Abstract
Sex-determining mechanisms are diverse among animal lineages and can be broadly divided into two major categories: genetic and environmental. In contrast to genetic sex determination (GSD), little is known about the molecular mechanisms underlying environmental sex determination (ESD). The Doublesex (Dsx) genes play an important role in controlling sexual dimorphism in genetic sex-determining organisms such as nematodes, insects, and vertebrates. Here we report the identification of two Dsx genes from Daphnia magna, a freshwater branchiopod crustacean that parthenogenetically produces males in response to environmental cues. One of these genes, designated DapmaDsx1, is responsible for the male trait development when expressed during environmental sex determination. The domain organization of DapmaDsx1 was similar to that of Dsx from insects, which are thought to be the sister group of branchiopod crustaceans. Intriguingly, the molecular basis for sexually dimorphic expression of DapmaDsx1 is different from that of insects. Rather than being regulated sex-specifically at the level of pre–mRNA splicing in the coding region, DapmaDsx1 exhibits sexually dimorphic differences in the abundance of its transcripts. During embryogenesis, expression of DapmaDsx1 was increased only in males and its transcripts were primarily detected in male-specific structures. Knock-down of DapmaDsx1 in male embryos resulted in the production of female traits including ovarian maturation, whereas ectopic expression of DapmaDsx1 in female embryos resulted in the development of male-like phenotypes. Expression patterns of another D. magna Dsx gene, DapmaDsx2, were similar to those of DapmaDsx1, but silencing and overexpression of this gene did not induce any clear phenotypic changes. These results establish DapmaDsx1 as a key regulator of the male phenotype. Our findings reveal how ESD is implemented by selective expression of a fundamental genetic component that is functionally conserved in animals using GSD. We infer that there is an ancient, previously unidentified link between genetic and environmental sex determination.
Author Summary
Sex determination is a fundamental biological process that can be broadly divided into two major categories. In genetic sex determination (GSD), sex-specific differentiation results from intrinsic genetic differences between males and females, whereas environmental sex determination (ESD) relies on environmental signals to induce male or female sex determination. In contrast to model organisms that utilize GSD system, environmental sex-determining organisms are poor genetic models. Therefore, although candidate genes involved in ESD have been found in vertebrates, their functions have remained largely unknown, impairing our understanding of ESD and making the comparison of sex-determining genes between both systems difficult. Here, we report the identification of a gene responsible for the production of males during environmental sex determination in the crustacean Daphnia. This gene is homologous to the Doublesex gene that is functionally conserved in animals that use GSD. Expression of Doublesex was increased primarily in male-specific structures. Gain- and loss-of-function analyses established that Daphnia Doublesex gene is a major effector that regulates the male phenotype in Daphnia. We infer that there is an ancient, previously unidentified link between genetic and environmental sex determination.
Introduction
Sex determination is a fundamental biological process. It affects not only the sexual differentiation of gonads, but also the development of most organs, and leads to sex-specific differences in behavior, physiology and morphology. Organisms have evolved a variety of different sex-determining systems [1], [2] that can be broadly divided phenomenologically into two categories: genetic and environmental [3]. Genetic sex determination (GSD) is attributed to the genetic segregation of genes, often residing on sex chromosomes that initiate alternate sex-determining developmental pathways. Environmental sex determination (ESD) is initiated by environmental cues that presumably trigger alternative genetic signals, which regulate male or female sex-determining genes [4]. Although GSD is a more prevalent system in animals, ESD is also phylogenetically widespread, occurring in such diverse taxa as rotifers, nematodes, crustaceans, insects, fishes, and reptiles [5]. Environmental cues involved in ESD include temperature, photoperiod, nutrition, and population density [5]. Temperature is the most widely studied environmental cue, particularly in the case of reptiles where the temperature at which the egg is incubated determines sex [6]. ESD has arisen repeatedly during evolution [7], which may imply the adaptive significance of this system in environments [8].
It has long been suggested that selection forces drove the transition between GSD and ESD [9], [10]. Previous experiments using a temperature-sensitive mutation created artificially in Drosophila melanogaster and Caenorhabditis elegans also demonstrated how GSD could rapidly evolve into ESD as a consequence of a mutation in a single control gene [11], [12]. In addition, orthologs of some genes involved in GSD have been examined in species that use ESD, especially in temperature-dependent sex-determining reptiles [13]. Some of those are expressed in the gonads during the temperature-sensitive period [13]. These observations have led to the hypothesis that both ESD and GSD should have the same origin and share similar genetic components in their sex-determining pathways [4]. However, a complete functional analysis of temperature dependent ESD has not yet been performed. Therefore, analyzing function of genes involved in ESD and unraveling the sex-determining pathways are crucial to understand the origin and evolution of sex-determining pathway.
The water flea Daphnia magna is a branchiopod crustacean, which is a common inhabitant in fresh water ponds in Europe and Asia. D. magna is known to switch between parthenogenetic and sexual reproduction when environmental quality declines [14]. Normal, healthy populations are entirely female. However, shortened photoperiod, a lack of food and/or increased population density, lead to the clonal production of males that are genetically identical to their sisters and mothers. First instar male juveniles are easily distinguished from the females by their elongated first antennae [15]. During maturation, daphnids undergo morphological sexual differentiation of various somatic tissues including the first thoracic leg that is armed with the copulatory hook in males, which becomes larger in the fifth instar [16]. Gonads develop and finally settle at both sides of the gut during embryogenesis in both males and females [17]. It has been reported that the gonads exhibit morphological sex differences in the first instar juveniles [18], [19]. The appearance of males allows sexual reproduction to occur [20], [21] when females begin producing haploid eggs requiring fertilization.
Recently, we and others have shown that juvenile hormone analogs (JHA) induce male production in cladoceran crustaceans without environmental cues [22], [23]. Interestingly, exposure of D. magna to JHA at the stage corresponding to the environmentally-sensitive period for the other cladoceran Moina sex determination [24], reliably produces exclusively male broods, suggesting that juvenile hormone could be a key molecule for understanding environmental sex determination [22], [25], [26]. Together with the growing genome and transcriptome resources for Daphnia [27]-[30], this system is ideal to study genes responsible for ESD.
Mechanisms underlying genetic sex-determining pathways have been extensively studied in model organisms such as D. melanogaster, C. elegans and mouse and key genes have been identified [31]-[33]. A Doublesex (Dsx) gene was originally identified in D. melanogaster as a critical transcription factor considered to be at the end of sex determination cascades in GSD, that directly targets genes conferring sexually dimorphic traits [34]. Dsx contains two conserved domains: one is the Dsx/Mab-3 (DM) domain at the N-terminus that is evolutionarily conserved even in vertebrates [35] and another is the oligomerization domain at the C-terminus [36]. Genes encoding DM-domain (DM-domain genes) were discovered to play a related role not only in C. elegans [37], [38], but even in vertebrates [39]. In contrast, results from numerous studies have shown that other genetic sex-determining genes are widely diverse among species [1], [2], [40].
