Abstract
This study explores the in vivo effects of the proposed transient receptor potential ankyrin 1 (TRPA1) agonist 4-oxo-2-nonenal (4-ONE). Pharmacological inhibitors and genetically modified mice were used to investigate the ability of 4-ONE to act via TRPA1 receptors and possible mechanisms involving transient receptor potential vanilloid 1 (TRPV1). We hypothesized that 4-ONE activates sensory nerves, via TRPA1 or possibly TRPV1, and thus triggers mechanical hyperalgesia, edema formation, and vasodilatation in mice. An automated dynamic plantar aesthesiometer was used to determine hind paw withdrawal thresholds, and a laser Doppler flowmeter was used to measure skin blood flow. Edema formation was determined by measuring paw weights and thickness. 4-ONE (10 nmol) triggers unilateral mechanical hyperalgesia, edema formation, and vasodilatation in mice and is shown here to exhibit TRPA1-dependent and -independent effects. Neurogenic vasodilatation and mechanical hyperalgesia at 0.5 h postinjection were significantly greater in TRPA1 wild-type (WT) mice compared with TRPA1 knockout (KO) mice. Edema formation throughout the time course as well as mechanical hyperalgesia from 1 to 4 h postinjection were similar in WT and TRPA1 KO mice. Studies involving TRPV1 KO mice revealed no evidence of TRPV1 involvement or interactions between TRPA1 and TRPV1 in mediating the in vivo effects of 4-ONE. Previously, 4-ONE was shown to be a potent TRPA1 agonist in vitro. We demonstrate its ability to mediate vasodilatation and certain nociceptive effects in vivo. These data indicate the potential of TRPA1 as an oxidant sensor for vasodilator responses in vivo. However, 4-ONE also triggers TRPA1-independent effects that relate to edema formation and pain.
Introduction
Transient receptor potential ankyrin 1 (TRPA1) is the most recently identified mammalian member of the TRP superfamily that contains six transmembrane domains with a pore-forming region located between the fifth and sixth transmembrane domains (Clapham, 2003). Functional TRPA1, which is likely to assemble as a tetramer, conducts cations such as Na+ and Ca2+ upon activation (Barritt and Rychkov, 2005). TRPA1 is primarily considered to be expressed in sensory neurons, where it is coexpressed in approximately 50% of all transient receptor potential vanilloid 1 (TRPV1)-positive sensory neurons that also contain and release the neuropeptides substance P and calcitonin gene-related peptide (CGRP) (Geppetti et al., 2008; Nassenstein et al., 2008; Streng et al., 2008).
TRPA1 was initially identified as the receptor for a variety of pungent compounds that can be extracted from wasabi [6-(methylsulfinyl)hexyl isothiocyanate], mustard oil (allyl isothiocyanate), cinnamon (cinnamaldehyde), or garlic (allicin and diallyl disulfide) (Bandell et al., 2004; Bautista et al., 2005, 2006; Macpherson et al., 2007b). It is now realized that TRPA1 can be activated by a range of compounds including reactive oxygen species such as hydrogen peroxide as well as products of lipid peroxidation such as 4-hydroxynonenal (4-HNE), and the prostaglandin metabolite 15-deoxy-Δ12,14-prostaglandin J2 (Trevisani et al., 2007; Andersson et al., 2008; Sawada et al., 2008; Taylor-Clark et al., 2008b). Activation is caused by the possession of an electrophilic carbon or sulfur (Macpherson et al., 2007a) that acts via covalent modifications of nucleophilic cysteine side chains in the intracellular N terminus of TRPA1 (Hinman et al., 2006; Macpherson et al., 2007a). Several TRPA1 agonists have been shown to trigger acute pain behaviors, hyperalgesia and neurogenic inflammation in animal and human studies (Eid et al., 2008). Our most recent evidence shows that mustard oil and cinnamaldehyde induce neurogenic vasodilatation in mouse skin that is TRPA1-dependent (Pozsgai et al., 2010). Far less is known about the potential of lipid peroxidation products to act as endogenous mediators of TRPA1-dependent peripheral responses. Evidence indicates that 4-HNE acts in vivo to mediate nociception and neurogenic inflammation via TRPA1 (Trevisani et al., 2007), and 4-ONE activates mouse bronchopulmonary fibers via TRPA1- and TRPV1-dependent mechanisms in vitro (Taylor-Clark et al., 2008a). It is noteworthy that 4-ONE has not previously been used in vivo. However, it has been shown to be the most potent activator of TRPA1 in vitro through study of TRPA1 expressed in Chinese hamster ovary cells with an EC50 for 4-ONE of 1.9 μM compared with an EC50 of 19.9 μM for 4-HNE (Andersson et al., 2008) and also in the mouse lung (Taylor-Clark, et al., 2008b). These in vitro data are consistent with the higher chemical reactivity of 4-ONE (Lin et al., 2005). Here, we have examined the dependence of 4-ONE on TRPA1 in vivo to influence peripheral nociceptive and peripheral vascular responses, which is of potential importance in understanding the response of the peripheral tissues to oxidants. We have also investigated the possible involvement of TRPV1 in these responses.
Materials and Methods
Animals.
