Abstract
Activators of AMP-activated protein kinase (AMPK) increase the expression of the human microsomal fatty acid ω-hydroxylase CYP4F2. A 24-h treatment of either primary human hepatocytes or the human hepatoma cell line HepG2 with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), which is converted to 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate, an activator of AMPK, caused an average 2.5- or 7-fold increase, respectively, of CYP4F2 mRNA expression but not of CYP4A11 or CYP4F3, CYP4F11, and CYP4F12 mRNA. Activation of CYP4F2 expression by AICAR was significantly reduced in HepG2 cells by an AMPK inhibitor, 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine (compound C) or by transfection with small interfering RNAs for AMPKα isoforms α1 and α2. A 2.5-fold increase in CYP4F2 mRNA expression was observed upon treatment of HepG2 cells with 6,7-dihydro-4-hydroxy-3-(2′-hydroxy[1,1′-biphenyl]-4-yl)-6-oxo-thieno[2,3-b]pyridine-5-carbonitrile (A-769662), a direct activator for AMPK. In addition, the indirect activators of AMPK, genistein and resveratrol increased CYP4F2 mRNA expression in HepG2 cells. Pretreatment with compound C or 1,2-dihydro-3H-naphtho[2,1-b]pyran-3-one (splitomicin), an inhibitor of the NAD+ activated deacetylase SIRT1, only partially blocked activation of CYP4F2 expression by resveratrol, suggesting that a SIRT1/AMPK-independent pathway also contributes to increased CYP4F2 expression. Compound C greatly diminished genistein activation of CYP4F2 expression. 7H-benz[de]benzimidazo[2,1-a]isoquinoline-7-one-3-carboxylic acid acetate (STO-609), a calmodulin kinase kinase (CaMKK) inhibitor, reduced the level of expression of CYP4F2 elicited by genistein, suggesting that CaMKK activation contributed to AMPK activation by genistein. Transient transfection studies in HepG2 cells with reporter constructs containing the CYP4F2 proximal promoter demonstrated that AICAR, genistein, and resveratrol stimulated transcription of the reporter gene. These results suggest that activation of AMPK by cellular stress and endocrine or pharmacologic stimulation is likely to activate CYP4F2 gene expression.
Introduction
Fatty acid ω-hydroxylases provide a means to remove potentially toxic, excess nonesterified fatty acids that can disrupt mitochondrial function and lead to cellular damage by catalyzing the first step in the formation of dicarboxylic acids. These dicarboxylic acids can be further degraded by peroxisomal β-oxidation for excretion as shorter chain dicarboxylic acids (Reddy and Mannaerts, 1994). In addition, ω-hydroxylases degrade signaling molecules such as prostanoids and leukotrienes. The major fatty acid ω-hydroxylases found in human liver and kidney are P450 4A11 and three members of the 4F P450 family, 4F2, 4F3B, and 4F11 (Lasker et al., 2000; Dhar et al., 2008). 4F2, 4F3B, and 4F11 exhibit highly similar amino acid sequences (≥87%) and display overlapping substrate profiles. 4F2, 4F3B, and 4F11 also provide pathways for the metabolic clearance of branched chain fatty acids, very long-chain saturated fatty acids, xenobiotic substrates such as dietary phytanic acid, some drugs, as well as excess amounts of vitamins E and K (Hsu et al., 2007a; Hardwick, 2008).
It is noteworthy that P450 4F2, as well as 4A11, 4F3B, and 4F11, catalyze the ω-hydroxylation of arachidonic acid to form 20-hydroxyeicosatetraenoic acid, which has been demonstrated to promote vasoconstriction and stimulate natriuresis in the kidney depending on the cellular site of its action (Capdevila and Falck, 2001; Miyata and Roman, 2005). Genetic association studies suggest a role for P450 4F2 in the maintenance of normal blood pressure and prevention of vascular disease (Fava et al., 2008; Fu et al., 2008, 2009; Ward et al., 2008). Increased risks for hypertension and vascular diseases have been reported for carriers of the relatively common minor allelic variant, 4F2 V433M (Stec et al., 2007), that exhibits lower catalytic efficiencies for 20-hydroxyeicosatetraenoic acid formation from arachidonic acid. Moreover, the 4F2 V433M allele seems to be associated with lower levels of expression in human liver microsomes (McDonald et al., 2009). The P450 4F2 V433M allelic variant has been associated with an increased dose requirement for anticoagulants, such as warfarin (Caldwell et al., 2008) and acenocoumarol (Pérez-Andreu et al., 2009). This phenomenon is thought to reflect the role of P450 4F2 in hepatic clearance of vitamin K (McDonald et al., 2009). Finally, the participation of P450s 4F2 and 4F3B in the biotransformation of very long-chain saturated fatty acid or phytanic acid in patients with X-linked adrenoleukodystrophy (Sanders et al., 2006) or Refsum's disease (Komen and Wanders, 2006), respectively, suggests that factors that modulate CYP4F2 expression may play an important role in alleviating these diseases and possibly other disorders of lipid metabolism.
AMP-activated protein kinase (AMPK) is known to play an important role in regulating fatty acid oxidation, and the present studies were designed to assess whether activation of AMPK alters the expression of CYP4F2 and other human fatty acid ω-hydroxylases. AMPK is activated when cellular AMP/ATP ratios are high and subsequently modulates metabolic processes to increase ATP production (Fogarty and Hardie, 2010). AMPK is a heterotrimeric enzyme consisting of a catalytic subunit α and two regulatory subunits β and γ. Increased consumption or reduced production of ATP leads to elevated AMP concentrations, and the binding of AMP to the γ subunit of AMPK activates the kinase. Activation of AMPK requires phosphorylation of Thr-172 on the α-subunit by upstream kinases. Thr-172 phosphorylation combined with AMP binding to the enzyme leads to a >1000-fold increase in kinase activity. Upon activation, AMPK phosphorylates key enzymes such as fatty acid synthase, acetyl-CoA carboxylase, and glycogen synthase kinase, which leads to decreased ATP utilization for fatty acid, sterol, and glycogen synthesis while increasing fatty acid oxidation and glycolysis for ATP production. In addition, AMPK modulates the activity of a number of transcription factors that serve to augment these metabolic changes (Cantó et al., 2010; Fogarty and Hardie, 2010).
In this study, we examined the effect of AICAR, a precursor of the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate (ZMP), on CYP4 gene expression in HepG2 cells and human hepatocytes as part of our ongoing studies to identify and characterize factors that govern the expression of CYP4 genes. Our results indicate that CYP4F2 mRNA, but not CYP4F3B, CYP4F11, CYP4F12, or CYP4A11, is increased after AICAR treatment. Using an AMPK chemical inhibitor, 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine (compound C), as well as AMPKα siRNA knockdown, we demonstrated that the increased CYP4F2 expression by AICAR depends on AMPK. In addition, resveratrol and genistein, which activate AMPK indirectly through independent pathways, were found to increase CYP4F2 expression.
Materials and Methods
Reagents and Antibodies.
AICAR was obtained from Toronto Research Chemicals Inc. (North York, ON, Canada). 6,7-Dihydro-4-hydroxy-3-(2′-hydroxy[1,1′-biphenyl]-4-yl)-6-oxo-thieno[2,3-b]pyridine-5-carbonitrile (A-769662) was obtained from Tocris Bioscience (Ellisville, MO). Resveratrol, genistein, compound C, 5-iodotubercidin, fulvestrant, and 1,2-dihydro-3H-naphtho[2,1-b]pyran-3-one (splitomicin) were purchased from EMD Biosciences (San Diego, CA). 7H-benz[de]benzimidazo[2,1-a]isoquinoline-7-one-3-carboxylic acid acetate (STO-609) and 17β-estradiol were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit antibodies recognizing total AMPKα, phospho-Ser79 acetyl-CoA carboxylase 1 (ACC), and total ACC were purchased from Cell Signaling Technology (Danvers, MA). β-Tubulin was used as a loading control, and a rabbit polyclonal antibody for β-tubulin was obtained from Thermo Fisher Scientific (Waltham, MA). Rabbit polyclonal antibody recognizing cytochrome P450 reductase (H-300) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-rabbit IgG (monoclonal clone RG-96) was obtained from Sigma-Aldrich. The Western Lightening Plus chemiluminescence reagent was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA).
Cell Culture.
The human hepatoma cell line, HepG2, was obtained from the American Type Culture Collection (Manassas, VA) and was maintained in Dulbecco's minimal essential medium (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Thermo Fisher Scientific). The medium was supplemented with 10 mM HEPES, minimal essential medium nonessential amino acid mixture, and penicillin/streptomycin. For drug treatment of nontransfected HepG2 cells, the cells were seeded at a density that reached 80 to 90% confluence after 24 h. Upon achieving the appropriate density, cells were incubated with fresh medium containing various concentrations of genistein, resveratrol, AICAR, or the appropriate solvent controls (DMSO for genistein and resveratrol; PBS for AICAR) as indicated in the figure legends.
For reporter gene transfection studies, HepG2 cells were transfected with CYP4F2-luciferase reporter constructs that have been described in detail elsewhere (Hsu et al., 2007b) together with an expression vector for β-galactosidase using jetPEI reagent (VWR, West Chester, PA) according to the manufacturer's protocol. After 24 h, media were replenished with fresh media containing either test compounds or corresponding vehicle. Cells were harvested for luciferase and β-galactosidase measurement 24 h later.