To understand the molecular and evolutionary relationships between GSD and ESD, we analyzed the function of two Dsx genes from D. magna using gene manipulations that we have developed [41]. We provide evidence that one of the homologs, termed DapmaDsx1, plays an important role in directing the major sexually dimorphic development of D. magna. Intriguingly, the function of Dsx is significantly conserved between Daphnia and genetic sex-determining insect species, which are thought to be the sister group of branchiopod crustaceans [42]; however, the factors that regulate Dsx gene expression are independently co-opted in each lineage. Our functional demonstration that Dsx controls sexual dimorphism in an environmental sex-determining organism supports the hypothesis that genetic and environmental sex determination are similar at their most fundamental level.
Results
Molecular Cloning of Two Doublesex Genes from Daphnia
In an effort to understand environmental sex determination, we previously identified three DM-domain genes from D. magna and showed that two of the three DM-domain genes have sex dimorphic gene expression pattern in adult gonads [43]. However, none of these DM-domain genes exhibited sexually dimorphic expression patterns during embryonic development, suggesting that they are not involved in sex determination (Figure S1). This result prompted us to search for other DM-domain genes that might be involved in Daphnia sex determination. Two additional DM-domain genes were found in the D. magna EST database [27] and we cloned and sequenced cDNAs encoding each.
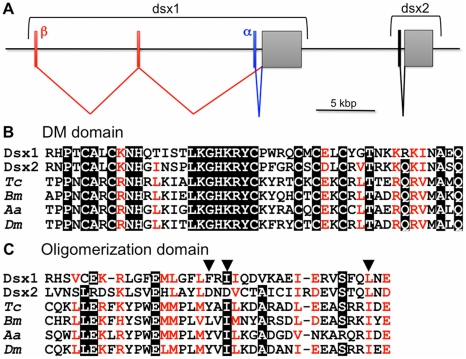
These newly identified DM-domain genes showed greater sequence similarity at the amino acid sequence level to known insect Dsx genes than to the previously identified Dsx-related genes. Therefore, these were designated Daphnia magna Dsx (DapmaDsx). One of the DapmaDsx genes (DapmaDsx1) encodes a protein of 330 amino acids from two mRNAs (DapmaDsx1-α and DapmaDsx1-β) that differ only in their 5'UTR (Figure 1A and Figure S2). The other (DapmaDsx2) encodes a protein of 314 amino acids (Figure S3). The predicted protein products of these two genes share 38% overall amino acid identity and both contain a DM-domain (Figure 1B). In addition, both genes also have an oligomerization domain that is characteristic for insect Dsx homologs (Figure 1C). Dimerization, which enhances specific DNA binding, is mediated by an extensive non-polar interface conserved within oligomerization domain. Importantly, in DapmaDsx2, two of three non-polar amino acids important in formation of the interface are substituted with the acidic amino acid, aspartic acid (Figure 1C). Phylogenetic analysis of these DM-domain genes confirmed that Daphnia Dsx genes are most closely related to the insect Dsx genes, but that the two Daphnia genes are paralogs that duplicated, forming a tandem gene cluster after the divergence of insects and crustaceans (Figure 1A and Figure S4).
Figure 1. Daphnia Dsx genes.
(A) Genomic organization of two dsx genes in Daphnia magna. Coding exons encoding Dsx1 and Dsx2 are indicated as grey boxes, respectively. Exons comprising DSX1-α, DSX1-β and DSX2 5'UTR are indicated as blue, red and black bars. (B, C) Alignment of deduced amino acid sequences of DM domains and oligomerization domains of dsx genes. Amino acid sequences were aligned using CLUSTAL-W. Identical amino acids are highlighted in black. Similar amino acids are shown in red. Positions of non-polar amino acids important in formation of the hydrophobic interface between oligomerization domains in Drosophila Dsx protein were indicated with solid triangles [36]. Tc, Tribolium castaneum: Bm, Bombyx mori; Aa, Aedes aegypti; Dm, Drosophila melanogaster.
Sexually Dimorphic Expression of Daphnia Dsx
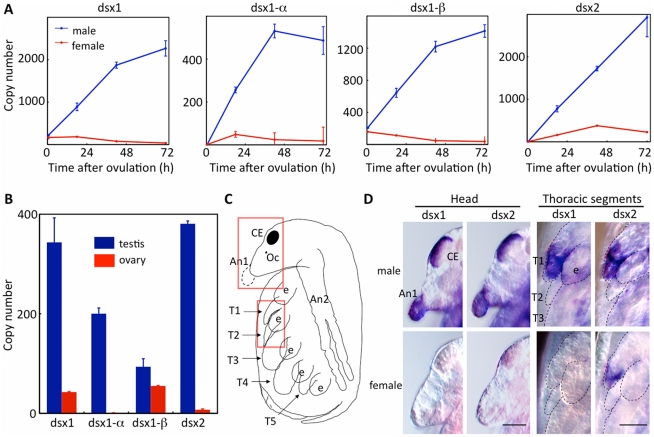
We next examined expression levels of DapmaDsx genes during development by quantitative real time PCR. Expression of both DapmaDsx1 and DapmaDsx2 genes increased over 72 h exclusively in male embryos (Figure 2A, dsx1, dsx2). This temporal expression correlates well with the development of sexually dimorphic organs (e.g., gonads and first antennae), in which morphological sex differences are observed at 72 h after ovulation when hatchlings begin to swim out from the brood chamber. This temporal expression pattern suggests that one or both of the Daphnia Dsx genes might play a critical role in sexual differentiation. To examine whether the two different DapmaDsx1 promoters are used in a temporally independent manner, the expression levels of DapmaDsx1-α and DapmaDsx1-β mRNAs were evaluated. Both mRNAs increased only in males during early development, suggesting that both promoters function primarily during male development (Figure 2A, dsx1-α). DapmaDsx1-β mRNA could be detected immediately post-ovulation in males and females (Figure 2A, dsx1-β), suggesting that maternal DapmaDsx1-β mRNA is transferred to ovulated eggs.
Figure 2. Temporal and spatial dimorphic gene expression of Dsx genes during development.
(A) Male and female embryos were obtained and gene expression levels of Dsx1 and Dsx2 were determined at 18 h, 42 h and 72 h after ovulation by quantitative RT-PCR using primers corresponding to Dsx1 and Dsx2 coding sequence (CDS). Dsx1-α and DSX1-β transcripts were also quantified using primers specific to each 5'UTR. Copy numbers were estimated by quantification compared with an external standard and dividing by the number of embryos used. Bars indicate S.E.M. (B) Expression of dsx genes in adult gonads was quantified. PCR primers corresponding to Dsx1 CDS, two types of 5'UTRs of Dsx1 gene and Dsx2 CDS were used for quantitative PCR. Bars indicate S.E.M. (C) Schematic illustration of D. magna late embryo. The red boxes indicate the areas shown in panel D. (D) Whole mount in situ hybridization in late embryos using DIG-labeled probes corresponding to Dsx1 and Dsx2. The heads and the thoracic segments are magnified. Bars indicate 50 µm (heads) and 100 µm (thoracic segments). CE: Compound eye, Oc: Ocellus, An1: First antennae, An2: Second antennae, e: Epipod, T1-5: First to fifth thoracic segments.
