Abstract
Diabetic neuropathy is a common cause of chronic pain that is not adequately relieved by conventional analgesics. The α2-adrenoceptors are involved in the regulation of glutamatergic input and nociceptive transmission in the spinal dorsal horn, but their functional changes in diabetic neuropathy are not clear. The purpose of the present study was to determine the plasticity of presynaptic and postsynaptic α2-adrenoceptors in the control of spinal glutamatergic synaptic transmission in painful diabetic neuropathy. Whole-cell voltage-clamp recordings of lamina II neurons were performed in spinal cord slices from streptozotocin-induced diabetic rats. The amplitude of glutamatergic excitatory postsynaptic currents (EPSCs) evoked from the dorsal root and the frequency of spontaneous EPSCs (sEPSCs) were significantly higher in diabetic than vehicle-control rats. The specific α2-adrenoceptor agonist 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline (UK-14304) (0.1–2 μM) inhibited the frequency of sEPSCs more in diabetic than vehicle-treated rats. UK-14304 also inhibited the amplitude of evoked monosynaptic and polysynaptic EPSCs more in diabetic than control rats. Furthermore, the amplitude of postsynaptic G protein-coupled inwardly rectifying K+ channel (GIRK) currents elicited by UK-14304 was significantly larger in the diabetic group than in the control group. In addition, intrathecal administration of UK-14304 increased the nociceptive threshold more in diabetic than vehicle-control rats. Our findings suggest that diabetic neuropathy increases the activity of presynaptic and postsynaptic α2-adrenoceptors to attenuate glutamatergic transmission in the spinal dorsal horn, which accounts for the potentiated antinociceptive effect of α2-adrenoceptor activation in diabetic neuropathic pain.
Introduction
Diabetic neuropathy is one of the most serious complications that afflicts people with diabetes and is frequently painful. Pain associated with diabetic neuropathy can occur either spontaneously or as a result of exposure to mildly painful stimuli (hyperalgesia) or stimuli not normally perceived as painful (allodynia) (Clark and Lee, 1995; Veves et al., 2008). The development of diabetic neuropathic pain is associated with increased nociceptive input, neuronal hyperactivity, and sustained stimulation of certain glutamate receptors in the spinal cord (Calcutt and Chaplan, 1997; Malcangio and Tomlinson, 1998; Chen and Pan, 2002; Wang et al., 2007; Li et al., 2010). Because diabetic neuropathic pain often is not adequately relieved by available analgesics, it represents an important unmet medical need.
Many experimental studies have demonstrated that intrathecal or systemic administration of α2-adrenoceptor agonists can produce a potent analgesic effect in neuropathic pain caused by traumatic nerve injury and diabetic neuropathy (Courteix et al., 1994; Yaksh et al., 1995; Calcutt and Chaplan, 1997; Buerkle and Yaksh, 1998; Pan et al., 1999; Omiya et al., 2008). In addition, clinical studies have shown that spinally administered α2-adrenoceptor agonists reduce the intractable neuropathic pain caused by nerve injury and cancer (Rauck et al., 1993; Hassenbusch et al., 2002). At the spinal level, the α2-adrenoceptor is present presynaptically at the primary afferent terminals and postsynaptically on dorsal horn neurons. Although the α2A-adrenoceptor subtype is located predominantly on the terminals of primary afferents (Sullivan et al., 1987; Roudet et al., 1994; Stone et al., 1998; Overland et al., 2009; Riedl et al., 2009), the α2C-adrenoceptor subtype is present mainly on spinal dorsal horn neurons (Stone et al., 1998). Stimulation of α2-adrenoceptors with clonidine dose-dependently reduces excitation of dorsal horn neurons evoked by primary afferent stimulation (Sullivan et al., 1987). The α2-adrenoceptor agonists can reduce glutamate release from primary afferents (Pan et al., 2002) and hyperpolarize the dorsal horn neurons through a postsynaptic action (North and Yoshimura, 1984). However, the plasticity and role of presynaptic and postsynaptic α2-adrenoceptors in the regulation of glutamatergic input to spinal dorsal horn neurons in diabetic neuropathic pain remain unknown.
Therefore, in this study, we used a rat model of diabetic neuropathy and electrophysiological approaches to determine the functional changes in presynaptic and postsynaptic α2-adrenoceptors in the control of spinal glutamatergic transmission and how these changes affect the antinociceptive effect of stimulation of α2-adrenoceptors at the spinal level in diabetic neuropathic pain.
Materials and Methods
Animal Model of Diabetic Neuropathic Pain.