Primary Human Hepatocyte Culture.
Primary human hepatocytes were obtained from CellzDirect (Tucson, AZ) or the Liver Tissue Cell Distribution System (Pittsburgh, Pennsylvania), the latter of which is funded by National Institutes of Health Contract N01-DK-7-0004/HHSN26700700004C. The anonymous donor subjects had no known history of overt drug or alcohol abuse or exposure to hepatitis B, hepatitis C, cirrhosis, biliary diseases, or HIV, and they were nonsmokers. Demographic information was as follows: subject Hu374, a 77-year-old woman; subject Hu419, a 53-year-old woman; subject HM1516, a 47-year-old woman; subject HM1521, a 47-year-old man; and subject HM1529, a 50-year-old woman. Hepatocytes were seeded onto collagen-coated six-well plates. Hepatocytes from the Liver Tissue Cell Distribution System (HM1516, HM1521, and HM1529) were maintained in Williams' medium E. Hepatocytes from CellzDirect (Hu374 and Hu419) were also overlaid with Matrigel and maintained in modified Chee's medium. All media were supplemented with dexamethasone (0.1 μM) and an insulin-transferrin-sodium selenite media supplement (Sigma-Aldrich). Media were changed daily. Two days after receipt, the cells were treated with AICAR or PBS and harvested 24 h later.
AMPK siRNA Study.
Human AMPKα1 ON-TARGETplus SMARTpool (α1b) and ON-TARGETplus Nontargeting pool siRNAs were obtained from Thermo Fisher Scientific/Dharmacon RNA Technologies (Lafayette, CO). AMPKα1 (α1a), AMPKα2 (α2a and α2b), DS Scrambled-Neg. (negative control), and HPRT-S1 DS (positive control) dicer-substrate siRNA duplexes were obtained from IDT Technology (San Diego, CA). Nucleotide sequences of the siRNA targeting AMPK are provided in Supplemental Table S1. HepG2 cells were transfected with siRNAs at a final concentration of 10 nM using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). The final concentration of siRNA was chosen based on significant silencing of AMPKα and HPRT expression with target siRNAs (AMPKα and HPRT) but without any apparent cytotoxicity. No significant difference was seen in the expression of AMPKα, CYP4F2, and HPRT between mock-transfected cells and cells transfected with nontarget siRNA. Twenty-four hours later, cell culture media were replaced with fresh media containing the test compounds. Total RNA was isolated 24 h later.
Preparation of P450 4F2/3 Antibody.
Recombinant human P450 4F2, 4F11, and 4F12 were derived from hemin-fortified suspension cultures of Spodoptera frugiperda (Sf9) insect cells that had been infected with the corresponding CYP4F cDNA-containing baculovirus constructs. After a 72-h infection period, Sf9 cells were harvested and lysed by sonication, and the recombinant P450 4Fs were purified to near homogeneity from the lysates using a combination of hydrophobic, adsorption, and/or metal ion affinity chromatography (Dhar et al., 2008). Additional details regarding CYP4F cDNA cloning, baculovirus construct preparation, and expressed enzyme purification will be published elsewhere. Polyclonal antibodies to recombinant P450 4F2 were raised in male New Zealand white rabbits. The cross-reactivity of the resultant antibody with P450 4F11 and 4F12 was then removed by back-adsorption of anti-P450 4F2 IgG against a Affi-Gel 10 agarose gel solid-phase support to which P450 4F11 and 4F12 Sf9 lysate proteins had been coupled. We previously used a similar procedure to remove the cross-reactivity of murine CYP4A proteins with antibodies to human P450 4A11 (Savas et al., 2009). The final back-absorbed antibody exhibited cross-reactivity with P450 4F2 and P450 4F3 and was designated as anti-P450 4F2/3 IgG (Supplemental Fig. S1).
Immunoquantitation: AMPKα, ΑCC, and β-Tubulin Protein Expression.
Whole-cell lysates from HepG2 cells were prepared as described previously (Nystrom and Lang, 2008). PhosphoSTOP phosphatase inhibitor cocktail and Complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) were also included during the preparation of lysates. Immunoblotting was performed using 10% NuPAGE Bis-Tris gels (Invitrogen). Nitrocellulose membranes were incubated with anti-AMPKα, anti-phospho-Ser79 ACC, and anti-total ACC and anti-β-tubulin antibodies overnight at 4°C according to the manufacturer's instructions.
Immunoquantitation: P450 4F2/3 Expression.
Immunoblotting of HepG2 microsomal proteins and Supersomes containing P450 4F2 or human cytochrome P450 reductase (CPR) (BD Gentest, Woburn, MA) was also performed using 10% NuPAGE Bis-Tris gels (Invitrogen) as described previously (Hsu et al., 2007b). Nitrocellulose membranes were incubated with anti-human P450 4F2/3 IgG and anti-CPR antibody. CPR protein levels were found to remain unaffected by treatments with AMPK activators by one-way analysis of variance and Dunnett's multiple comparison tests and were thus used as a control (i.e., internal standard) for microsomal protein content. After reaction with peroxidase-conjugated anti-rabbit IgG, the blots were developed using chemiluminescence (Western Lightening Plus; PerkinElmer Life and Analytical Sciences).
Total RNA Isolation, cDNA Generation, and Real-Time PCR.
Total RNA was isolated using TRIzol reagent (Invitrogen). First-strand cDNAs were generated by reverse transcriptase using the RevertAid First Strand cDNA Synthesis Kit (MBI Fermentas, Hanover, MD). Amplicons corresponding to CYP4F2, CYP4F3, AMPKα1, AMPKα2, and PPIA mRNAs were generated by reverse transcription (RT)-PCR from HepG2 cells, subcloned into the pCRII-TOPO vector (Invitrogen), and the cloned sequences were confirmed by DNA sequencing. The PCR primer sets used are listed in Supplemental Table S1. Serial dilutions of each amplicon were used as a reference to determine the mRNA copy number in each RT sample. For real-time thermal cycling, triplicate aliquots of serially diluted amplicon or RT sample were used in a reaction mixture that contained 250 nM of each primer with the Maxima SYBR Green Master Mix (MBI Fermentas). A BioRad iCYCLER iQ real-time PCR instrument (Bio-Rad Laboratories, Hercules, CA) was used. The PCR conditions were 10 min at 95°C, followed by 50 to 60 cycles of (95°C for 20 s, 60°C for 30 s, and 72°C for 30 s). Melting curves for the final products were analyzed to insure product uniformity and absence of primer-dimer or other nonspecific products. Because CYP4F2 and CYP4F3B have highly similar nucleotide sequences, the CYP4F2 primer sets were checked with the CYP4F3 plasmid to rule out the potential for PCR cross-reaction. The same was performed for the CYP4F3, CYP4F11, and CYP4F12 primer sets with the CYP4F2 plasmid. Copy numbers were calculated by mapping the threshold cycle to the corresponding plasmid copy number on standard curves. Analyses of additional gene expression by qPCR used PPIA amplicons as the normalization control and the primer sets listed in Supplemental Table S1. The mRNA levels of examined genes were normalized to PPIA mRNA levels, which were not affected by the treatments of AMPK activators. For each independent experiment, triplicate samples were used for each treatment.
Statistical Analysis.
Results were analyzed using Prism (GraphPad Software Inc., San Diego, CA) or Excel (Microsoft, Redmond, WA). Statistical significance was determined by Student's t test or analysis of variance.
Results
AICAR Increases CYP4F2 Expression in HepG2 Cells.
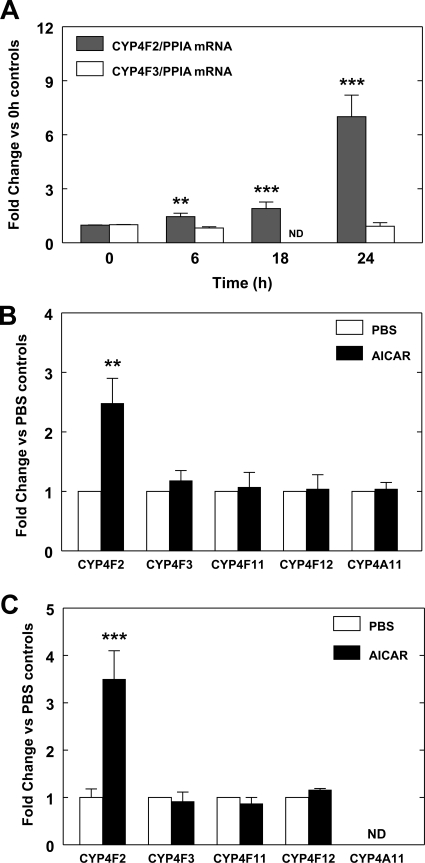
To test whether activation of AMPK modulates CYP4 gene expression in HepG2 cells, we examined the effects of AICAR, a compound widely used to activate AMPK (Fogarty and Hardie, 2010), on CYP4 expression. The human hepatoma-derived cell line, HepG2, expresses CYP4F2 and CYP4F3B constitutively and has been used to characterize the increased expression of CYP4F2 by statins (Hsu et al., 2007b). Treatment of HepG2 cells with AICAR led to a time-dependent increase in the expression of CYP4F2 mRNA that was elevated by an average of 7-fold at 24 h, but remained relatively low until 18 h (Fig. 1A). Likewise, the expression of the CYP4F2 gene in cultured human hepatocytes was increased by AICAR to 1.7 - 4.1-fold (mean, 2.5-fold), but no effect was seen on the expression of CYP4F3, CYP4F11, CYP4F12, and CYP4A11 genes (Fig. 1B). Significant expression of the CYP4A11 gene was not evident in HepG2 cells as reported previously (Hsu et al., 2007b), and the expression of the CYP4F3, CYP4F11, and CYP4F12 genes was not significantly affected by AICAR in HepG2 cells (Fig. 1C). These results indicate that AICAR increases CYP4F2 expression and suggest that the HepG2 cell line could serve as a suitable model system for studying the effect of AICAR on CYP4F2 expression.