We next examined whether DapmaDsx genes are expressed in male specific structures. Both DapmaDsx1 and DapmaDsx2 genes were highly expressed in the testis (Figure 2B). Whereas DapmaDsx1-α mRNA was expressed exclusively in the testis, DapmaDsx1-β mRNA was expressed in both testis and ovary. Whole mount in situ hybridization showed that DapmaDsx1 and DapmaDsx2 could both be detected in the first antennae and the first thoracic segments (Figure 2C and 2D), both of which are known to show sexually dimorphic characteristics. DapmaDsx2 is also expressed in female first thoracic segments though apparently more weakly than in males. Male-specific expression was also observed in the compound eye, whose sex difference has not been reported in this species to date. Taken together, these male specific expression patterns are regulated temporally and spatially, supporting strongly the involvement of DapmaDsx genes in male differentiation in Daphnia.
Daphnia Dsx1 Is Necessary for Male Trait Development
The sexually dimorphic expression of DapmaDsx mRNAs led us to hypothesize that the expression level of the DapmaDsx transcripts could mediate sex determination. To test this hypothesis, we established a technique to introduce exogenous genes into ovulated eggs and developed a dsRNA-based gene knockdown technique for D. magna [41]. Eggs induced to become males by fenoxycarb exposure to the mother during a critical stage of oocyte development [22], [26] were injected with Dsx-specific, or control dsRNAs, grown to the swimming juveniles and evaluated the phenotypes.
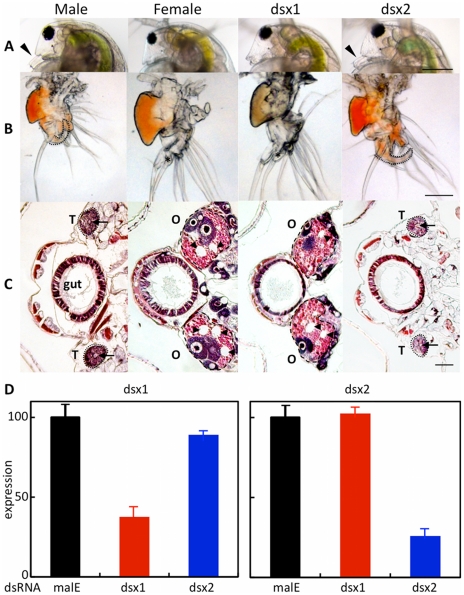
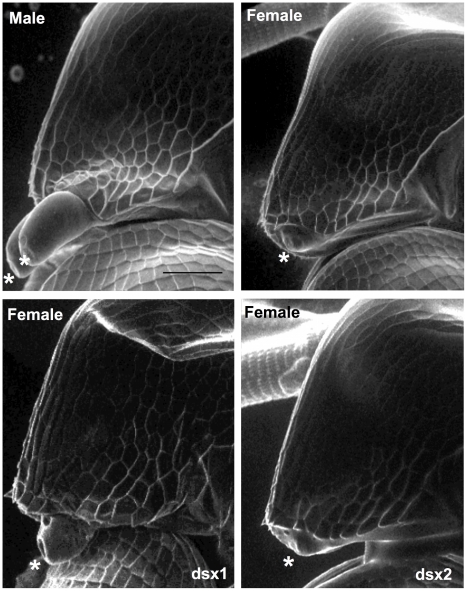
At the third instar stage, microinjection of the DapmaDsx1-specific dsRNAs resulted in development of the shortened first antenna whose length was the same as that of females in all of the DapmaDsx1-dsRNA-injected juveniles (Figure 3A). At the fifth instar stage, we dissected the feminized daphnids and found that the first thoracic appendage lacks a hook used in clasping the females and has a female-like long filament instead (Figure 3B). Correspondingly, repression of DapmaDsx1 resulted in the development of ovaries during which oocytes accumulate yolk granules and lipid droplets as well as those of wild-type females (Figure 3C). In contrast, microinjection of DapmaDsx2-dsRNAs did not induce the formation of either female-like somatic or gonadal tissues (Figure 3A–3C, Table 1).
Figure 3. Dimorphic development of Daphnia magna.
Eggs induced to become males were obtained from D. magna. After injection of the synthesized dsRNA, sexually dimorphic phenotypes were examined at the fifth or sixth instar except first antennae (third instar). The first two columns represent normal male and female phenotypes, respectively. The third and fourth columns represent phenotypes of individuals injected with #1-dsRNA of dsx1 and dsx2, respectively. (A): Lateral view of the head. Arrowheads indicate the first antennae. (B): First thoracic limb. Dotted line shows the outline of the stout chitinized hook. A female-type long filament corresponding to the hook is labeled with an asterisk. (C): Gonad. Daphnids were embedded in paraffin and sectioned, following by standard hematoxylin and eosin staining. Dorsal is left, ventral is right. Dotted circled lines show gonads at both sides of a gut. T and O indicate testis and ovary, respectively. Arrowheads indicate large lipid droplets lying among the eosinophilic yolk granules. Arrows indicate lumens into which the mature spermatozoa are released. (D): Gene expression profile of Dsx1 and Dsx2 in embryos injected with dsRNA of Dsx1 (left panel) and in dsRNA of Dsx2 (right panel). The MalE gene from E. coli was used as a control gene. Bars in (A), (B) and (C) indicate 200, 100, 50 µm, respectively.
Table 1. Summary of RNA interference using dsRNA.
| dsRNA | Sex | Short first antennae | Ovarian development | First leg with a hook |
| malE* | ♂ | 0% (0/40) | 0% (0/31) | 100% (13/13) |
| ♀ | 100% (45/45) | 100% (28/28) | 0% (17/17) | |
| dsx1-#1 | ♂ | 100% (48/48)** | 98% (47/48)** | 0% (13/13)** |
| dsx1-#2 | ♂ | 100% (39/39)** | 41% (7/17)** | 0% (17/17)** |
| dsx2-#1 | ♂ | 0% (0/36) | 0% (0/36) | 100% (15/15) |
| dsx2-#2 | ♂ | 0% (0/45) | 0% (0/24) | 100% (18/18) |
*malE gene from E. coli was used as control.
**P<0.05 versus malE (Fisher's exact probability test).
The quantity of DapmaDsx1 and DapmaDsx2 mRNA was decreased to 40% and 20%, respectively, relative to control-dsRNA-injected embryos. Reduction of transcripts from either of the two DapmaDsx genes did not change the mRNA level of the other (Figure 3D), suggesting that the DapmaDsx genes do not regulate each other's expression or stability. We confirmed the specificity of the knockdown by using non-overlapping dsRNAs for each DapmaDsx gene (Figure S5). As expected, only DapmaDsx1 RNAi induced the same phenotypic changes (Table 1). Since the size of testes appears to be somewhat reduced following the DapmaDsx2-dsRNA injection (Figure 3C), we cannot rule out some low-level requirement for DapmaDsx2 function. However, these results suggest that DapmaDsx1 is necessary for sex determination.
To ask whether expression of DapmaDsx1 or Dsx2 might be sufficient to trigger male development in females, we developed a technique for transient transgenesis in Daphnia embryos by microinjection of capped, polyadenylated mRNAs into ovulated eggs. When the DapmaDsx1 or DapmaDsx2 mRNA was introduced into female eggs, only DapmaDsx1 mRNA partially induced elongation of the first antenna in females at 72 h after microinjection (Figure 4 and Table 2). Unfortunately, due to the transient nature of this method, the masculinization of the first antennae was observed only in the first instar juveniles, whose gonads were too small to evaluate the sex-reversal. Taken together, these data show that although they are similar in amino acid sequence and expression pattern, DapmaDsx1 and not DapmaDsx2 plays a primary role for male trait development.