All experiments were carried out in accordance with the UK Home Office Animals (Scientific Procedures) Act of 1986. Female CD1 mice (25–30 g) were purchased from Charles River (Margate, Kent, UK). We used age- and sex-matched male and female wild-type (WT) and TRPV1 knockout (KO) mice on a C57BL6/129SVJ strain (Clark et al., 2007) as well as WT and TRPA1 KO mice on a mixed genetic background as described previously (Kwan et al., 2006; Andersson et al., 2008; Starr et al., 2008) and WT and CGRP KO mice on a C57BL/6 strain (Grant et al., 2005; Starr et al., 2008); all mice were bred in-house. Mice were housed in a climatically controlled environment, maintained on a 12:12-h light/dark cycle, with access to food and water ad libitum. Anesthesia for nonrecovery procedures was induced by an intraperitoneal injection of a ketamine (75 mg · kg−1) + medetomidine (1mg · kg−1) mixture. Recovery procedures were conducted under 2% isoflurane anesthesia. Intraplantar injections were administered using 30G BD microfine syringes, and intraperitoneal injections were administered using 27G needles. In total, 65 CD1 mice, 47 WT and TRPA1 KO mice, 45 WT and TRPV1 KO mice, and 26 WT and CGRP KO mice were used for this study.
Mechanical Hyperalgesia.
A dynamic plantar aesthesiometer (Ugo Basile, Comerio, Italy) was used to test paw withdrawal thresholds in the hind paws of mice. Mice were placed on a wire-mesh platform, and a metal filament was directed under the hind paws that exerted an increasing upward pressure at a preset rate (10 g/s) until the paw was withdrawn. Measurements were taken in triplicate, and the average was taken as paw withdrawal threshold in grams. Measurements were taken at baseline and at several time points after the intraplantar injection of 4-ONE (1–30 nmol in 50 μl) in ipsilateral paws and vehicle (1% ethanol in saline) in contralateral paws, according to a randomized injection pattern. A significant reduction in paw withdrawal thresholds (g) compared with baseline values was defined as mechanical hyperalgesia. In some experiments, 4-(4-chlorophenyl)-3-methyl-3-buten-2-one oxime (AP18) (25 nmol in 25 μl of a 1 mM solution; Petrus et al., 2007; Fernandes, et al., 2010), a small-molecule TRPA1 receptor antagonist, was given intraplantarly 15 min before 4-ONE or vehicle injections. The injection pattern was always randomized, and all measurements were carried out by an observer blinded to treatment regimes and genotype of the mice.
Measurement of Paw Thickness.
Paw thickness was measured to determine the development of edema in the hind paws. Engineer's calipers were used to measure paw thickness in millimeters both before any injections and at several time points postinjection. Alternatively, weights (mg) of vehicle- and drug-treated paws were compared to determine paw mass as an assessment of paw swelling (Gühring et al., 2000).
Laser Doppler Flowmetry.
Cutaneous blood flow in mouse hind paws was assessed using a two-channel laser Doppler flowmeter (Moor Instruments, Devon, UK) connected to a PowerLab (ADInstruments Ltd., Chalgrove, Oxfordshire, UK). Blood flow probes were placed above the plantar surface of the hind paw of anesthetized mice. Baseline measurements were obtained to ensure similar blood flow, and 4-ONE (10 nmol in 50 μl) or vehicle (1% ethanol in saline) was injected into either hind paw. Changes in blood flow upon these treatments were followed for 0.5 h. Results were expressed as area under the recorded flux versus time for the entire recording period.
Reagents.
Chemicals used were: 4-oxo-2-nonenal (C9H14O2) (Cayman Europe, Tallinn, Estonia); AP18 (C11H12CINO) (Maybridge Chemicals, Trevillet, UK); ketamine (C13H16ClNOHCl) and medetomidine (C13H16N2) (Pfizer Central Research, Sandwich, Kent, UK); 0.9% saline [sodium chloride (HCl) pyrogen free] (Baxter Healthcare, Berkshire, UK); DMSO [(CH3)2SO] (Sigma-Aldrich, Poole, Dorset, UK); ethanol (C2H6O) (BDH, Poole, Dorset, UK) and Tween 80 (C24H44O6) (Sigma-Aldrich).
Statistical Analysis.
Results are presented as mean ± S.E.M. Prism software (GraphPad Software Inc., San Diego, CA) was used for two-sampled paired or unpaired Student's t tests or one-way or two-way analysis of variance followed by Bonferroni's multiple comparison test or a repeated-measures analysis of variance followed by Dunnet's comparison test as required by the data. Values of p <0.05 were considered statistically significant.
Results
4-ONE Induces Dose- and Time-Dependent Mechanical Hyperalgesia and Edema Formation in CD1 Mice.
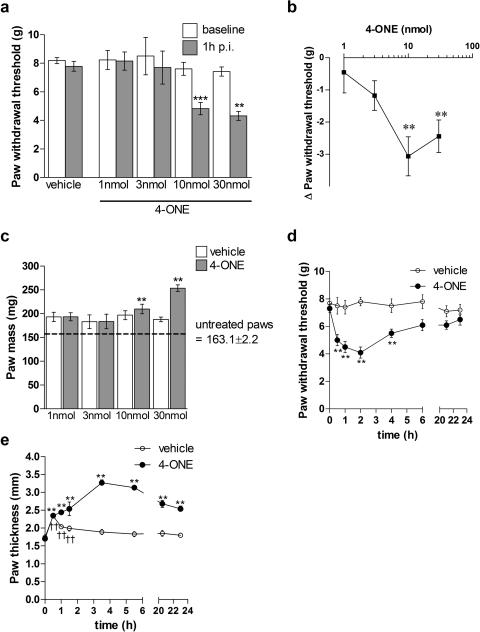
The effects of intraplantar injections of 1 nmol to 30 nmol 4-ONE in 50 μl in the ipsilateral paw or 50 μl of vehicle, 1% ethanol in saline, in the contralateral paw were examined. Intraplantar injections of 10 and 30 nmol 4-ONE induced a significant reduction in paw withdrawal thresholds compared with respective baseline values, thus signifying the development of mechanical hyperalgesia (Fig. 1, a and b). Lower doses had no observed effect. Likewise, only the higher doses of 10 or 30 nmol 4-ONE were seen to induce a significant increase in paw mass compared with that of vehicle-treated paws (Fig. 1c). It has to be noted that both vehicle- and 4-ONE-treated paws at all concentrations showed a larger paw mass than untreated paws, which was probably caused by an injection volume artifact. Furthermore, 4-ONE could elicit a significant decrease in withdrawal threshold when injected into both hind paws of the same mouse (data not shown).
