Abstract
Withdrawal-related anxiety is cited as a major contributor to relapse in recovering alcoholics. Changes in lateral/basolateral amygdala (BLA) neurotransmission could directly influence anxiety-like behaviors after chronic ethanol exposure and withdrawal. We have shown that these treatments enhance BLA glutamatergic function and neurotransmission. However, the BLA GABAergic system tightly controls the expression of anxiety-like behavior, and additional neuroadaptations in this system are potentially important as well. The intrinsic BLA GABAergic system consists of at least two populations of interneurons: local feed-back interneurons scattered throughout the region and feed-forward interneurons concentrated within groups found in the lateral/paracapsular region of the BLA. In the present study, we found that withdrawal from chronic ethanol robustly decreased presynaptic function at feed-forward GABA synapses but did not alter neurotransmitter release from local interneurons. Differential presynaptic changes at these synapses were complemented by decreased zolpidem sensitivity at feed-forward synapses and decreased midazolam sensitivity at local synapses. Consistent with this, chronic ethanol/withdrawal decreased expression of GABA α1-subunit total protein and increased surface expression of α4-subunit protein. We also found transient increases in GABA-receptor-associated protein levels and persistent increases in γ2-subunit and gephyrin proteins that would suggest alterations in GABAA receptor trafficking that might help regulate changes in α4-subunit localization. These data together suggest that chronic ethanol and withdrawal differentially modulate local and lateral paracapsular cell GABAergic synapses via distinct presynaptic and postsynaptic mechanisms. These findings extend our understanding of the neurobiological mechanisms governing changes in anxiety-like behavior after chronic ethanol exposure and withdrawal.
Introduction
Dependence-associated anxiety is a significant risk factor for relapse in human alcoholics. This withdrawal (WD)-related anxiety has been recapitulated in many different rodent models of ethanol dependence. Although many brain regions are likely to regulate withdrawal-associated anxiety, the amygdala plays an important role in both fear-learning and innate anxiety-like behaviors across many species (Davis et al., 2010) and seems to regulate the expression of withdrawal anxiety as well (Läck et al., 2007). The lateral and basolateral subdivisions of the amygdala serve as a primary input into the fear/anxiety circuit and are critically important to drug-related behaviors such as relapse (See, 2005). The lateral/basolateral amygdala (BLA) neurotransmitter systems that are altered by ethanol exposure and help regulate withdrawal-associated behaviors such as anxiety have only recently been explored.
We have previously shown that chronic ethanol/withdrawal produces robust increases in glutamatergic synaptic and receptor function measured from BLA principal neurons (Läck et al., 2007, 2009), which may contribute to the expression of withdrawal anxiety. However, intoxicated animals express decreased, not increased, anxiety-like behavior immediately after a chronic exposure despite exposure-induced increases in glutamatergic synaptic function. Because BLA glutamatergic synaptic responses are relatively insensitive to acute ethanol (Läck et al., 2008), the maintenance of an anxiolytic phenotype suggests contributions by neurotransmitter systems other than glutamate. In this regard, the expression of anxiety-like behavior (Sanders and Shekhar, 1995) and the activity of BLA principal neurons (Woodruff et al., 2006) are tightly regulated by GABAergic neurotransmission. Given that GABAergic neurotransmission in this brain region is robustly enhanced by acute ethanol (Silberman et al., 2008), we hypothesized that exposure-related changes in the BLA GABAergic system might ultimately influence the expression of anxiety-like behavior in intoxicated animals and during withdrawal.
There is an extensive literature demonstrating that chronic ethanol exposure results in robust changes to GABAA receptor pharmacology, expression, and function. Several excellent reviews on this topic are available (Siggins et al., 2005; Kumar et al., 2009). In general, chronic ethanol exposure seems to alter GABAA receptor subunit composition in a manner that changes the pharmacology and biophysical properties of the channel. Studies demonstrating altered lateral amygdala GABAA receptor pharmacology and mRNA expression (Floyd et al., 2004) in nonhuman primates after long-term ethanol self-administration are consistent with this. Likewise, chronic exposure to a liquid ethanol diet increased the functional expression of GABAA receptors measured in acutely isolated rat BLA neurons (McCool et al., 2003). Although these data suggest that chronic ethanol may modulate the BLA GABA system, GABAergic synaptic neurotransmission has not been specifically examined.
The GABAergic system in the BLA is comprised of at least two anatomically and functionally distinct populations of interneurons. Lateral paracapsular cells (LPCs) are GABAergic interneurons concentrated in “islands” along the external capsule and provide feed-forward inhibitory synapses onto the distal dendrites of BLA principal neurons (Marowsky et al., 2005). In contrast, local interneurons are scattered throughout the subdivision and provide feed-back inhibitory synapses onto perisomatic areas of BLA principal neurons (Woodruff and Sah, 2007). A similar dichotomy of GABAergic neurocircuitry has also been found in the hippocampus (Weiner et al., 1997; Poelchen et al., 2000) and the cerebellum (Mameli et al., 2008), and the acute effects of ethanol are distinct at these different GABAergic inputs. Nevertheless, these anatomically distinct BLA GABAergic synapses arising from different interneuron populations can be independently activated during in vitro electrophysiological recordings using specific placement of the stimulating electrodes (Silberman et al., 2008; Diaz et al., 2011). This provides the opportunity to study the effects of chronic ethanol and withdrawal on distinct populations of BLA GABAergic synapses.
Materials and Methods
Animals.
All animal procedures were performed in accordance with protocols approved by the Wake Forest University School of Medicine Animal Care and Use Committee and were consistent with the National Institutes of Health animal care and use policy. Male Sprague-Dawley periadolescent rats (∼5 weeks of age; 120–150 g) (McCutcheon and Marinelli, 2009) were obtained from Harlan (Indianapolis, IN) and housed in an animal care facility at 23°C with a 12-h light/dark cycle and given food and water without restriction. Rats were weighed daily to ensure that ≥80% of their free-feeding weight was maintained during vapor chamber ethanol exposure.
Chronic Ethanol Exposure.