Figure 4. Elongation of 1st antenna by the expression of Dsx1 gene.
The mRNA was injected to embryos within one hour after ovulation and observed using electron microscope after 72 h. Male and female indicate normal phenotype of each sex. Dsx1 and Dsx2 indicate mRNA of Dsx1 and Dsx2, which were injected to female eggs, respectively. Asterisk indicates first antennae. Bar indicates 100 µm.
Table 2. Summary of effects caused by ectopic expression of Dsx genes in female embryos.
| mRNA | Amounts (pg) | Eggs injected | Juveniles | Viability | Long first antennae |
| dsx1-α | 1000 | 128 | 81 | 63% | 21% (17/81)* |
| dsx1-β | 1000 | 124 | 98 | 79% | 8% (8/98)* |
| dsx2 | 500 | 94 | 54 | 58% | 0% (0/54) |
| egfp | 1000 | 60 | 49 | 82% | 0% (0/49) |
Equal molar amounts of dsx1-α, dsx1-β, and dsx2 mRNA were injected. egfp was used as control. * P<0.05 versus egfp (Fisher's exact probability test).
Daphnia Dsx1 Gene Lacks the Phylogenetically Conserved Splicing Variants
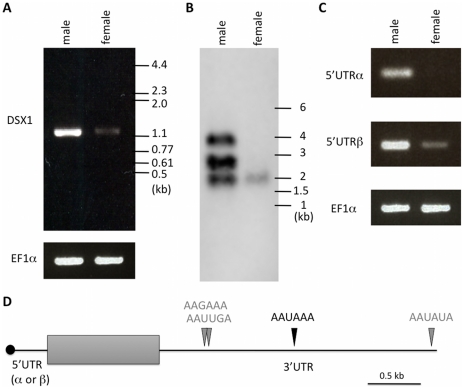
Recent phylogenetic analyses [44]-[46] and developmental genetics [47], [48] suggest that insects may be a sister group to branchiopod crustaceans, a group that includes daphnids (water fleas) and brine shrimp Artemia [42]. As it is known that insect Dsx genes express sex-specific variants in their coding sequences, we next examined whether the sex-specific splicing of the Daphnia Dsx1 gene also occurs. Only a single amplified cDNA could be detected from either male or female by RT-PCR with primers to amplify a coding sequence, although PCR products obtained from females were very faint (Figure 5A). We sequenced the cDNA fragments and confirmed no sex differences of the fragments.
Figure 5. Structure of Dsx1 mRNAs expressed in males and females.
(A) RT-PCR using oligonucleotides corresponding to 5'- and 3'-ends of Dsx1 CDS. The amplified cDNAs were resolved by agarose gel electrophoresis. (B) A northern blot probed for dsx1 mRNAs. Migration of markers with lengths indicated (kb) is shown at the right. (C) RT-PCR using oligonucleotides to amplify 5'UTR-α and -β of Dsx1 gene. The amplified cDNAs were resolved by agarose gel electrophoresis. (D) Schematic illustration of Dsx1 mRNAs with alternative isoforms due to usage of alternative promoters and polyadenylation signals. A grey box shows protein coding region; black line represents untranslated regions. Canonical and non-canonical functional polyadenylation signals identified by 3'RACE are indicated with black and grey arrowheads.
Further, to find altered DapmaDsx1 mRNA length and abundance, we used a northern blot with an antisense probe hybridized to the coding region. In male adults, three transcripts of approximately 4 kb, 2.8 kb, and 2 kb were detected. Of the three, 2.8 kb transcripts were the most abundant. In contrast, only faint 2 kb transcripts were detected in female adults (Figure 5B). As length differences between 5'UTR-α and -β were only 112 bases, one explanation for large differences of the transcript lengths might be the 3'UTR lengths. To determine the 3'end of DapmaDsx1 mRNAs, 3'RACE was performed. We identified four tandem polyadenylation sites located downstream of the stop codon and found that alternative usage of those sites was correlated well with production of the three DapmaDsx1 transcripts. The canonical AAUAAA signal was used for 2.8 kb transcripts, the variant AAUAUA for the 4-kb, and for the shortest 2 kb transcripts the two variant signals were used (AAGAAA or AAUUGA, Figure 5D, Figure S2). Interestingly, RT-PCR analysis with primers to discriminate between 5'UTR-α and -β showed that female 2 kb transcripts were only DapmaDsx1-β mRNAs with the shortest 3'UTR that presumably were supplied to eggs as maternal mRNAs (Figure 5C).
Discussion
We report here that gain- and loss-of-function analyses in environmental sex-determining Daphnia have allowed us to characterize the function of DapmaDsx1 in sexual dimorphism and provide insight into the molecular relationship between GSD and ESD. We and others previously showed that exposure to juvenile hormone analogs reliably produces male Daphnia [25], [49]. This finding enabled us to examine genes in embryos directed to either male or female. Together with the growing genome and transcriptome resources and gene manipulation techniques for Daphnia [27]-[30], , this species is a first crustacean model that provides novel insights into understanding evolution of the sex-determining pathway.
The molecular mechanisms of genetic sex determination have been well studied in a few model organisms, such as the mouse, fruit fly and nematode. DM-domain genes are highly conserved and involved in sexual differentiation of these species [38]. The DM-domain gene was also identified as a sex-determining gene in some populations of fish of the genus Medaka [51]. Moreover, recently, molecular analyses of GSD in the frog Xenopus laevis [52] and chicken Gallus gallus [53] demonstrate deep conservation of DM-domain genes in GSD. To our knowledge, this study is the first in vivo demonstration that the Dsx gene, a fundamental genetic component that is functionally conserved in animals using GSD, can also implement ESD. Interestingly, in reptiles with temperature-dependent sex determination, the Dsx ortholog, Dmrt1 is regulated by temperature [54]-[56]. These indicate that DM-domain genes also play an important role in environmental sex determining organisms, supporting the hypothesis that both ESD and GSD have the same origin and share similar genetic components in their sex determining pathways.
Crustacean and Insect Doublesex Share Molecular Characteristics to Function as a Major Effector of Sexual Dimorphism
DapmaDsx1, one of two Dsx homologs from Daphnia, shares several important characteristic features of Drosophila Dsx protein to function as a major effector of sexual differentiation [40], [57], [58]. First, DapmaDsx1 protein is composed of two domains, the phylogenetically conserved DM-domain [35] and the insect-specific oligomerization domain. In contrast, the DapmaDsx2 protein appears to be unable to regulate sexual differentiation and contains mutations at important amino acids of oligomerization domain [36], suggesting that this domain might be necessary for establishing sexual dimorphic traits. Second, male-specific expression of DapmaDsx1 is regulated temporally and spatially during development. Although it has long been believed that Drosophila Dsx gene was cell-autonomously expressed in all cells [59], Robinette et al. [60] recently reported that Drosophila also exclusively expressed Dsx in sexually dimorphic tissues and cells. Third, and perhaps most importantly, knock-down of DapmaDsx1 in male embryos resulted in the production of female traits including ovarian maturation whereas ectopic expression of DapmaDsx1 in female embryos resulted in partial masculinization of the first antennae. These results suggest that Dsx gene expression in sexually dimorphic tissues is a key process to induce sexual differentiation in crustacean Daphnia and insects.
