Abstract
Evidence suggests that elevations in extracellular serotonin (5-HT) in the brain can diminish stimulant effects of dopamine (DA). To assess this proposal, we evaluated the pharmacology of amphetamine analogs (m-fluoroamphetamine, p-fluoroamphetamine, m-methylamphetamine, p-methylamphetamine), which display similar in vitro potency as DA releasers (EC50 = 24–52 nM) but differ in potency as 5-HT releasers (EC50 = 53–1937 nM). In vivo microdialysis was used to assess the effects of drugs on extracellular DA and 5-HT in rat nucleus accumbens, while simultaneously measuring ambulation (i.e., forward locomotion) and stereotypy (i.e., repetitive movements). Rats received two intravenous injections of drug, 1 mg/kg at time 0 followed by 3 mg/kg 60 min later. All analogs produced dose-related increases in dialysate DA and 5-HT, but the effects on DA did not agree with in vitro predictions. Maximal elevation of dialysate DA ranged from 5- to 14-fold above baseline and varied inversely with 5-HT response, which ranged from 6- to 24-fold above baseline. All analogs increased ambulation and stereotypy, but drugs causing greater 5-HT release (e.g., p-methylamphetamine) were associated with significantly less forward locomotion. The magnitude of ambulation was positively correlated with extracellular DA (p < 0.001) and less so with the ratio of DA release to 5-HT release (i.e., percentage DA increase divided by percentage 5-HT increase) (p < 0.029). Collectively, our findings are consistent with the hypothesis that 5-HT release dampens stimulant effects of amphetamine-type drugs, but further studies are required to address the precise mechanisms underlying this phenomenon.
Introduction
Monoamine transporter proteins play a fundamental role in limiting the extent of nonsynaptic volume transmission in the central nervous system (Rice and Cragg, 2008; Vizi et al., 2010). Amphetamine-type stimulants target these transporters by acting as substrates and triggering the efflux of monoamine transmitters, especially dopamine (DA) and norepinephrine (NE) (Rothman and Baumann, 2003; Fleckenstein et al., 2007). Drug-induced increases in extracellular monoamines produce a spectrum of effects in animals that includes forward locomotion, stereotypy, and reward-relevant behaviors such as drug self-administration (for review see Rothman and Baumann, 2006; Newman and Rothman, 2007). Substantial evidence indicates that DA, rather than NE, is the most critical mediator of reward-relevant properties of stimulant drugs (Wise, 2008). In humans, certain stimulant drugs are commonly abused (e.g., cocaine and methamphetamine), whereas others are prescribed as efficacious treatments for psychiatric conditions such as attention-deficit hyperactivity disorder (e.g., methylphendiate and amphetamine). It is noteworthy that stimulant medications are among the most promising candidates for treating cocaine and methamphetamine dependence (Grabowski et al., 2004; Mooney et al., 2009).
Given the widespread therapeutic use of stimulants, it seems prudent to evaluate strategies for decreasing the abuse liability of these agents. Studies dating back to the 1980s (Lyness, 1983) demonstrate that increasing serotonergic tone can decrease stimulant self-administration. In addition, drug treatments that simultaneously elevate extracellular DA and 5-HT are not as readily self-administered as drug treatments that elevate DA alone (Roberts et al., 1999; Rothman et al., 2005; Wee and Woolverton, 2006; Howell et al., 2007). In a representative study, Wee and Woolverton (2006) examined the self-administration of mixtures containing the 5-HT releaser fenfluramine and the DA releaser (+)-amphetamine. Those investigators found that increasing the relative amount of fenfluramine (i.e., increasing 5-HT release) markedly decreased the reinforcing potency of the mixture. Such studies support the idea that elevation of extracellular 5-HT concentrations in the brain can reduce the reward-relevant properties of stimulants such as (+)-amphetamine.
To further explore the interactions between DA and 5-HT release in the brain, we have characterized the pharmacology of amphetamine analogs derived from an extensive library of phenylethylamine compounds (i.e., PAL compounds). In particular, we have focused on four PAL analogs that display similar in vitro potency as DA releasers but differ in potency as 5-HT releasers (Wee et al., 2005) (see Fig. 1 for structures). The selectivity for DA release versus 5-HT release for these drugs ranges from 80-fold DA-selective for m-fluoroamphetamine (PAL-353) to nonselective for p-methylamphetamine (PAL-313) (see Table 1). Various behavioral effects of these analogs in rats and nonhuman primates have been reported (Wee et al., 2005; Negus et al., 2007; Kimmel et al., 2009; Wellman et al., 2009), and Kimmel et al. (2009) used in vivo microdialysis in squirrel monkey nucleus accumbens to demonstrate that PAL-313 evokes significantly less DA release than PAL-353. It is noteworthy that PAL-313 supports less self-administration behavior in rhesus monkeys compared with other PAL analogs, but the precise role of DA and 5-HT in mediating differences in behavioral responsiveness to these drugs remains unclear.
Fig. 1.
Chemical structures of PAL analogs tested in this study. Amphetamine is shown for comparison.
TABLE 1.