Ethanol exposure was accomplished via an ethanol vapor chamber as described previously (Läck et al., 2007, 2009). In brief, rats were housed in groups of four in large, standard polycarbonate cages. To achieve the ethanol exposure, these home cages were placed in large, custom-built Plexiglas chambers (Triad Plastics, Winston-Salem, NC) similar to those described previously (Läck et al., 2009). At the beginning of the light cycle (lights on at 9:00 PM), animals were exposed to either ethanol vapor or only room air [control (CON) group] for 12 h during the light cycle for 10 days. Using calibrated pressure gauges, we mixed ethanol vapor with room air to achieve the desired vapor concentration (∼45 mg EtOH/liter air) in the ethanol chamber. Vapor levels were tested daily. Animals receiving the chronic intermittent ethanol (CIE) vapor were further divided into two experimental groups: some animals were sacrificed immediately after the last ethanol exposure while they were still intoxicated (CIE group); the remaining animals remained in the chamber but were withdrawn from ethanol for 24 h before sacrifice (WD group). Blood was collected at sacrifice from the CIE group, and blood ethanol levels were 194 ± 11 mg/dl (n = 45) as determined by a commercially available alcohol dehydrogenase assay (Genzyme, Cambridge, MA).
Slice Preparation.
Animals were anesthetized with halothane and decapitated according to a protocol approved by the Institutional Animal Care and Use Committee. The brains were quickly removed and incubated in ice-cold sucrose/artificial cerebrospinal fluid (aCSF) equilibrated with 95% O2 and 5% CO2 containing 180 mM sucrose, 30 mM NaCl, 4.5 mM KCl, 1 mM MgCl2·6H2O, 26 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM glucose, and 0.1 mM ketamine. Coronal brain slices (400 μm) were prepared using a Vibratome Series 3000 (Vibratome, St. Louis, MO) and submerged in room-temperature (∼25°C), oxygenated standard aCSF containing 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 26 mM NaHCO3, 10 mM glucose, and 2 mM CaCl2·2H2O. Slices were maintained in aCSF for ∼1 h before recording. All experiments were performed 1 to 4 h after preparation of the BLA slices. All chemicals for slice preparation and electrophysiology were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Whole-Cell Patch-Clamp Electrophysiology.
Methods used for whole-cell patch-clamp electrophysiology were similar to those described previously (Läck et al., 2007). Slices were placed in a recording chamber and perfused with room-temperature aCSF at a rate of 2 ml/min. Patch electrodes were filled with an internal solution containing 122 mM Cs-gluconate, 10 mM CsCl, 10 mM HEPES, 1 mM EGTA, 5 mM NaCl, 0.1 mM CaCl2, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 2 mM N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide (QX314)-(Cl) and had an open tip of 8 to 12 MΩ. All recordings were made from principal BLA neurons as suggested by their initial membrane resistance of <50 MΩ (Läck et al., 2007). Inclusion criteria for analysis was that access resistance and baseline holding currents did not change more than 20% throughout the duration of any experiment. Analysis of holding currents revealed a significant treatment-dependent decrease in holding current in neurons recorded from CIE and WD slices relative to the CON neurons (one-way ANOVA, F = 4.16, df = 2, P < 0.05; data not shown). Data were acquired at 10 kHz with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) and analyzed using Clampex software (Molecular Devices). Electrically evoked GABA-inhibitory postsynaptic currents (IPSCs) were pharmacologically isolated with the glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (20 μM) and d/l-2-amino-5-phosphono-pentanoic acid (50 μM).
For some experiments, GABAergic IPSCs were electrically evoked using platinum/iridium concentric bipolar stimulating electrodes (FHC Inc., Bowdoinham, ME) with an inner pole diameter of 25 μm. Stimulating electrodes were placed in the external capsule to stimulate paracapsular GABAergic synapses and within the BLA, medial to the recording site, to stimulate local GABAergic synapses (Silberman et al., 2008; Diaz et al., 2011). Stimulation intensities used at both stimulation sites achieved a GABAergic response of ∼100 pA; this level of stimulation represents less than 20% of electrically evoked maximum responses (data not shown) and preferentially activates these distinct interneuron populations (Silberman et al., 2008). For electrically evoked experiments, the LPCs and local IPSCs both were measured in a given BLA principal neuron. The consecutive order of stimulation sites was alternated between neurons, and, in some cases, these sites were consecutively stimulated during the same recording epoch. BLA neurons were maintained at a holding potential of −10 mV.
Paired-Pulse Ratios.
Paired-electrical stimuli were given to each stimulation site at interpulse intervals of 50 and 250 ms. These intervals were chosen to examine treatment-related changes in GABA release probability (50 ms) and presynaptic autoreceptor function (250 ms). A normalized paired-pulse ratio for each stimulation site was calculated as [amplitude IPSC2 − amplitude IPSC1]/amplitude IPSC1. These ratios were expressed as means ± S.E.M. and compared across treatment groups using one-way ANOVA and a Newman-Keuls post hoc test with P < 0.05 considered statistically significant.
Spontaneous GABAergic Synaptic Events.
Miniature IPSCs (mIPSCs) were acquired at 20 kHz and filtered at 2 kHz. For these experiments, we used a holding membrane potential of −60mV and an internal solution consisting of 135 mM KCl, 10 mM HEPES, 2 mM MgCl2, 0.5 mM EGTA, 5 mM Mg-ATP, 1 mM Na-GTP, and 1 mM QX314-(Cl), pH 7.25, osmolarity 280 to 290 mOsm. After the onset of the recordings, 1 μM tetrodotoxin (TTX) was applied for >5 min before recording spontaneous activity. mIPSCs were recorded for 1 min after a baseline period (∼5 min). Event amplitude, frequency, charge transfer, and decay time (including τ measures) were measured using MiniAnalysis (Synaptosoft, Decatur, GA). Median values of these measures from individual cells were averaged within treatment groups (Läck et al., 2007), reported as mean ± S.E.M., and analyzed using one-way ANOVA with Newman-Keuls post hoc test. P < 0.05 was considered statistically significant.
Zolpidem, Midazolam, and Ethanol Pharmacology.
After collecting a baseline of evoked GABA-IPSCs, 100 nM zolpidem (a GABAA α1-subunit-selective modulator), 1 μM midazolam (a benzodiazepine allosteric modulator), or 80 mM ethanol was perfused onto slices until the drug effect reached steady state (typically within 10 min). Drug effects were calculated as percentage change from baseline ± S.E.M. and subjected to one-way ANOVA with Newman-Keuls post hoc test. P < 0.05 was considered statistically significant.