Factors Regulating Expression of Doublesex Are Independently Co-Opted during Evolution in Each Lineage
In the fruit fly D. melanogaster, Dsx is spliced in the coding region by the Tra protein in a sex-dependent manner [34]. The female-specific RNA produced by alternative splicing is a functional mediator of Tra activity [61]. The female-specific splice variant of the Tra homologs encodes a functional protein not only in the Mediterranean fruit fly [62] and the house fly [63], but also in the honeybee Apis mellifera [64]. In all insect species studied to date (except the silkworm Bombyx mori) [65], Tra regulates sex-specific splicing of Dsx, which produces different mRNAs and proteins, resulting in sex-specific transcriptional activation and repression [66] (Figure 6). Sex-specific splicing of the Dsx gene by the Tra protein might be ancestral in insects. In contrast, DapmaDsx genes do not encode sex-specific Dsx proteins, but instead exhibit sexually dimorphic differences in the abundance of its transcripts. Interestingly, Daphnia has a homolog of the Tra protein but the D. magna Tra gene does not display any detectable sexually dimorphic differences in expression or splicing patterns [26]. We also performed knock-down of the D. magna Tra gene, but could not find any effect for development of sexually dimorphic traits (data not shown). This is consistent with the apparent lack of a sex-specific splicing in the Dsx1 gene. Although it is not yet clear if juvenile hormone directly activates the transcription of DapmaDsx genes, this remains an interesting possibility for future study. We found several motifs in promoter regions of the Dsx1 and Dsx2 genes, which resemble JH-responsive elements previously reported in D. melanogaster [67] and D. magna [68] (data not shown), suggesting that these motifs possibly function as elements to regulate the JH-dependent gene expression. The detection of unfavorable environmental conditions by Daphnia could be transmitted to the endocrine system, leading to the release of juvenile hormone to convey the environmental signals to sexually dimorphic cells. This would be a simple and elegant type of sex determination cascade. Understanding the molecular nature by which the transcription of the DapmaDsx genes is regulated remains an important future goal that will greatly enhance our understanding of not only sex determination, but also invertebrate hormonal systems.
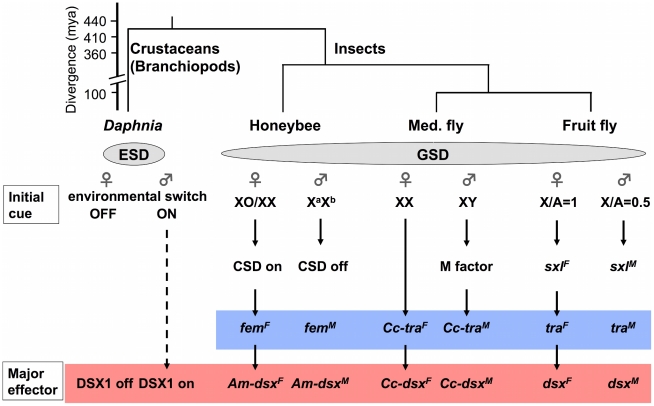
Figure 6. Simplified view of sex-determining pathways in the branchiopod crustacean Daphnia and insects.
An ESD pathway in Daphnia is compared with GSD pathways in insect model species, honeybee (Apis mellifera [64]), Mediterranean fruit fly (Med. fly, Ceratitis capitata, [62]) and fruit fly (Drosophila melanogaster [78]). Conserved Doublesex and Transformer homologs are indicated with red and blue boxes, respectively. Phylogenetic relationships among the four species are shown above the pathways [42], [64], [71]. CSD, complementary sex determiner; fem, feminizer; Am, Apis mellifera: Cc, Ceratits capitata; sxl, sex lethal; mya, million years ago.
Interestingly, expression of the DapmaDsx1 gene utilizes alternative polyadenylation at tandem poly(A) sites, which can yield transcripts that have identical protein-coding sequences but different 3'UTR sequences. Alternative polyadenylation is often associated with tissue- or developmental stage-dependent gene expression [69], [70]. We found that female DapmaDsx1-β mRNAs exclusively use the most promoter-proximal polyadenylation signals. The presence of alternative polyadenylation sites in Dsx genes has been reported in three insects, D. melanogaster, the phorid fly Megaselia scalaris and the mosquito Anopheles gambiae, indicating that regulation of the 3'UTR length might be a common mode to regulate expression of Dsx genes.
Despite having last shared a common ancestor with insects about 400 million years ago [42], [71] and differences of the initial cue to determine sex, the DapmaDsx1 maintained the domain structure essential for establishing sexual dimorphism, while regulation of its expression by other factors became complex and diverse. This is consistent with the prediction that new signals are co-opted upstream of a cascade during the course of evolution [72], [73]. Thus, we have established that there were no boundaries between GSD and ESD in evolution of sex-determining genes at their most fundamental level.
Materials and Methods
Daphnia Strain and Culture Conditions
The Daphnia magna strain (NIES clone) was obtained from the National Institute for Environmental Studies (NIES; Tsukuba, Japan) and maintained as described previously [25]. In order to obtain male embryos, adult D. magna (about 2 weeks of age) were treated with a synthetic juvenile hormone mimic, fenoxycarb (1 µg/L), and eggs ovulated into the brood chamber were collected.
Cloning of DSX-Like Genes
The amino acid sequence of the Drosophila melanogaster Dsx gene was retrieved from NCBI database (http://www.ncbi.nlm.nih.gov/) and used to search the D. magna EST database for related sequences. Two EST sequences were identified to have similarities with the Drosophila Dsx gene. The harvested daphnids were briefly washed and homogenized using the Physcotron NS-310E (Nichion, Tokyo, Japan). Total RNA was extracted with TRIzol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). Poly (A)+ RNA was isolated from purified total RNA using Fast Track (Invitrogen) and converted to cDNA using Superscript III and random primers (both Invitrogen) according to the manufacturer's protocol. cDNAs corresponding to the EST sequences were obtained by PCR, and full length cDNAs were obtained by RACE (Cap Fishing, SeeGene, Seoul, South Korea) using the oligonucleotide sequences shown in Table S1. Sequence data from this article have been deposited with the DDBJ/EMBL/Genbank Data Libraries under Accession No. AB569296, AB569297, AB569298.
Phylogenetic Analysis of the DM Domain Genes
A phylogenetic tree of DM domain genes including newly cloned D. magna Dsx genes was constructed using amino acid sequences of DM-domain genes used in the previous study [43] and insect Dsx genes listed in Table S2. A multiple alignment was constructed using Clustal W [74] with the following settings (pairwise alignment parameters: gap opening penalty 6.00, gap extension penalty 0.21, identity protein weight matrix; multiple alignment parameters: gap opening penalty 10.00, gap extension penalty 0.24, delay divergent cutoff 30%, gap separation distance 4). Phylogenetic reconstruction was performed using the p-distance algorithm and the neighbor-joining method implemented in MEGA version 4 [75]. The phylogenetic tree was rooted to vertebrate DMRT7 outgroups (mouse; NP_082008, bovine; NP_0010332710, data not shown).