In vitro potency of PAL analogs as releasers of monoamine neurotransmitters
These data are adapted from Wee et al. (2005) and Rothman et al. (2005). DA/NE ratio was calculated by EC50−1 for DA release/EC50−1 for NE release; DA/5-HT ratio was calculated by EC50−1 DA release/EC50−1 for 5-HT release. Higher ratios indicate higher DA selectivity.
| Drug | EC50 (nM ± S.D.) |
DA/NE Ratio | DA/5-HT Ratio | ||
|---|---|---|---|---|---|
| [3H]DA | [3H]NE | [3H]5-HT | |||
| (+)-Amphetamine | 8.0 ± 0.43 | 7.2 ± 0.44 | 1756 ± 94 | 0.91 | 219 |
| PAL-353 | 24.2 ± 1.1 | 16.1 ± 1.7 | 1937 ± 202 | 0.67 | 80 |
| PAL-303 | 51.5 ± 1.7 | 28.0 ± 1.8 | 939 ± 76 | 0.56 | 18 |
| PAL-314 | 33.3 ± 1.3 | 18.3 ± 1.4 | 218 ± 22 | 0.55 | 6.5 |
| PAL-313 | 44.1 ± 2.6 | 22.2 ± 1.3 | 53.4 ± 4.1 | 0.50 | 1.2 |
Few published studies have reported the effects of PAL compounds on extracellular DA and 5-HT in reward-relevant brain regions, and no experiments have assessed neurochemical and behavioral effects of these analogs in the same experimental subjects. To this end, we used in vivo microdialysis to examine the effects of PAL compounds on extracellular DA and 5-HT in the nucleus accumbens of rats that were housed in chambers equipped with photobeams to allow the assessment of motor activity (Zolkowska et al., 2009). Using these in vivo methods enabled us to examine relationships between neurochemistry and behavior in the same groups of animals. We found that the potency of compounds to release DA in vitro does not necessarily predict the magnitude of increase in extracellular transmitter in vivo. Furthermore, analogs producing greater 5-HT release were associated with less DA release and reduced forward locomotion. The findings are consistent with the notion that increased serotonergic tone attenuates stimulant effects mediated by DA.
Materials and Methods
Animals and Surgery.
Male Sprague-Dawley rats weighing 300 to 350 g were housed under conditions of controlled temperature (22 ± 2°C) and humidity (45 ± 5%) with food and water freely available. Rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and procedures were carried out in accordance with the Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program. Lights were on from 7:00 AM to 7:00 PM and experiments were carried out between 9:00 AM and 2:00 PM. Rats received sodium pentobarbital (60 mg/kg i.p.) for surgical anesthesia. Indwelling catheters made of Silastic medical-grade tubing (Dow Corning, Midland, MI) were implanted into the right jugular vein to allow for intravenous drug administration. Intracerebral guide cannulae made of plastic (CMA 12; CMA/Microdialysis, Acton, MA) were implanted above the nucleus accumbens (n = 7 rats/drug), according to stereotaxic coordinates: 1.6 mm lateral and 1.6 mm anterior to bregma, and 6.0 mm below the surface of dura (Paxinos, 1982). Guide cannulae were secured to the skull using stainless-steel anchor screws and dental acrylic. Animals were individually housed postoperatively and allowed 7 to 10 days for recovery.
Drugs and Reagents.
The synthesis of PAL-series compounds was as described previously (Wee et al., 2005). All drugs were dissolved in saline immediately before use and administered intravenously in a volume of 1 ml/kg. Doses are expressed as the HCl salt. Sources of reagents required for microdialysis and HPLC methods have been described previously (Baumann et al., 2008; Zolkowska et al., 2009).
Microdialysis Methods.
In vivo microdialysis sampling was carried out as described with minor modifications (Zolkowska et al., 2009). On the evening before an experiment, rats were moved to the testing room and briefly anesthetized with the short-acting barbiturate methohexital (5 mg/kg i.v.). A plastic collar was placed around the neck of each rat, dialysis probes (CMA/12; CMA Microdialysis) were inserted into guide cannulae, and extension tubes were attached to jugular catheters. The probe exchange surface was 2 × 0.5 mm. Each rat was placed into its own activity field arena (Coulbourn Instruments, Allentown, PA) and connected to a tethering system that allowed motor activity within the container. Tubing for microdialysis lines and catheter extensions was connected to a fluid swivel (Instech Laboratories, Plymouth Meeting, PA). Probes were perfused overnight with artificial cerebrospinal fluid containing 150.0 mM Na, 3.0 mM K, 1.4 mM Ca, 0.8 mM Mg, 1.0 mM P, and 155 mM Cl (Harvard Bioscience, Holliston, MA), pumped at a flow rate of 0.6 μl/min. On the morning of the experiment, dialysate samples were collected at 20-min intervals. Samples were immediately assayed for DA and 5-HT by HPLC-ECD as described below. Once three stable baseline samples were obtained, rats received two sequential intravenous injections of test drug, 1 mg/kg at time 0 followed by 3 mg/kg 60 min later. Microdialysis samples were collected throughout the postinjection period for 120 min. At the end of the experiments, rats were euthanized with CO2, and brains were removed and stored in 10% formalin. Brains were sectioned on a cryostat and mounted on glass slides. The placement of microdialysis probe tips within the nucleus accumbens was verified by visual inspection, and only rats with correct placements were included in data analyses.
Analysis of DA and 5-HT.