Western Blots.
Lysis buffer [50 mM Tris, pH 7.4, 0.5% SDS, 1 mM EDTA, pH 8, and protease inhibitors for mammalian tissue (Sigma-Aldrich)] was added to BLA dissected from CON, CIE, and WD coronal slices at 5 μl/mg tissue, and tissue was disrupted by brief sonication and incubated at 4°C on a rotisserie mixer for 2 h. Protein yield was quantified using the BCA assay (Thermo Fisher Scientific, Waltham, MA). Fifteen micrograms of total protein was loaded onto 4 to 20% SDS precast polyacrylamide gels (Thermal Fisher Scientific), separated, and transferred to a nitrocellulose membrane (Hybond N; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The membrane was blocked with 10% nonfat dry milk in Tris-buffered saline (TBS-T; 150 mM NaCl, 5.2 mM Na2HPO4, 1.7 mM KH2PO4, 0.05% Tween 20). Blots were incubated overnight at 4°C in TBS-T/0.5% nonfat dry milk containing a rabbit polyclonal primary antibody: GABAA α1-ubunit, 1:3000 dilution (Millipore Bioscience Research Reagents, Temecula, CA); GABAA α4-subunit-N terminus, 1:650 dilution (PhosphoSolutions, Aurora, CO); GABAA γ2-subunit, 1:500 dilution (Sigma-Aldrich); gephyrin, 0.5 μg/ml (Millipore Bioscience Research Reagents); and GABA receptor-associated protein (GABA-RAP), 1:300 (Novus Biologicals, Inc., Littleton, CO). After extensive washing with TBS-T, the blots were exposed to a goat anti-rabbit horseradish peroxidase-labeled secondary antibody (1:3000 dilution; Sigma-Aldrich) for 1 h at room temperature with agitation. Detection of bound secondary antibody was performed using SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific). To normalize expression between experiments, the blots were probed with mouse monoclonal antibody directed against β-actin, 1:50,000 to 1:100,000 dilution (Millipore Bioscience Research Reagents) followed by peroxidase-labeled goat anti-mouse secondary antibody, 1:10,000 dilution (Sigma-Aldrich). Band intensity was quantified from digital images captured on a charge-coupled device camera using a Bio-Rad ChemiDoc XRS Imaging System with Quantity One Analysis Software (Bio-Rad Laboratories, Hercules, CA).
Surface Cross-Linking with Bis(sulfosuccinimidyl)suberate.
To examine surface expression of specific GABAA receptor subunits, we used the membrane-impermeable cross-linker bis(sulfosuccinimidyl)suberate (BS3; Thermo Fisher Scientific) as described previously (Grosshans et al., 2002) with slight modifications. Slices from individual CON, CIE, and WD animals were allowed to stabilize for 1 h after preparation and subsequently transferred to aCSF ± 1 mg/ml BS3 and allowed to incubate for 1 h at 4°C. We then rinsed slices three times with aCSF containing 20 mM Tris, pH 7.4. The BLA was dissected and frozen until tissue was used for Western blot analysis. Western blot methods were the same as described above except that 20 μg of total protein was loaded onto precast 8 to 16% SDS-polyacrylamide gels. BS3-insensitive intracellular protein was compared with total protein from the untreated samples collected in parallel to calculate the percentage of protein found on the surface for each animal. Percentages of “cell surface” receptors from individual animals were averaged within the treatment groups and compared using one-way ANOVA with Newman-Keuls multiple comparisons post-test.
Results
Differential Presynaptic Adaptations at Paracapsular and Local GABAergic Synapses.
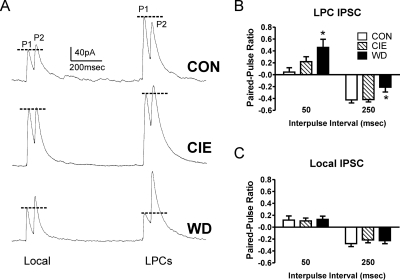
To measure presynaptic changes from both paracapsular and local synapses, we used two stimulating electrodes, one placed along the border of the BLA within the external capsule and one placed within the BLA medial to the recording site. This arrangement functionally separates GABAergic synapses from paracapsular and local interneurons (Silberman et al., 2008, 2009). To measure presynaptic function from each synapse, we used paired electrical stimuli and calculated the ratio between the first and second synaptic response (see Materials and Methods; Fig. 1A). At the paracapsular GABAergic synapses, we found ethanol exposure/withdrawal significantly increased the paired-pulse ratio at the 50-ms interstimulus interval (Fig. 1B; one-way ANOVA, F = 4.53, df = 2, P < 0.05) and significantly decreased the ratio at the 250-ms interstimulus interval (one-way ANOVA, F = 3.90, df = 2, P < 0.05). Neuman-Kuels multiple comparison post-test indicated that ratios obtained from BLA neurons in the WD group were significantly different from the CON group at both stimulus intervals (P < 0.05). With the 250-ms interval, WD paracapsular response ratios were also significantly different from the CIE group (P < 0.05). In contrast to the effects of withdrawal at the paracapsular GABAergic inputs, there were no treatment effects on the paired-pulse ratio recorded from local GABAergic synapses (Fig. 1C; one-way ANOVA, P > 0.05 at both interstimulus intervals). These data suggest that withdrawal from chronic ethanol exposure differentially modulates the presynaptic function of paracapsular and local GABAergic inputs onto BLA pyramidal neurons.
Fig. 1.
Chronic ethanol and withdrawal differentially modulate LPC and local interneuron synaptic responses to paired electrical stimuli. A, sample paired-pulse traces from local and LPC neurons across treatment groups. P1 and P2 denote the first and second response, respectively. Stimulation artifacts have been removed for clarity. Dotted lines are used to compare the peak of the first response to the second. These continuous traces sampled both local and LPC GABAergic synapses in the same BLA principal neuron using two stimulating electrodes (see Materials and Methods). B, normalized paired-pulse ratios (see Materials and Methods) from LPC interneurons across the treatment groups at 50- and 250-ms interpulse intervals show significant changes in WD-treated neurons (n = 11) relative to CON (n = 21) (one-way ANOVA; *, P < 0.05 with Newman-Keuls multiple comparison post-test). There was no significant difference between CON and CIE neurons (n = 17). C, paired-pulse ratios measured in the same neurons as in B were evoked from local interneurons and were not different between the treatment groups at either stimulus interval.