Fig. 1.
AICAR elevates PPIA normalized CYP4F2 expression in HepG2 cells and human hepatocytes. A, HepG2 cells were treated with 0.5 mM AICAR for various times as indicated. Cells were harvested, and RNA was isolated and subjected to qPCR analysis as described under Materials and Methods. The mRNA levels of CYP4F2 and CYP4F3 were normalized to PPIA mRNA levels, which were not affected by the AICAR treatment. For each independent experiment, triplicate samples were used for each treatment and triplicate determinations were performed for each sample. The fold change for PPIA-normalized CYP4F2 and CYP4F3 mRNA levels in each independent experiment was determined by comparing the mean value obtained from cells harvested at various time points to the mean obtained from the cells harvested at the zero time point. Mean values and standard errors were determined from four independent experiments. Statistically significant differences between the zero time point and each time point of AICAR treatments are indicated: **, p < 0.01; ***, p < 0.001. ND, not determined. B, primary human hepatocytes were treated with 0.5 mM AICAR for 24 h. RNA was isolated and subjected to qPCR, and the data were analyzed as described above. The basal PPIA normalized CYP4F2 expression level varied over a 10-fold range for hepatocytes obtained from five tissue donors. The fold increase for PPIA normalized CYP4F2, CYP4F3, CYP4F11, CYP4F12, and CYP4A11 mRNA expression was determined by comparing the mean value obtained from hepatocytes treated with AICAR to the mean obtained from PBS-treated cells. Mean values and standard errors were determined for hepatocytes from five different tissue donors. A statistically significant difference between PBS and AICAR treatment was seen for CYP4F2: **, p < 0.01. C, HepG2 cells were treated with 0.5 mM AICAR for 24 h. The fold increase for PPIA normalized CYP4F2, CYP4F3, CYP4F11, CYP4F12, and CYP4A11 mRNA expression by AICAR was determined by comparison with the cells treated with PBS. Mean values and standard errors were determined from at least four independent experiments. Statistically significant differences between the PBS and AICAR treatments are indicated: ***, p < 0.001. ND, not determined.
In addition, we found that actinomycin D, an inhibitor of transcription, blocked the increase of CYP4F2 expression regardless of whether it was coadministered with AICAR or given 6 h after AICAR treatment, thus indicating that increased CYP4F2 expression does not involve stabilization of existing CYP4F2 mRNA (Supplemental Fig. S2A).
The Effect of AICAR on CYP4F2 Expression Is Mediated through AMPK.
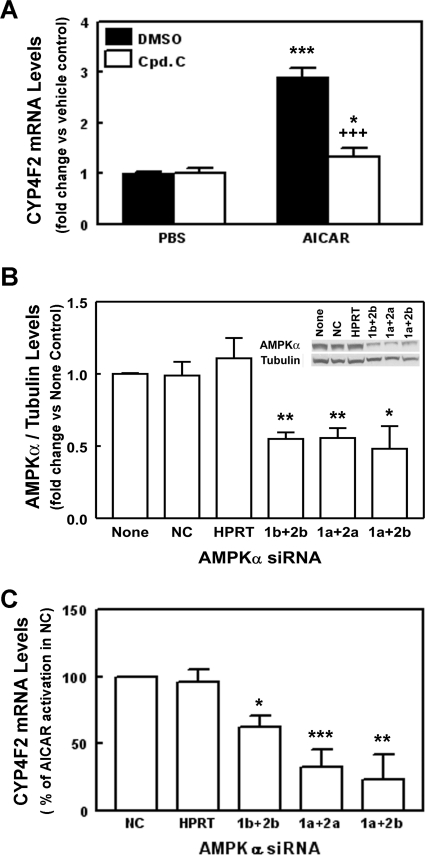
AICAR is converted by adenosine kinase to ZMP, which activates AMPK by mimicking the actions of AMP as an allosteric activator of the enzyme (Henin et al., 1995). To confirm that the enhancement of CYP4F2 expression by AICAR depends on ZMP formation, 5-iodotubercidin was used to block the conversion of AICAR to ZMP in HepG2 cells. In the presence of 5-iodotubercidin, the induction of CYP4F2 transcripts by AICAR was inhibited to levels seen for the vehicle control (Supplemental Fig. S2B), suggesting that the effect of AICAR on CYP4F2 mRNA expression requires its conversion to ZMP. The activation of AMPK by AICAR in HepG2 cells was supported by the observations that the phosphorylated states of ACC, a target for AMPK phosphorylation, were elevated by the AICAR treatment (Supplemental Fig. S3). In addition, expression of small heterodimer partner (SHP) was elevated after AICAR treatment in HepG2 cells (Supplemental Fig. S4). AMPK was shown to mediate the stimulation of SHP expression by AMPK activators (Kim et al., 2008). Confirmation of AMPK's role in mediating the AICAR-increased CYP4F2 gene expression was provided by compound C, an AMPK inhibitor. When HepG2 cells were pretreated with compound C, the increased CYP4F2 mRNA expression elicited by AICAR was decreased by 82% (Fig. 2A). Likewise, compound C blocked the increase of SHP expression in response to AICAR treatment (Supplemental Fig. S4).
Fig. 2.
AMPK mediates the stimulatory effects of AICAR on PPIA-normalized CYP4F2 mRNA expression in HepG2 cells. A, HepG2 cells were pretreated with either 10 μM compound C (an AMPK inhibitor) in DMSO or DMSO alone for 30 min before treatment with AICAR (0.5 mM) for 24 h. The cells were harvested, RNA was isolated, and CYP4F2 and PPIA mRNA expression was assessed using qPCR. Data were analyzed as described in the legend to Fig. 1. Results are expressed as fold change in PPIA-normalized CYP4F2 mRNA levels by AICAR relative to the vehicle control in the same experiment. Data represent means and standard errors determined from four independent experiments. Statistically significant differences between PBS and AICAR treatments are shown: *, p < 0.05; ***, p < 0.001. Significant difference between DMSO and compound C treatments in the presence of AICAR are shown: +++, p < 0.001. B and C, HepG2 cells were cotransfected with both AMPKα1 (1) and AMPKα2 (2) siRNAs as described under Materials and Methods. For each AMPKα isoform, two different siRNAs (a or b) were used. Twenty-four hours later, the culture media were replaced with fresh media containing 0.5 mM AICAR or PBS. Cells were harvested for total RNA isolation after another 24 h. In addition, a siRNA targeting HPRT (HPRT) and a nontargeting/scrambled siRNA control (NC) were transfected into the HepG2 cells to monitor transfection efficiency and cellular cytotoxicity. All siRNAs were used at a final concentration of 10 nM. After 48 h of transfection, HPRT siRNA reduced the corresponding PPIA-normalized HPRT mRNA levels by 80% relative to the scrambled siRNA controls (NC) (Supplemental Fig. S5A). No significant difference in the expression of HPRT was found between mock-transfected cells (None) and cells transfected with the NC siRNA. B, effects of AMPKα siRNAs on the β-tubulin-normalized AMPKα protein levels. Whole-cell lysates (80–100 μg of protein per sample) were subjected to immunoblotting with anti-human AMPKα (AMPKα) and anti-β-tubulin (Tubulin) and analyzed as described under Materials and Methods. The effect of AMPKα siRNA knockdown on β-tubulin-normalized AMPKα protein expression was determined by comparing the expression of β-tubulin-normalized AMPKα in the mock-transfected control (None), which was set as 1. Means and standard errors were determined from three independent experiments. Statistically significant differences between NC and AMPK or HPRT targeting siRNAs are indicated as: *, p < 0.05; **, p < 0.01. A representative immunoblot is shown as an insert. C, effects of AMPKα siRNAs on AICAR elevated PPIA-normalized CYP4F2 mRNA expression. The effect of AMPKα siRNA knockdown on PPIA-normalized CYP4F2 mRNA expression was determined by comparing the mean fold change produced by AICAR in the presence of AMPK siRNAs to the mean fold change produced by AICAR in the presence of the NC siRNA, which was set as 100%, in the same experiment. Because no significant differences in AMPKα protein/RNA and CYP4F2 expression were noted between cells transfected with NC siRNA and mock-transfected cells (None), the data obtained from the NC siRNA-treated cells was used as control. Means and standard errors were determined from three independent experiments. Statistically significant differences from NC are indicated: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
RNA interference studies using AMPK siRNAs were performed to corroborate the role of AMPK in the increased CYP4F2 expression by AICAR. Because mRNAs for AMPKα isoforms, α1 and α2, are expressed in HepG2 cells, different pairs of siRNAs for the AMPKα1 and AMPKα2 isoforms, 1a + 2a, 1a + 2b, and 1b + 2b, were cotransfected into HepG2 cells. No significant difference was seen between cells transfected with nontargeting/scrambled siRNA control (NC) and mock-transfected cells with regard to the expression of hypoxanthine phosphoribosyl transferase (HPRT), CYP4F2, and AMPKα1 and AMPKα2 mRNAs (Supplemental Fig. S5A). Similar results were seen for the AMPKα protein expression (Fig. 2B). These observations indicate that the concentration of nontargeting siRNA used for transfection did not affect the expression of AMPKα, CYP4F2, and HPRT. When the AMPKα protein expression was examined in HepG2 cells at 48 h after transfection of the AMPKα siRNAs, the protein level was reduced to 30 to 60% of the level seen with NC or mock-transfected cells (Fig. 2B). The expression of mRNAs for the AMPKα1 and AMPKα2 isoforms was also reduced to 40 to 70% of control values at 48 h after transfection (Supplemental Fig. S5B). When CYP4F2 mRNA levels were examined, it was found that these AMPK siRNAs did not significantly affect CYP4F2 expression in the absence of AICAR. However, the increased CYP4F2 expression noted upon AICAR treatment was significantly reduced to 30 to 70% by each combination of siRNAs (Fig. 2C). The decrease in CYP4F2 transcript levels was similar to the effects of these probes on the expression of mRNAs and protein for the AMPKα1 and AMPKα2 isoforms. No effect on CYP4F2 mRNA was observed for scrambled control siRNA or a siRNA targeting HPRT, although the latter suppressed the expression of HPRT mRNA to more than 80% of control values (Supplemental Fig. S5A). Such results further support the obligatory role of AMPK in mediating the enhancement of CYP4F2 expression by AICAR.