Aliquots of dialysate (5 μl) were injected onto a microbore C18 column that was coupled to an amperometric detector (model LC-4C; BAS Bioanalytical Systems, Inc., West Lafayette IN) with a glassy carbon electrode set at a potential of +650 mV relative to Ag/AgCl reference. Mobile phase consisting of 150 mM monochloroacetic acid, 150 mM sodium hydroxide, 2.5 mM sodium octanesulfonic acid, 250 μM disodium EDTA, 6% methanol, and 6% acetonitrile per liter of water (final pH 5.3) was pumped at 60 μl/min using a syringe pump (model 260D; ISCO, Lincoln NE). Chromatographic data were acquired on-line and exported to a Millennium software system (Waters, Milford, MA) for peak amplification, integration, and analysis. A monoamine standard mix containing DA, 5-HT, and their respective acid metabolites was injected before and after the experiment to insure validity of constituent retention times. Peak heights of unknowns were compared with peak heights of standards, and the lower limit of assay sensitivity (3× baseline noise) was 50 fg/5 μl per sample.
Locomotor Measures.
Locomotor measures were obtained as described with minor modifications (Baumann et al., 2008). During the overnight acclimation period and while undergoing microdialysis, each rat was housed within a square Plexiglas arena (43 cm length × 43 cm width × 43 cm height) that was equipped with an activity monitoring system (Tru Scan; Coulbourn Instruments). A sensor ring lined with photobeams spaced 2.54 cm apart was positioned in the horizontal plane to allow for real-time monitoring of various motor parameters. Activity was monitored in 20-min bins, beginning 60 min before intravenous drug injections and continuing for 120 min thereafter. Ambulation and stereotypy were quantified separately; ambulation is defined as the total distance traveled in the horizontal plane (measured in cm), whereas stereotypy is defined as the number of photobeam breaks less than ± 1.5 beam spaces and back to the original point that do not exceed 2 s apart (measured in number of events).
Data Analyses.
Group data are expressed as mean ± S.E.M. for n = 7 rats/group. Neurotransmitter and behavioral data from individual rats were normalized to percentage of control values (i.e., percentage basal) using the averaged raw data from three preinjection time points as basal, or 100%. In this manner, each rat served as its own control. Normalized group data were evaluated by a two-factor analysis of variance (ANOVA) (drug, time). Subsequently, the individual time-effect curve for each drug was assessed by one-factor ANOVA; if this analysis demonstrated a significant main effect, data from time points after 1 mg/kg (20, 40, and 60 min) and 3 mg/kg (80, 100, and 120 min) were compared with time 0 values by separate one-factor ANOVAs followed by Dunnett's post hoc test. Mean drug effects were then calculated at each dose of drug by taking the average value for data collected after 1 mg/kg (20, 40, and 60 min) and 3 mg/kg (80, 100, and 120 min) for each parameter (Matthews et al., 1990). Mean drug effects were evaluated by one-factor ANOVA followed by Newman-Keul's post hoc test. Relationships between DA, 5-HT, and motor parameters were assessed by the Pearson correlation coefficient. Specifically, mean values for DA, 5-HT, or the ratio of DA/5-HT (i.e., percentage basal DA response divided by percentage basal 5-HT response) at each time point after drug injection were plotted against corresponding mean values for ambulation and stereotypy to generate plots (i.e., six time points postinjection, four drugs, for a total of 24 data points per plot). P < 0.05 was chosen as the minimum level for statistical significance.
Results
Effects of PAL Analogs on Dialysate Neurotransmitters.
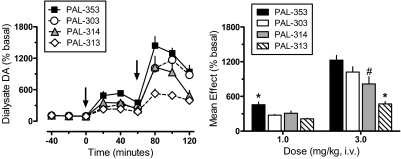
Figure 2 depicts the effects of PAL compounds on extracellular DA in rat nucleus accumbens. A two-factor ANOVA indicated significant main effects of drug (F3,216 = 30.92, p < 0.0001) and time (F8,216 = 94.91, p < 0.001) on dialysate DA response, with an interaction between the two factors (F24,216 = 4.60, p < 0.0001). One-factor ANOVA evaluating the time-effect data for each compound (Fig. 2, left) demonstrated significant dose-related increases in DA compared with preinjection control values for PAL-353 (F8,54 = 36.50, p < 0.0001), p-fluoroamphetamine (PAL-303) (F8,54 = 53.54, p < 0.0001), m-methylamphetamine (PAL-314) (F8,54 = 16.53, p < 0.0001), and PAL-313 (F8,54 = 26.60, p < 0.0001). All drugs significantly elevated dialysate DA above preinjection control at 20 and 40 min after 1 mg/kg and at all time points after 3 mg/kg (Dunnett's, p < 0.05). One-factor ANOVA comparing the mean rise in dialysate DA for all compounds (Fig. 2, right) revealed marked differences between drugs after administration of 1 mg/kg (F3,24 = 11.31, p < 0.0001) and 3 mg/kg (F3,24 = 12.79, p < 0.0001). Specifically, at the 1 mg/kg dose PAL-353 had greater effects on DA than other compounds. At the 3 mg/kg dose, PAL-313 produced a smaller rise in DA than other compounds, whereas effects of PAL-314 were less than those evoked by PAL-353 (Newman-Keul's, p < 0.05).
Fig. 2.