Fig. 1.
4-ONE induced pain-related behaviors and increase in paw thickness are dose- and time-dependent in CD1 mice. a, paw withdrawal thresholds (g) were determined in hind paws of female CD1 mice after the intraplantar injections of 4-ONE (1–30 nmol/50 μl per paw) or vehicle (1% ethanol in saline) at 1 h postinjection. Results are shown as mean ± S.E.M. **, p < 0.01; ***, p < 0.001 versus baseline (n = 4–7). b, the same data are shown here as the difference (Δ) in paw withdrawal thresholds (g) between 1 h postinjection and baseline values for 4-ONE (1–30 nmol/50 μl per paw)-treated hind paws. Results are shown as mean ± S.E.M. **, p < 0.01 versus baseline (n = 4–7). c, paw mass (mg) of vehicle and 4-ONE (1–30 nmol/50 μl per paw)-treated hind paws as well as untreated hind paws were taken at 1 h postinjection as a measure of edema formation. Results are shown as mean ± S.E.M. **, p < 0.01 versus contralateral vehicle treated paws (n = 4–7). d, paw withdrawal thresholds (g) of vehicle and 4-ONE (10 nmol/50 μl)-treated hind paws were measured throughout a 24-h time course. Results are shown as mean ± S.E.M. **, p < 0.01 versus baseline (n = 8). e, paw thickness (mm) of vehicle and 4-ONE (10 nmol/50 μl)-treated hind paws was measured throughout a 24-h time course. Results are shown as mean ± S.E.M. **, p < 0.01 versus baseline for 4-ONE-treated paws; ††, p < 0.01 versus baseline for vehicle-treated paws (n = 8).
A dose of 4-ONE (10 nmol) was then chosen to study the time-dependent effects of 4-ONE. Paw withdrawal thresholds (g) were measured at baseline and throughout a 24-h time course. There was a significant reduction in paw withdrawal thresholds from 0.5 to 4 h postinjection of 4-ONE-treated paws compared with baseline values. This demonstrates that 10 nmol 4-ONE induced a unilateral mechanical hyperalgesia that had an immediate onset and lasted until 4 h postinjection (Fig. 1d). Paw thickness (mm) was followed throughout the time course. Both 4-ONE- and vehicle-treated paws showed significant initial increase in paw thickness until 1.5 h postinjection compared with baseline paw thickness, because of an injection volume artifact. It is noteworthy that 4-ONE induced a significant increase in paw thickness throughout the 24-h time course, whereas paw thickness of vehicle-treated mice returned to baseline values by 1.5 h postinjection (Fig. 1e).
4-ONE Has Both TRPA1-Dependent and -Independent Effects.
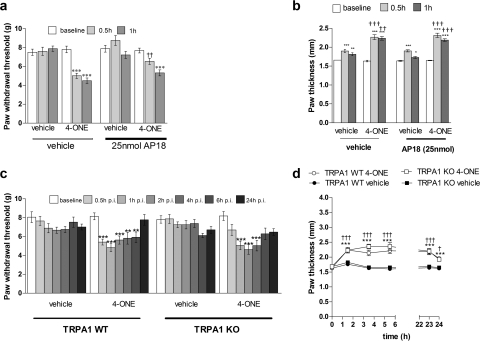
To determine whether the in vivo effects of 4-ONE were mediated by TRPA1, we used both the established TRPA1 antagonist AP18 and WT and TRPA1 KO mice. 4-ONE induced a significant reduction in paw withdrawal thresholds (g) at 0.5 and 1 h postinjection when paws were pretreated with the vehicle for AP18. However, in paws that were pretreated with the TRPA1 antagonist AP18, 4-ONE induced mechanical hyperalgesia at 1 h postinjection but not at 0.5 h postinjection (Fig. 2a). In addition, there was no significant difference between paw thickness (mm) of vehicle- and 4-ONE-treated hind paws (Fig. 2b). To determine the selectivity of the dose of TRPA1 antagonist used, we tested it in WT and TRPA1 KO mice. Here, the response to 4-ONE in WT mice was significantly attenuated at 0.5 h, and a similar response was observed in TRPA1 KO mice, whether they received the antagonist or not (Fig. 3c). Again, 4-ONE-induced edema formation was not affected by deletion or antagonism of TRPA1 (Fig. 3d). Higher doses were not used, because preliminary evidence suggested that higher doses induced a nonspecific inflammation.
Fig. 2.
