imax = 1/[1 + (EC50 /C)−n], where I is the response produced by C concentration of agonist or blocker, Imax is the response at 100 μM ATP, EC50 (for activation) is the concentration of a half-maximal response, and n is the Hill coefficient (slope factor). For kinetics experiments we used single, mostly lifted-up cells. However, statistical comparison showed no difference in lifted-up and attached cells, and the data from lifted-up and attached cells were pooled together where appropriate. Statistical analysis was performed using t test and one-way ANOVA. Significance is defined as p < 0.05.
Materials.
ATP disodium salt, ethanol (190 proof, USP), and trypsin were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of reagent grade.
Results
Ethanol Inhibits P2X4Rs Expressed in HEK293 Cells.
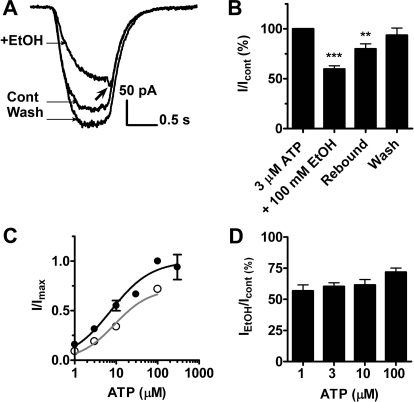
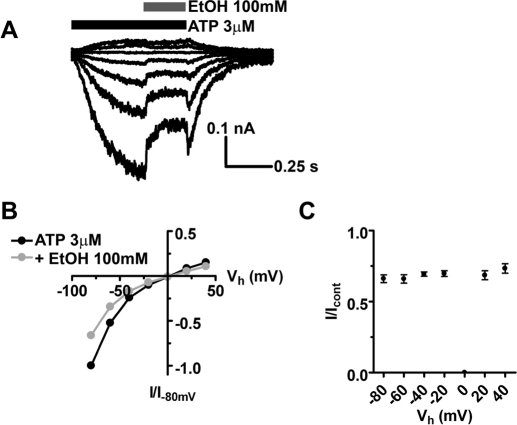
In the first investigation, ethanol (100 mM) significantly inhibited 3 μM ATP (∼EC30) currents to 59.8 ± 3.2% of control with full recovery to 93.6 ± 7.3% upon washout (Fig. 1, A and B). Ethanol applied in the absence of ATP did not significantly alter baseline current (data not shown). To gain insight into the mechanism of ethanol action, we next conducted ATP concentration response studies in the presence and absence of 100 mM ethanol. As shown in Fig. 1C, ATP elicited a robust current in a concentration-dependent manner with EC50 = 7.5 μM and Hill coefficient = 0.9. This finding agrees with previous published studies in oocytes and HEK293 cells (Xiong et al., 2000; Jelínková et al., 2006; Popova et al., 2010). Coapplication of 100 mM ethanol with ATP resulted in the ATP concentration-response curve with EC50 = 9.2 μM, Hill coefficient = 1.0. It is noteworthy that the degree of ethanol inhibition was not significantly different when tested at lower versus higher concentrations (e.g., 1 μM versus 100 μM ATP; Fig. 1D). Because of the lack of concentration dependence, we elected to use 3 μM ATP for the remainder of our experiments because it yielded reasonable amplitudes of ATP currents without causing appreciable desensitization (when application was not prolonged), and these currents were reliably inhibited by 100 mM ethanol. The lack of an obvious ATP EC dependence differed with previous work on P2X4Rs when expressed in oocytes where exposure to 100 μM ATP surmounted the ethanol effect to a greater extent than at lower concentrations of ATP (Xiong et al., 2000; Davies et al., 2005; Popova et al., 2010).
Fig. 1.
Ethanol inhibits ATP currents of P2X4Rs expressed in HEK293 cells. A, shown are 3 μM ATP-induced currents ± 100 mM ethanol. Arrow shows the characteristic rebound of current at the end of ATP/ethanol application. B, bar graph of the data analyzed from several experiments (mean ± S.D., n = 8). Ethanol inhibited 3 μM ATP-induced currents (59.8 ± 3.2% of control, P < 0.001, t test) followed by recovery (93.6 ± 7.3%). Current rebound reached 80.0 ± 5.0% of the control (P < 0.01, t test). C, ethanol (100 mM) right-shifts the concentration-response curve for P2X4R (control, ●, EC50 = 7.5 μM, Hill coefficient = 0.9, n = 4–12; in the presence of ethanol, ○, EC50 = 9.2 μM, Hill coefficient = 1.0). D, the extent of 100 mM ethanol inhibition of P2X4-mediated currents is similar at different ATP concentrations ranging from 56.8 ± 4.8 to 71.9 ± 3.3% of the control (n = 4–10, P > 0.05, ANOVA).
It is noteworthy that in the first studies a consistent and significant “rebound” of the ATP-gated currents at the end of the ethanol/ATP applications appeared to occur (Fig. 1A, arrow). That is, at the termination of ethanol/ATP application there was a reduction in the degree of ethanol inhibition (shifting from approximately 60% of the control ATP current back to 80.0 ± 5.0%; Fig. 1B). This effect was more visible when cells were in a lifted-up mode. We reasoned that the rebound was probably caused by ethanol washing off faster than ATP, resulting in an immediate relief from the inhibition. To test this notion, we next varied the timing and order of ethanol and ATP exposures using a three-barrel flow pipe.