Effects of PAL analogs on extracellular DA in nucleus accumbens of rats undergoing in vivo microdialysis. Rats received intravenous injection of 1 mg/kg of drug at time 0, followed by 3 mg/kg at 60 min. Extracellular DA was determined by HPLC-ECD as described under Materials and Methods; mean basal concentration of DA was 1.58 ± 0.12 pg/5 μl for n = 28 rats. Left, for time course data, dialysate DA values are mean ± S.E.M. expressed as a percentage of three preinjection baseline samples (% basal). Arrows indicate times of drug injection. Right, for mean effect data, DA values are mean ± S.E.M. expressed as the average DA response determined from three postinjection samples after each dose. n = 7 rats/group. *, p < 0.05 compared with all other groups; #, p < 0.05 compared with PAL-353.
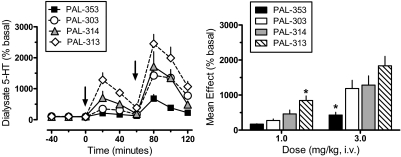
The effects of PAL compounds on dialysate 5-HT are shown in Fig. 3. Two-factor ANOVA indicated significant main effects of drug (F3,216= 28.81, p < 0.0001) and time (F8,216 = 45.00, p < 0.001), with an interaction between the two factors (F24,216 = 3.48, p < 0.0001). One-factor ANOVA assessing the time-effect data for each compound (Fig. 3, left) showed significant dose-related increases in dialysate 5-HT compared with preinjection control values for PAL-353 (F8,54 = 12.11, p < 0.0001), PAL-303 (F8,54 = 12.98, p < 0.0001), PAL-314 (F8,54 = 6.45, p < 0.0001), and PAL-313 (F8,54 = 20.92, p < 0.0001). All drugs significantly elevated dialysate 5-HT above preinjection control at 20 and 40 min after 1 mg/kg (Dunnett's, p < 0.05). PAL-353, PAL-303, and PAL-314 increased 5-HT at 80 and 100 min, whereas PAL-313 increased 5-HT at all time points after 3 mg/kg (Dunnett's, p < 0.05). A one-factor ANOVA comparing the mean elevation of dialysate 5-HT across treatments (Fig. 3, right) revealed significant differences between drugs after administration of 1 mg/kg (F3,24 = 11.66, p < 0.0001) and 3 mg/kg (F3,24 = 7.09, p < 0.001). At the 1 mg/kg dose, PAL-313 had greater effects on 5-HT than all other compounds, whereas PAL-353 had significantly smaller effects than other drugs at 3 mg/kg (Newman-Keul's, p < 0.05).
Fig. 3.
Effects of PAL analogs on extracellular 5-HT in nucleus accumbens of rats undergoing in vivo microdialysis. Rats received intravenous injection of 1 mg/kg of drug at time 0, followed by 3 mg/kg at 60 min. Extracellular 5-HT was determined by HPLC-ECD as described under Materials and Methods; mean basal concentration of 5-HT was 0.19 ± 0.03 pg/5 μl for n = 28 rats. Left, for time course data, dialysate 5-HT values are mean ± S.E.M. expressed as a percentage of three preinjection baseline samples (% basal). Arrows indicate times of drug injection. Right, for mean effect data, 5-HT values are mean ± S.E.M. expressed as the average 5-HT response determined from three postinjection samples after each dose. n = 7 rats/group. *, p < 0.05 compared with all other groups.
Effects of PAL Analogs on Motor Activation.
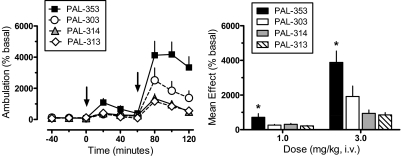
Figure 4 illustrates the effects of PAL compounds on ambulation (i.e., forward locomotion) in rats undergoing microdialysis in nucleus accumbens. A two-factor ANOVA indicated significant main effects of drug (F3,216 = 26.95, p < 0.0001) and time (F8,216 = 31.10, p < 0.0001) on ambulatory response, with an interaction between the two factors (F24,216 = 4.59, p < 0.001). One-factor ANOVA evaluating the time-effect data for each compound (Fig. 4, left) showed significant dose-related increases in locomotion compared with preinjection control values for PAL-353 (F8,54 = 13.87, p < 0.0001), PAL-303 (F8,54 = 6.34, p < 0.0001), PAL-314 (F8,54 = 9.64, p < 0.0001), and PAL-313 (F8,54 = 20.66, p < 0.0001). All drugs significantly stimulated ambulation above preinjection control by 20 min after 1 mg/kg and at all time points after 3 mg/kg (Dunnett's, p < 0.05). One-factor ANOVA comparing the mean magnitude of ambulation for all compounds (Fig. 4, right) revealed striking differences between drugs after administration of 1 mg/kg (F3,24 = 4.14, p < 0.01) and 3 mg/kg (F3,24 = 9.19, P < 0.0001). In particular, PAL-353 evoked much greater levels of ambulation at both doses compared with other drugs (Newman-Keul's, p < 0.05).
Fig. 4.
Effects of PAL analogs on ambulation in rats undergoing in vivo microdialysis. Rats received intravenous injection of 1 mg/kg of drug at time 0, followed by 3 mg/kg at 60 min. Ambulation was determined as described under Materials and Methods; mean basal value for ambulation was 109 ± 7 cm/20 min for n = 28 rats. Left, for time course data, ambulation values are mean ± S.E.M. expressed as a percentage of three preinjection baseline samples (% basal). Arrows indicate times of drug injection. Right, for mean effects data, ambulation values are the mean ± S.E.M. expressed as the average ambulation response determined from three postinjection samples after each dose. n = 7 rats/group. *, p < 0.05 compared with all other groups.