Quantitative PCR
Embryos were obtained from D. magna at two weeks of age. Ovulation occurred just after molting and was assigned to be 0 h. The embryos were collected 18 h, 42 h and 72 h after ovulation. Gonads were isolated and specific mRNAs were quantified as described previously [43]. The oligonucleotide sequences for PCR were indicated in Table S3.
Whole Mount In Situ Hybridization
Templates for the probe preparation were synthesized by PCR using gene-specific primers containing the T7 polymerase promoter sequence at their 5'-ends (Table S4). DIG-labeled probes were prepared as described by Butler et al. [76] and subjected to alkaline hydrolysis. Whole mount in situ hybridization was performed as described by Sagawa et al. [17]. Both antisense and sense probes were used to confirm the specificity of staining.
Double-Strand RNA (dsRNA) Preparation
Double-stranded RNA was synthesized using the MEGAscript high yield transcription kit (Ambion, Austin, TX, USA). Templates were prepared by PCR using gene-specific primers with the T7 polymerase promoter sequence at their 5'-ends (Table S5). The synthesized RNAs were purified using phenol/chloroform. Following ethanol precipitation, the RNA was resuspended in DNase/RNase-free distilled water (Invitrogen, Tokyo, Japan) and annealed [41]. Sequences corresponding to each dsRNAs were shown in Figure S2. dsRNA lengths were: Dsx1-#1, 778 bp: Dsx1-#2, 579 bp: Dsx2-#1, 703 bp: Dsx2-#2: 448 bp.
Synthesis of Capped and Polyadenylated mRNAs
DSX1-α, DSX1-β and DSX2 cDNAs were subcloned into pCS2 vector and used for RNA synthesis. To synthesize the control RNA, pEGFP-C1 vector was used. In vitro transcription with T7 RNA polymerase and poly-A tail addition were performed according to the manufacturers' protocol using commercial kits [mMESSAGE mMACHINE, and Poly(A) Tailing kit, respectively, Ambion]. Templates were prepared by PCR using primers corresponding to the 5'- and 3'-ends of the mRNA sequences. The T7 polymerase promoter sequence was attached to the 5' end of the forward primer.
Microinjection
Eggs were obtained from D. magna at two weeks of age just after the ovulation and placed in ice-cold M4 media [77] containing 40 mM sucrose (M4-sucrose). The synthesized dsRNA (1 mg/ml) containing 1 mM Chromeo 494 fluorescent dye (Active Motif Chromeon GmbH, Tegernheim, Germany) or mRNA was injected and incubated in a 96-well plate for appropriate times [41]. Equal molar amounts of DSX1-α, DSX1-β and Dsx2 mRNA were injected for the gain-of-function study. Injection volume was approximately 0.3 nL.
Microscopy
Embryos were dissected off the yolk, and photographed with a Zeiss Axioplan 2 Imaging microscope (Zeiss, Oberkochen, Germany). Adults and juveniles were observed and photographed using a Leica MZ APO dissecting microscope (Leica Microsystems Heidelberg GmbH, Mannheim, Germany). mRNA-injected juveniles were directly observed using an environmental scanning electron microscope (XL30 ESEM; Philips, Hillsboro, OR, USA).
Northern Blot Analysis
3 µg and 10 µg of male and female poly (A)+ RNA were used respectively. The RNAs were separated by electrophoresis on a 1.0% formaldehyde–agarose gel and then transferred to positively charged nylon membranes (Hybond-N+; GE Healthcare, Little Chalfont, England). RNA probes were prepared with a DIG RNA labeling kit (Roche Diagnostic GmbH, Manheim, Germany). Primers to amplify templates for the probe preparation were (5'-3'): forward: AAGAATTGTCCGTGGGGGCAC and reverse: TAATACGACTCACTATAGGGAAAGTTTGGTGTAGGGAG. The membranes were hybridized with DIG-labeled RNA probes for 11 hr at 68 °C with DIG easy hyb (Roche Diagnostic). DIG-labeled RNA was detected with an alkaline phosphatase-conjugated anti-DIG antibody using CDP star (Roche Diagnostic) according to the manufacture's protocol.
Supporting Information
Temporal embryonic gene expression profile of DM-domain genes. Embryonic gene expression levels of dmrt11E, dmrt93B, and dmrt99B in male (blue line) and female (red line) Daphnia at developmental points after ovulation. Copy numbers were estimated by quantification of plasmid DNA for each DM-domain gene and divided by the number of individuals examined. Bars indicate S.E.M.
(3.46 MB TIF)
Nucleotide and deduced amino acid sequences of dsx1 cDNA. Nucleotide sequences of UTR-α (A), UTR-β (B) are indicated. Nucleotide sequences of the common region including ORF and 3'UTR (C) are indicated with deduced amino acid sequence. Sequences corresponding to the #1-dsRNA and #2-dsRNA are highlighted in red and green. Squares indicate polyadenylation signals identified by 3'RACE.
(5.44 MB TIF)
Nucleotide and deduced amino acid sequences of dsx2 cDNA. The deduced amino acid sequence is shown below the nucleotide sequence. Sequences corresponding to the #1-dsRNA and #2-dsRNA are highlighted in red and green. The square indicates the putative polyadenylation signal.
(3.15 MB TIF)
Phylogenetic tree connecting the dsx genes. Branch lengths are proportional to the evolutionary distances. Numbers in each branch represent the bootstrap values obtained by Neighbor-Joining.
(8.92 MB TIF)
Shortened first antenna in males induced by RNAi using #2-dsRNAs of dsx1 and dsx2, respectively. The phenotype was examined at the third instar. Arrowheads indicate the first antenna. Bar indicates 200 µm.
(1.48 MB TIF)
Primer sequences for 5'RACE and 3'RACE.
(0.03 MB DOC)
Accession numbers of insect Dsx genes used for phylogenetic analysis.
(0.03 MB DOC)
Primer sequences for quantitative real-time PCR.
(0.03 MB DOC)
Primer sequences for probe preparation.
(0.03 MB DOC)
Primer sequences for probe preparation.
(0.03 MB DOC)
Acknowledgments
Genomic sequence data were produced by the Center for Genomics and Bioinformatics at Indiana University and distributed via wFleaBase in collaboration with the Daphnia Genomics Consortium, http://daphnia.cgb.indiana.edu. We thank Dr. John Colbourne, the Center for Genomics and Bioinformatics, Indiana University, Indiana, USA; Dr. Bruce Blumberg, University of California, Irvine, California, USA; and Dr. David Zarkower, University of Minnesota, Minneapolis, Minenesota, USA, for their critical readings of this manuscript. We also thank Dr. Yasuhiro Shiga, Tokyo University of Pharmacy and Life Science, Tokyo, Japan, for sharing the in situ hybridization protocol and NIBB Center for Analytical Instruments for XL30 ESEM.
Footnotes
The authors have declared that no competing interests exist.