The Onset and Offset of Ethanol Effect on P2X4R Channels Is Rapid.
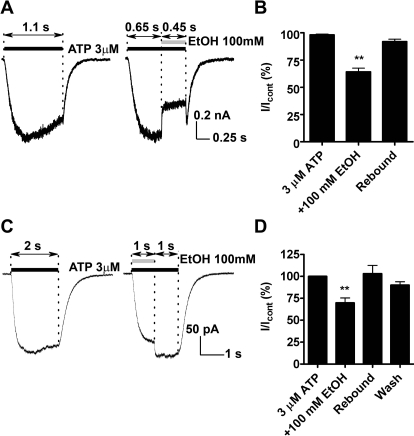
As illustrated in Fig. 2A, in the presence of ATP, ethanol inhibition of P2X4R currents and washout was rapid with rates of onset comparable with the rates of solution exchange (∼10–100 ms depending on lifted or attached cell mode). When applied with a delay to allow full channel opening, ethanol continued to caused inhibition of the ATP-gated current to the similar degree as when using a simultaneous coapplication protocol (Figs. 2, A and B and 1, A and B). In addition, simultaneous termination of both ethanol and ATP application resulted in a similar “rebound” as previously noted (Fig. 2A, right). Comparison of the two traces shown in Fig. 2A, illustrates that the maximum point of the rebound is similar in magnitude to the level of the ATP-activated currents at this time point when tested in the absence of ethanol. This finding adds support to the hypothesis that the rebound is a result of faster ethanol washing out compared with ATP.
Fig. 2.
Ethanol inhibition of ATP currents in P2X4Rs is rapid. A, left, 3 μM ATP-induced currents. Right, the same cell showing 100 mM ethanol inhibition of ATP-induced currents. In both traces ATP was applied for 1.1 s. Ethanol was added at 650 ms of ATP application when the current reached the peak. B, bar graph of the data presented as mean ± S.D. (n = 7). Ethanol inhibition reached 64.3 ± 3.4% of the control (P < 0.01). Rebound was 89.4 ± 3.4% of the control. C, left, ATP was applied for 2 s. Right, ethanol was added along with ATP during the first second. D, bar graph of the data presented as mean ± S.D. (n = 5). Ethanol inhibition reached 69.7 ± 5.6% of the control (P < 0.01). Rebound was 103 ± 9.5% of the control. No statistical differences were found between the control and rebound conditions. Only cells in the lifted-up mode were used. Statistics were assessed with repeated-measures ANOVA.
To further test this hypothesis, we changed the order of ethanol and increased the time of ethanol and ATP application. Changing the order of drug application did not affect the magnitude or the duration of the ethanol effect (Fig. 2C). However, continued application of ATP after termination of ethanol application resulted in ATP currents that were similar in magnitude compared with that of the control after the rebound (Fig. 2B). It is noteworthy that changing the order of drug application did not significantly alter the degree of ethanol inhibition or the rebound effect. A comparison of the degree of ethanol inhibition and rebound (where applicable) between the three protocols did not reveal any significant differences (Figs. 1B and 2, B and D). Taken together, the findings support the hypothesis that the observed “rebound” is caused by ethanol rapidly washing out from the receptor before ATP unbinding. Moreover, the experiments demonstrated that the time frame of our solution exchange was sufficient to allow equilibration of ethanol at its site of action. Overall, our results suggest that the effects of ethanol, as measured in P2X4Rs, are attributable to ethanol acting as a fast channel inhibitor.
Ethanol Affects P2X4R Channels in Open State.
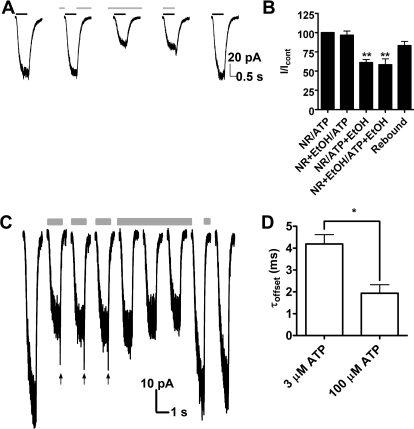
We next investigated whether the effects of ethanol are use-dependent by comparing the degree of ethanol-induced inhibition using several different drug application protocols (Fig. 3). This included protocols with and without ethanol pretreatment before coapplication with agonist. Pretreatment of ethanol for 30 to 60 s before ethanol/ATP application did not significantly change the magnitude of ethanol inhibition (illustrated in Fig. 3A, third and fourth trace, and B). In addition, there was no subsequent inhibition after ethanol was applied during the rest and the deactivation but not open states of the channel (Fig. 3A, second trace and B). It is noteworthy that leaving ethanol in the buffer while washing out ATP resulted in the absence of the rebound (Fig. 3A, third trace and C). This observation provides additional support for the hypothesis that ethanol interacts with the channel faster than ATP.
Fig. 3.