Evidence suggests that tonic GABAB receptor activity regulates LPC GABAergic synapses in the BLA (Silberman et al., 2008, 2009). Although this modulation did not involve apparent presynaptic mechanisms in naive animals, it is possible that chronic ethanol exposure and withdrawal altered the presynaptic contributions by GABAB receptors at LPC synapses. To test this directly, we measured the effects of the GABAB antagonist CGP55845 [2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride] (10 μM; Tocris Bioscience, Ellisville, MO) on paired-pulse responses from LPC synapses using the 250-ms interstimulus interval. In these experiments, the paired-pulse ratio at 250 ms in WD neurons was not significantly different ± CGP55845 (baseline = −0.27 ± 0.15, +CGP55845 = −0.32 ± 0.11, P > 0.05, paired t test, n = 9 cells). Likewise, CGP55845 did not significantly alter the 250-ms ratio in either CON (n = 4) or CIE (n = 7) neurons (not shown, P > 0.05, paired t test). Together, these data are very similar to that reported previously in a study using the GABAB antagonist SCH50911 (2-[(2S)-(+)−5,5-dimethylmorpholin-2-yl]acetic acid) (Silberman et al., 2009) and suggest that treatment-related changes in LPC paired-pulse ratios at the 250-ms stimulus interval are not related to changes in GABAB receptor function.
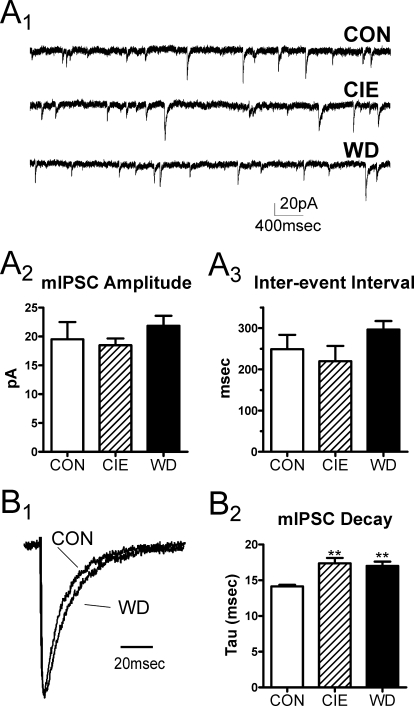
To confirm that presynaptic transmitter release from local synapses was not altered by chronic ethanol or withdrawal, we quantified the frequency and amplitude of spontaneous IPSCs in the presence of 1 μM TTX. In BLA principal neurons, mIPSCs arise solely from the GABAergic terminals of local feed-back interneurons (Silberman et al., 2009). Consistent with the paired-stimulus data, we found no treatment-related changes in mIPSC frequency (Fig. 2A; one-way ANOVA, P > 0.05, F = 1.33, df = 2). There was a trend of increased mIPSC amplitude in the WD group (21.85 ± 1.74 pA, n = 7) relative to CON (17.75 ± 1.61 pA, n = 5) and CIE (18.49 ± 1.17 pA, n = 5), but this did not reach statistical significance (one-way ANOVA, P > 0.05). However, we found decay times from WD mIPSCs were significantly longer than both CON and CIE mIPSCs (Fig. 2B; one-way ANOVA, F = 6.21, df = 2, P < 0.05, using Newman-Keuls multiple comparison test). This was paralleled by a significant increase in the mIPSC charge transfer (area) in the WD group (data not shown one-way ANOVA; F = 5.14, df = 2, P < 0.05 compared with CIE from Newman-Keuls multiple comparisons test). These data indicate that withdrawal from chronic ethanol may change postsynaptic GABAA receptor function.
Fig. 2.
Chronic ethanol and withdrawal differentially modulate presynaptic and postsynaptic properties of mIPSCs. A1, exemplar traces of mIPSCs collected from individual principal cells at each exposure group (CON, CIE, and WD). A2 and A3, mIPSC amplitudes (A2) and interevent intervals (A3) were not significantly different between CON (n = 7), CIE (n = 5), or WD (n = 7) BLA neurons. B1 and B2, mIPSCs from each neuron were averaged to yield a normalized mIPSC current. B1, exemplar traces from representative CON and WD neurons are shown. The normalized mIPSC decays were fit to a single exponential; amplitudes from these representative traces have been normalized to emphasize the decay phase of the response. B2, time constants (Tau) were significantly larger in both CIE and WD mIPSCs relative to CON (one-way ANOVA; **, P < 0.01 relative to CON with Newman-Keuls multiple comparison post-test).
Withdrawal Decreases Benzodiazepine Sensitivity at Local Synapses and Alters α4-Subunit Expression and Localization.
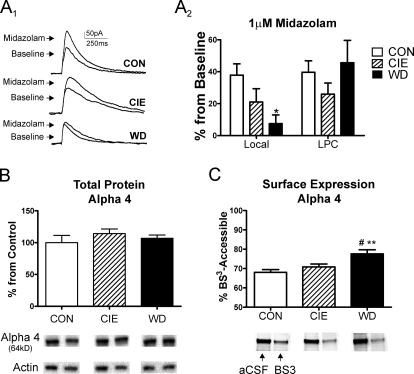
The WD-related changes in mIPSC kinetics suggest that chronic ethanol exposure or withdrawal might alter the properties of postsynaptic GABAA receptors. To examine this in more detail, we characterized the pharmacological properties of LPC and local GABAergic synapses in the CON, CIE, and WD treatment groups using the benzodiazepine midazolam (1 μM). There was no apparent treatment-related alteration in the percentage effect of midazolam at LPC synapses (Fig. 3A2, one-way ANOVA, P > 0.05, F = 1.05, df = 2). In contrast, we did find that midazolam modulation of the local GABAergic IPSC was significantly reduced in WD slices relative to CON and CIE (Fig. 3, A1 and A2; one-way ANOVA, F = 4.76, df = 2, P < 0.05 relative to CON from Newman-Keuls multiple comparison post hoc analysis). These data suggest that withdrawal decreases the contribution by benzodiazepine-sensitive GABAA receptors at local but not LPC GABAergic inputs.