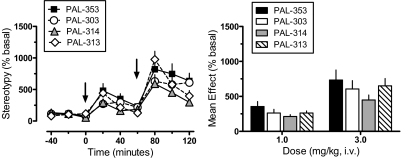
The effects of PAL compounds on stereotypy (i.e., repetitive movements) are shown in Fig. 5. Two-factor ANOVA indicated significant main effects of drug (F3,216 = 5.28, p < 0.001) and time (F8,216 = 38.32, p < 0.001), but no interaction between factors (F24,216 = 1.35, p = 0.135, NS). One-factor ANOVA assessing the time-effect data for each compound (Fig. 5, left) showed significant dose-related increases in stereotypy compared with preinjection control values for PAL-353 (F8,54 = 7.87, p < 0.0001), PAL-303 (F8,54 = 8.33, p < 0.0001), PAL-314 (F8,54 = 11.14, p < 0.0001), and PAL-313 (F8,54 = 19.76, p < 0.0001). All drugs evoked stereotypy that was above preinjection control at 20 min after 1 mg/kg and at all time points after 3 mg/kg (Dunnett's, p < 0.05). One-factor ANOVA comparing the mean magnitude of stereotypy across treatments (Fig. 5, right) revealed no significant differences between drugs after administration of 1 mg/kg (F3,24 = 1.46, p = 0.252, NS) or 3 mg/kg (F3,24 = 1.16, p < 0.345, NS).
Fig. 5.
Effects of PAL analogs on stereotypy in rats undergoing in vivo microdialysis. Rats received intravenous injection of 1 mg/kg of drug at time 0, followed by 3 mg/kg at 60 min. Stereotypy was determined as described under Materials and Methods; mean basal value for stereotypy was 116 ± 9 episodes/20 min for n = 28 rats. Left, for time course data, stereotypy values are mean ± S.E.M. expressed as a percentage of three preinjection baseline samples (% basal). Arrows indicate times of drug injection. Right, for mean effect data, stereotypy values are the mean ± S.E.M. expressed as the average stereotypy response determined from three postinjection samples after each dose. n = 7 rats/group.
Correlation Analyses.
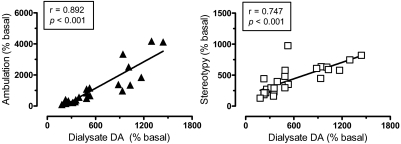
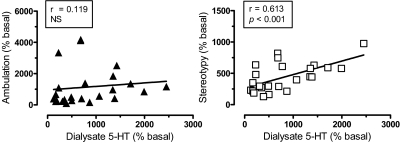
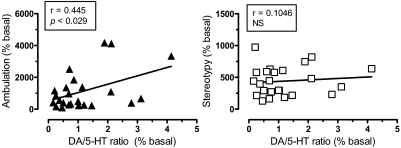
In an effort to examine possible relationships between DA, 5-HT, and motor responses, Pearson correlation analyses were carried out on mean group data from all time points after drug administration (i.e., 20–120 min postinjection). The data in Fig. 6 show that dialysate DA response across all drug treatments displayed significant positive correlations with ambulation (r = 0.892, p < 0.001) and stereotypy (r = 0.747, p < 0.001). As depicted in Fig. 7, dialysate 5-HT was positively correlated with stereotypy (r = 0.613, p < 0.001) but not ambulation (r = 0.119, NS). Wee et al. (2005) provided evidence that the ratio of in vitro potency for DA release versus 5-HT release might predict the reinforcing efficacy of PAL compounds. Here, we calculated in vivo DA/5-HT ratios by dividing the percentage increase in DA by the percentage increase in 5-HT at each time point after injection, and these values were plotted against the corresponding values for ambulation and stereotypy. Figure 8 demonstrates that DA/5-HT ratio was positively correlated with ambulation (r = 0.445, p < 0.029) but not with stereotypy (r = 0.1406, NS).
Fig. 6.
Correlation of dialysate DA with ambulation (left) and stereotypy (right). Mean group data for dialysate DA, ambulation, and stereotypy at each time point after drug treatment (i.e., from 20–120 min postinjection for each drug) were used to construct correlation plots. Dialysate DA is positively correlated with ambulation and stereotypy (p < 0.001) based on Pearson's coefficient (r).
Fig. 7.
Correlation of dialysate 5-HT with ambulation (left) and stereotypy (right). Mean group data for dialysate 5-HT, ambulation, and stereotypy at each time point after drug treatment (i.e., from 20–120 min postinjection for each drug) were used to construct correlation plots. Dialysate 5-HT is positively correlated with stereotypy (p < 0.001) based on Pearson's coefficient (r).
Fig. 8.
Correlation of the ratio of DA/5-HT response with ambulation (left) and stereotypy (right). Mean group data for DA/5-HT ratio, ambulation, and stereotypy at each time point after drug treatment (i.e., from 20–120 min postinjection for each drug) were used to construct correlation plots. The ratio of DA/5-HT response was positively correlated with ambulation (p < 0.029) based on Pearson's coefficient (r).