Ethanol affects P2X4R in an open state. A, traces showing the effect of different ethanol application sequences under 3 μM ATP. Black bars denote ATP and gray bars denote ethanol applications. B, bar graph showing ethanol pretreatment effects before coapplication with ATP. Pretreatment of ethanol did not affect the degree of inhibition [61.2 ± 3.9% of the control without pretreatment (P < 0.01, n = 6) versus 58.4 ± 7.5% of the control (P < 0.01, n = 5) with pretreatment]. Rebound was 83.1 ± 5.5% (P > 0.05, n = 5). C, ethanol does not exhibit use-dependent effect in consecutive applications. Arrows show rebound. Black and gray bars denote where ATP and ethanol were applied. Description of pretreatment and treatment solution constituents are separated by a slash. NR, normal Ringer (bath). ATP applications are not shown for clarity. Statistics were assessed with ANOVA and Dunnett's post-test. D, ethanol exhibits use-dependent effect. τoffset = 4.2 ± 0.4 ms at 3 μM ATP (n = 9) versus 1.9 ± 0.4 ms at 100 μM ATP (n = 3). P < 0.05, t test.
Extending our investigation to gain further insight into the mechanism of ethanol inhibition, we performed a series of multiple applications of ethanol in the presence of ATP. As shown in Fig. 3C, there was no significant change in the magnitude of ethanol inhibition from one application of ethanol to the next, confirming that the onset of ethanol action is too fast to resolve the question of use dependence using multiple applications. As such, we tested whether the speed of ethanol offset of inhibition depended on the ATP concentration. This was accomplished by estimating the offset rates at 3 and 100 μM ATP by monoexponentionally fitting the rebound curve. Indeed, as shown in Fig. 3D, the τoffset was significantly lower at high agonist concentration [τoffset = 4.2 ± 0.4 ms at 3 μM ATP (n = 9) versus 1.9 ± 0.4 ms at 100 μM ATP (n = 3)]. This result suggested a use-dependent action of ethanol when the channel is in the conducting (open) state (i.e., higher percentage of open channels allows for faster equilibration of ethanol).
Ethanol Does Not Affect Kinetics of P2X4R Currents.
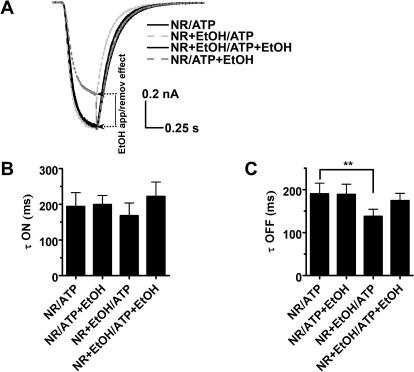
We also investigated the kinetics of current activation and deactivation using different ethanol application protocols. In accordance with previous observations (Li et al., 1998), ethanol did not change the rate of activation (Fig. 4, A and B). On the other hand, ethanol did not affect the rate of deactivation either (Fig. 4, A and C). These results contrast previous findings in bullfrog DRG neurons demonstrating that 50 to 100 mM ethanol accelerates channel deactivation (Li et al., 1994, 1998). It should be noted that DRG neurons express different P2X subunits, thus allowing for multiple receptor phenotypes (Kobayashi al., 2005). The small, but statistically significant, difference for deactivation time constant τoff in the case when ethanol was present only in the bathing solution probably was caused by a rapid effect of ethanol during the course of deactivation as shown in Fig. 4A. These data also confirmed a rapid noncompetitive onset of ethanol action during channel open state but did not distinguish whether the action of ethanol is caused by an open-channel block mechanism.
Fig. 4.
Ethanol does not affect activation and deactivation kinetics of P2X4R currents. A, representative traces of currents normalized to peak value. B, ethanol does not significantly affect current activation rates. τon (ms) values for different pretreatment/treatment protocols were: NR/ATP, 194 ± 39; NR/ATP + EtOH, 199 ± 25; NR + EtOH/ATP, 168 ± 35; and NR + EtOH/ATP + EtOH, 222 ± 40. C, ethanol has little effect on the rates of current deactivation. τoff (ms): NR/ATP, 190 ± 25; NR/ATP + EtOH, 189 ± 23; NR + EtOH/ATP, 138 ± 17; NR + EtOH/ATP + EtOH, 174 ± 17. Significant difference was found only for the pair τoff control and τoff in the presence of ethanol during ethanol pretreatment (P < 0.01, Dunnett's multiple comparison test). Currents were elicited by 3 μM ATP (n = 6).
Ethanol Effect on P2X4Rs Is Voltage-Independent.
Because our studies suggested that ethanol acts similarly to what one would expect from an open-channel blocker and because open-channel blockers commonly exhibit voltage-dependent block (Hille, 2001), we investigated the effect of ethanol on current-voltage relationship. In this experiment, we used a series of rapid ethanol applications where ethanol was applied during the same ATP pulse at different holding potentials (Fig. 5, A and B). Ethanol did not shift the reversal potential of ATP currents (∼0 mV). There was no effect of ethanol at 0 mV caused by the absence of visible current. The inhibitory effect of ethanol on P2X4R currents was voltage-independent (Fig. 5C). This outcome was not surprising given the lack of charge of ethanol molecules. Variability of ethanol effect on a cell-to-cell basis averaged from all tested voltages ranged from 55.8 to 74.8%; however, no significant difference was found (data not shown). Overall, these results are in accordance with earlier investigations of ethanol inhibition of recombinant and native P2XRs (Xiong et al., 2000).