Fig. 3.
Midazolam sensitivity of local IPSCs and α4-subunit expression are modulated by chronic ethanol exposure/withdrawal. A1 and A2, midazolam sensitivity decreases at local GABAergic synapses but not paracapsular GABAergic inputs. A1, exemplar traces of the effects of midazolam (1 μM) on electrically evoked GABA IPSCs from local interneurons from CON-, CIE-, and WD-treated slices. A2, summary of data shows that the midazolam sensitivity of local IPSCs (Local) was significantly decreased in principal neurons from WD slices (n = 9; one-way ANOVA; *, P < 0.05 versus CON, Newman-Keuls multiple comparison post-test) relative to CON (n = 10) and CIE (n = 9) BLA neurons. The effect of midazolam on evoked-IPSCs from LPC interneurons was not altered in principal neurons from CIE or WD. B, total α4 protein levels were not significantly changed across treatment groups (n = 4 animals each). Representative immunoreactive bands from α4 total protein and β-actin correspond to the treatment groups in the bar graphs. C, surface-accessible, BS3-sensitive α4-subunit protein levels were significantly elevated in WD BLA (n = 4 animals in each group), compared with CON BLA (one-way ANOVA; **, P < 0.01 with Newman-Keuls multiple comparison posttest) and CIE BLA (#, P < 0.05, Newman-Keuls). Representative aCSF-treated and BS3-treated samples from each treatment are shown below the bar graph. The intracellular, BS3-insensitive pool of α4 subunit protein is shown (see Materials and Methods for details).
GABAA receptors containing the α4-subunit are insensitive to benzodiazepines (Wisden et al., 1991), so we examined α4-subunit expression and localization using Western analysis of BLA tissue. There was no treatment-related change in total α4-subunit protein expression (Fig. 3B; one-way ANOVA, P > 0.05). GABAA subunit immunoreactivity was normalized to β-actin, and there was no significant differences in β-actin expression between CON (100.0 ± 3.8%), CIE (90.0 ± 4.0%), and WD (90.0 ± 10.7%) samples (n = 4 animals per treatment, P > 0.05 one-way ANOVA). Because the midazolam pharmacology suggested increased contributions by α4-contraining receptors, we measured the expression of α4-subunit protein that was accessible to the membrane-impermeant cross-linking agent BS3 (see Materials and Methods). WD caused a significant increase in the BS3-accessible α4-subunit protein (Fig. 3D) compared with both CON and CIE (one-way ANOVA, F = 8.42, df = 2; P < 0.01 compared with CON; P < 0.05 compared with CIE; Newman-Keuls multiple comparisons post-test). To ensure that BS3 exposure did not interact with intracellular proteins, we compared β-actin levels from aCSF-exposed and BS3-exposed samples and found no significant effect of the BS3 treatment on β-actin immunoreactivity (BS3 = 90 ± 9% compared with 100 ± 11% aCSF; n = 4 each; P > 0.05 paired t test). These data suggest that WD does not alter total α4-subunit protein levels in the BLA but instead increases the amount of BS3-accessible α4-subunit protein found on the cell surface. The functional decrease of local IPSC midazolam sensitivity is consistent with this interpretation.
Withdrawal-Induced Changes in α1-Subunit Function and Expression at LPC Synapses.
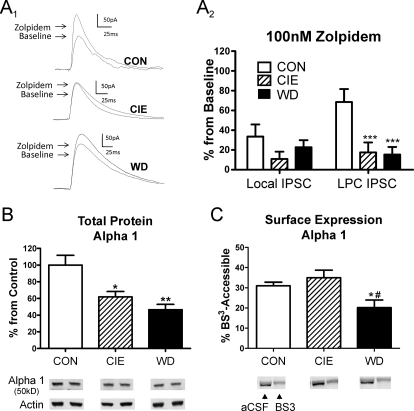
Changes in GABAA α4-subunit expression frequently are associated with alterations in the α1-subunit as well. To examine this directly, we used the nonbenzodiazepine allosteric modulator zolpidem, which is selective for GABAA α1-containing receptors. Zolpidem (100 nM) robustly increased electrically evoked GABAergic responses from both lateral paracapsular (Fig. 4A1) and local interneurons. We were surprised to find that CIE and WD significantly diminished the percentage effect of zolpidem at the paracapsular synapses (Fig. 4A2; one-way-ANOVA, F = 12.55, df = 2; P < 0.001 compared with CON, Newman-Keuls multiple comparison post-test) but not at local GABAergic synapses (one-way ANOVA, P > 0.05, F = 2.57). These data illustrate that chronic ethanol exposure and withdrawal diminish the functional contributions of zolpidem-sensitive GABAA receptors specifically at BLA LPC GABAergic synapses.
Fig. 4.
Zolpidem sensitivity and α1-subunit expression are robustly diminished by chronic ethanol and withdrawal. A1 and A2, zopidem (100 nM) modulation of LPC IPSCs is decreased in CIE and WD BLA principal neurons. A1, sample traces from LPC inputs illustrate the treatment effect. A2, summary of zolpidem data shows that CIE (LPC: n = 7, local: n = 9) and WD (LPC: n = 9, local: n = 9) significantly diminish modulation at LPC but not local IPSCs relative to CON (LPC: n = 9, local: n = 9; one-way ANOVA; ***, P < 0.001 versus CON, Newman-Keuls multiple comparison post-test). B, total α1-subunit protein levels were significantly reduced in CIE and WD BLA relative to CON (n = 4 animals per group; one-way ANOVA, *, P < 0.05; **, P < 0.01 versus CON with Newman-Keuls post hoc). Representative immunoreactive bands from total α1 protein and β-actin correspond to the treatment groups in the bar graphs. C, BS3-sensitive, surface accessible α1-subunit protein levels were significantly reduced in WD BLA relative to both CON and CIE (n = 4 animals/treatment; one-way ANOVA, *, P < 0.05 compared with CON; #, P < 0.05 compared with CIE, Newman-Keuls post hoc). Representative aCSF-treated and BS3-treated samples from each treatment are shown below the bar graph. The intracellular, BS3-insensitive pool of α1-subunit protein is shown (see Materials and Methods for details).