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) initially weighing 200 to 220 g were used in this study. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ; 60 mg/kg; Sigma-Aldrich, St. Louis, MO) freshly dissolved in 0.9% sterile saline (Chen and Pan, 2002; Wang et al., 2007; Li et al., 2010). Diabetes was confirmed in STZ-injected rats by measuring the blood glucose concentration. Glucose levels in blood obtained from the tail vein were assayed using ACCU-CHEK test strips (Roche Diagnostics, Indianapolis, IN). The blood glucose level was measured 2 weeks after STZ administration, and only rats with high levels (> 300 mg/dl) were used for the diabetic groups. Age-matched vehicle-injected rats were used as controls. The low dose of STZ was used to minimize the effects of STZ administration on the overall health of the animal (Chen and Pan, 2002; Chen et al., 2009). This model of neuropathic pain mimics the symptoms of neuropathy in diabetic patients, with alterations in pain sensitivity and poor responses to μ opioid administered systemically or intrathecally (Courteix et al., 1993; Malcangio and Tomlinson, 1998; Zurek et al., 2001; Chen and Pan, 2002). It has been shown that early insulin intervention can impede the development of painful diabetic neuropathy induced by STZ in rats (Sasaki et al., 1998; Hoybergs and Meert, 2007). Neuropathic pain in diabetic rats was confirmed by examining nociceptive thresholds using an analgesimeter (Ugo Basile, Comerio, Italy), and all diabetic rats developed mechanical hyperalgesia in our study. All of the diabetic rats remained relatively healthy, although their growth rate was reduced (body weight gain: 5 g/week in the diabetic group versus 20 g/week in the nondiabetic group). The final electrophysiological and behavioral experiments were performed on rats 3 weeks after STZ or vehicle treatment. The experiments were performed according to National Institutes of Health guidelines and approved by the Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Intrathecal Cannulation and Behavioral Assessment of Nociception.
Intrathecal catheters (PE-10 polyethylene tubing) were inserted in diabetic and control rats during isoflurane-induced anesthesia. The catheter was advanced 8 cm caudally through an incision in the cisternal membrane and secured to the musculature at the incision site (Chen and Pan, 2001, 2006). The rats were allowed to recover for at least 5 days before drug testing began. Only rats with no evidence of neurological deficit after catheter insertion were used in the study. Drugs for intrathecal injections were dissolved in normal saline and administered in a volume of 5 μl followed by a 10-μl flush with normal saline.
The nociceptive mechanical threshold was measured using an Ugo Basile analgesimeter to apply a noxious pressure to a hindpaw. By pressing a pedal that activated a motor, the force increased at a constant rate on the linear scale. When the rat responded by withdrawal of the paw or vocalization, the pedal was immediately released and the nociceptive threshold was read on a scale. A cutoff of 400 g was used to avoid tissue injury (Chen and Pan, 2002, 2006). Both hindpaws were tested in each rat, and the mean value was used as the nociceptive withdrawal threshold.
Spinal Cord Slice Preparation.
The rats were anesthetized with 2 to 3% isoflurane, and the lumbar segment of the spinal cord was removed by means of laminectomy. The spinal cord segment at the L5 and L6 levels was immediately placed in ice-cold sucrose-artificial cerebrospinal fluid (aCSF) presaturated with 95% O2 and 5% CO2. The sucrose-aCSF contained 234 mM sucrose, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2 PO4, 12 mM glucose, and 25 mM NaHCO3. The tissue was then placed in a shallow groove formed in a gelatin block and glued to the stage of a vibratome. Transverse spinal cord slices (400 μm) were cut in the ice-cold sucrose-aCSF and then preincubated in Krebs' solution oxygenated with 95% O2 and 5% CO2 at 34°C for at least 1 h before they were transferred to the recording chamber. The Krebs' solution contained 117 mM NaCl, 3.6 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2 PO4, 11 mM glucose, and 25 mM NaHCO3. The perfusion buffer had been optimized to best preserve the spinal cord tissue for the in vitro recording. On the basis of our experience, the high glucose level (11 mM) in the buffer is required to maintain the viability of spinal slices in vitro at 34 to 36°C. If the glucose level in the buffer is lowered to 4 to 5 mM, we can obtain stable whole-cell recordings only at room temperature (24–25°C).
Electrophysiological Recordings.
Excitatory postsynaptic currents (EPSCs) were recorded using the whole-cell voltage-clamp method as described previously (Wang et al., 2007; Li et al., 2010). Each spinal cord slice was placed in a glass-bottomed chamber and fixed with parallel nylon threads supported by a U-shaped stainless-steel weight. The slice was continuously perfused with Krebs' solution at 5.0 ml/min at 34°C maintained by an inline solution heater and a temperature controller. Neurons in the lamina II of the spinal cord slice were identified with differential interference contrast/infrared illumination on a fixed-stage microscope (BX50WI; Olympus, Tokyo, Japan).
Monosynaptic or polysynaptic EPSCs were evoked by electrical stimulation through a bipolar stimulation electrode placed on the dorsal root. We used fixed stimulation intensity (0.2 ms and 0.6 mA) to evoke EPSCs from primary afferents in both diabetic and control rats. At the stimulation intensity used, both A- and C-afferent fibers that were in close contact with the electrode tip were stimulated (Wang et al., 2007; Zhou et al., 2010). The evoked EPSCs were considered to be monosynaptic if 1) the latency was constant with repeated electrical stimulation at 0.1 Hz, and 2) there was no conduction failure or changes in the latency when the stimulation frequency was increased to 20 Hz (stimulus train duration, 1 s) (Wang et al., 2007; Li et al., 2010). In contrast, evoked EPSCs were considered to be polysynaptic if the latency was variable and conduction failure occurred at a higher stimulation frequency (20 Hz). The electrode for the whole-cell recordings was pulled from borosilicate glass capillaries. The impedance of the pipette was 5 to 8 MΩ when filled with an internal solution containing 110 mM Cs2SO4, 5 mM tetraethylammonium, 2 mM MgCl2, 0.5 mM CaCl2, 5 mM HEPES, 5 mM EGTA, 5 mM ATP-Mg, 0.5 mM Na-GTP, and 10 mM lidocaine N-ethyl bromide that had been adjusted to pH 7.2 to 7.3 with 1 M CsOH (290–300 mOsm). In the recordings of spontaneous EPSCs (sEPSCs) or evoked EPSCs, 1 mM guanosine 5′-O-(2-thiodiphosphate), a general G protein inhibitor (Zhou et al., 2008, 2010), was included in the pipette internal solution to block the potential postsynaptic effect produced by the α2-adrenoceptor agonist. Miniature EPSCs (mEPSCs) were recorded in the presence of 1 μM tetrodotoxin (TTX).