This study was partly supported through grants from Grants-in-Aid from Ministry of Education, Culture, Sport, Science, and Technology (to HW), grants from Ministry of the Environment of Japan, and a grant of Long-Range Research Initiative (LRI) by Japan Chemical Industry Association (JCIA) (to TI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marin I, Baker BS. The evolutionary dynamics of sex determination. Science. 1998;281:1990–1994. doi: 10.1126/science.281.5385.1990. [DOI] [PubMed] [Google Scholar]
- 2.Zarkower D. Establishing sexual dimorphism: conservation amidst diversity? Nat Rev Genet. 2001;2:175–185. doi: 10.1038/35056032. [DOI] [PubMed] [Google Scholar]
- 3.Bull JJ. Sex determining mechanisms: an evolutionary perspective. Experientia. 1985;41:1285–1296. doi: 10.1007/BF01952071. [DOI] [PubMed] [Google Scholar]
- 4.Crews D, Bull JJ. Mode and tempo in environmental sex determination in vertebrates. Semin Cell Dev Biol. 2009;20:251–255. doi: 10.1016/j.semcdb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Korpelainen H. Sex-Ratios and conditions required for environmental sex determination in animals. Biol Rev Camb Philos Soc. 1990;65:147–184. doi: 10.1111/j.1469-185x.1990.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 6.Bull JJ, Vogt RC. Temperature-dependent sex Determination in turtles. Science. 1979;206:1186–1188. doi: 10.1126/science.505003. [DOI] [PubMed] [Google Scholar]
- 7.Organ CL, Janes DE. Evolution of sex chromosomes in Sauropsida. Integ Comp Biol. 2008;48:512–519. doi: 10.1093/icb/icn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner DA, Shine R. The adaptive significance of temperature-dependent sex determination in a reptile. Nature. 2008;451:566-U565. doi: 10.1038/nature06519. [DOI] [PubMed] [Google Scholar]
- 9.Bull JJ. Evolution of environmental sex determination from genotypic sex determination. Heredity. 1981;47:173–184. [Google Scholar]
- 10.Bulmer MG, Bull JJ. Models of polygenic sex determination and sex-ratio control. Evolution. 1982;36:13–26. doi: 10.1111/j.1558-5646.1982.tb05005.x. [DOI] [PubMed] [Google Scholar]
- 11.Epper F, Bryant PJ. Sex-specific control of growth and differentiation in the Drosophila genital disk, studied using a temperature-sensitive transformer-2 mutation. Dev Biol. 1983;100:294–307. doi: 10.1016/0012-1606(83)90224-5. [DOI] [PubMed] [Google Scholar]
- 12.Hodgkin J. Exploring the envelope: Systematic alteration in the sex-determination system of the nematode Caeraorhabditis elegans. Genetics. 2002;162:767–780. doi: 10.1093/genetics/162.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoemaker CM, Crews D. Analyzing the coordinated gene network underlying temperature-dependent sex determination in reptiles. Semin Cell Dev Biol. 2009;20:293–303. doi: 10.1016/j.semcdb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebert PDN. The population biology of Daphnia (Crustacea, Daphnidae). Biol Rev. 1978;53:387–426. [Google Scholar]
- 15.Olmstead AW, LeBlanc GA. Effects of endocrine-active chemicals on the development of sex characteristics of Daphnia magna. Environ Toxicol Chem. 2000;19:2107–2113. [Google Scholar]
- 16.Mitchell SE. Intersex and male development in Daphnia magna. Hydrobiologia. 2001;442:145–156. [Google Scholar]
- 17.Sagawa K, Yamagata H, Shiga Y. Exploring embryonic germ line development in the water flea, Daphnia magna, by zinc-finger-containing VASA as a marker. Gene Expr Patterns. 2005;5:669–678. doi: 10.1016/j.modgep.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Zaffagnini F. Reproduction in Daphnia. Mem Inst Ital Idrobiol. 1987;45:245–284. [Google Scholar]
- 19.Ojima Y. A cytological study on the development and maturation of the parthenogenetic and sexual eggs of Daphnia pulex (Crustacea–Cladocera). Kwansei Gakuen Univ Ann Stud. 1958;6:123–176. [Google Scholar]
- 20.Stross RG, Hill JC. Diapause induction in Daphnia requires two stimuli. Science. 1965;150:1462–1464. doi: 10.1126/science.150.3702.1462. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PD. Genotypic characteristics of cyclic parthenogens and their obligately asexual derivatives. Experientia Suppl. 1987;55:175–195. doi: 10.1007/978-3-0348-6273-8_8. [DOI] [PubMed] [Google Scholar]
- 22.Olmstead AW, Leblanc GA. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J Exp Zool. 2002;293:736–739. doi: 10.1002/jez.10162. [DOI] [PubMed] [Google Scholar]
- 23.Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T. Production of male neonates in four cladoceran species exposed to a juvenile hormone analog, fenoxycarb. Chemosphere. 2005;60:74–78. doi: 10.1016/j.chemosphere.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 24.Banta AM, Brown LA. Control of sex in cladocera. III. Localization of the critical period for control of sex. Proc Natl Acad Sci U S A. 1929;15:71–81. doi: 10.1073/pnas.15.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T. Juvenile hormone agonists affect the occurrence of male Daphnia. Chemosphere. 2003;53:827–833. doi: 10.1016/S0045-6535(03)00761-6. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Kobayashi K, Oda S, Tatarazako N, Watanabe H, et al. Sequence divergence and expression of a transformer gene in the branchiopod crustacean, Daphnia magna. Genomics. 2010;95:160–165. doi: 10.1016/j.ygeno.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe H, Tatarazako N, Oda S, Nishide H, Uchiyama I, et al. Analysis of expressed sequence tags of the water flea Daphnia magna. Genome. 2005;48:606–609. doi: 10.1139/g05-038. [DOI] [PubMed] [Google Scholar]
- 28.Colbourne JK, Singan VR, Gilbert DG. wFleaBase: The Daphnia genome database. BMC Bioinformatics. 2005;6:45. doi: 10.1186/1471-2105-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw JR, Pfrender ME, Eads BD, Klaper R, Callaghan A, Colson I, Jansen B, Gilbert D, Colbourne JK. Daphnia as an emerging model for toxicological genomics. Adv Exp Biol. 2008;2:165–219. 327-328. [Google Scholar]
- 30.Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, et al. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutt C, Nothiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development. 2000;127:667–677. doi: 10.1242/dev.127.4.667. [DOI] [PubMed] [Google Scholar]
- 32.Zarkower D. Somatic sex determination. WormBook. 2006:1–12. doi: 10.1895/wormbook.1.84.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Burtis KC, Baker BS. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell. 1989;56:997–1010. doi: 10.1016/0092-8674(89)90633-8. [DOI] [PubMed] [Google Scholar]
- 35.Volff JN, Zarkower D, Bardwell VJ, Schartl M. Evolutionary dynamics of the DM domain gene family in metazoans. J Mol Evol. 2003;57(Suppl 1):S241–249. doi: 10.1007/s00239-003-0033-0. [DOI] [PubMed] [Google Scholar]
- 36.Bayrer JR, Zhang W, Weiss MA. Dimerization of doublesex is mediated by a cryptic ubiquitin-associated domain fold - Implications for sex-specific gene regulation. J Biol Chem. 2005;280:32989–32996. doi: 10.1074/jbc.M507990200. [DOI] [PubMed] [Google Scholar]
- 37.Shen MM, Hodgkin J. mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell. 1988;54:1019–1031. doi: 10.1016/0092-8674(88)90117-1. [DOI] [PubMed] [Google Scholar]
- 38.Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, et al. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- 39.Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams TM, Carroll SB. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nature Rev Genet. 2009;10:883–883. doi: 10.1038/nrg2687. [DOI] [PubMed] [Google Scholar]