4-ONE has both TRPA1 receptor-dependent and -independent in vivo effects. a, hind paws were pretreated with the TRPA1 receptor antagonist AP18 (25 nmol/25 μl) or vehicle (25 μl of 1% DMSO, 0.5% Tween 80 in PBS) and were then treated with 4-ONE (10 nmol/25 μl) or vehicle (25 μl of 1% ethanol in saline). Paw withdrawal thresholds (g) were measured at 0.5 and 1 h postinjection in CD1 mice. Results are shown as mean ± S.E.M. ***, p < 0.001 versus baseline; ††, p < 0.01 versus 4-ONE-treated paws in vehicle-treated mice (n = 9–10). b, paw thickness (mm) at baseline and 0.5 and 1 h postinjection are shown as a measure of edema formation. Results are shown as mean ± S.E.M. ***, p < 0.001 versus baseline values; †††, p < 0.001 versus vehicle-pretreated paws at corresponding time points postinjection (n = 8–9). c, paw withdrawal thresholds (g) were measured throughout a 24-h time course in WT and TRPA1 KO mice after the intraplantar injection of vehicle or 4-ONE (10 nmol). Results are shown as mean ± S.E.M. **, p < 0.01; ***, p < 0.001 versus baseline (n = 8). d, paw thickness (mm) was measured in WT and TRPA1 KO mice after the intraplantar injection of vehicle or 4-ONE (10 nmol) throughout the 24-h time course. Results are shown as mean ± S.E.M. **, p < 0.01 and ***, p < 0.001 versus baseline for TRPA1 WT mice; †, p < 0.05 and †††, p < 0.001 versus baseline for TRPA1 KO mice (n = 13).
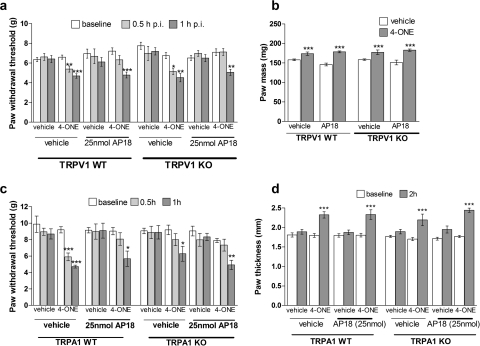
Fig. 3.
Lack of interplay between the TRPA1 and TRPV1 receptors in mediating 4-ONE-induced mechanical hyperalgesia. a, WT and TRPV1 KO mice were pretreated with the TRPA1 receptor antagonist AP18 (25 nmol/25 μl) or vehicle (25 μl of 1% DMSO, 0.5% Tween 80 in PBS) before intraplantar 4-ONE (10 nmol/25 μl) or vehicle (25 μl of 1% ethanol in saline). Paw withdrawal thresholds (g) were measured at 0.5 and 1 h postinjection. Results are shown as mean ± S.E.M. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus baselines (n = 6–11). b, paw mass (mg) as a measure of 4-ONE-induced edema formation was taken at 1 h postinjection. Results are shown as mean ± S.E.M. ***, p < 0.001 versus vehicle-treated hind paws (n = 6–11). c, paw withdrawal thresholds (g) after intraplantar injections of 4-ONE (10 nmol/25 μl) or vehicle (25 μl of 1% ethanol in saline) and the local pretreatment with AP18 (25 nmol/25 μl) or vehicle (25 μl of 1% DMSO, 0.5% Tween 80 in PBS) in WT and TRPA1 KO mice. Results are shown as mean ± S.E.M. (n = 5–6). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus respective baseline values. d, paw thickness (mm) of vehicle and 4-ONE (10 nmol/25 μl)-treated hind paws after the local pretreatment with AP18 (25 nmol/25 μl) or vehicle (25 μl of 1% DMSO, 0.5% Tween 80 in PBS) in WT and TRPA1 KO mice. Results are shown as mean ± S.E.M. (n = 5–6). ***, p < 0.001 versus respective baseline values.
These results were followed up using WT and TRPA1 KO mice that were given intraplantar injections of 4-ONE (10 nmol) or vehicle. In WT mice, intraplantar 4-ONE induced a significant reduction in paw withdrawal thresholds from 0.5 h postinjection until 6 h postinjection (Fig. 2c). There was no change in paw withdrawal thresholds in vehicle-treated paws. The mechanical hyperalgesia seemed more sustained in TRPA1 WT mice than in CD1 mice (Fig. 1a). We were surprised to find that in TRPA1 KO mice intraplantar injections of 4-ONE triggered significant mechanical hyperalgesia at 1, 2, and 4 h postinjection but not at 0.5 and 6 h postinjection (Fig. 2c). 4-ONE induced a significant increase in paw thickness compared with baseline values in both WT and TRPA1 KO mice (Fig. 2d).
TRPV1 Receptors Are Not Involved in Mediating 4-ONE-Induced Responses.
The above findings led to an investigation of the possible contribution of TRPV1 receptors to 4-ONE-induced in vivo responses in WT and TRPV1 KO mice. Previous evidence demonstrated that TRPA1 and TRPV1 receptors can be colocalized in sensory nerves and that 4-ONE activities may involve a TRPV1 component (Taylor-Clark et al., 2008a). In addition, to investigate a possible interaction of the TRPA1 and TRPV1 receptors these mice were pretreated with the TRPA1 receptor antagonist AP18. WT and TRPV1 KO mice pretreated with vehicle developed mechanical hyperalgesia at 0.5 and 1 h after the injection of 4-ONE. On the other hand, AP18 pretreatment in WT and TRPV1 KO mice blocked 4-ONE-induced mechanical hyperalgesia at 0.5 h postinjection but not at 1 h postinjection (Fig. 3a). Vehicle injections did not change paw withdrawal thresholds in any of the treatment conditions. Paw weights were taken at 1 h postinjection to determine 4-ONE-induced edema formation, and it was seen that 4-ONE-induced edema formation is independent of TRPV1 as well as TRPA1 receptors (Fig. 3b).