We recorded G protein-coupled inwardly rectifying K+ channel (GIRK) currents elicited by 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline (UK-14304; also known as brimonidine) as a measure of postsynaptic α2-adrenoceptor activity in the spinal cord. GIRK currents were recorded from lamina II neurons at a holding potential of −60 mV using a pipette internal solution containing 135.0 mM potassium gluconate, 5.0 mM KCl, 2.0 mM MgCl2, 0.5 mM CaCl2, 5.0 mM HEPES, 5.0 mM EGTA, 5.0 mM ATP-Mg, and 0.5 mM Na-GTP; the solution was adjusted to pH 7.2 to 7.4 with 1 M KOH (290–300 mΩsm) (Zhou et al., 2008, 2010). Recordings of EPSCs or GIRK currents began approximately 6 min after whole-cell access was established and the current reached a steady state. The input resistance was monitored, and the recording was abandoned if it changed by more than 15%. All signals were recorded using an amplifier (MultiClamp 700B; Molecular Devices, Sunnyvale, CA) at a holding potential of −60 mV, filtered at 1 to 2 kHz, digitized at 10 kHz, and stored in a computer using pCLAMP 9.2 (Molecular Devices).
Yohimbine and guanosine 5′-O-(2-thiodiphosphate) were obtained from Sigma-Aldrich. UK-14304 was purchased from Tocris Bioscience (Ellisville, MO). TTX was obtained from Ascent Scientific (Princeton, NJ).
Data Analysis and Statistics.
Data are presented as means ± S.E.M. In general, two to three neurons were recorded from each rat, and at least five rats were used for each group. The amplitude of evoked EPSCs and GIRK currents were analyzed using Clampfit (Molecular Devices). The frequency and amplitude of sEPSCs and mEPSCs were analyzed off-line using a peak detection program (MiniAnalysis; Synaptosoft, Decatur, GA). Detection of events was accomplished by setting a threshold above the noise level. The sEPSCs and mEPSCs were detected by the fast rise time of the signal over an amplitude threshold above the background noise. We manually excluded the event when the background noise was erroneously identified as an sEPSC by the program. The cumulative probability of the amplitude and interevent interval of sEPSCs and mEPSCs was compared by using the Komogorov-Smirnov test, which estimates the probability that two cumulative distributions are similar. The effects of UK-14304 on GIRK currents, evoked EPSCs, sEPSCs, and mEPSCs were determined using repeated-measures analysis of variance (ANOVA), and the differences between the control and diabetic groups were tested using two-way ANOVA with Bonferroni's post hoc test. The effect of drug treatments on the paw withdrawal threshold was determined by using repeated-measures ANOVA followed by Dunnett's post hoc test. P < 0.05 was considered to be statistically significant.
Results
Increased Effect of UK-14304 on Glutamatergic Input to Spinal Dorsal Horn Neurons in Diabetic Rats.
Three weeks after induction of diabetes, the diabetic rats showed significantly lower paw withdrawal thresholds (94.73 ± 4.11 g) in response to the pressure stimulus applied to the hindpaw than the age-matched control rats treated with vehicle (134.14 ± 4.48 g; P < 0.05, t test). The baseline frequency, but not the amplitude, of glutamatergic sEPSCs of lamina II neurons was significantly higher in diabetic than in vehicle control rats (Fig. 1).
Fig. 1.
Effects of UK-14304 on glutamatergic sEPSCs of spinal lamina II neurons in control and diabetic rats. A, original traces show sEPSCs during baseline control, application of 0.1, 0.5, and 1 μM UK-14304, and washout in one lamina II neuron from a control rat and another from a diabetic rat. B, cumulative probability plots of sEPSCs of the same neuron in A showing the distribution of the interevent interval and amplitude of sEPSCs during control, application of UK-14304, and washout. C, summary data compare the effect of 0.1 to 2 μM UK-14304 on the frequency of sEPSCs in 16 neurons from control rats and 21 neurons from diabetic rats. Note that the baseline frequency of sEPSCs of lamina II neurons was significantly higher in diabetic than in control rats. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. #, P < 0.05 compared with the corresponding value in the control group.