Discussion
A major aim of this study was to determine whether 5-HT release in the brain can influence dopaminergic effects of amphetamine-type stimulants. A large body of evidence supports an inhibitory role for 5-HT in modulating DA-mediated motor stimulation and reinforcement (Rothman and Baumann, 2006), but determining interactions between these transmitters is complicated by the existence of multiple 5-HT receptor subtypes that differentially affect DA transmission across brain regions (Alex and Pehek, 2007; Bubar and Cunningham, 2008). Here, we assessed the in vivo effects of amphetamine analogs that have similar in vitro potency as DA releasers but vary in potency as 5-HT releasers (see Table 1). All of the PAL drugs produced elevations in extracellular concentrations of DA and 5-HT in the nucleus accumbens, but effects on dialysate DA did not agree with in vitro DA release data. Specifically, drug-induced increases in extracellular DA varied inversely with increases in 5-HT, suggesting that 5-HT is capable of reducing DA release and its associated hyperactivity. In agreement with our previous work, dialysate DA responses in the nucleus accumbens were positively correlated with stereotypy and ambulation, whereas 5-HT was correlated only with stereotypy (Baumann et al., 2008; Zolkowska et al., 2009). It is noteworthy that the ratio of DA/5-HT responses across all drug treatments was positively correlated with ambulation, suggesting an inhibitory role for 5-HT in modulating motor activity.
Our microdialysis study is the first to assess the effects of PAL analogs on extracellular DA and 5-HT in rat nucleus accumbens. All drugs evoked dose-related increases in dialysate DA, and the rank order of DA response magnitude at 3 mg/kg was PAL-353 = PAL-303 > PAL-314 > PAL-313. This relationship does not agree with in vitro results showing the drugs have similar DA-releasing ability (see Table 1). The microdialysis data are consistent with the report of Marona-Lewicka et al. (1995), who demonstrated that p-fluoroamphetmine (PAL-303) increases extracellular DA in rat striatum in a manner similar to (+)-amphetamine. Our results also agree with Kimmel et al. (2009), who showed that PAL-353 increases dialysate DA in squirrel monkey nucleus accumbens to a much greater extent than PAL-313, but no measures of extracellular 5-HT were reported by those investigators. We found the rank order of 5-HT response magnitude at 3 mg/kg to be PAL-313 > PAL-314 = PAL-303 > PAL-353, and this relationship generally agrees with in vitro 5-HT release data. A comparison of the data in Figs. 2 and 3 shows the magnitude of DA response was diminished as 5-HT response increased. These neurochemical findings agree with the notion that 5-HT can attenuate DA release produced by amphetamine-type stimulants, although our results are correlative and do not directly address causative relationships between 5-HT and DA.
The precise mechanism that might be responsible for an inhibitory effect of 5-HT is not known, but the data of Bankson and Yamamoto (2004) could be relevant. Those investigators showed that elevation of extracellular 5-HT produced by the amphetamine analog, 3,4-methylendioxymethamphetamine (MDMA), increases GABA release in the ventral tegmental area (VTA) by activating 5-HT2C receptors. Elevation of GABA inhibits the activity of VTA DA cells, thereby limiting the ability of MDMA to release DA from nerve terminals in the nucleus accumbens. It generally is assumed that DA-releasing effects of amphetamines are independent of calcium or impulse firing (i.e., nonexocytotic) (see Westerink et al., 1989), but a number of studies have shown that a component of amphetamine-induced DA release is calcium-dependent, as determined in vitro (Crespi et al., 1997) and in vivo (Gray et al., 1999). Thus, we speculate that increases in 5-HT release produced by PAL drugs serve to reduce concurrent DA release from the nucleus accumbens via activation of 5-HT2C receptors in the VTA. Substantial evidence supports the feasibility of this proposal (Di Giovanni et al., 2008; Di Matteo et al., 2008), and future studies should address this hypothesis.
Based on the present data alone it is impossible to discount alternative mechanisms, unrelated to 5-HT release, which could explain discrepancies between in vitro and in vivo effects of PAL analogs on DA function. For instance, certain amphetamine analogs (e.g., PAL-313) might have direct receptor actions that reduce DA release, and the role of NE in modulating effects of PAL drugs is unexplored. PAL analogs may vary in their molecular interactions with DA transporters, thereby causing differences in conformational preference (Liang et al., 2009), oligomer formation (Chen and Reith, 2008), or protein trafficking (Furman et al., 2009), which could influence ensuing DA release. It also seems possible that PAL analogs might display differences in pharmacokinetic parameters. In rats, amphetamine-type drugs are metabolized predominantly via p-hydroxylation of the phenyl ring (Green et al., 1986; Law et al., 2000). Because all of the PAL analogs tested exhibited ring substitution at the m- or p-position, the efficiency or degree of metabolism may be different for each drug. There currently is no evidence to support or refute any of these alternative explanations.