4-ONE-Induced Vasodilatation Depends on TRPA1 and CGRP.
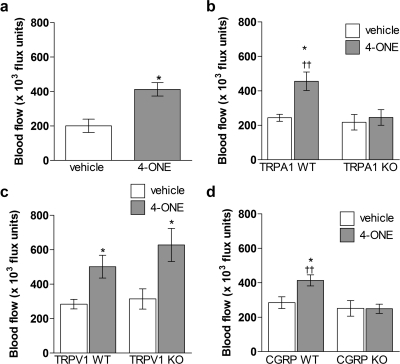
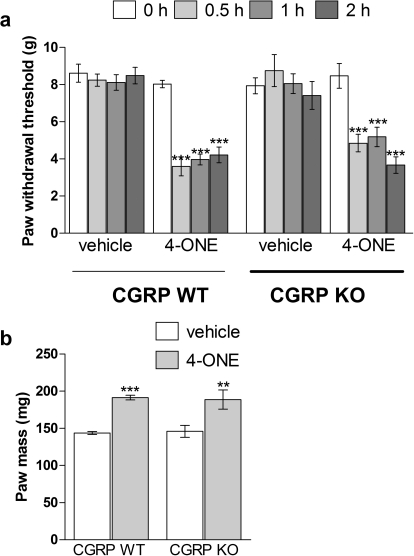
The effect of 10 nmol 4-ONE on hind paw blood flow was investigated. Previous studies have shown that topical application of mustard oil induces a potent TRPA1-dependent vasodilatation (Pozsgai et al., 2010), but 4-ONE is not absorbed into the skin via topical application. Here, intraplantar injections of 50 μl of 4-ONE or vehicle were given into either hind paw, and plantar blood flow was measured for 30 min. In genetically unaltered CD1 mice 10 nmol 4-ONE induced a significant and sustained increase in blood flow compared with vehicle injections (Fig. 4a). A range of genetically modified mice were then used to investigate signaling mechanisms involved in 4-ONE-induced vasodilatation. It is noteworthy that 4-ONE induced a vasodilatation measured over 0.5 h that depended on TRPA1 but was independent of TRPV1 (Fig. 4, b and c). In addition, 4-ONE induced a vasodilatation in CGRP WT mice that was not observed in CGRP KO mice, further pointing toward the activation of peripheral sensory nerves by 4-ONE (Fig. 4d). It is noteworthy that in both WT and CGRP KO mice 10 nmol 4-ONE triggered a significant reduction in paw withdrawal thresholds compared with baseline values from 0.5 to 2 h postinjection (Fig. 5a). In addition, 10 nmol 4-ONE triggered a significant increase in paw mass at 2 h postinjection in both WT and CGRP KO mice (Fig. 5a). This indicates that, although CGRP is pivotal for the development of 4-ONE-induced vasodilatation, it is not involved in mediating pain signals.
Fig. 4.
4-ONE-induced vasodilatation depends on the TRPA1 receptor as well as CGRP release. Blood flow (×103 flux units) was measured for 0.5 h after the intraplantar injection of 4-ONE (10 nmol/50 μl) or vehicle in the hind paws of CD1 mice (a), WT and TRPA1 KO mice (b), WT and TRPV1 KO mice (c), and WT and CGRP KO mice (d). Results are shown as mean ± S.E.M. In a, *, p < 0.05 versus vehicle-treated paws (n = 7). In b, *, p < 0.05 versus vehicle-treated hind paw; ††, p < 0.01 versus 4-ONE-treated hind paw in TRPA1 KO mice (n = 6–9). In c, *, p < 0.05 versus vehicle-treated hind paws (n = 6–8). In d, *, p < 0.05 versus vehicle-treated hind paw; ††, p < 0.01 versus 4-ONE-treated hind paw in CGRP KO mice (n = 9–17).
Fig. 5.
Effect of CGRP deletion on 4-ONE-induced mechanical hyperalgesia. a, paw withdrawal thresholds (g) were determined in hind paws of WT and CGRP KO mice after the intraplantar injections of 4-ONE (10 nmol/50 μl) or vehicle (1% ethanol in saline). Measurements were taken at baseline, 0.5, 1, and 2 h postinjection. Results are shown as mean ± S.E.M. ***, p < 0.001 versus baseline (n = 6–7). b, paw mass (mg) as a measure of 4-ONE-induced edema formation was taken at 2 h postinjection. Results are shown as mean ± S.E.M. **, p < 0.01; ***, p < 0.001 versus vehicle-treated hind paws (n = 6–7).