Fig. 5.
Lack of voltage dependence of ethanol inhibitory effect in P2X4Rs. A, representative traces of ATP-induced currents in the presence of ethanol at different holding potentials. B, current-voltage (I/V) relationship for control (3 μM ATP) and ethanol - affected (100 mM) current peak amplitudes. C, the effect of 100 mM ethanol at different holding potentials averaged from 5 to 10 cells each point. Inhibited current was 66.2 ± 2.8% (n = 10) of control at −80 mV, 69.4 ± 1.5% (n = 10) of control at −40 mV, and 68.6 ± 3.1% (n = 6) at 20 mV. No significant differences were found in the presence of ethanol in the voltage range test (P > 0.05, ANOVA).
Residues 331 and 336 in P2X4Rs Play a Role in Channel Functioning and Ethanol Inhibition.
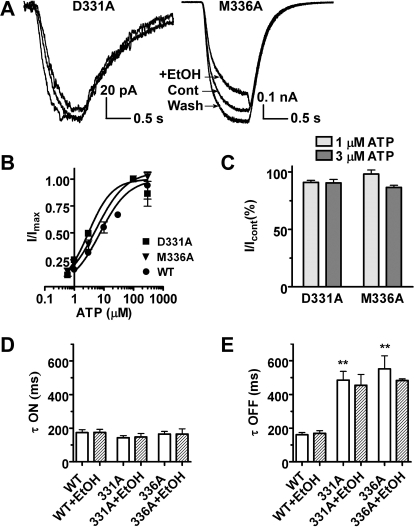
Previous investigations found that amino acid substitutions at nonconserved positions 331 (i.e., D331) and 336 (i.e., M336) in P2X4Rs significantly reduced or eliminated the effects of ethanol when tested in Xenopus oocytes using two-electrode voltage-clamp analysis (Popova et al., 2010). It is noteworthy that these mutations did not seem to be involved in agonist action and did not significantly alter basic receptor function. Mutants were slightly more sensitive to ATP than the WT receptor with EC50 = 3.0 μM, Hill coefficient = 1.08 for D331A and EC50 = 5.5 μM, Hill coefficient = 0.86 for M336A (Fig. 6B) but they were much less sensitive to 100 mM ethanol at ATP concentrations 1 and 3 μM (Fig. 6, A and C). To gain insight into the biophysical changes that the mutations might cause, we tested whether residues D331 and M336 are important for channel operation using 3 μM ATP as the agonist. We found that substituting alanine at either position 331 or 336 did not significantly alter the activation rate of the mutant receptors compared with WT (Fig. 6D) [τon for 331A, 143 ± 12 ms (n = 10); 336A, 165 ± 16 ms (n = 10); and WT, 174 ± 18 ms (n = 14)]. However, the current deactivation rate, i.e., kinetics of rapid channel closing upon agonist removal, was significantly altered in the mutant receptors compared with WT P2X4Rs (Fig. 6E) [τoff for 331A, 485 ± 52 ms (n = 10); 336A, 454 ± 65 ms (n = 10); and WT, 161 ± 14 ms (n = 14)]. Ethanol did not change the kinetic properties of activation and deactivation of the mutants compared with WT receptors (Fig. 6, D and E). In agreement with our findings in oocytes (Popova et al., 2010), current results support the hypothesis that positions 331 and 336 are important for the action of ethanol in P2X4R. Moreover, our new findings suggest that the amino acid residues at positions 331 and 336 play a role in channel functioning.
Fig. 6.
P2X4R mutants D331A and M336A reveal altered gating and decreased ethanol effect. A, representative traces showing ethanol effects on 3 μM ATP-activated currents. B, concentration-response curves for mutants: D331A, EC50 = 3.0 μM, Hill coefficient = 1.08; and M336A, EC50 = 5.5 μM, Hill coefficient = 0.86. C, bar graph showing the effect of ethanol on mutant P2X4R current amplitude. For D331A the residual current was 90.9 ± 1.9% (n = 5) at 1 μM ATP and 90.4 ± 3.1% (n = 9) at 3 μM ATP. For M336A the residual current was 98.1 ± 3.6% (n = 6) and 86.5 ± 1.9% (n = 12) at 1 and 3 μM ATP, respectively. P < 0.05 compared with ethanol effects in the WT P2X4R ANOVA. D and E, bar graphs showing activation (D) and deactivation (E) kinetics of WT and mutant P2X4R currents in the absence and presence of ethanol. P < 0.01 for τoff of both mutants versus WT (ANOVA, Dunnett's post-test).