A-769662, a Direct Activator of AMPK, Increases the Expression of CYP4F2 mRNA in HepG2 Cells.
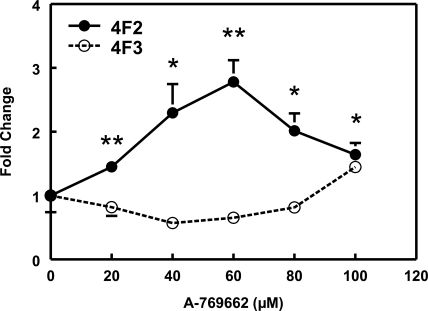
As mentioned previously, both adenosine transporter and adenosine kinase are required for transport and transformation of AICAR to ZMP to mimic the effect of AMP on AMPK activation. In contrast to AICAR, A-769662, a small-molecule thienopyridone, has been reported to activate AMPK directly in cell-free assays (Göransson et al., 2007). When HepG2 cells were exposed to various concentrations of A-769662 for 24 h, CYP4F2 mRNA expression was found to increase 2.2- to 3.6-fold (average 2.8-fold) at 60 μM A-769662 (Fig. 3). In addition, A-769662 treatment elevated SHP expression, and this effect was abolished by compound C treatment (Supplemental Fig. S4).
Fig. 3.
A-769662 increases PPIA-normalized CYP4F2 mRNA expression in HepG2 cells. A-769662 displays a dose-dependent increase of PPIA-normalized CYP4F2 expression in HepG2 cells at 24 h after treatment. PPIA-normalized CYP4F2 and CYP4F3 mRNA levels were expressed relative to the corresponding controls in the same experiment as described in the legend to Fig. 1. Means and standard errors were determined from at least three independent experiments. Statistically significant differences compared with DMSO (0 μM) treatments are indicated: *, p < 0.05; **, p < 0.01.
AMPK Mediates the Increased Expression of CYP4F2 by Resveratrol and Genistein.
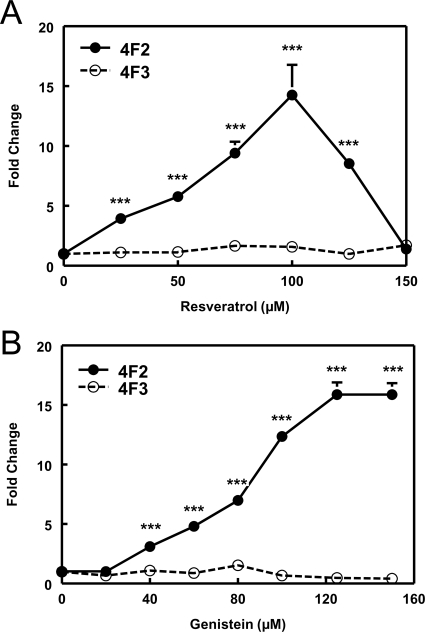
Resveratrol (Hou et al., 2008) has been reported to activate AMPK indirectly. As such, CYP4F2 mRNA expression was found to exhibit a dose-dependent increase in response to resveratrol. In contrast, resveratrol had little effect on CYP4F3B mRNA expression (Fig. 4A). Resveratrol at 75 μM was chosen for further studies to optimize the response without producing overt cell cytotoxicity. At this concentration, resveratrol increased CYP4F2 expression 3.7- to 11-fold (average ∼7-fold) in HepG2 cells. The activation of AMPK by resveratrol in HepG2 cells was demonstrated by increased phosphorylation of ACC (Supplemental Fig. S3).
Fig. 4.
Resveratrol and genistein increase PPIA-normalized CYP4F2 mRNA expression in HepG2 cells. A, resveratrol elicits a dose-dependent increase of PPIA-normalized CYP4F2 expression in HepG2 cells at 24 h after treatment. PPIA-normalized CYP4F2 and CYP4F3 mRNA levels were expressed relative to the corresponding controls in the same experiment as described in the legend to Fig. 1. Means and standard errors were determined from at least three independent experiments. Statistically significant differences compared with DMSO (0 μM) treatments are indicated: ***, p < 0.001. B, genistein dose-response curve for PPIA-normalized CP4F2 and CYP4F3 mRNA expression in HepG2 cells was determined after a 24-h exposure. The PPIA-normalized CYP4F2 and CYP4F3 mRNA levels were expressed relative to the corresponding controls (0 μM) in the same experiment. Means and standard errors were determined from at least three independent experiments. Statistically significant differences between DMSO and genistein treatments are indicated: ***, p < 0.001.
Genistein, a dietary isoflavone and phytoestrogen, has been reported to activate AMPK indirectly (Hwang et al., 2005). In HepG2 cells, genistein elicited a dose-dependent increase in CYP4F2 mRNA expression, but, similar to resveratrol, had no effect on CYP4F3B mRNA (Fig. 4B). A final concentration of 100 μM genistein was chosen for further studies based on its efficacy and lack of apparent toxicity. This concentration of the isoflavone, which is similar to that used in other studies of AMPK activation (Hwang et al., 2005), produced a 5.5- to 23-fold (average ∼13-fold) increase in CYP4F2 expression in HepG2 cells. Genistein treatment also increased ACC phosphorylation in HepG2 cells consistent with activation of AMPK by this isoflavone (Supplemental Fig. S3).
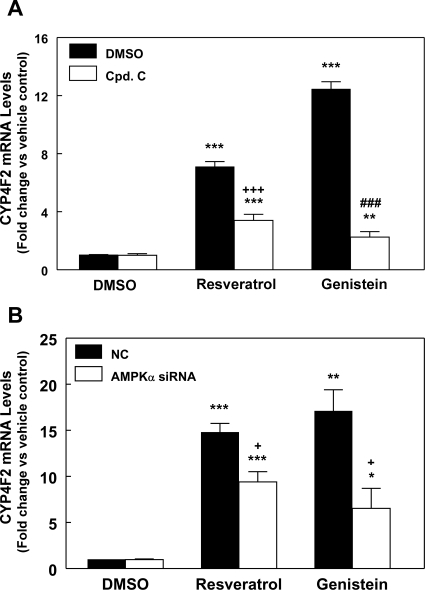
Upon pretreatment of HepG2 cells with compound C, an AMPK inhibitor, the increase in CYP4F2 expression mediated by resveratrol was diminished to ∼50% of that seen in the absence of compound C. In contrast, the induction of CYP4F2 expression by genistein was reduced more extensively to 16% when the cells were pretreated with compound C (Fig. 5A). The expression of AMPK known target gene SHP was also elevated by genistein and resveratrol. This stimulation of expression was blocked by compound C treatment (Supplemental Fig. S4). These results indicate that the effect of genistein on CYP4F2 expression is mediated largely by AMPK, whereas resveratrol induces CYP4F2 expression through AMPK-dependent and -independent pathways in approximately equal proportions. Similar results were also obtained when the cells were transfected with AMPKα siRNAs (Fig. 5B).
Fig. 5.