Discussion
This study has examined the in vivo effects of 4-ONE, a selective TRPA1 agonist in vitro (Andersson et al., 2008; Taylor-Clark et al., 2008a). We hypothesized that activation of TRPA1, which is expressed mainly on peptidergic C and Aδ-fibers, by 4-ONE would trigger the development of mechanical hyperalgesia and neurogenic edema and also lead to vasodilatation in vivo. Here, we demonstrate that 4-ONE (10 nmol) induced a dose- and time-dependent unilateral mechanical hyperalgesia and edema formation in vivo, with only a component of the mechanical hyperalgesia mediated by TRPA1 and no evidence for a role of TRPA1 in inflammatory swelling. We present novel evidence that 4-ONE has the ability to trigger TRPA1-dependent neurogenic vasodilatation in vivo.
4-ONE is an electrophilic ketoaldehyde that is derived from oxidized ω-6-polyunsaturated fatty acids such as arachidonic acid and can function as a mediator of oxidative stress (Lin et al., 2005). 4-ONE can form stable Michael adducts with cysteine and lysine residues in vitro and activate TRPA1 because of its electrophilic properties (Uchida, 2000; Lin et al., 2005; Andersson et al., 2008). These amino acids are commonly found in proteins and can thus be targeted by 4-ONE. 4-ONE is broken down enzymatically in vitro and in vivo, leading to the formation of reactive metabolites such as 4-HNE or 4-oxo-2-nonen-1-ol (Kuiper et al., 2008; Shimozu et al., 2009). In addition, it can form conjugates with other proteins such as glutathione (Kuiper et al., 2008). It is possible that 4-ONE metabolites or reactions with other proteins contribute to the TRPA1-independent effects seen in the present study. However, 4-ONE has been shown to selectively activate TRPA1 in dissociated dorsal root ganglion neurons in vitro from WT but not TRPA1 KO mice (Andersson et al., 2008). In addition, Taylor-Clark et al. (2008a) demonstrated that 4-ONE activates vagal bronchopulmonary C-fibers in vitro via TRPA1. In the present study, we have extended knowledge to demonstrate that 4-ONE induces unilateral mechanical hyperalgesia, edema formation, and vasodilatation in mice; 10 nmol 4-ONE was chosen for all detailed studies, and it was shown that 4-ONE-induced mechanical hyperalgesia is present from 0.5 h postinjection until 4 h postinjection. Similar results were previously seen with the related TRPA1 agonist 4-HNE, where the substantially higher dose of 150 nmol 4-HNE triggered unilateral mechanical hyperalgesia and edema in the rat hind paw (Trevisani et al., 2007). It is evident that 4-ONE is more potent at inducing mechanical hyperalgesia than 4-HNE in vivo, in keeping with in vitro data (Lin et al., 2005; Andersson et al., 2008). However, it seems that although 4-HNE displays a selectivity for TRPA1 in vitro and in vivo, 4-ONE-induced in vivo responses are only partially mediated by the TRPA1 receptor. It is noteworthy that Taylor-Clark et al. (2008) suggested that 4-ONE has the ability to activate TRPV1 receptors in vitro; however, we did not observe TRPV1 receptor-dependent responses using the present protocols in vivo.
Hyperalgesia develops as a result of activation of sensory C-fibers that express a range of receptors and ion channels, including TRPA1 and TRPV1, to be able to integrate various noxious stimuli. We hypothesized that 4-ONE induces mechanical hyperalgesia as well as edema formation by activating TRPA1 and possibly TRPV1 that are coexpressed on these sensory C-fibers. Petrus et al. (2007) have discovered a small-molecule TRPA1 receptor antagonist, AP18. This TRPA1 antagonist has been shown to block human and mouse TRPA1 in vitro and coinjection with 1 mM AP18 reduces mustard oil-induced nocifensive behavior in vivo (Petrus et al., 2007; C. Gentry, personal communication). We demonstrated that local pretreatment with 25 μl of a 1 mM AP18 solution inhibits 4-ONE-induced mechanical hyperalgesia at 0.5 h postinjection but not at 1 h postinjection (Fig. 2a). It is noteworthy that Petrus et al. only showed early time points when they examined the inhibition of TRPA1 activation. In their studies, coinjection with 1 mM AP18 inhibited the acute nocifensive behavior induced by cinnamaldehyde during the first 5 min after injection (Petrus et al., 2007), and AP18 partially blocked the early phase of bradykinin-evoked mechanical hyperalgesia, which is thought to be mediated by activation of TRPA1 (Petrus et al., 2007; Wang et al., 2008). This indicates that AP18 has a short half-life in vivo; however, intraplantar injections of 1 mM AP18 that were given 24 h after complete Freund's adjuvant treatment were able to reverse complete Freund's adjuvant-induced mechanical hyperalgesia at 1 and 2 h postinjection (Petrus et al., 2007; Fernandes et al., 2010). In the present study, TRPA1 KO mice did not develop 4-ONE-induced mechanical hyperalgesia at 0.5 h postinjection although mechanical hyperalgesia was observed from 1 to 4 h postinjection in TRPA1 KO mice. This indicates that TRPA1 is not a necessary prerequisite for 4-ONE-induced mechanical hyperalgesia, although a TRPA1 component exists. There was no difference in 4-ONE-induced edema formation between WT and TRPA1 KO mice, indicating that 4-ONE-induced edema formation is independent of TRPA1. In addition, pretreatment with the TRPA1 antagonist AP18 at a dose shown here to be selective blocked 4-ONE-induced mechanical hyperalgesia at 0.5 h postinjection, consistent with the results obtained in TRPA1-deficient mice. Thus these results indicate that 4-ONE is able to act via TRPA1-dependent and -independent pathways to mediate mechanical hyperalgesia.