To determine changes in the activity of α2-adrenoceptors in the control of glutamatergic input to spinal dorsal horn neurons in diabetic neuropathy, we compared the inhibitory effects of UK-14304 on the frequency of sEPSCs of lamina II neurons in diabetic and control rats. We selected UK-14304 for our study because it is a highly potent and selective α2-adrenoceptor agonist (Buerkle and Yaksh, 1998). Unlike clonidine and dexmedetomidine, the effect of UK-14304 is fast and can be easily washed out upon bath application. Bath application of 0.1 to 2 μM UK-14304 decreased the frequency, but not the amplitude, of sEPSCs in all 21 lamina II neurons from diabetic rats in a concentration-dependent manner (Fig. 1). The cumulative probability analysis of sEPSCs revealed that the distribution pattern of the interevent interval of sEPSCs was shifted toward the right (i.e., reduced frequency of sEPSCs) in response to UK-14304 treatment. However, the distribution pattern of the amplitude of sEPSCs was not significantly changed (Fig. 1B). UK-14304 also inhibited the frequency, but not the amplitude, of sEPSCs in 16 lamina II neurons from control rats. However, the inhibitory effect of UK-14304 was nearly maximal at 0.5 μM. Because the baseline frequency of sEPSCs was significantly different between the diabetic and control rats, we normalized the effect of UK-14304 to the baseline of sEPSCs in each group. UK-14304 caused a significantly greater decrease in the frequency of sEPSCs in diabetic than in control rats (Fig. 1C). Similar to what we reported previously (Wang et al., 2007; Li et al., 2010), bath application of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione, a non-N-methyl-d-aspartate receptor antagonist, eliminated sEPSCs in all neurons examined in control and diabetic rats (data not shown).
To further determine whether the effect of the α2-adrenoceptor agonist on spinal glutamatergic transmission occurs through a presynaptic site of action, we compared the effects of UK-14304 on sEPSCs and mEPSCs in the same neurons obtained from spinal cord slices of vehicle-control and diabetic rats. Bath application of 1 μM UK-14304 had a similar inhibitory effect on the frequency, but not the amplitude, of sEPSCs and mEPSCs in 14 lamina II neurons from control rats and 11 lamina II neurons from diabetic rats (Fig. 2).
Fig. 2.
Comparison of the effects of UK-14304 on sEPSCs and mEPSCs of spinal lamina II neurons in control and diabetic rats. A, original traces show sEPSCs during baseline control and application of 1 μM UK-14304, 0.5 μM TTX, and 1 μM UK-14304 plus 0.5 μM TTX in one lamina II neuron from a control rat and another from a diabetic rat. B, summary data compare the effects of 1 μM UK-14304 on the frequency of sEPSCs and mEPSCs (in the presence of TTX) in 14 neurons from control rats and 11 neurons from diabetic rats. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. #, P < 0.05 compared with TTX only.
Increased Effect of UK-14304 on Glutamate Release from Primary Afferent Terminals in Diabetic Rats.
To determine the activity of α2-adrenoceptors on primary afferent terminals in the regulation of glutamatergic synaptic input to dorsal horn neurons in diabetic neuropathy, we next examined the effects of UK-14304 on monosynaptic and polysynaptic EPSCs evoked from the dorsal root. The baseline amplitude of both monosynaptic and polysynaptic EPSCs of spinal lamina II neurons was significantly higher in diabetic than control rats (Fig. 3).
Fig. 3.
Effects of UK-14304 on monosynaptic EPSCs of lamina II neurons evoked from primary afferents in control and diabetic rats. A, original recordings show evoked monosynaptic EPSCs during baseline control, application of 0.1 to 2 μM UK-14304, and washout in one neuron from a control rat and another from a diabetic rat. B, summary data compare the effect of UK-14304 on the peak amplitude of evoked monosynaptic EPSCs in 13 neurons from control rats and 13 neurons from diabetic rats. C, summary data show that 2 μM yohimbine abolished the inhibitory effect of 1 μM UK-14304 on evoked monosynaptic EPSCs in six neurons from diabetic rats. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. #, P < 0.05 compared with the corresponding value in the control group.
Bath application of 0.1 to 2 μM UK-14304 concentration-dependently reduced the peak amplitude of monosynaptic EPSCs in most neurons (13/14, 92.3%) from diabetic rats (Fig. 3). Although UK-14304 (0.5–2 μM) also significantly inhibited the amplitude of monosynaptic EPSCs in 13 of 15 neurons (86.7%) tested in the control group, its effect was nearly maximal at 0.5 μM. Because the baseline amplitude of the evoked EPSCs was significantly different between the two groups, we normalized the effect of UK-14304 to the baseline control of evoked EPSCs in each group. At concentrations of 1 and 2 μM, UK-14304 had a significantly greater effect on the amplitude of monosynaptic EPSCs in diabetic than control rats (Fig. 3). Furthermore, blocking α2-adrenoceptors with yohimbine (2 μM) (Pan et al., 2002) abolished the inhibitory effect of 1 μM UK-14304 on monosynaptic EPSCs in all neurons from diabetic rats (Fig. 3C).
In separate groups of lamina II neurons, bath application of 0.1 to 2 μM UK-14304 inhibited the peak amplitude of evoked polysynaptic EPSCs in the control and diabetic groups in a dose-dependent manner (Fig. 4). When the effect of UK-14304 on evoked EPSCs was normalized to the baseline control, it also produced a significantly greater effect on the amplitude of polysynaptic EPSCs in diabetic rats than in control rats (Fig. 4B).
Fig. 4.
Effects of UK-14304 on polysynaptic EPSCs of lamina II neurons evoked from primary afferents in control and diabetic rats. A, original recordings show evoked polysynaptic EPSCs during control, application of 0.1 to 2 μM UK-14304, and washout in one neuron from a control rat and another from a diabetic rat. B, summary data compare the effect of 0.1 to 2 μM UK-14304 on the peak amplitude of evoked polysynaptic EPSCs in 14 neurons from control rats and 15 neurons from diabetic rats. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. #, P < 0.05 compared with the corresponding value in the control group.