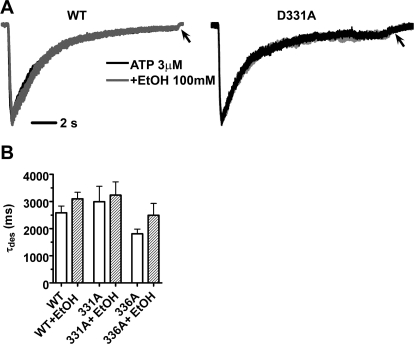
We continued this line of investigation to determine whether mutations at positions 331 and 336 and ethanol have an effect on channel desensitization, i.e., channel closing occurring during prolonged exposure to agonist. ATP (3 μM) was applied for 15 s, and decaying currents fit monoexponentially in the presence and absence of ethanol. As shown, there was not a significant difference in the rate of desensitization between WT and 331A or 336A mutant P2X4Rs [τdes 2586 ± 246% (n = 7), 2992 ± 569% (n = 6), and 1810 ± 177% (n = 6), respectively (Fig. 7B)]. This finding suggests that these mutations do not prevent the channel from proceeding normally into the desensitized state (occurring in the continuous presence of agonist). That is, substitution of alanine at either position 331 or 336 did not seem to alter the mechanism of channel desensitization compared with WT P2X4Rs. As expected, we found that the residual deactivation rate occurring after ATP removal still did proceed at a slower rate (Fig. 7A, arrows). Inclusion of ethanol in the experimental paradigm did not significantly alter channel desensitization (Fig. 7A). This latter finding suggests that ethanol does not inhibit P2X4R by increasing channel desensitization.
Fig. 7.
Ethanol does not change the rate of desensitization of P2X4Rs. A, P2X4 currents normalized to peak value in the presence and absence of 100 mM ethanol upon 15-s ATP application in WT (left) and D331A mutant (right) P2X4Rs. Arrows mark the course of slow deactivation. B, bar graph of desensitization rates of WT, D331A, and M336A in the absence and presence of ethanol. For WT, D331A, and M336A τdes was 2586 ± 246% (n = 7), 2992 ± 569% (n = 6), and 1810 ± 177% (n = 6), respectively (P > 0.05, ANOVA, Dunnett's post-test).
P2X4Rs Overexpressed in Rat Hippocampal Neurons Are Inhibited by Ethanol.
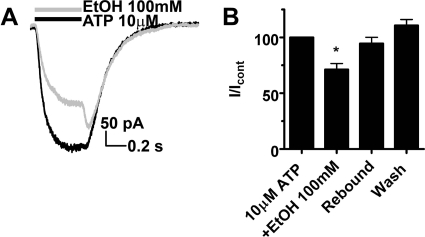
To begin investigating the physiological consequences stemming from ethanol effects on P2XRs, we overexpressed P2X4R in cultured E18 rat hippocampal neurons using a recombinant lentiviral system. Pilot studies determined that cultured neurons at this stage (days in vitro 9–14) expressed negligible levels of functioning P2XRs. Application of 100 μM ATP did not evoke any measureable currents when tested in noninfected neurons (data not shown). Thus this system allows for testing of recombinant P2XRs in a neuronal environment without the complications of native P2XRs as would be found when testing adult, freshly cultured hippocampal neurons (Li et al., 2000; Cunha, 2000; Pankratov, 2009). Results from this experiment provide initial insight into the biophysical properties and susceptibility to ethanol of P2X4Rs when expressed in neurons. Application of 10 μM ATP induced a robust response in lentivirus-transduced neurons, being 78.9 ± 2.4% (n = 3) of maximal (100 μM) response (Fig. 8). Ethanol (100 mM) significantly reduced ATP-gated currents [residual current was 71.3 ± 5.4% (n = 4)]. A prominent rebound to 94.6 ± 5.6% of control was apparent after ethanol removal. Overall, the findings in E18 neurons transduced with P2X4Rs resulted in a similar degree and pattern of ethanol inhibition compared with those of transfected HEK293 cells.
Fig. 8.
Ethanol inhibits ATP-gated currents in neurons transduced with P2X4R encoding recombinant lentivirus. A, shown are 10 μM ATP-induced currents ± 100 mM ethanol. ATP was applied for 500 ms. B, bar graph showing 100 mM ethanol inhibition of ATP-induced currents to 71.3 ± 5.4% (n = 4) with the rebound level of 94.6 ± 5.6% of the control. P < 0.05, compared with control, t test. The degree of ethanol inhibition and the typical rebound were similar to those found in HEK293 cells expressing P2X4Rs (Fig. 1).
Discussion
The current study represents the first comprehensive investigation focusing on the mechanism of ethanol action on P2X4Rs expressed/overexpressed in a mammalian cell system using whole-cell patch-clamp analysis. It is noteworthy that findings from the current investigation and previous studies demonstrate a similar degree of ethanol inhibition in both oocyte and mammalian expression systems (Xiong et al., 1999, 2000, 2005; Davies et al., 2002, 2005; Popova et al., 2010). Our data also support the previous evidence that ethanol directly interacts with P2XRs (Li et al., 1994). Furthermore, this interaction occurs at concentrations similar to those that modulate other ligand-gated ion channels that are known targets of alcohol action (Yu et al., 1996; Mihic et al., 1997; Peoples et al., 1997).