We next investigated whether TRPV1 underlies the TRPA1-independent effects of 4-ONE. It is well documented that TRPV1 acts as a molecular integrator of several noxious stimuli and thus plays a key role in sensing tissue injury and inflammation (Caterina et al., 1997). Indeed, Taylor-Clark et al. (2008a) have demonstrated that 100 μM 4-ONE can activate TRPV1 expressed in human embryonic kidney 293 cells. TRPA1 and TRPV1 share some activation pathways, and a functional interaction between these receptors has indeed been shown before (Akopian et al., 2007; Ruparel et al., 2008; Salas et al., 2009). We thus pretreated TRPV1 WT and KO mice with the TRPA1 antagonist AP18 to investigate a possible interaction or compensation reaction between the two ion channels. In vehicle-pretreated hind paws of TRPV1 WT and KO mice 4-ONE induced mechanical hyperalgesia and edema independently of TRPV1. Moreover, at 0.5 h postinjection AP18 pretreatment inhibited 4-ONE-induced mechanical hyperalgesia in both TRPV1 WT and KO mice. Antagonism of TRPA1 receptors in TRPV1 KO mice does not lead to a significant additional reduction of 4-ONE-induced hyperalgesia and edema. These data do not support the hypothesis that the presence of one active receptor can compensate for the inactivation of the other to mediate the 4-ONE-induced in vivo effects. However, it is possible that there is an interaction of the receptors on the molecular level that cannot be detected using the present experimental design. By comparison, there is evidence that TRPV1 and TRPA1 can interact with each other in other systems, such as craniofacial muscles (Ro et al., 2009).
Finally, we investigated whether 4-ONE can, like the exogenous agonists mustard oil and cinnamaldehyde (Pozsgai et al., 2010), influence peripheral blood flow in vivo. We have shown previously that activation of TRPV1 by capsaicin triggers neurogenic vasodilatation in the mouse in vivo (Grant et al., 2002; Starr et al., 2008). Unlike mustard oil and capsaicin, 4-ONE cannot be prepared for topical administration and was therefore injected intraplantarly. 4-ONE induced TRPA1-dependent vasodilatation in vivo that depends on the release of CGRP, although CGRP was found not to be involved in mediating mechanical hyperalgesia and inflammatory swelling. Moreover, we were unable to demonstrate an involvement of TRPV1 in vasodilator responses, in support of present results that indicated that 4-ONE induced TRPA1-dependent mechanical hyperalgesia independently of TRPV1. Although we believe that this is the first in vivo evidence to show the ability of a putative endogenous activator of TRPA1 to mediate increased blood flow, there is a growing body of evidence to indicate the potential of TRPA1 to mediate vascular relaxant activity. Garlic compounds, which were shown to activate TRPA1, trigger dose-dependent relaxation of rat mesenteric arteries (Bautista et al., 2005), and cinnamaldehyde mediates TRPA1-dependent vasodilatation in mice mesenteric arteries (Pozsgai et al., 2010). In addition, activation of TRPA1 by mustard oil was shown to induce vasorelaxation of rat cerebral arteries via an endothelial cell-dependent mechanism (Earley et al., 2009). It is noteworthy that our investigation of a TRPA1 agonist that was sufficiently soluble for systemic intravenous administration (cinnamaldehyde) has not revealed a systemic hypotensive response that may be hypothesized from the results discussed here, but there is evidence for involvement in cardiovascular reflex responses (Pozsgai et al., 2010). This suggests that we are at an early stage in understanding the pathophysiological significance of TRPA1 channels in the cardiovascular system, with the possibility of different regional effects depending on site of channel and presentation of activating agent.
In summary, the present study set out to investigate the potential of 4-ONE to activate TRPA1 in vivo and trigger nociceptive and peripheral cardiovascular responses. We present novel data to show that 4-ONE induces mechanical hyperalgesia and edema formation as well as neurogenic vasodilatation in vivo. However, it is clear that 4-ONE displays TRPA1-independent effects in vivo, in addition to specific TRPA1-dependent actions. We were not able to show an interaction between TRPA1 and TRPV1 receptors in mediating 4-ONE-induced responses. This study highlights the importance of in vivo research, because previous in vitro experiments suggested that 4-ONE could be a selective TRPA1 agonist for in vivo studies. The present study supports the concept that TRPA1 receptors can act as oxidant sensors in vivo and provides evidence that this leads to increased blood flow, in addition to nociceptive responses.
Acknowledgments
We thank Patrick Fox for expert technical assistance and Drs. Kelvin Kwan and David Corey for the TRPA1 KO mice.
This work was supported by the British Heart Foundation [Grant PG/06/030/20579]; an Integrative Mammalian Biology capacity building award from the Biotechnology and Biological Sciences Research Council [Award BB/E527098/1]; and Arthritis Research UK [Grant 19296]. D.A. holds a London Law Trust/King's College London Medal Fellowship.