Increased GIRK Currents Elicited by UK-14304 in Spinal Dorsal Horn Neuron in Diabetic Rats.
GIRK channels are present postsynaptically in spinal lamina II neurons (Marker et al., 2006; Zhou et al., 2008). To determine the diabetes-induced changes in the activity of postsynaptic α2-adrenoceptors in the spinal dorsal horn, we measured GIRK currents elicited by UK-14304 in lamina II neurons of diabetic and control rats. Bath application of 0.1 to 2 μM UK-14304 readily produced GIRK currents in a concentration-dependent manner in a small population of lamina II neurons (22/90, 24.4%) from diabetic rats (Fig. 5, A and B). These concentrations of UK-14304 also elicited GIRK currents in 18 of 82 (22.0%) lamina II neurons from control rats, but its effect was nearly maximal at 0.5 μM. By comparison, UK-14304 at 1 and 2 μM elicited significantly larger GIRK currents in diabetic than in control rats (Fig. 5B). In another six lamina II neurons tested from diabetic rats, yohimbine (2 μM) completely blocked the UK-14304 (1 μM)-elicited GIRK currents (Fig. 5C).
Fig. 5.
Changes in the GIRK currents elicited by UK-14304 in lamina II neurons in control and diabetic rats. A, representative traces show the GIRK currents produced by bath application of 0.5 to 2 μM UK-14304 in one neuron from a control rat and another from a diabetic rat. B, summary data show the difference in the peak amplitude of UK-14304-elicited GIRK currents in 18 neurons from control rats and 22 neurons from diabetic rats. C, summary data show that 2 μM yohimbine blocked the GIRK currents elicited by 1 μM UK-14304 in six lamina II neurons from diabetic rats. Data are presented as means ± S.E.M. *, P < 0.05 compared with the corresponding value in the control group. #, P < 0.05 compared with UK-14304 alone.
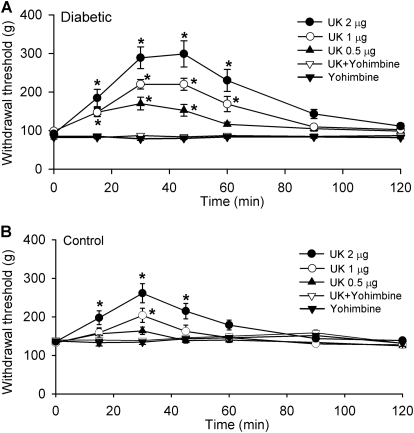
Increased Spinal Effect of UK-14304 on Mechanical Nociception in Diabetic Rats.
In addition, to determine the functional activity of α2-adrenoceptors at the spinal level in nociceptive control in diabetic neuropathy, we compared the dose-response effects of intrathecal injection of UK-14304 on the nociceptive withdrawal threshold of the rat hindpaw in both control and diabetic rats. The baseline pressure withdrawal threshold was significantly lower in diabetic than in control rats, which is suggestive of mechanical hyperalgesia (Fig. 6). For intrathecal injections, both UK-14304 and yohimbine were dissolved in saline. Intrathecal administration of 0.5 to 2 μg of UK-14304 (n = 8 rats in each dose group) significantly increased the paw withdrawal threshold in response to noxious mechanical stimulus in diabetic rats (Fig. 6). The effect of UK-14304 appeared 15 min after intrathecal injection, and its effect lasted for approximately 60 min in diabetic rats. However, intrathecal injection of UK-14304 produced only a transient, small effect on the nociceptive withdrawal threshold at 1 and 2 μg in control rats (n = 8 rats in each dose group; Fig. 6). Intrathecal pretreatment with 30 μg of yohimbine 15 min before injection of 2 μg of UK-14304 abolished the antinociceptive effect of UK-14304 in both the diabetic and control groups (Fig. 6). In another seven rats, intrathecal injection of 30 μg of yohimbine alone had no significant effect on the paw withdrawal threshold in either diabetic or control rats.
Fig. 6.
Effects of intrathecal injection of UK-14304 on the nociceptive withdrawal threshold in diabetic and control rats. A, effect of intrathecal administration of 0.5 to 2 μg of UK-14304 on the mechanical withdrawal threshold in diabetic rats. B, effect of intrathecal injection of 0.5 to 2 μg of UK-14304 on the nociceptive withdrawal threshold in control rats. Note that intrathecal injection of 30 μg of yohimbine blocked the antinociceptive effect of 2 μg of UK-14304 in control and diabetic rats. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control (time 0).
Discussion
The major objective of our study was to determine how painful diabetic neuropathy affects the activity of presynaptic and postsynaptic α2-adrenoceptors in the spinal dorsal horn. We found that the α2-adrenoceptor agonist UK-14304 inhibited the frequency of sEPSCs more in diabetic than in control rats. In addition, the inhibitory effect of UK-14304 on monosynaptic and polysynaptic EPSCs evoked from primary afferents was significantly greater in diabetic than in control rats. Furthermore, the amplitude of GIRK currents elicited by UK-14304 in dorsal horn neurons was significantly larger in the diabetic than in the control group. The presynaptic and postsynaptic effects of UK-14304 were completely antagonized by the α2-adrenoceptor antagonist yohimbine. In addition, intrathecal administration of UK-14304 increased the nociceptive withdrawal threshold more in diabetic rats than in control rats. Collectively, our electrophysiological data suggest that diabetic neuropathy up-regulates α2-adrenoceptors on primary afferent terminals and interneurons in the spinal dorsal horn. The profound antinociceptive effect of α2-adrenoceptor agonists on diabetic neuropathic pain probably results from the inhibition of glutamatergic transmission caused by stimulation of presynaptic and postsynaptic α2-adrenoceptors at the spinal level.