We observed that all PAL analogs increased ambulation and stereotypy, but the extent of ambulation varied with drug treatment. It is noteworthy that the level of stereotypy was similar across treatment groups, indicating that differences in ambulation cannot be explained on the basis of physiological antagonism whereby enhanced stereotypy reduces ambulation. The rank order of ambulatory response magnitude at 3 mg/kg was PAL-353 > PAL-303 > PAL314 = PAL-313. This relationship parallels nucleus accumbens DA elevations shown in Fig. 2 and confirms prior results that relied on behavioral scoring methods to reveal differences in locomotor sensitivity to PAL drugs (Rothman and Baumann, 2006). It is well established that DA nerve terminals in the nucleus accumbens are critical substrates of the behavioral-activating effects of amphetamine-type stimulants (Swerdlow et al., 1986; Ikemoto and Panksepp, 1999). Figure 6 shows that ambulation evoked by PAL analogs was strongly correlated with nucleus accumbens DA (p < 0.001), an observation consistent with data showing positive correlations between locomotor and dialysate DA responses evoked by stimulants such as methamphetamine and MDMA in rats (Baumann et al., 2008; Zolkowska et al., 2009). Ambulation was not correlated with nucleus accumbens 5-HT but was significantly correlated with the ratio of DA/5-HT responses across all drug treatments. One hypothesis to explain the combined neurochemical and motor effects of PAL drugs is that increasing 5-HT release reduces ongoing DA release and its associated hyperactivity by activation of 5-HT2C receptors (Bankson and Cunningham, 2002; Fletcher et al., 2006). A comparison of data in Figs. 2 and 4 suggests that an additional 5-HT mechanism, independent of DA, also may contribute to diminished behavioral effects of PAL-314 and PAL-313, because ambulatory responses to these drugs were lower than predicted based on the concurrent elevations in dialysate DA.
The present behavioral data are reminiscent of those reported by Wellman et al. (2009), who compared locomotor and hypophagic effects of (+)-amphetamine, PAL-353, PAL-313, and the preferential 5-HT releaser 1-naphthyl-2-aminopropane (PAL-287) (Rothman et al., 2005) in male rats. Those investigators found that PAL-353 produced robust hyperactivity similar to (+)-amphetamine, whereas PAL-313 was much less potent and had effects similar to PAL-287 (Rothman et al., 2005). Wellman et al. also discovered that all test drugs exerted similar effects on hypophagia despite differences in locomotor activity. The rat behavioral data suggest that increasing 5-HT-releasing properties of amphetamine analogs can diminish motor stimulant actions without altering potential therapeutic effects such as appetite suppression. It is instructive to compare behavioral effects of PAL analogs in rats to the effects of these drugs in nonhuman primate models of addiction. In squirrel monkeys, PAL-353 and (+)-amphetamine markedly increase rates of responding in a stimulus termination procedure, whereas PAL-313, PAL-287, and the 5-HT releaser fenfluramine do not (Kimmel et al., 2009). In rhesus monkeys, the rank order of potency to produce cocaine-appropriate responding in a drug discrimination assay is PAL-353 > PAL-314 > PAL-287 > fenfluramine (Negus et al., 2007). As noted previously, PAL-313 engenders significantly less self-administration behavior compared with other PAL analogs tested in the present study (Wee et al., 2005). Collectively, such findings indicate that 5-HT release attenuates stimulant properties of amphetamine-type drugs in a consistent manner across a variety of behavioral assays in diverse species.
Clinical trials conducted over the last decade reveal that monoamine releasers are promising treatment adjuncts for cocaine addiction (Mooney et al., 2009; Herin et al., 2010). Negus et al. (2007) have provided compelling evidence in rhesus monkeys to show that DA-selective agents, such as (+)-amphetamine and (+)-methamphetamine, might be more efficacious medications than 5-HT-selective or nonselective monoamine releasers. On the other hand, adding 5-HT-releasing properties to DA-releasing medications could have two desirable outcomes for certain patients: 1) reduction in the stimulant side effects of medications (Rothman et al., 2005) and 2) restoration of serotonergic deficits in the brain of chronic cocaine and methamphetamine users (Baumann and Rothman, 1998; Rothman et al., 2006). Although no mixed DA/5-HT releasers are clinically available at the present time, a viable treatment approach might be to administer a DA releaser in conjunction with a serotonergic medication. Examples of this combination strategy include the use of the (+)-amphetamine with the 5-HT precursor l-5-hydroxytryptophan (Rothman et al., 2006; Baumann et al., 2010). A similar combination, phentermine and l-5-hydroxytryptophan, is being used in humans to treat obesity (Hendricks et al., 2009; Rothman, 2010). Given the inhibitory role of 5-HT2C receptors in modulating DA function, another possibility might be to administer (+)-amphetamine with a selective 5-HT2C agonist, such as locaserin (Smith et al., 2009). Taken together with other published data, the present findings in rats provide additional support for the hypothesis that pharmacological treatments that increase extracellular DA and 5-HT should be examined as possible medications for stimulant addiction.
This work was supported by in part by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse; and the National Institutes of Health National Institute on Drug Abuse [Grant 5R01DA012970-10].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.176271.
ABBREVIATIONS:
- DA
- dopamine
- HPLC-ECD
- high-pressure liquid chromatography with electrochemical detection
- 5-HT
- serotonin
- MDMA
- 3,4-methylenedioxymethamphetamine
- NE
- norepinephrine
- PAL
- phenylethylamine library
- PAL-353
- m-fluoroamphetamine
- PAL-303
- p-fluoroamphetamine
- PAL-314
- m-methylamphetamine
- PAL-313
- p-methylamphetamine
- PAL-287
- 1-naphthyl-2-aminopropane
- VTA
- ventral tegmental area
- ANOVA
- analysis of variance
- NS
- not significant.