To examine the mechanism responsible for decreased zolpidem sensitivity at LPC synapses, we measured total α1-subunit protein expression and localization with Western analysis. In contrast to the α4 data, α1-like immunoreactivity was significantly decreased in both CIE and WD groups compared with CON (Fig. 4B; one-way-ANOVA, F = 10.35, df = 2, P < 0.01; P < 0.05, CIE compared with CON; P < 0.01, WD compared with CON from Newman-Keuls multiple comparison post-test). BS3-sensitive, surface-accessible α1-like immunoreactivity was also significantly decreased but only in the WD group (Fig. 4C; one-way ANOVA, F = 5.39, df = 2; P < 0.05 from Newman-Keuls multiple comparison post hoc analysis). Together, these biochemical data suggest that chronic ethanol exposure and withdrawal cause a robust decrease in the expression of total α1 GABAA receptor subunit protein that is complemented during WD by decreased levels of α1-containing receptors at the cell surface. The changes in zolpidem pharmacology suggest these alterations are more robustly expressed at LPC synapses relative to local GABAergic inputs.
Molecular Mechanisms Regulating Postsynaptic Subunit Changes during CIE and WD.
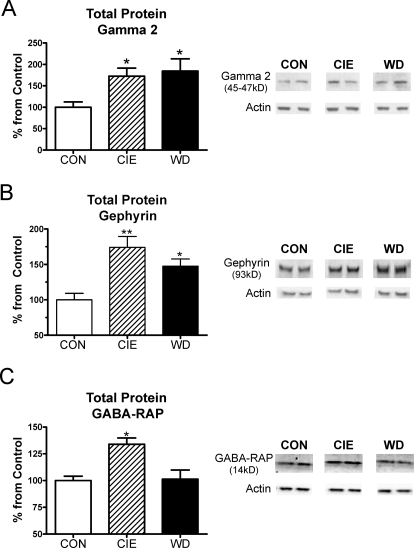
The reciprocal regulation of GABAA receptor subunit pharmacology and expression suggest complex molecular mechanisms might be involved. To test this, we examined GABAA-associated proteins involved with receptor localization and trafficking. CIE and WD significantly increased total γ2-subunit protein expression (Fig. 5A; one-way ANOVA, F = 4.742, df = 2, P < 0.05, relative to CON from Newman-Keuls multiple comparison test). It is noteworthy that neither α2 (CON = 100 ± 3%, CIE = 79 ± 4%, and WD = 88 ± 16%; n = 4 animals each, P > 0.05 one-way ANOVA) nor α3 (CON = 100 ± 7%, CIE = 91 ± 5%, and WD = 93 ± 11%, n = 4, P > 0.05) total protein levels were altered by CIE or WD (not shown). We likewise found that the expression levels of total gephyrin protein was significantly increased during CIE and WD (Fig. 5B; one-way ANOVA, F = 9.94, df = 2; CIE, P < 0.01; WD, P < 0.05, Newman-Keuls multiple comparison post-test). Finally, GABA-RAP levels were significantly increased during CIE (Fig. 5C; one-way ANOVA, P < 0.05 compared with CON from Newman-Keuls multiple comparisons post hoc analysis) but returned to CON levels when measured after 24 h of withdrawal.
Fig. 5.
CIE and WD significantly alter protein expression of several GABAA receptor association proteins. A, total γ2-subunit protein levels were robustly elevated in CIE and WD slices relative to CON (n = 4 animals/treatment; one-way ANOVA, *, P < 0.05 versus CON from Newman-Keuls post-test). B, total gephryin protein levels were also significantly increased in both CIE (**, P < 0.01 relative to CON) and WD BLA slices (*, P < 0.05 versus CON, Newman-Keuls post hoc). C, total GABA-RAP expression was significantly increased in CIE slices (*, P < 0.05, Newman-Keuls post hoc) but returned to CON levels by 24 h of withdrawal. Representative protein and β-actin immunoreactivity from each treatment group is shown at right.
Lack of Tolerance to Acute Ethanol during CIE and WD.
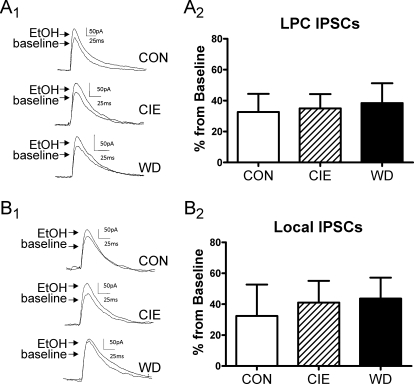
At local synapses we found that neither CIE nor WD altered the efficacy of 80 mM ethanol relative to CON BLA neurons (Fig. 6, A1 and A2; one-way ANOVA, P > 0.05, F = 0.11, df = 2). Likewise at LPC GABAergic synapses, the effect of 80 mM ethanol was not altered by CIE or WD at LPC synapses (Fig. 6, B1 and B2; one-way ANOVA, P > 0.05, F = 0.06, df = 2). These data suggest that there is no tolerance to acute effects of ethanol on BLA GABAergic transmission in CIE and WD animals.
Fig. 6.
CIE and WD treatments do not alter acute ethanol modulation of BLA GABAergic synapses. Graphs demonstrate that the effect of 80 mM acute ethanol on electrically evoked GABAergic transmission from LPC (A) and local (B) synapses is different between CON (n = 6 at each input), CIE (n = 11), and WD (n = 10) principal neurons (P > 0.05, one-way ANOVA). Sample traces correspond to the bar graphs.
Discussion
In the lateral/basolateral amygdala, the GABAergic system is robustly modulated by chronic alcohol and withdrawal. This modulation is characterized by increased paired-pulse ratios at feed-forward GABAergic synapses and differential alterations of postsynaptic contributions by α1- and α4-containing receptors at feed-forward LPC and feed-back local inputs, respectively. These functional measures are complemented by changes in total subunit protein expression and/or surface localization of these subunits. Although receptor benzodiazepine pharmacology is altered by chronic ethanol and withdrawal, the facilitating effects of acute ethanol on GABAergic transmission at both synapses remain intact.