The spinal dorsal horn is a critical site for the transmission and modulation of nociception. Glutamate released from the central terminals of primary afferents and glutamatergic interneurons is an important neurotransmitter in the spinal dorsal horn. It has been reported that the basal glutamate concentrations in the CSF, measured using a dialysis catheter placed in the intrathecal space, is lower in diabetic rats than in control rats (Malmberg et al., 2006). However, the sources of glutamate in the CSF cannot be determined by the dialysis technique. In our study, the frequency of glutamatergic sEPSCs and the amplitude of monosynaptic and polysynaptic EPSCs evoked from primary afferents were significantly higher in diabetic than in control rats. Our data suggest that glutamate release from the primary afferents to spinal dorsal horn neurons is increased in diabetic neuropathic pain. The enhanced glutamate input probably contributes to the hyperexcitability of the dorsal horn neurons and the maintenance of diabetic neuropathic pain (Chen and Pan, 2002; Wang et al., 2007; Chen et al., 2009). Thus, effective reduction in glutamatergic input to spinal dorsal horn neurons represents an important therapeutic strategy for alleviating diabetic neuropathic pain.
At the spinal level, one of the important G protein-coupled receptors that regulate nociceptive transmission is the α2-adrenoceptor (Pan et al., 2008). We found in the present study that activation of α2-adrenoceptors with UK-14304 significantly inhibited glutamatergic sEPSCs and monosynaptic and polysynaptic EPSCs evoked from primary afferents in control rats. UK-14304 also significantly reduced the frequency of mEPSCs, suggesting that α2-adrenoceptors are present at presynaptic terminals in the spinal cord. These presynaptic effects of UK-14304 are similar to the effects we observed with another α2-adrenoceptor agonist, clonidine (Pan et al., 2002). Because the baseline sEPSC frequency and evoked EPSC amplitude were significantly different between the diabetic and control rats, we normalized the effect of UK-14304 to the baseline of sEPSCs and evoked EPSCs. It is noteworthy that we found that UK-14304 inhibited sEPSCs and evoked EPSCs more in diabetic than in control rats. Our findings suggest that the α2-adrenoceptor at the primary afferent terminals is up-regulated in diabetic neuropathy. The presynaptic effect of α2-adrenoceptor agonists probably results from inhibition of voltage-gated Ca2+ channels present on primary afferent terminals. In this regard, α2-adrenoceptor agonists can inhibit voltage-gated Ca2+ channels in the locus coeruleus neurons and sympathetic neurons (Lipscombe et al., 1989; Ingram et al., 1997).
In addition to the presynaptic sites of actions, stimulation of α2-adrenoceptors could directly suppress the excitability of spinal dorsal horn neurons through activation of GIRK channels (North and Yoshimura, 1984; Mitrovic et al., 2003). Because the analgesic effect produced by the α2-adrenoceptor agonist is reduced in GIRK2-knockout mice (Mitrovic et al., 2003), the postsynaptic effect of α2-adrenoceptor agonists probably is involved in their antinociceptive effect on painful diabetic neuropathy. We found in this study that the GIRK currents elicited by UK-14304 were present in approximately 20% of the lamina II neurons examined in control and diabetic rats. Moreover, the amplitude of the UK-14304-produced GIRK currents in lamina II neurons was significantly larger in diabetic rats than in control rats. These data suggest that the postsynaptic α2-adrenoceptor activity is also increased in interneurons in the spinal dorsal horn in diabetic neuropathy. The mechanisms underlying increased α2-adrenoceptor activity in the spinal cord in diabetic neuropathy are not fully known. It has been reported that diabetic neuropathy reduces the norepinephrine release and causes a compensatory up-regulation of the α2-adrenoceptor in the spinal cord (Omiya et al., 2008). Nevertheless, because the endogenous ligand for α2-adrenoceptors may be reduced in diabetic rats, this could explain why the baseline glutamate release at the spinal level is still elevated in diabetic rats. The role of α2-adrenoceptors in the control of spinal glutamatergic transmission in other types of neuropathic pain has not been determined. Results from the [35S]GTPγS binding study suggest that nerve injury also increases α2-adrenoceptor activity in the spinal cord (Bantel et al., 2005). However, it has been reported that nerve injury decreases presynaptic α2-adrenoceptors but increases postsynaptic α2-adrenoceptors in the spinal cord (Stone et al., 1999).