Parts of this work have previously been presented as abstracts at the following meetings: Graepel R, Andersson D, Bevan S, and Brain S (2008) 4-ONE, a product of lipid peroxidation, triggers mechanical hyperalgesia and edema independently of the TRPV1 receptor, European Neuropeptide Club and European Opioid Conference Joint Meeting, 8–11 April 2008, Ferrara, Italy; Graepel R, Andersson D, Bevan S, and Brain S (2008) 4-ONE, a product of lipid peroxidation, triggers mechanical hyperalgesia and edema, British Pharmacological Society 6th James Black Conference, 16–17 August 2008, St. Andrews, UK; Graepel R, Andersson D, Bevan S, and Brain S (2008) 4-ONE, a product of lipid peroxidation, triggers mechanical hyperalgesia and edema independently of the TRPV1 receptor, National Symposium, Centre for Integrative Mammalian Physiology and Pharmacology, 30 September 2008, London, UK; Graepel R, Andersson D, Bevan S, and Brain S (2009) Vascular effects of TRPA1 receptor activation, European Neuropeptide Festival, 20–23 July 2009, Salzburg, Austria; Graepel R, Andersson D, Bevan S, and Brain S (2009) 4-ONE, a product of lipid peroxidation, has both TRPA1 receptor dependent and independent effects, British Pharmacological Society 7th James Black Conference, 1–3 September 2009, London, UK.
This work has also been presented in a thesis: Graepel R (2009) An Investigation of the TRPA1 and TRPV1 Receptor in Peripheral Nociception and Vascular Responses. King's College London, London, United Kingdom.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172403.
ABBREVIATIONS:
- TRP
- transient receptor potential
- TRPA1
- TRP ankyrin 1
- TRPV1
- TRP vanilloid 1
- 4-HNE
- 4-hydroxynonenal
- 4-ONE
- 4-oxo-2-nonenal
- CGRP
- calcitonin gene-related peptide
- WT
- wild type
- KO
- knockout
- DMSO
- dimethyl sulfoxide
- PBS
- phosphate-buffered saline
- AP18
- 4-(4-chlorophenyl)-3-methyl-3-buten-2-one oxime.
Authorship Contributions
Participated in research design: Graepel, Bevan, and Brain.
Conducted experiments: Graepel, Fernandes, and Aubdool.
Contributed new reagents or analytic tools: Andersson and Bevan.
Performed data analysis: Graepel, Fernandes, and Aubdool.
Wrote or contributed to the writing of the manuscript: Graepel, Aubdool, Andersson, Bevan, and Brain.
Other: Brain acquired funding for the research.
References
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. (2007) Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 583:175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. (2008) Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28:2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857 [DOI] [PubMed] [Google Scholar]
- Barritt G, Rychkov G. (2005) TRPs as mechanosensitive channels. Nat Cell Biol 7:105–107 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102:12248–12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824 [DOI] [PubMed] [Google Scholar]
- Clapham DE. (2003) TRP channels as cellular sensors. Nature 426:517–524 [DOI] [PubMed] [Google Scholar]
- Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. (2007) The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 21:3747–3755 [DOI] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Crnich R. (2009) Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res 104:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. (2008) HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland A, Mountford D, Keeble J, Malcangio M, et al. (2010) A distinct role for TRPA1, in addition to TRPV1, in TNFα-induced inflammatory hyperalgesia and CFA-induced monoarthritis. Arthritis Rheumatism doi:10.1002/art.30150 [DOI] [PubMed] [Google Scholar]
- Geppetti P, Nassini R, Materazzi S, Benemei S. (2008) The concept of neurogenic inflammation. BJU Int 101(Suppl 3):2–6 [DOI] [PubMed] [Google Scholar]
- Grant AD, Gerard NP, Brain SD. (2002) Evidence of a role for NK1 and CGRP receptors in mediating neurogenic vasodilatation in the mouse ear. Br J Pharmacol 135:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Pinter E, Salmon AM, Brain SD. (2005) An examination of neurogenic mechanisms involved in mustard oil-induced inflammation in the mouse. Eur J Pharmacol 507:273–280 [DOI] [PubMed] [Google Scholar]
- Gühring H, Görig M, Ates M, Coste O, Zeilhofer HU, Pahl A, Rehse K, Brune K. (2000) Suppressed injury-induced rise in spinal prostaglandin E2 production and reduced early thermal hyperalgesia in iNOS-deficient mice. J Neurosci 20:6714–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. (2006) TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA 103:19564–19568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper HC, Miranda CL, Sowell JD, Stevens JF. (2008) Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J Biol Chem 283:17131–17138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289 [DOI] [PubMed] [Google Scholar]
- Lin D, Lee HG, Liu Q, Perry G, Smith MA, Sayre LM. (2005) 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem Res Toxicol 18:1219–1231 [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. (2007a) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445:541–545 [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. (2007b) An ion channel essential for sensing chemical damage. J Neurosci 27:11412–11415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. (2008) Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586:1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain 3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. (2010) Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res 87:760–768 [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee JS, Zhang Y. (2009) Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 144:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. (2008) Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 135:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. (2009) TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. (2008) Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci 27:1131–1142 [DOI] [PubMed] [Google Scholar]
- Shimozu Y, Shibata T, Ojika M, Uchida K. (2009) Identification of advanced reaction products originating from the initial 4-oxo-2-nonenal-cysteine michael adducts. Chem Res Toxicol 22:957–964 [DOI] [PubMed] [Google Scholar]
- Starr A, Graepel R, Keeble J, Schmidhuber S, Clark N, Grant A, Shah AM, Brain SD. (2008) A reactive oxygen species-mediated component in neurogenic vasodilatation. Cardiovasc Res 78:139–147 [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Högestätt ED, Zygmunt PM. (2008) Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53:391–399 [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. (2008a) Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 586:3447–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. (2008b) Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73:274–281 [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, et al. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104:13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. (2000) Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med 28:1685–1696 [DOI] [PubMed] [Google Scholar]
- Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. (2008) Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 131:1241–1251 [DOI] [PubMed] [Google Scholar]