On the other hand, it does not seem that the action of ethanol can be explained by changes in membrane fluidity. Evidence has been presented over the past 30 years that the effects of alcohols on membrane lipid order are unable to account for ion channel modulation (e.g., equivalent to a <1°C change in temperature; reviewed in Franks and Lieb, 1994), and a number of studies support a direct interaction of alcohols with ion channels. For example, alcohol “cutoffs” for P2X or NMDA receptor modulation differ markedly (Li et al., 1994; Peoples and Weight, 1995; Asatryan et al., 2010). Moreover, mutations in alcohol-sensitive sites of GABAA, glycine, NMDA, nicotinic acetylcholine, and P2XRs alter ethanol sensitivity in a manner that is inconsistent with lipid disordering (e.g., Mihic et al., 1997; Ren et al., 2003; Popova et al., 2010). Finally, sulfhydryl-containing alcohol analogs directly bind to, and persistently modify, putative sites of action on GABAA and glycine receptors (e.g., Mascia et al., 2000; Crawford et al., 2007).
Taken together, the findings suggest that the mechanism of ethanol effect on P2X4Rs is similar in different mammalian systems in vitro and may be relevant in vivo. Taken together, results from the current investigation provide new insights into the biophysical properties of P2X4Rs and mechanism of ethanol action on these receptors.
Ethanol Is a Rapid Inhibitor of P2X4 Channel in Open State.
We explored different possibilities that could explain inhibitory ethanol action on P2X4Rs: 1) decrease in affinity or efficacy of agonist, i.e., ATP binding or channel gating; 2) stabilization of desensitized state; 3) change in ion permeability; and 4) open-channel block.
An interesting first outcome of the present study was that the magnitude of ethanol inhibition did not depend on the concentration of ATP tested being similar across a broad range of ATP concentrations (1–100 μM). In contrast, in previous investigations using oocytes and ATP-sensitive neurons, the action of ethanol was prominent at low ATP concentrations and insignificant at high saturating concentrations of ATP (Xiong et al., 1999, 2000, 2005; Davies et al., 2002, 2005; Popova et al., 2010). This scenario contradicts earlier suggestions that the mechanism of ethanol action is a result of an allosteric decrease in ATP affinity, i.e., that interaction with ethanol changes the conformation of the receptor, reshaping the ATP binding site (Li et al., 1998). Another fact that speaks against the mechanism of a decrease in ATP affinity is that, in this case, the channel deactivation would be accelerated. However, in our experiments τoff (as well as τon) did not change under ethanol exposure (Fig. 4).
Next, the experiments with different application protocols confirmed the idea of a rapid rate of ethanol association/dissociation with its interaction locus. The speed of onset and offset of ethanol effect was comparable with the limits of our perfusion system, i.e., in the millisecond range.
Different offset times of ethanol inhibition at high (100 μM) and low (3 μM) ATP concentrations may suggest that action of ethanol is use-dependent. This serves as evidence that ethanol shares some properties of an open-channel blocker. On the other hand, ethanol apparently did not stabilize the desensitized state (Fig. 7) and did not change the ion permeability ratio (Fig. 5). Thus, it is plausible to suggest that the open state of the P2X4 channel plays the predominant role for ethanol effects, whereas resting and desensitized states do not support ethanol interaction with the channel.
Such a mode of action is expected from a low-affinity modulator with easy access to an interaction locus, possibly through the ion channel pore or the lipid bilayer. We would like to use the data from our rapid ethanol applications to patch-clamped preparations as a means to discriminate between pathways for ethanol entry into P2X4 binding sites from the ion pore versus the lipid bilayer. However, even though we have achieved very rapid kinetics, the equilibration of a small molecule, such as ethanol, with a lipid bilayer is likely to be even faster, in a microsecond range (Trudell and Hubbell, 1976; Xu et al., 1995; Cheng et al., 2008). Indeed, the consensus is that there are multiple pathways for entry of ethanol into binding sites in P2X4R and that what we refer to as “binding” is actually a series of rapid bounces of individual ethanol molecules on and off of putative binding sites or “interaction pockets.”
D331 and M336 Residues Participate in P2X4 Channel Gating and Ethanol Blocking Effect.
The current study provided new information regarding changes in receptor function caused by mutations that resulted in a decrease of ethanol effectiveness on P2X4Rs expressed in oocytes (Popova et al., 2010). In agreement with the oocyte data, the mutations did not significantly alter basic receptor function, namely, current amplitudes and dose-response relationship characteristics. On the other hand, both M336A and D331A mutants revealed significantly slower deactivation course. The significant change in gating properties of the mutants along with presumed location of the residues 331 and 336 at the ectodomain-TM2 domain interface suggests that these sites may be located near the P2X4R gate. This notion is strongly supported by crystallographic investigations and modeling data (Kawate et al., 2009; Asatryan et al., 2010). Aligning the sequence of zebrafish P2X4 with rat P2X4 suggests that rat M336 (zebrafish L339-L340) is a welcome door of the gate in the rat orthologue with its large hydrophobic side chain capable of occluding the pore and thus serving a putative function of L340 in zebrafish P2X4. Residue D331 (zN334) may serve a similar function of regulating ion flow at the entrance of a channel pathway. Structural data suggest there is an “extracellular vestibule” at the ectodomain-TM2 interface that may concentrate and direct ions into the pore. It is noteworthy that a similar mechanism was proposed for acid-sensing ion channels (Jasti et al., 2007). Residue D331 is apparently located at the very beginning of the TM2 domain, thus it is indeed positioned at the ectodomain-TM2 interface, whereas M336 is rather positioned at the TM2 vestibule-gate interface.