Studies using α2-adrenoceptor subtype-knockout mice have shown that the α2A-adrenoceptor is involved primarily in the analgesic effect produced by α2-adrenoceptor agonists (Stone et al., 1997; Fairbanks et al., 2002; Mansikka et al., 2004). Moreover, the α2C-adrenoceptors, but not the α2B-adrenoceptors, in the spinal cord contribute to the analgesic effect produced by moxonidine, an imidazoline/α2-adrenoceptor agonist (Fairbanks et al., 2002). The mRNA of all three α2-adrenoceptor subtypes is expressed in the human spinal cord and dorsal root ganglion (Smith et al., 1995; Ongioco et al., 2000). However, very little mRNA of the α2B-adrenoceptor is detected in the rat spinal dorsal horn (Shi et al., 1999). Capsaicin treatment in neonatal rats or resiniferatoxin treatment in adult rats removes transient receptor potential cation channel V1-expressing sensory neurons and induces a large reduction in the α2A-adrenoceptor, but not the α2C-adrenoceptor, immunoreactivity in the spinal dorsal horn (Stone et al., 1998; Chen et al., 2007). Therefore, the α2A-adrenoceptor is located predominantly on the central terminals of primary afferents, whereas the α2C-adrenoceptor subtype is located postsynaptically on spinal dorsal horn neurons. Because our electrophysiological data suggest that the activity of both presynaptic and postsynaptic α2-adrenoceptors is increased in diabetic rats, it is possible that both the α2A- and α2C-adrenoceptors in the spinal cord are up-regulated in painful diabetic neuropathy.
We found that intrathecal injection of UK-14304 profoundly increased the paw withdrawal threshold in response to a noxious mechanical stimulus in diabetic rats. By comparison, intrathecal administration of UK-14304 produced only a small and transient effect on mechanical nociception in control rats. These behavioral data provide further functional evidence for the up-regulation of α2-adrenoceptors in the spinal cord in diabetic neuropathy. The combined presynaptic (α2A-adrenoceptors) and postsynaptic (α2C-adrenoceptors) effects could account for the profound antinociceptive effect of α2-adrenoceptor agonists on diabetic neuropathic pain. By inhibiting increased glutamatergic input from primary afferents and direct hyperpolarization of dorsal horn neurons, the α2-adrenoceptor agonist can reduce central sensitization and nociceptive transmission at the spinal level in diabetic neuropathic pain.
In summary, we provide electrophysiological data showing that the α2-adrenoceptor is up-regulated on primary afferent terminals and dorsal horn interneurons in painful diabetic neuropathy. Our study provides new evidence for the important role of presynaptic and postsynaptic α2-adrenoceptors in the control of glutamatergic input and nociceptive transmission at the spinal level in diabetic neuropathic pain. The α2-adrenoceptor agonists may represent a class of nonopioid analgesics for effective treatments of painful diabetic neuropathy.
This study was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM64830], the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS45602], and the N.G. and Helen Hawkins Endowment (to H.L.P.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.176586.
ABBREVIATIONS:
- STZ
- streptozotocin
- GIRK
- G protein-coupled inwardly rectifying K+ channels
- EPSC
- excitatory postsynaptic current
- mEPSC
- miniature EPSC
- sEPSC
- spontaneous EPSC
- TTX
- tetrodotoxin
- UK-14304
- 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline
- aCSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: S.-R. Chen and Pan.
Conducted experiments: S.-R. Chen, H. Chen, and Yuan.
Performed data analysis: S.-R. Chen, H. Chen, Yuan, and Pan.
Wrote or contributed to the writing of the manuscript: S.-R. Chen and Pan.
References
- Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. (2005) Spinal nerve ligation increases α2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res 1038:76–82 [DOI] [PubMed] [Google Scholar]
- Buerkle H, Yaksh TL. (1998) Pharmacological evidence for different α2-adrenergic receptor sites mediating analgesia and sedation in the rat. Br J Anaesth 81:208–215 [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Chaplan SR. (1997) Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol 122:1478–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Pan HL. (2001) Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology 95:525–530 [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. (2002) Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol 87:2726–2733 [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. (2006) Loss of TRPV1-expressing sensory neurons reduces spinal μ opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol 95:3086–3096 [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HM, Richardson TE, Pan HL. (2007) Potentiation of spinal α(2)-adrenoceptor analgesia in rats deficient in TRPV1-expressing afferent neurons. Neuropharmacology 52:1624–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Samoriski G, Pan HL. (2009) Antinociceptive effects of chronic administration of uncompetitive NMDA receptor antagonists in a rat model of diabetic neuropathic pain. Neuropharmacology 57:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Jr, Lee DA. (1995) Prevention and treatment of the complications of diabetes mellitus. N Engl J Med 332:1210–1217 [DOI] [PubMed] [Google Scholar]
- Courteix C, Bardin M, Chantelauze C, Lavarenne J, Eschalier A. (1994) Study of the sensitivity of the diabetes-induced pain model in rats to a range of analgesics. Pain 57:153–160 [DOI] [PubMed] [Google Scholar]
- Courteix C, Eschalier A, Lavarenne J. (1993) Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain 53:81–88 [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. (2002) α(2C)-adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther 300:282–290 [DOI] [PubMed] [Google Scholar]
- Hassenbusch SJ, Gunes S, Wachsman S, Willis KD. (2002) Intrathecal clonidine in the treatment of intractable pain: a phase I/II study. Pain Med 3:85–91 [DOI] [PubMed] [Google Scholar]