Activation of PPIA-normalized CYP4F2 mRNA expression by resveratrol and genistein in HepG2 cells is mediated by AMPK. A, HepG2 cells were pretreated with compound C (10 μM) or DMSO for 30 min before addition of resveratrol (75 μM), genistein (100 μM), or DMSO. After 24 h, the cells were harvested, total RNA was isolated, and the RNA was then subjected to qPCR analysis. Data were analyzed as described in the legend to Fig. 1. Means and standard errors were determined from at least three independent experiments. Statistically significant differences between DMSO and resveratrol/genistein treatments are depicted: **, p < 0.01 and ***, p < 0.001. Significant difference between DMSO and compound C treatments in the presence of resveratrol is indicated: +++, p < 0.001. Significant difference between DMSO and compound C treatments in the presence of genistein is indicated: ###, p < 0.001. B, effects of AMPKα siRNAs on the genistein- or resveratrol-stimulated PPIA-normalized CYP4F2 mRNA expression. HepG2 cells were transfected with the combination of 1a + 2b AMPKα siRNAs, and the cells were treated with genistein or resveratrol as described in the legend to Fig. 2B. Data were analyzed as described in the legend to Fig. 1. Means and standard errors were determined from three independent experiments. Statistically significant differences between DMSO and resveratrol or genistein treatments are indicated: *, p < 0.05; **, p < 0.01; ***, p < 0.001). Significant difference between cells transfected with NC siRNA and AMPKα siRNAs is indicated: +, p < 0.05.
AMPK Activators Elevate P450 4F2 Protein Expression.
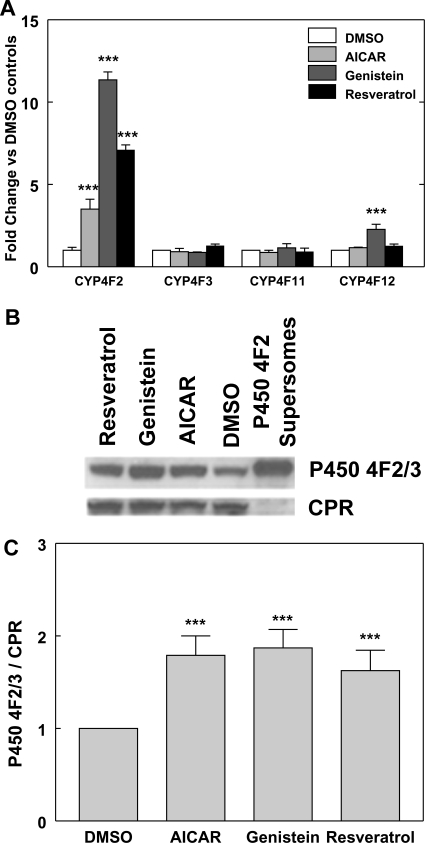
As shown in Figs. 1 and 6A, CYP4F3B, CYP4F11, and CYP4F12 mRNA expression was not affected by AICAR treatment. We also examined the effects of genistein and resveratrol on CYP4F11 and CYP4F12 mRNA expression. Resveratrol did not elicit significant effects on CYP4F11 and CYP4F12 mRNA expression as seen with AICAR. Genistein also did not have an effect on CYP4F11 mRNA expression, whereas the expression of CYP4F12 mRNA was elevated approximately 2-fold (Fig. 6A). This stimulatory effect of genistein on CYP4F12 mRNA expression is ∼11% of that observed for CYP4F2 mRNA expression. Because CYP4F2 and CYP4F12 mRNAs are expressed at similar levels in HepG2 cells (Hart et al., 2010), a more specific antibody was developed, which recognizes P450 4F2 and 4F3B, but not 4F11 and 4F12 (Supplemental Fig. S1). Immunoblots probed with this P450 4F2/3 antibody showed that P450 4F2/3 protein expression was elevated 1.6- to 1.9-fold in HepG2 cells treated with AICAR, genistein, or resveratrol for 24 h compared with control values (Fig. 6C). Because CYP4F3B mRNA expression was not significantly affected by AMPK activators, the enhancement of total P450 4F2/3 protein noted in response to AICAR, genistein, and resveratrol stems mainly from the increase in P450 4F2 expression. Although the increase in P450 4F2 protein expression observed with AMPK activators is less than that noted with CYP4F2 mRNA, this difference may arise from a masking effect of P450 4F3B, which is also recognized by the 4F2/4F3 antibody but cannot be resolved electrophoretically from CYP4F2. Therefore, the actual fold increase of P450 4F2 protein levels by AMPK activators is likely to be underestimated. In addition, it should be noted that the increased expression of CYP4F2 mRNA elicited by AICAR is a delayed response and did not become apparent until after 18 h of treatment (Fig. 1A). Because the samples used for CYP4F2 protein analysis were derived from cells treated with AMPK activators for 24 h, a larger enhancement of P450 4F2 protein expression by AMPK activators may have occurred at a later time point.
Fig. 6.
P450 4F2 protein expression is elevated by treatment with AICAR, genistein, or resveratrol. A, effects of AMPKα activators on the expression of CYP4F mRNA expression in HepG2 cells. HepG2 cells were treated with resveratrol (75 μM), genistein (100 μM), AICAR (0.5 mM), or DMSO. After 24 h, the cells were harvested, total RNA was isolated, and the RNA was then subjected to qPCR analysis. Data were analyzed as described in the legend to Fig. 1. Means and standard errors were determined from at least three independent biological replicates. Statistically significant differences between DMSO and AMPK activator treatments are indicated: ***, p < 0.001. B, microsomes (30–70 μg of protein) prepared from HepG2 cells that were treated with AICAR (0.5 mM), genistein (100 μM), or resveratrol (75 μM) for 24 h were subjected to immunoblotting with anti-human P450 4F2/3 IgG (P450 4F2/3) and anti-P450 reductase (CPR) as described under Materials and Methods. The P450 4F2/3 antibody recognizes both 4F2 and 4F3, but not 4F11 or 4F12 (Supplemental Fig. S1). P450 4F2 Supersomes (20 fmol) were included as positive controls. A representative immunoblot is shown. C, exposed films of the P450 4F2/3 immunoblots were scanned, and the signals were analyzed with ImageQuant 5.2 software (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Standard curves were obtained with P450 4F2 Supersomes to ensure the linearity of immunochemical staining. P450 4F2/3 immunostaining intensity was normalized against that of CPR, which was not affected by treatment with AMPK activators. The normalized P450 4F2/3 signals from the HepG2 cells treated with test compounds were compared with the corresponding DMSO controls and are expressed as fold change. The means and standard errors are determined from at least four independent experiments. Statistically significant differences from DMSO controls are indicated: ***, p < 0.001.
Role of Upstream Kinases in AMPK-Mediated Induction of CYP4F2 Expression.
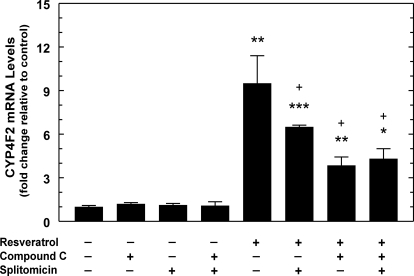
Small molecules can activate AMPK indirectly by altering cellular AMP/ATP ratios and stimulating upstream kinases such as LKB1 or Ca2+/calmodulin kinase kinase (CaMKK) (Fogarty and Hardie, 2010). Resveratrol is thought to activate AMPK by stimulating the NAD+- dependent deacetylase SIRT1, which, in turn, deacetylates LKB1 to increase the enzyme's activity (Hou et al., 2008). To test whether the effect of resveratrol depended on SIRT1 activation, we treated HepG2 cells with the SIRT1 inhibitor splitomicin (Bedalov et al., 2001). Splitomicin reduced the effect of resveratrol on CYP4F2 expression to approximately 60%, which accounts for 55% of the AMPK-dependent activation seen for the experiment depicted in Fig. 7. The inhibitory effect of splitomicin ranges from 55 to 80% of the AMPK-dependent effect of resveratrol. Moreover, the effect of splitomicin was not additive with compound C, suggesting that SIRT1/LKB1 participates in the AMPK-mediated response to resveratrol, but not in the AMPK-independent response (Fig. 7).
Fig. 7.
Involvement of SIRT1 in the activation of PPIA-normalized CYP4F2 mRNA expression by resveratrol in HepG2 cells. HepG2 cells were pretreated with SIRT1 inhibitor splitomicin (100 μM) or DMSO for 24 h. Compound C (10 μM) or DMSO was added to the medium 30 min before addition of resveratrol (75 μM) or vehicle control (DMSO), and the cells were harvested 24 h later. Total RNA was isolated for qPCR assays, and data were analyzed as described in the legend to Fig. 1. Two independent experiments were performed, of which one is shown. Means and standard errors were determined from triplicate samples. Statistically significant differences from DMSO controls are indicated: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significant differences for the treatments with compound C and/or splitomicin in the presence of resveratrol are indicated: +, p < 0.05.
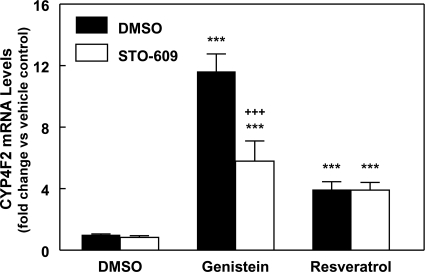
The induction of CYP4F2 expression in HepG2 cells by genistein was not affected by splitomicin treatment (Supplemental Fig. S6), which suggests that the effect of genistein is not mediated through activation of SIRT1 as seen for resveratrol. The role of the AMPK upstream kinase, CaMKK, was also examined. The genistein-mediated increase of CYP4F2 mRNA levels in HepG2 cells was reduced to ∼53% by treating the cells with the CaMKK-specific inhibitor STO-609 (Fig. 8). However, STO-609 treatment did not significantly affect the increase of CYP4F2 expression elicited by resveratrol. These results suggest that the CaMKK pathway contributes to AMPK-mediated induction of CYP4F2 in response to genistein. We also examined whether the estrogenic effects of genistein contributed to its ability to enhance CYP4F2 expression. Fulvestrant, an estrogen receptor-specific antagonist, did not affect the increase of CYP4F2 expression elicited by genistein. Moreover, the estrogen receptor agonist, 17β-estradiol, did not stimulate the expression of the CYP4F2 gene in HepG2 cells (Supplemental Fig. S7).