Our paired-pulse findings suggest that withdrawal from chronic ethanol suppresses presynaptic function at BLA paracapsular GABAergic. Because synaptic responses to closely paired electrical stimuli are modulated presynaptic environments (reviewed in Zucker and Regehr, 2002), the WD-dependent increase in the paired-pulse ratio at LPC inputs may reflect decreased GABA release from these synapses. It is noteworthy that these presynaptic effects were specific to paracapsular interneurons because the paired-pulse ratio did not change at local GABAergic synapses. Current evidence suggests that LPC interneurons are the predominant source of feed-forward inhibition in the BLA (Marowsky et al., 2005), whereas local interneurons seem to provide the dominant source of feed-back inhibition in this brain region (Lang and Paré, 1998). Anatomical data suggest that lateral paracapsular synapses are segregated to the distal dendritic processes of BLA principal neurons (Marowsky et al., 2005), and pharmacological evidence is consistent with this hypothesis (Silberman et al., 2008, 2010; Diaz et al., 2011). This suggests that dominant contributions by the perisomatic GABAergic synapses arising from local interneurons would probably mask any spontaneous activity arising from LPC interneuron synapses (Muller et al., 2006). The precise mechanisms governing down-regulation of GABA release from feed-forward LPC synapses are therefore not currently clear.
Our presynaptic findings contrast to some degree with previous work in the central amygdala and hippocampus. For example, chronic ethanol exposure increases GABAergic neurotransmission in the central amygdala (Roberto et al., 2003). However, the neuroanatomy of the BLA and central amygdala GABAergic synapses differ considerably. There are also numerous GABAergic neuronal populations within the central amygdala (Pitkänen and Amaral, 1994), and chronic ethanol modulation of these distinct neuronal phenotypes remains to be specifically examined. In contrast, chronic ethanol exposure decreases mIPSC frequency and amplitude in hippocampal neurons (Cagetti et al., 2003). Methodological differences between these studies and the current work might play some role in these apparent regional differences.
It is worth noting that the results from the paired-pulse 250 experiments at LPC synapses could suggest changes in autoreceptor function. In fact, tonic GABAB receptor activity regulates electrically evoked IPSCs from LPC synapses (Silberman et al., 2008), but this does not seem to involve presynaptic mechanisms (Silberman et al., 2009). Similar to this, we found no treatment-dependent changes in the sensitivity of paired-pulse responses to a GABAB antagonist. This suggests that the treatment-related changes in paired-pulse ratios at LPC synapses do not involve GABAB receptors. Likewise, the acute effects of ethanol on LPC GABAergic responses do not seem to be presynaptic (Silberman et al., 2009). The specific mechanisms underlying changes in paired-pulse responses at longer interstimulus intervals remain to be precisely identified.
Although presynaptic neuroadaptations in the GABA system seem to occur exclusively at lateral paracapsular synapses, postsynaptic alterations occur at both GABAergic inputs. At LPC synapses, we found that CIE and WD decreased zolpidem sensitivity. This was associated with a decrease in both total and surface (BS3)-accessible α1-subunit immunoreactivity. Although Western analysis does not specifically measure changes in GABAA receptor subunits at synaptic sites, the coincidental depression of both zolpidem modulation and α1-subunit expression/localization measured are remarkably consistent. There are several potential mechanisms that could alter surface expression of GABAA receptor subunit proteins. The BS3-resistant populations might consist of mature subunit proteins sequestered to intracellular compartments or may be related to immature forms of the subunit protein that have yet to reach the cell surface. Chronic ethanol modulation of either process would alter the quantity of BS3-accessible protein. In addition, BLA α1-mRNA decreases after long-term exposure/withdrawal in nonhuman primates (Floyd et al., 2004) and rats (Falco et al., 2009). Although these findings are similar to other brain regions (Cagetti et al., 2003), their apparent functional segregation to one subtype of BLA GABAergic input suggests that generalized decreases in GABAA-subunit protein or mRNA expression might have a more localized impact at specific synapses. Because there was no significant exposure-related change in the midazolam modulation at LPC GABAergic synapses, contributions by additional benzodiazepine-sensitive α-subunits might compensate for the loss of α1-containing receptors. The lack of any treatment effect on α2- or α3-total protein expression is consistent with this.
In contrast to the LPC inputs, we found a decrease in midazolam modulation by chronic ethanol exposure/withdrawal at local BLA GABAergic synapses. This suggests increased functional contributions by benzodiazepine-insensitive receptors at these inputs. The GABAA α4-subunit confers a benzodiazepine-insensitive phenotype to the channel, and α4 expression is robustly modulated by chronic ethanol. In the hippocampus, CIE exposure causes a “switch” from benzodiazepine-sensitive GABAA receptors to more insensitive receptors (Liang et al., 2006), and this functional alteration is associated with down-regulation of α1- subunit protein and up-regulation of α4-subunit protein (Cagetti et al., 2003). The decreased midazolam sensitivity of local BLA GABA synapses is likewise consistent with increased contributions by α4-containing receptors. Our biochemical data support this by showing increased levels of plasma membrane-associated α4-subunit protein (as measured by BS3-accessible protein). Whether this represents an increased accumulation of mature, α4-containing protein specifically at synaptic sites or increased maturation rates for newly synthesized α4-subunits is not yet clear. It is also worth noting that there was a trend toward a decrease in zolpidem sensitivity at local GABAergic inputs during CIE that did not reach statistical significance. However, it remains to be established whether longer exposures/withdrawals might spread contributions by benzodiazepine-insensitive subunit contributions to LPC GABAergic synapses or decrease zolpidem-sensitive subunit contributions at local GABAergic inputs.