- Hoybergs YM, Meert TF. (2007) The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett 417:149–154 [DOI] [PubMed] [Google Scholar]
- Ingram S, Wilding TJ, McCleskey EW, Williams JT. (1997) Efficacy and kinetics of opioid action on acutely dissociated neurons. Mol Pharmacol 52:136–143 [DOI] [PubMed] [Google Scholar]
- Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. (2010) Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem 112:162–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Kongsamut S, Tsien RW. (1989) α-Adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature 340:639–642 [DOI] [PubMed] [Google Scholar]
- Malcangio M, Tomlinson DR. (1998) A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain 76:151–157 [DOI] [PubMed] [Google Scholar]
- Malmberg AB, O'Connor WT, Glennon JC, Ceseña R, Calcutt NA. (2006) Impaired formalin-evoked changes of spinal amino acid levels in diabetic rats. Brain Res 1115:48–53 [DOI] [PubMed] [Google Scholar]
- Mansikka H, Lähdesmäki J, Scheinin M, Pertovaara A. (2004) α(2A) adrenoceptors contribute to feedback inhibition of capsaicin-induced hyperalgesia. Anesthesiology 101:185–190 [DOI] [PubMed] [Google Scholar]
- Marker CL, Luján R, Colón J, Wickman K. (2006) Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci 26:12251–12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. (2003) Contribution of GIRK2-mediated postsynaptic signaling to opiate and α2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci USA 100:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Yoshimura M. (1984) The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol 349:43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiya Y, Yuzurihara M, Suzuki Y, Kase Y, Kono T. (2008) Role of α2-adrenoceptors in enhancement of antinociceptive effect in diabetic mice. Eur J Pharmacol 592:62–66 [DOI] [PubMed] [Google Scholar]
- Ongioco RR, Richardson CD, Rudner XL, Stafford-Smith M, Schwinn DA. (2000) α2-Adrenergic receptors in human dorsal root ganglia: predominance of α2b and α2c subtype mRNAs. Anesthesiology 92:968–976 [DOI] [PubMed] [Google Scholar]
- Overland AC, Kitto KF, Chabot-Doré AJ, Rothwell PE, Fairbanks CA, Stone LS, Wilcox GL. (2009) Protein kinase C mediates the synergistic interaction between agonists acting at α2-adrenergic and δ-opioid receptors in spinal cord. J Neurosci 29:13264–13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HL, Chen SR, Eisenach JC. (1999) Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology 90:509–514 [DOI] [PubMed] [Google Scholar]
- Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. (2008) Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther 117:141–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YZ, Li DP, Pan HL. (2002) Inhibition of glutamatergic synaptic input to spinal lamina II(o) neurons by presynaptic α(2)-adrenergic receptors. J Neurophysiol 87:1938–1947 [DOI] [PubMed] [Google Scholar]
- Rauck RL, Eisenach JC, Jackson K, Young LD, Southern J. (1993) Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology 79:1163–1169; discussion 27A [PubMed] [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Doré AJ, Taylor AM, Ribeiro-da-Silva A, Elde RP, Wilcox GL, Stone LS. (2009) Coexpression of α2A-adrenergic and δ-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol 513:385–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudet C, Mouchet P, Feuerstein C, Savasta M. (1994) Normal distribution of α2-adrenoceptors in the rat spinal cord and its modification after noradrenergic denervation: a quantitative autoradiographic study. J Neurosci Res 39:319–329 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yasuda H, Maeda K, Kikkawa R. (1998) Hyperalgesia and decreased neuronal nitric oxide synthase in diabetic rats. Neuroreport 9:243–247 [DOI] [PubMed] [Google Scholar]
- Shi TJ, Winzer-Serhan U, Leslie F, Hökfelt T. (1999) Distribution of α2-adrenoceptor mRNAs in the rat lumbar spinal cord in normal and axotomized rats. Neuroreport 10:2835–2839 [DOI] [PubMed] [Google Scholar]
- Smith MS, Schambra UB, Wilson KH, Page SO, Hulette C, Light AR, Schwinn DA. (1995) α2-Adrenergic receptors in human spinal cord: specific localized expression of mRNA encoding α2-adrenergic receptor subtypes at four distinct levels. Brain Res Mol Brain Res 34:109–117 [DOI] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hökfelt T, Riedl MS, Elde R. (1998) Differential distribution of α2A and α2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci 18:5928–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. (1997) The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci 17:7157–7165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Vulchanova L, Riedl MS, Wang J, Williams FG, Wilcox GL, Elde R. (1999) Effects of peripheral nerve injury on α2A and α2C adrenergic receptor immunoreactivity in the rat spinal cord. Neuroscience 93:1399–1407 [DOI] [PubMed] [Google Scholar]
- Sullivan AF, Dashwood MR, Dickenson AH. (1987) α2-Adrenoceptor modulation of nociception in rat spinal cord: location, effects and interactions with morphine. Eur J Pharmacol 138:169–177 [DOI] [PubMed] [Google Scholar]
- Veves A, Backonja M, Malik RA. (2008) Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med 9:660–674 [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. (2007) Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol 579:849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Pogrel JW, Lee YW, Chaplan SR. (1995) Reversal of nerve ligation-induced allodynia by spinal α2 adrenoceptor agonists. J Pharmacol Exp Ther 272:207–214 [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. (2008) Sustained inhibition of neurotransmitter release from nontransient receptor potential vanilloid type 1-expressing primary afferents by μ-opioid receptor activation-enkephalin in the spinal cord. J Pharmacol Exp Ther 327:375–382 [DOI] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci 30:4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek JR, Nadeson R, Goodchild CS. (2001) Spinal and supraspinal components of opioid antinociception in streptozotocin induced diabetic neuropathy in rats. Pain 90:57–63 [DOI] [PubMed] [Google Scholar]