Fig. 8.
CaMKK/AMPK pathway participates in genistein-mediated activation of PPIA-normalized CYP4F2 mRNA expression in HepG2 cells. HepG2 cells were pretreated with 1 μM STO-609 (a specific calmodulin kinase kinase inhibitor) or DMSO for 1 h before treatment for 24 h with genistein (100 μM), resveratrol (75 μM), or vehicle control (DMSO). RNA was isolated and subjected to qPCR, and the data were analyzed as given in the legend to Fig. 1. Means and standard errors were determined from three independent experiments. Statistically significant differences between DMSO and treatments are indicated: ***, p < 0.001. Significant difference between DMSO and STO-609 treatments in the presence of genistein is indicated: +++, p < 0.001.
Role of SREBP-2 in the AMPK-Mediated Activation of CYP4F2 Expression.
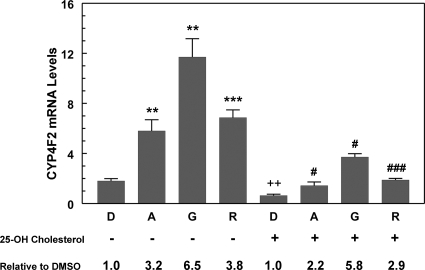
Genistein has been reported to activate the transcription of SREBP-regulated genes as well as increase the abundance of the mature, active form of SREBP-2 in nuclei of HepG2 cells (Mullen et al., 2004). SREBP-2 could also be activated because of the inhibitory effects of AMPK activation on cholesterol synthesis. AMPK can phosphorylate and inactivate HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. The generation of the mature, active form of SREBP-2 would increase if disruption of cholesterol synthesis significantly lowered cellular sterol concentrations. Mullen et al. (2004) cultured HepG2 cells in the absence of serum to further reduce cellular sterol levels and demonstrated that supplementation of cultures with 25-hydroxycholesterol suppressed the effects of genistein on SREBP-2 activation. Because CYP4F2 gene transcription is activated by SREBPs (Hsu et al., 2007b), the effect of genistein as well as other activators of AMPK on CYP4F2 expression could reflect, in part, SREBP-2 activation. To test this hypothesis, HepG2 cells cultured in media containing serum (as in the other experiments described here) were treated with either 25-hydroxycholesterol or vehicle control. We first examined the expression of SREBP-2 target genes, such as low-density lipoprotein receptor and HMG-CoA reductase. In the absence of 25-hydroxycholesterol, the mRNA levels of these target genes were modestly elevated by the three AMPK activators, ∼1.6-fold for AICAR and resveratrol and ∼2-fold for genistein. Addition of 25-hydroxycholesterol suppressed the increased expression observed in response to AMPK activators (Supplemental Fig. S8). These results suggested that SREBP-2 activation was suppressed by the addition of 25-hydroxycholesterol. Then, the effect of 25-hydroxycholesterol on the expression of CYP4F2 mRNA was examined. In the absence of AMPK activators, 25-hydroxycholesterol suppressed the expression of CYP4F2 mRNA by approximately 2-fold (Fig. 9). Nevertheless, each of the three activators of AMPK produced relative increases of CYP4F2 expression that were 70 to 90% of that seen in the absence of 25-hydroxycholesterol. Although this observation does not preclude contribution of SREBP-2 activation to the overall effect of AMPK activators on the CYP4F2 gene expression, the robust response noted in the presence of 25-hydroxycholesterol is highly suggestive that an additional pathway is involved.
Fig. 9.
Effect of 25-hydroxycholesterol on the AMPK activator-mediated increase of PPIA normalized CYP4F2 mRNA expression in HepG2 cells. HepG2 cells were incubated with 25-hydroxycholesterol (25-OH cholesterol) (5 μg/ml) or vehicle control (DMSO, D) together with 0.5 mM AICAR (A), 100 μM genistein (G), or 75 μM resveratrol (R) for 24 h in Dulbecco's minimal essential medium containing 10% fetal bovine serum. The cells were harvested for total RNA isolation and subjected to qPCR analysis. At least four independent experiments were performed, and a representative experiment is shown. Means and standard errors were determined from triplicate samples for each treatment. Statistically significant differences between DMSO and treatments in the absence of 25-OH cholesterol (−) are indicated: **, p < 0.01; ***, p < 0.001. Significant difference between DMSO controls in the presence or absence of 25-OH cholesterol is indicated: ++, p < 0.01. Significant differences between DMSO and treatments in the presence of 25-OH cholesterol (+) are indicated: #, p < 0.05; ###, p < 0.001.
Transcriptional Activation of CYP4F2 Gene Expression by AMPK Activators.
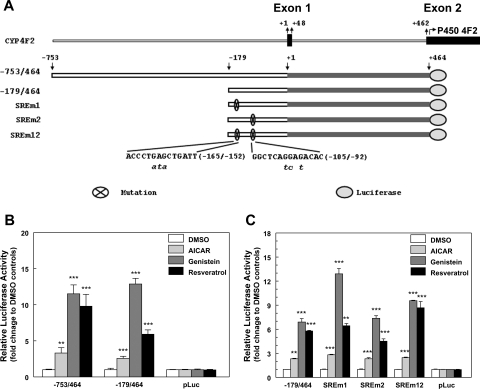
To test more directly whether AMPK activation leads to increased CYP4F2 gene transcription, we examined the effects of AMPK activators on expression of reporter constructs harboring CYP4F2 proximal promoter regions in HepG2 cells (Fig. 10). Treatment of AMPK activators increased the expression of the luciferase reporter gene, and this increase depended on the presence of the CYP4F2 promoter (Fig. 10B). These results provide evidence that the activation of CYP4F2 gene expression by AMPK activators results from increased promoter activity, and that the CYP4F2 reporter constructs can be used to delineate the underlying mechanisms. Two SREBP binding sites (SRE) have been identified in the −179/−6 promoter region of the CYP4F2 gene (Hsu et al., 2007b). When reporter constructs containing the CYP4F2 (−179/464) proximal promoter region and reporters bearing disruptive mutations in the SREs were introduced into HepG2 cells, the effect of AMPK activators on CYP4F2 reporter activity was not significantly altered by the presence of SRE mutations (Fig. 10C). The disruptive mutations were shown previously to block transcriptional activation of the CYP4F2 reporter construct by an HMG-CoA reductase inhibitor, lovastatin (Hsu et al., 2007b). This suggests that additional cis-acting control elements contribute to the effects of AMPK activators on the transcription of the reporter gene. Work is in progress to identify these response elements and the cognate transcription factors.
Fig. 10.
Activators of AMPK increase transcription of CYP4F2 reporter constructs in HepG2 cells. A, a schematic of the CYP4F2 gene is shown to indicate the location of fragments relative to the start sites of transcription (+1) and translation (+463, ATG). Site-directed mutagenesis was performed to mutate the identified SREBP binding sites in the CYP4F2 −179/464 reporter and the mutations are shown below in lowercase italic bold. The SREm1 construct contains the mutations in the −169/−145 SRE site, the SREm2 contains the mutations in the −109/−83 SRE site, and SREm12 contains mutations in both SRE sites (Hsu et al., 2007b). B, CYP4F2 reporter constructs, −753/+464 and −179/+464, or the control luciferase vector (pLuc) were transfected into HepG2 cells. A β-galactosidase expression vector (pCMV-β-Gal) was also transfected to normalize transfection efficiency. After 24 h, cells were treated with AICAR (0.5 mM), genistein (100 μM), resveratrol (75 μM), or DMSO alone. Cells were then harvested after 24 h, and luciferase and β-galactosidase activities were assessed. The fold activation by AMPK activators for each reporter is expressed relative to the data obtained from cells treated with DMSO. At least three independent experiments were performed, and a representative experiment is shown. Means and standard errors were determined for triplicate samples. Statistically significant differences from DMSO are indicated: **, p < 0.01; ***, p < 0.001. C, the CYP4F2 −179/+464 reporter and mutants in which the two SREBP binding sites (SRE) were mutated were transfected into HepG2 cells. After transfection, the cells were treated with three activators as described in B. At least four independent experiments were performed, and a representative experiment is shown. Means and standard errors were determined from three replicate samples. Statistically significant differences from DMSO for each reporter construct are indicated: **, p < 0.01; ***, p < 0.001.