Structural and electrophysiology data could be interpreted as evidence of participation of mutated residues in channel gating or as a consequence of significant difference in sensitivity to agonist. In the case of mutations it is often not possible to distinguish between the changes of affinity (i.e., binding) and efficacy (i.e., gating) without single-channel recordings (Colquhoun, 1998). A change in deactivation would generally indicate a change in agonist binding versus gating, although both entities are intrinsically interdependent (Li et al., 1997a,b; Weight et al., 1999). However, altered affinity could also be secondary to changes in gating based on the facts that 1) D331 and M336 are not conserved residues that would be expected for the ATP binding site; 2) dose-response curves normalized to the peak current do not show a dramatic change in EC50 although they are slightly shifted to the left; and 3) the presumed position of these residues in the channel pore, as based on modeling data, is more consistent with the regulation of gating.
Based on the findings from the current investigation, it is plausible to propose that ethanol affects P2X4 channel functioning through interaction with residues 331 and 336. Our modeling data also show that it is possible for these residues to be part of the same putative alcohol interaction pocket (Asatryan et al., 2010). However, we cannot distinguish between the possibilities of ethanol direct interaction with D331/M336 residues and ethanol interaction with separate loci, which change channel conformation upon mutations in these positions and render the channel invulnerable to ethanol.
Multiple Interaction Sites of Ethanol in P2XRs.
Collectively, our data hint that ethanol may be acting as an open-channel blocker for P2X4Rs. Additional support for this hypothesis comes from evidence that EtOH action is highly impaired by mutations at positions 331 and 336, which apparently constitute an important part of the channel pore. We cannot resolve the question of mechanism completely without single-channel experiments that lie beyond the scope of the present work; however, comparison with the well investigated ethanol action on NMDA receptors also supports the hypothesis of open-channel block. Similar to our findings, rapid ethanol action on NMDA receptors was characterized by a noncompetitive type of concentration-response curve and lack of effect on deactivation and desensitization (Weight et al., 1991; Lovinger, 1995; Peoples et al., 1997; Wirkner et al., 2000). However, the effect of ethanol on peak and plateau phases of the current as well as during different application protocols was not in agreement with open-channel block and this was confirmed by single-channel experiments (Wright et al., 1996).
There is also building evidence that amino acids in the extracellular domain may differentially regulate action of ethanol, suggesting that ethanol may also associate with pockets within the extracellular loop (Xiong et al., 2005; Yi et al., 2009). Each of the P2X subunits and assembled channels possess a unique structure of both an extracellular loop and a pore with the help of nonconserved residues. This structural tuning leads to unique gating properties. Thus, it is reasonable to assume that ethanol may have at least two interaction sites or regions in P2XRs. One would be located in the extracellular loop and implicate the allosteric nature of ethanol's effect on the ATP binding site that is also located in the ectodomain. A second site could be located close to a gating region. Superposition of native differences in structure of loci and affinity of ethanol for these loci may explain the difference in apparent mechanism of ethanol action on different subunits, their orthologues, and native P2X channels.
Acknowledgments
We thank Miriam Fine for conducting the molecular biology work and Jim Trudell for helpful scientific discussions.
This work was supported by the National Institutes of Health National Institutes on Alcohol Abuse and Alcoholism [Grants KO1-AA017243, RO1-AA013992, RO1-AA03972, RO-1-AA015203-01A1] (to L.A., D.L.D., R.L.A., and R.W.P., respectively); Ruth L. Kirschstein National Service Awards [Awards F31-AA017029, F31-AA018926 (to M.P. and L.W., respectively)]; Integrative Neurosciences Initiative on Alcoholism [Pilot Project AA013517] (to D.L.D.); and the University of Southern California School of Pharmacy.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.176990.
ABBREVIATIONS:
- P2XR
- P2X receptor
- WT
- wild type
- TM
- transmembrane
- ANOVA
- analysis of variance
- HEK
- human embryonic kidney
- NMDA
- N-methyl-d-aspartate
- DRG
- dorsal root ganglia
- E18
- embryonic day 18
- GFP
- green fluorescent protein
- EtOH
- ethanol
- NR
- normal Ringer.
Authorship Contributions
Participated in research design: Ostrovskaya, Asatryan, Alkana, and Davies.
Conducted experiments: Ostrovskaya, Asatryan, Wyatt, and Li.
Performed data analysis: Ostrovskaya.
Wrote or contributed to the writing of the manuscript: Ostrovskaya, Asatryan, Wyatt, Popova, Li, Alkana, Peoples, and Davies.
Other: Asatryan, Wyatt, Popova, Alkana, and Davies acquired funding for the research.