Authorship Contributions
Participated in research design: Baumann, Woolverton, Blough, and Rothman.
Conducted experiments: Clark.
Contributed new reagents or analytic tools: Blough.
Performed data analysis: Baumann, Wee, and Rothman.
Wrote or contributed to the writing of the manuscript: Baumann, Clark, Woolverton, Wee, Blough, and Rothman.
References
- Alex KD, Pehek EA. (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. (2002) Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology 26:40–52 [DOI] [PubMed] [Google Scholar]
- Bankson MG, Yamamoto BK. (2004) Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J Neurochem 91:852–859 [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. (2008) Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav 90:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. (1998) Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry 44:578–591 [DOI] [PubMed] [Google Scholar]
- Baumann MH, William Z, Zolkowska D, Rothman RB. (2010) Serotonin (5-HT) precursor loading with 5-hydroxy-L-tryptophan (5-HTP) reduces locomotor activation produced by (+)-amphetamine in the rat. Drug Alcohol Depend doi:10.1016/j.drugalcdep.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Progr Brain Res 172:319–346 [DOI] [PubMed] [Google Scholar]
- Chen N, Reith ME. (2008) Substrates dissociate dopamine transporter oligomers. J Neurochem 105:910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. (1997) Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol 121:1735–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Esposito E. (2008) Serotonin-dopamine interaction: electrophysiological evidence. Progr Brain Res 172:45–71 [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. (2008) Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Progr Brain Res 172:7–44 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. (2007) New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 47:681–698 [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Sinyard J, Higgins GA. (2006) The effects of the 5-HT(2C) receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology 187:515–525 [DOI] [PubMed] [Google Scholar]
- Furman CA, Chen R, Guptaroy B, Zhang M, Holz RW, Gnegy M. (2009) Dopamine and amphetamine rapidly increase dopamine transporter trafficking to the surface: live-cell imaging using total internal reflection fluorescence microscopy. J Neurosci 29:3328–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464 [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. (1999) The κ-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem 73:1066–1074 [DOI] [PubMed] [Google Scholar]
- Green CE, LeValley SE, Tyson CA. (1986) Comparison of amphetamine metabolism using isolated hepatocytes from five species including human. J Pharmacol Exp Ther 237:931–936 [PubMed] [Google Scholar]
- Hendricks EJ, Rothman RB, Greenway FL. (2009) How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 17:1730–1735 [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. (2010) Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann N Y Acad Sci 1187:76–100 [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. (2007) Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:757–765 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev 31:6–41 [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Manvich DF, Blough BE, Negus SS, Howell LL. (2009) Behavioral and neurochemical effects of amphetamine analogs that release monoamines in the squirrel monkey. Pharmacol Biochem Behav 94:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MY, Slawson MH, Moody DE. (2000) Selective involvement of cytochrome P450 2D subfamily in in vivo 4-hydroxylation of amphetamine in rat. Drug Metab Dispos 28:348–353 [PubMed] [Google Scholar]
- Liang YJ, Zhen J, Chen N, Reith ME. (2009) Interaction of catechol and non-catechol substrates with externally or internally facing dopamine transporters. J Neurochem 109:981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness WH. (1983) Effect of l-tryptophan pretreatment on d-amphetamine self administration. Substance Alcohol Actions/Misuse 4:305–312 [PubMed] [Google Scholar]
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE. (1995) Psychostimulant-like effects of p-fluoroamphetamine in the rat. Eur J Pharmacol 287:105–113 [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. (1990) Analysis of serial measurements in medical research. BMJ 300:230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. (2009) Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 101:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. (2007) Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther 320:627–636 [DOI] [PubMed] [Google Scholar]
- Newman AH, Rothman RB. (2007) Addiction, in Comprehensive Medicinal Chemistry II (Williams M. ed) pp 169–192, Elsevier, Amsterdam [Google Scholar]
- Paxinos G. (1982) The Rat Brain in Stereotaxic Coordinates. Academic Press, New York [Google Scholar]
- Rice ME, Cragg SJ. (2008) Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev 58:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. (1999) Self-administration of cocaine analogs by rats. Psychopharmacology 144:389–397 [DOI] [PubMed] [Google Scholar]
- Rothman RB. (2010) Treatment of obesity with “combination” pharmacotherapy. Am J Ther 17:596–603 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2006) Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann NY Acad Sci 1074:245–260 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. (2006) Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci 27:612–618 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH. (2005) Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther 313:1361–1369 [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR. APD356–004 Study Group (2009) Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 17:494–503 [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. (1986) The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav 25:233–248 [DOI] [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A. (2010) Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br J Pharmacol 160:785–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. (2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854 [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. (2006) Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav 84:337–343 [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davis KW, Clifford PS, Rothman RB, Blough BE. (2009) Changes in feeding and locomotion induced by amphetamine analogs in rats. Drug Alcohol Depend 100:234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink BH, Hofsteede RM, Tuntler J, de Vries JB. (1989) Use of calcium antagonism for the characterization of drug-evoked dopamine release from the brain of conscious rats determined by microdialysis. J Neurochem 52:722–729 [DOI] [PubMed] [Google Scholar]
- Wise RA. (2008) Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14:169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. (2009) Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329:738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]