Discussion
This study demonstrates that the expression of CYP4F2 in HepG2 cells is increased after exposure to three distinct AMPK activators, namely AICAR, resveratrol, and genistein, and that activation of CYP4F2 expression is inhibited by an AMPK inhibitor, compound C. siRNAs targeting mRNAs encoding the alternative AMPKα subunits 1 and 2 suppressed the enhancement of CYP4F2 mRNA expression by these three AMPK activators. Furthermore, an elevated expression of CYP4F2 mRNA was also seen in HepG2 cells when the cells were treated with A-769662, which activates AMPK directly. The increased CYP4F2 mRNA and protein expression elicited by activators of AMPK seems to reflect transcriptional activation of the corresponding gene, as shown by the stimulatory effects of each AMPK activator on reporter gene transcription directed by the proximal promoter region of the human CYP4F2 gene and inhibition of the responses of the native gene to activators of AMPK by actinomycin D.
Changes in gene expression after activation of AMPK could reflect phosphorylation of transcription factors and consequent effects on their activity. For example, phosphorylation of HNF4α by AMPK leads to decreased stability and loss of function (Hong et al., 2003). Likewise, the DNA binding activity of the carbohydrate response element binding protein is inactivated after phosphorylation by AMPK (Kawaguchi et al., 2002). It is possible that the increased expression of CYP4F2 shown here reflects inactivation or activation of a transcription factor involved in this P450's regulation by AMPK-mediated phosphorylation. Some transcription factors are elevated after AMPK activation, including the SHP (Kim et al., 2008) and early growth response 1 (Egr-1) (Berasi et al., 2006). Preliminary evidence suggests that Egr-1 is unlikely to mediate the induction of the CYP4F2 expression. When Egr-1 was overexpressed in HepG2 cells, the basal activities of CYP4F2 gene reporters were suppressed but the responses to AMPK activators were retained. SHP acts generally to inhibit other transcription factors. The activation of SHP expression by AMPK activation is thought to be mediated by upstream stimulus factor 1 (Kim et al., 2008). The effects of upstream stimulus factor 1 on CYP4F2 expression have yet to be investigated.
Certain of the effects of AMPK activation noted on gene transcription are believed to be secondary responses to altered cellular metabolism (Leff, 2003). Activation of AMPK inhibits cellular sterol synthesis, and if cellular sterol levels become sufficiently depleted, SREBP-2 will undergo proteolytic activation to increase the expression of enzymes and receptors involved in cholesterol synthesis and disposition (Amemiya-Kudo et al., 2002). In our previous study, we showed that lovastatin, an HMG-CoA reductase inhibitor, induced CYP4F2 expression through activation of SREBP-2 (Hsu et al., 2007b). Nevertheless, in HepG2 cells supplemented with the exogenous sterol 25-hydroxycholesterol, which inhibits SREBP-2 activation, the overall expression of the CYP4F2 gene was found to be diminished, whereas responses to AICAR, resveratrol and genistein were retained (Fig. 9). This diminished CYP4F2 expression suggests that SREBP plays a role in regulating the basal expression of the CYP4F2 gene and potentially contributes, at least in part, to the response to activators of AMPK. However, the robust response noted upon supplementation of the cells with AMPK activators in the presence of 25-hydroxycholesterol suggests that additional mechanisms of gene transactivation are probably involved. The role for SREBP in CYP4F2 regulation was also tested in transient transfection studies (Fig. 10C), where it was shown that mutations that blocked transcriptional activation of the CYP4F2 reporter construct by lovastatin (Hsu et al., 2007b), an HMG-CoA reductase inhibitor, did not significantly block activation of the reporter gene response to AMPK activators. These results suggest the involvement of other cis-acting elements and additional regulatory mechanisms for enhanced CYP4F2 gene transcription observed in response to activation of AMPK.
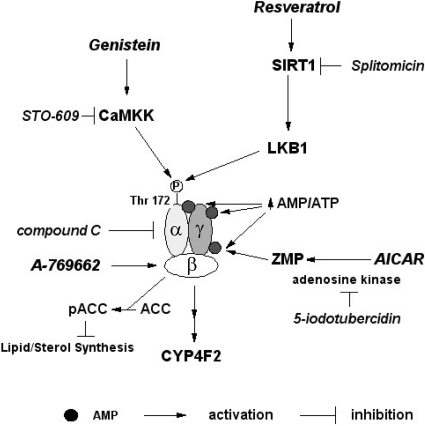
Our results suggest that the expression of CYP4F2 may become elevated in human liver under conditions of stress that increase the AMP/ATP ratio or that activate AMPK via a pharmacological activation of upstream kinases (Fogarty and Hardie, 2010). As summarized in Fig. 11, the AMPK activators examined in this study increase the phosphorylation of AMPK through different mechanisms. AICAR serves as a precursor for ZMP, which activates AMPK by acting as an allosteric activator that stabilizes the phosphorylated state of the enzyme (Viollet et al., 2009). In contrast to AICAR, A-769662 can activate AMPK directly in both cell-free assays and intact cells by allosterically activating AMPK and inhibiting its dephosphorylation (Göransson et al., 2007). LKB1 serves as a constitutively active upstream kinase for activation of AMPK by phosphorylation of the threonine residue found at position 172 in the α-subunit activation loop. The induction of CYP4F2 expression by resveratrol seems to be mediated by both AMPK-dependent and -independent mechanisms based on the partial inhibition observed with compound C. The AMPK-dependent component can be attributed (55–80%) primarily to resveratrol-mediated activation of SIRT1, which, in turn, activates LKB1 by deacetylation of the kinase (Hou et al., 2008). Compound C substantially inhibited the induction of CYP4F2 by genistein, suggesting that AMPK largely mediated the response to genistein. Although genistein was reported previously to activate AMPK (Hwang et al., 2005), the mechanism for this activation was unknown. A screen of several inhibitors suggests that CaMKKβ, an AMPK upstream kinase, contributes to the activation of AMPK by genistein. The regulation of AMPK activity by elevated ratios of AMP to ATP and upstream kinase-dependent phosphorylation contributes to the maintenance of cellular metabolic capacity in response to stresses including fasting, osmotic shock, exercise, hypoxia, and hypoglycemia or to those that result from exposure to therapeutic agents such as metformin, thiazolidinediones, genistein, and resveratrol (Fogarty and Hardie, 2010).
Fig. 11.
Regulatory pathways implicated in the stimulation of CYP4F2 gene expression by AICAR, A-769662, genistein, and resveratrol. AMPK plays an important role in regulating fatty acid oxidation, is activated when cellular AMP/ATP ratios are high, and subsequently modulates metabolic processes to increase ATP production (Fogarty and Hardie, 2010). AMPK is a heterotrimeric enzyme consisting of a catalytic subunit α and two regulatory subunits β and γ. Activation of AMPK requires phosphorylation of Thr-172 on the α-subunit by upstream kinases such as LKB1 or Ca2+/CaMKK. AMP binding to the γ-subunit allosterically increases Thr-172 phosphorylation, thereby significantly increasing kinase activity. Upon activation, AMPK phosphorylates key enzymes in fatty acid, sterol and glycogen synthesis such as ACC to decrease ATP utilization. The expression of CYP4F2 is increased at 24 h after treatment with three distinct AMPK activators, namely genistein, resveratrol, and AICAR. These effects are inhibited by compound C, an AMPK inhibitor, and by siRNAs targeting the mRNAs encoding the alternative α-subunits 1 and 2. A-769662 is a direct activator of AMPK that binds to the β-subunit. In contrast, AICAR acts indirectly after its conversion by adenosine kinase to ZMP, an AMP analog. 5-Iodotubercidin, an adenosine kinase inhibitor, blocks the increased CYP4F2 expression by AICAR (Henin et al., 1995). Reservatrol has been demonstrated to activate AMPK indirectly by stimulation of the NAD+-activated deacetylase SIRT1, which in turn activates the AMPK upstream kinase LKB1. Splitomicin, a SIRT1 inhibitor, only partially blocked activation of CYP4F2 expression by resveratrol. CaMKK is also an AMPK upstream kinase. STO-609, a CaMKK inhibitor, reduced the level of expression of CYP4F2 elicited by genistein.
CYP4F2 has the capacity to oxidize excess nutritive and non-nutritive substrates such as fatty acids, leukotrienes, prostanoids, and lipid-soluble vitamins, thereby facilitating their catabolism. Excess nonesterified fatty acids are thought to underlie lipotoxicity, which can disrupt mitochondrial function and potentially reduce ATP synthesis (Weinberg, 2006). The induction of CYP4F2 in response to AMPK activation augments the capacity of hepatocytes to ω-oxidize excess nonesterified fatty acids. Thus, the up-regulation of CYP4F2 gene expression is analogous to the protection provided by the induction of CYP4A P450s mediated by the nuclear receptor PPARα, when insulin levels decline and fatty acids are released from adipocytes (Hsu et al., 2007a). Under the latter conditions, hepatic lipid metabolism leads to the export of nutrients to other tissues. AMPK is unlikely to be activated unless the liver is stressed by high levels of fatty acids and/or ATP levels fall. Thus, the activation of AMPK may trigger a defensive mechanism that involves the induction of CYP4F2 gene expression, which can then eliminate excess fatty acids that can disrupt mitochondrial function and reduce ATP production. Likewise, drugs and other xenobiotics can disrupt mitochondrial function, which can lead to liver injury (Labbe et al., 2008). In the latter case, AMPK activation seems to contribute to more generalized increase in capacity for P450-catalyzed oxidizations of xenobiotics mediated by the nuclear receptor constitutive androstane receptor (Rencurel et al., 2006). In this sense, the regulation of CYP4F2 is complementary to that of CYP4A11 because CYP4F2 expression is increased during lipogenesis when SREBPs are activated or by cellular stresses, whereas CYP4A11 is induced when hepatic lipid metabolism is increased to provide nutrients to other tissues. Activation of CYP4F2 under these conditions is likely to contribute to the >10-fold variation in CYP4F2 expression noted in liver and kidney tissue samples (Hirani et al., 2008).