In addition to these shifts in pharmacological sensitivity, it is worth noting that we found that the decay times of TTX-insensitive spontaneous IPSCs were modestly, but significantly, increased after chronic ethanol exposure/withdrawal. This finding contrasts with previous results in hippocampus showing significantly shorter decay times after CIE exposure (Cagetti et al., 2003). Given the coincidence between altered midazolam sensitivity at local synapses and the changes in mIPSC decay kinetics, α4-containing receptors may contribute to longer decay times measured in the current work. In support of this hypothesis, deactivation times of recombinant receptors after very brief (∼1 ms) applications of saturating GABA concentrations are longer in α4-containing receptors relative to α1-containing GABAA receptors (Lagrange et al., 2007). Unlike the conditions needed to produce receptor desensitization (prolonged exposure to high agonist concentrations), the constraints associated with this brief GABA application are similar to central GABAergic synapses where GABA concentrations are 1.2 to 5 mM and clearance rates are estimated to be <1 ms (Overstreet and Westbrook, 2003; Barberis et al., 2004). We believe the slower mIPSC decay times after ethanol exposure/withdrawal are consistent with slower deactivation caused by increased α4-subunit contributions at the local GABAergic synapses. Given the paucity of pharmacological tools, neither the current study nor previous studies can rule out the possibility that altered mIPSC kinetics may be related to GABAA subunits other than α4.
The precise molecular mechanism for altered functional contributions by zolpidem- and midazolam-sensitive GABAA receptors is not totally clear. However, the increased levels of BLA γ2-subunit and gephyrin protein during CIE and WD may suggest a potential mechanism. γ2-Subunits are important for GABAA receptor clustering at synaptic specializations (Schweizer et al., 2003), apparently via their close association with the postsynaptic density protein gephyrin (Essrich et al., 1998). We also found a significant increase in GABA-RAP expression; but this was transient and found only in the BLA from the CIE slices. GABA-RAP interacts with gephyrin (Kneussel and Betz, 2000) within intracellular compartments and dissociates from GABAA receptors once they are found at the synapse (Kneussel and Betz, 2000). In our system, GABA-RAP might help regulate the exposure-related trafficking of GABAA receptors to and from intracellular pools to the plasma membrane (Kittler et al., 2001). Withdrawal could then consolidate these receptors at synaptic sites in a GABA-RAP-independent fashion.
In the BLA, the GABA and glutamate systems work intimately together to regulate BLA-mediated behaviors. Although glutamatergic function is elevated during CIE (Läck et al., 2007, 2009), both the chronic ethanol-resistant local GABAergic synapses and the continued sensitivity of GABAergic neurotransmission to acute ethanol seem sufficient to offset this elevated glutamatergic function while the animal is intoxicated. During withdrawal, however, the BLA GABA system is markedly suppressed as a result of decreased GABAergic inhibition from LPC synapses and the notable absence of any ethanol in the system. Under these conditions, enhanced glutamatergic function would tend to drive the observed increases in anxiety-like behaviors (Läck et al., 2007). Our study therefore emphasizes the importance of the BLA GABA system and its control over the BLA principal neurons during chronic alcohol and withdrawal.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants R01/=/AA014445, U01/=/AA016671, F31/=/AA017576].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.177121.
ABBREVIATIONS:
- BLA
- lateral/basolateral amygdala
- BS3
- bis(sulfosuccinimidyl)suberate
- CIE
- chronic intermittent ethanol
- GABA-RAP
- GABA receptor-associated protein
- LPC
- lateral paracapsular cell
- IPSC
- inhibitory postsynaptic current
- mIPSC
- miniature IPSC
- WD
- withdrawal
- aCSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- TTX
- tetrodotoxin
- CON
- control
- QX314
- N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide
- TBS-T
- Tris-buffered saline
- CGP55845
- 2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride
- SCH50911
- 2-[(2S)-(+)−5,5-dimethylmorpholin-2-yl]acetic acid.
Authorship Contributions
Participated in research design: Diaz and McCool.
Conducted experiments: Diaz, Christian, and Anderson.
Wrote or contributed to the writing of the manuscript: Diaz, Christian, Anderson, and McCool.
References
- Barberis A, Petrini EM, Cherubini E. (2004) Presynaptic source of quantal size variability at GABAergic synapses in rat hippocampal neurons in culture. Eur J Neurosci 20:1803–1810 [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. (2003) Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63:53–64 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. (2010) Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. (2011) Dopamine D3-like receptors modulate anxiety-like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology doi:10.1038/npp.2010.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci 1:563–571 [DOI] [PubMed] [Google Scholar]
- Falco AM, Bergstrom HC, Bachus SE, Smith RF. (2009) Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav 96:169–173 [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. (2004) Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther 311:1071–1079 [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. (2002) LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci 5:27–33 [DOI] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. (2001) The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci 18:13–25 [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. (2000) Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations. J Physiol 525(Part 1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology 205:529–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. (2008) Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology 55:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Christian DT, Diaz MR, McCool BA. (2009) Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol 43:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. (2007) Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol 98:3185–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. (2007) Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol 578:655–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Paré D. (1998) Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience 83:877–889 [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. (2006) Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci 26:1749–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. (2008) Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther 327:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. (2005) A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48:1025–1037 [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. (2003) Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res 963:165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. (2009) Age matters. Eur J Neurosci 29:997–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. (2006) Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol 494:635–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. (2003) Synapse density regulates independence at unitary inhibitory synapses. J Neurosci 23:2618–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. (1994) The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci 14:2200–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchen W, Proctor WR, Dunwiddie TV. (2000) The in vitro ethanol sensitivity of hippocampal synaptic γ-aminobutyric acid(A) responses differs in lines of mice and rats genetically selected for behavioral sensitivity or insensitivity to ethanol. J Pharmacol Exp Ther 295:741–746 [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA 100:2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. (1995) Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav 52:701–706 [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Lüscher B. (2003) The γ2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci 24:442–450 [DOI] [PubMed] [Google Scholar]
- See RE. (2005) Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol 526:140–146 [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. (2005) The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther 107:80–98 [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. (2010) Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of β3 adrenoceptor activation. Neuropsychopharmacology 35:1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. (2009) Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology 56:886–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. (2008) Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther 324:251–260 [DOI] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. (1997) Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol 77:1306–1312 [DOI] [PubMed] [Google Scholar]
- Wisden W, Gundlach AL, Barnard EA, Seeburg PH, Hunt SP. (1991) Distribution of GABAA receptor subunit mRNAs in rat lumbar spinal cord. Brain Res Mol Brain Res 10:179–183 [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Monyer H, Sah P. (2006) GABAergic excitation in the basolateral amygdala. J Neurosci 26:11881–11887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. (2007) Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci 27:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405 [DOI] [PubMed] [Google Scholar]