References
- Asatryan L, Popova M, Perkins D, Trudell JR, Alkana RL, Davies DL. (2010) Ivermectin antagonizes ethanol inhibition in purinergic P2X4 receptors. J Pharmacol Exp Ther 334:720–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. (2008) Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacology 55:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. (1996) An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J 15:55–62 [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. (2008) Unresolved issues and controversies in purinergic signalling. J Physiol 586:3307–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MH, Coalson RD, Cascio M. (2008) Molecular dynamics simulations of ethanol binding to the transmembrane domain of the glycine receptor: implications for the channel potentiation mechanism. Proteins 71:972–981 [DOI] [PubMed] [Google Scholar]
- Colquhoun D. (1998) Binding, gating, affinity, and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol 125:924–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DK, Trudell JR, Bertaccini EJ, Li K, Davies DL, Alkana RL. (2007) Evidence that ethanol acts on a target in loop 2 of the extracellular domain of α1 glycine receptors. J Neurochem 102:2097–2109 [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA. (2000) ATP as a presynaptic modulator. Life Sci 68:119–137 [DOI] [PubMed] [Google Scholar]
- Davies DL, Asatryan L, Kuo ST, Woodward JJ, King BF, Alkana RL, Xiao C, Ye JH, Sun H, Zhang L, et al. (2006) Effects of ethanol on adenosine 5′-triphosphate-gated purinergic and 5-hydroxytryptamine3 receptors. Alcohol Clin Exp Res 30:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. (2005) Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology 49:243–253 [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. (2002) Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol Clin Exp Res 26:773–778 [PubMed] [Google Scholar]
- Franks NP, Lieb WR. (1994) Molecular and cellular mechanisms of general anaesthesia. Nature 367:607–614 [DOI] [PubMed] [Google Scholar]
- Hille B. (2001) Ion Channels of Excitable Membranes, Sinauer Associates, Sunderland, MA [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449:316–323 [DOI] [PubMed] [Google Scholar]
- Jelínková I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, Zemková H. (2006) Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun 349:619–625 [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. (2001) Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2:165–174 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. (2005) Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol 481:377–390 [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1994) Alcohol action on a neuronal membrane receptor: evidence for a direct interaction with the receptor protein. Proc Natl Acad Sci USA 91:8200–8204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1997a) Inhibition of ATP-activated current by zinc in dorsal root ganglion neurones of bullfrog. J Physiol 505:641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1997b) Mg2+ inhibition of ATP-activated current in rat nodose ganglion neurons: evidence tat Mg2+ decreases the agonist affinity of the receptor. J Neurophysiol 77:3391–3395 [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. (1998) Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br J Pharmacol 123:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiong K, Weight FF. (2000) Ethanol inhibition of adenosine 5′-triphosphate-activated current in freshly isolated adult rat hippocampal CA1 neurons. Neurosci Lett 295:77–80 [DOI] [PubMed] [Google Scholar]
- Lovinger DM. (1995) Developmental decrease in ethanol inhibition of N-methyl-d-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther 274:164–172 [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389:385–389 [DOI] [PubMed] [Google Scholar]
- North RA. (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. (2009) P2X receptors and synaptic plasticity. Neuroscience 158:137–148 [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. (1995) Cutoff in potency implicates alcohol inhibition of N-methyl-d-aspartate receptors in alcohol intoxication. Proc Natl Acad Sci USA 92:2825–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF. (1997) Ethanol inhibition of N-methyl-d-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol 122:1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt LR, Li K, Alkana RL, Davies DL. (2010) A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem 112:307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Honse Y, Peoples RW. (2003) A site of alcohol action in the fourth membrane-associated domain of the N-methyl-d-aspartate receptor. J Biol Chem 278:48815–48820 [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. (2001) Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci 21:641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stühmer W. (1996) P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA 93:3684–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, et al. (2009) Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol 7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274:6653–6659 [DOI] [PubMed] [Google Scholar]
- Trudell JR, Hubbell WL. (1976) Localization of molecular halothane in phospholipid bilayer model nerve membranes. Anesthesiology 44:202–205 [DOI] [PubMed] [Google Scholar]
- Weight FF, Li C, Peoples RW. (1999) Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors). Neurochem Int 35:143–152 [DOI] [PubMed] [Google Scholar]
- Weight FF, Lovinger DM, White G. (1991) Alcohol inhibition of NMDA channel function. Alcohol Alcohol Suppl 1:163–169 [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. (2000) Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol 362:568–576 [DOI] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, Weight FF. (1996) Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res 738:249–256 [DOI] [PubMed] [Google Scholar]
- Xiong K, Hu XQ, Stewart RR, Weight FF, Li C. (2005) The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br J Pharmacol 145:576–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. (2000) Inhibition by ethanol of rat P2X4 receptors expressed in Xenopus oocytes. Br J Pharmacol 130:1394–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. (1999) Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol 81:2088–2094 [DOI] [PubMed] [Google Scholar]
- Xu Y, Tang P, Zhang W, Firestone L, Winter PM. (1995) Fluorine-19 nuclear magnetic resonance imaging and spectroscopy of sevoflurane uptake, distribution, and elimination in rat brain. Anesthesiology 83:766–774 [DOI] [PubMed] [Google Scholar]
- Yi CL, Liu YW, Xiong KM, Stewart RR, Peoples RW, Tian X, Zhou L, Ai YX, Li ZW, Wang QW, et al. (2009) Conserved extracellular cysteines differentially regulate the inhibitory effect of ethanol in rat P2X4 receptors. Biochem Biophys Res Commun 381:102–106 [DOI] [PubMed] [Google Scholar]
- Yu D, Zhang L, Eiselé JL, Bertrand D, Changeux JP, Weight FF. (1996) Ethanol inhibition of nicotinic acetylcholine type α7 receptors involves the amino-terminal domain of the receptor. Mol Pharmacol 50:1010–1016 [PubMed] [Google Scholar]