Supplementary Material
Acknowledgments
This article is dedicated to the memory of Keith J. Griffin, who unexpectedly passed away on July 27, 2010, for his contributions to the studies described.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant HD004445].
Part of this work was presented previously: Hsu M-H, Savas Ü, Griffin KJ, and Johnson EF (2008) Induction of human CYP4F2 expression by genistein: role of AMPK-activated protein kinase, at the International Symposium on Microsomes and Drug Oxidations 17th Meeting; 2008 July 6–10; Saratoga Springs, NY, International Society for the Study of Xenobiotics, Washington, DC; Hsu M-H, Savas Ü, Griffin KJ, and Johnson EF (2008) Role of AMP-activated protein kinase in regulation of human CYP4F2, at the International Society for the Study of Xenobiotics 15th North American Regional Meeting; 2008 Oct 12–16; San Diego, CA. International Society for the Study of Xenobiotics, Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175851.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
ABBREVIATIONS:
- AMPK
- AMP-activated protein kinase
- A-769662
- 6,7-dihydro-4-hydroxy-3-(2′-hydroxy[1,1′-biphenyl]-4-yl)-6-oxo-thieno[2,3-b]pyridine-5-carbonitrile
- ACC
- acetyl-CoA carboxylase 1
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- CaMKK
- calmodulin kinase kinase
- compound C
- 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine
- CPR
- cytochrome P450 reductase
- DMSO
- dimethyl sulfoxide
- Egr-1
- early growth response 1
- HPRT
- hypoxanthine phosphoribosyl transferase
- LKB1
- liver kinase B1
- NC
- nontargeting/scrambled siRNA control
- PBS
- phosphate-buffered saline
- PPIA
- cyclophilin A
- qPCR
- quantitative polymerase chain reaction
- RT
- reverse transcription
- SHP
- small heterodimer partner
- siRNA
- small interfering RNA
- SIRT1
- NAD+-activated deacetylase or sirtuin
- SREBP
- sterol regulatory element binding protein
- SRE
- SREBP response element
- STO-609
- 7H-benz[de]benzimidazo[2,1-a]isoquinoline-7-one-3-carboxylic acid acetate
- splitomicin
- 1,2-dihydro-3H-naphtho[2,1-b]pyran-3-one
- ZMP
- 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate.
Authorship Contributions
Participated in research design: Hsu and Johnson.
Conducted experiments: Hsu, Savas, and Lasker.
Contributed new reagents or analytic tools: Lasker.
Performed data analysis: Hsu and Johnson.
Wrote or contributed to the writing of the manuscript: Hsu, Savas, Lasker, and Johnson.
Other: Johnson acquired funding for the research.
References
- Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, et al. (2002) Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res 43:1220–1235 [PubMed] [Google Scholar]
- Bedalov A, Gatbonton T, Irvine WP, Gottschling DE, Simon JA. (2001) Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci USA 98:15113–15118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasi SP, Huard C, Li D, Shih HH, Sun Y, Zhong W, Paulsen JE, Brown EL, Gimeno RE, Martinez RV. (2006) Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J Biol Chem 281:27167–27177 [DOI] [PubMed] [Google Scholar]
- Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, et al. (2008) CYP4F2 genetic variant alters required warfarin dose. Blood 111:4106–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. (2010) Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR. (2001) The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochem Biophys Res Commun 285:571–576 [DOI] [PubMed] [Google Scholar]
- Dhar M, Sepkovic DW, Hirani V, Magnusson RP, Lasker JM. (2008) Omega oxidation of 3-hydroxy fatty acids by the human CYP4F gene subfamily enzyme CYP4F11. J Lipid Res 49:612–624 [DOI] [PubMed] [Google Scholar]
- Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, Engström G, Berglund G, Minuz P, Melander O. (2008) The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension 52:373–380 [DOI] [PubMed] [Google Scholar]
- Fogarty S, Hardie DG. (2010) Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta 1804:581–591 [DOI] [PubMed] [Google Scholar]
- Fu Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Shindo A, Ohta M, Soma M, Aoi N, Sato M, et al. (2008) A haplotype of the CYP4F2 gene is associated with cerebral infarction in Japanese men. Am J Hypertens 21:1216–1223 [DOI] [PubMed] [Google Scholar]
- Fu Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Shindo A, Ohta M, Soma M, Aoi N, Sato M, et al. (2009) A haplotype of the CYP4F2 gene associated with myocardial infarction in Japanese men. Mol Genet Metab 96:145–147 [DOI] [PubMed] [Google Scholar]
- Göransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. (2007) Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 282:32549–32560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick JP. (2008) Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol 75:2263–2275 [DOI] [PubMed] [Google Scholar]
- Hart SN, Li Y, Nakamoto K, Subileau EA, Steen D, Zhong XB. (2010) A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab Dispos 38:988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Gruber HE, Van den Berghe G. (1995) Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J 9:541–546 [DOI] [PubMed] [Google Scholar]
- Hirani V, Yarovoy A, Kozeska A, Magnusson RP, Lasker JM. (2008) Expression of CYP4F2 in human liver and kidney: assessment using targeted peptide antibodies. Arch Biochem Biophys 478:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YH, Varanasi US, Yang W, Leff T. (2003) AMP-activated protein kinase regulates HNF4α transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem 278:27495–27501 [DOI] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, et al. (2008) SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283:20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MH, Savas U, Griffin KJ, Johnson EF. (2007a) Human cytochrome p450 family 4 enzymes: function, genetic variation and regulation. Drug Metab Rev 39:515–538 [DOI] [PubMed] [Google Scholar]
- Hsu MH, Savas U, Griffin KJ, Johnson EF. (2007b) Regulation of human cytochrome P450 4F2 expression by sterol regulatory element-binding protein and lovastatin. J Biol Chem 282:5225–5236 [DOI] [PubMed] [Google Scholar]
- Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ. (2005) Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochem Biophys Res Commun 338:694–699 [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. (2002) Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 277:3829–3835 [DOI] [PubMed] [Google Scholar]
- Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, et al. (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57:306–314 [DOI] [PubMed] [Google Scholar]
- Komen JC, Wanders RJ. (2006) Identification of the cytochrome P450 enzymes responsible for the omega-hydroxylation of phytanic acid. FEBS Lett 580:3794–3798 [DOI] [PubMed] [Google Scholar]
- Labbe G, Pessayre D, Fromenty B. (2008) Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol 22:335–353 [DOI] [PubMed] [Google Scholar]
- Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. (2000) Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem 275:4118–4126 [DOI] [PubMed] [Google Scholar]
- Leff T. (2003) AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem Soc Trans 31:224–227 [DOI] [PubMed] [Google Scholar]
- McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. (2009) CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol 75:1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata N, Roman RJ. (2005) Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41:175–193 [DOI] [PubMed] [Google Scholar]
- Mullen E, Brown RM, Osborne TF, Shay NF. (2004) Soy isoflavones affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. J Nutr 134:2942–2947 [DOI] [PubMed] [Google Scholar]
- Nystrom GJ, Lang CH. (2008) Sepsis and AMPK activation by AICAR differentially regulate FoxO-1, -3 and -4 mRNA in striated muscle. Int J Clin Exp Med 1:50–63 [PMC free article] [PubMed] [Google Scholar]
- Pérez-Andreu V, Roldán V, Antón AI, García-Barberá N, Corral J, Vicente V, González-Conejero R. (2009) Pharmacogenetic relevance of CYP4F2 V433M polymorphism on acenocoumarol therapy. Blood 113:4977–4979 [DOI] [PubMed] [Google Scholar]
- Reddy JK, Mannaerts GP. (1994) Peroxisomal lipid metabolism. Annu Rev Nutr 14:343–370 [DOI] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da Silva Xavier G, Rutter GA, Viollet B, Meyer UA. (2006) Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol 70:1925–1934 [DOI] [PubMed] [Google Scholar]
- Sanders RJ, Ofman R, Duran M, Kemp S, Wanders RJ. (2006) Omega-oxidation of very long-chain fatty acids in human liver microsomes. Implications for X-linked adrenoleukodystrophy. J Biol Chem 281:13180–13187 [DOI] [PubMed] [Google Scholar]
- Savas U, Machemer DE, Hsu MH, Gaynor P, Lasker JM, Tukey RH, Johnson EF. (2009) Opposing roles of peroxisome proliferator-activated receptor alpha and growth hormone in the regulation of CYP4A11 expression in a transgenic mouse model. J Biol Chem 284:16541–16552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec DE, Roman RJ, Flasch A, Rieder MJ. (2007) Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 30:74–81 [DOI] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, Andreelli F, Foretz M. (2009) AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 196:81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD. (2008) A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51:1393–1398 [DOI] [PubMed] [Google Scholar]
- Weinberg JM. (2006) Lipotoxicity. Kidney Int 70:1560–1566 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.