- 41.Kato Y, Shiga Y, Kobayashi K, Tokishita S, Yamagata H, et al. Dev Genes Evol In press; 2011. Development of an RNA interference method in the cladoceran crustacean Daphnia magna. [DOI] [PubMed] [Google Scholar]
- 42.Glenner H, Thomsen PF, Hebsgaard MB, Sorensen MV, Willerslev E. The origin of insects. Science. 2006;314:1883–1884. doi: 10.1126/science.1129844. [DOI] [PubMed] [Google Scholar]
- 43.Kato Y, Kobayashi K, Oda S, Colbourne JK, Tatarazako N, et al. Molecular cloning and sexually dimorphic expression of DM-domain genes in Daphnia magna. Genomics. 2008;91:94–101. doi: 10.1016/j.ygeno.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Regier JC, Shultz JW. Molecular phylogeny of the major arthropod groups indicates polyphyly of crustaceans and a new hypothesis for the origin of hexapods. Mol Biol Evol. 1997;14:902–913. doi: 10.1093/oxfordjournals.molbev.a025833. [DOI] [PubMed] [Google Scholar]
- 45.Regier JC, Shultz JW, Kambic RE. Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc Biol Sci. 2005;272:395–401. doi: 10.1098/rspb.2004.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mallatt J, Winchell CJ. Ribosomal RNA genes and deuterostome phylogeny revisited: more cyclostomes, elasmobranchs, reptiles, and a brittle star. Mol Phylogenet Evol. 2007;43:1005–1022. doi: 10.1016/j.ympev.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 47.Averof M, Akam M. Hox genes and the diversification of insect and crustacean body plans. Nature. 1995;376:420–423. doi: 10.1038/376420a0. [DOI] [PubMed] [Google Scholar]
- 48.Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385:627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- 49.Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T. Production of male neonates in Daphnia magna (Cladocera, Crustacea) exposed to juvenile hormones and their analogs. Chemosphere. 2005;61:1168–1174. doi: 10.1016/j.chemosphere.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 50.Kato Y, Kobayashi K, Watanabe H, Iguchi T. Introduction of foreign DNA into the water flea, Daphnia magna, by electroporation. Ecotoxicology. 2010;19:589–592. doi: 10.1007/s10646-010-0460-9. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 52.Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci U S A. 2008;105:2469–2474. doi: 10.1073/pnas.0712244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461:267–271. doi: 10.1038/nature08298. [DOI] [PubMed] [Google Scholar]
- 54.Kettlewell JR, Raymond CS, Zarkower D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis. 2000;26:174–178. [PubMed] [Google Scholar]
- 55.Murdock C, Wibbels T. Dmrt1 expression in response to estrogen treatment in a reptile with temperature-dependent sex determination. J Exp Zool B Mol Dev Evol. 2006;306:134–139. doi: 10.1002/jez.b.21076. [DOI] [PubMed] [Google Scholar]
- 56.Shoemaker C, Ramsey M, Queen J, Crews D. Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Dev Dyn. 2007;236:1055–1063. doi: 10.1002/dvdy.21096. [DOI] [PubMed] [Google Scholar]
- 57.Hildreth PE. Doublesex, recessive gene that transforms both males and females of Drosophila into intersexes. Genetics. 1965;51:659–678. doi: 10.1093/genetics/51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliver B, Kim YJ, Baker BS. Sex-lethal, master and slave: a hierarchy of germ-line sex determination in Drosophila. Development. 1993;119:897–908. doi: 10.1242/dev.119.3.897. [DOI] [PubMed] [Google Scholar]
- 59.Baker BS, Ridge KA. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics. 1980;94:383–423. doi: 10.1093/genetics/94.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinett CC, Vaughan AG, Knapp JM, Baker BS. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKeown M, Belote JM, Baker BS. A molecular analysis of transformer, a gene in Drosophila melanogaster that controls female sexual differentiation. Cell. 1987;48:489–499. doi: 10.1016/0092-8674(87)90199-1. [DOI] [PubMed] [Google Scholar]
- 62.Pane A, Salvemini M, Delli Bovi P, Polito C, Saccone G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development. 2002;129:3715–3725. doi: 10.1242/dev.129.15.3715. [DOI] [PubMed] [Google Scholar]
- 63.Hediger M, Henggeler C, Meier N, Perez R, Saccone G, et al. Molecular characterization of the key switch F provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics. 2010;184:155–170. doi: 10.1534/genetics.109.109249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gempe T, Hasselmann M, Schiott M, Hause G, Otte M, et al. Sex determination in honeybees: Two separate mechanisms induce and maintain the female pathway. Plos Biol. 2009;7:e1000222. doi: 10.1371/journal.pbio.1000222. doi: 10.1371/journal.pbio.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujii T, Shimada T. Sex determination in the silkworm, Bombyx mori: A female determinant on the W chromosome and the sex-determining gene cascade. Semin Cell Dev Biol. 2007;18:379–388. doi: 10.1016/j.semcdb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 66.Verhulst EC, van de Zande L, Beukeboom LW. Insect sex determination: it all evolves around transformer. Curr Opin Genet Dev. 2010;20:376–383. doi: 10.1016/j.gde.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem. 2007;282:37605–37617. doi: 10.1074/jbc.M704595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorr TA, Rider CV, Wang HY, Olmstead AW, LeBlanc GA. A candidate juvenoid hormone receptor cis-element in the Daphnia magna hb2 hemoglobin gene promoter. Mol Cell Endocrinol. 2006;247:91–102. doi: 10.1016/j.mce.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 69.Hughes TA. Regulation of gene expression by alternative untranslated regions. Trends in Genetics. 2006;22:119–122. doi: 10.1016/j.tig.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Ji Z, Lee JY, Pan ZH, Jiang BJ, Tian B. Progressive lengthening of 3' untranslated regions of mRNAs by alternative polyadenylation during mouse embryonic development. Proc Natl Acad Sci U S A. 2009;106:7028–7033. doi: 10.1073/pnas.0900028106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- 72.Wilkins AS. Moving up the hierarchy - a hypothesis on the evolution of a genetic sex determination pathway. Bioessays. 1995;17:71–77. doi: 10.1002/bies.950170113. [DOI] [PubMed] [Google Scholar]
- 73.Nothiger R, Steinmannzwicky M. A single principle for sex determination in insects. Cold Spring Harb Symp Quant Biol. 1985;50:615–621. doi: 10.1101/sqb.1985.050.01.074. [DOI] [PubMed] [Google Scholar]
- 74.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 76.Butler K, Zorn AM, Gurdon JB. Nonradioactive in situ hybridization to Xenopus tissue sections. Methods. 2001;23:303–312. doi: 10.1006/meth.2000.1142. [DOI] [PubMed] [Google Scholar]
- 77.Elendt BP, Bias WR. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing. Effects of the optimization of culture conditions on life history parameters of D. magna. Water Res. 1990;24:1157–1167. [Google Scholar]
- 78.Pomiankowski A, Nothiger R, Wilkins A. The evolution of the Drosophila sex-determination pathway. Genetics. 2004;166:1761–1773. doi: 10.1534/genetics.166.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Temporal embryonic gene expression profile of DM-domain genes. Embryonic gene expression levels of dmrt11E, dmrt93B, and dmrt99B in male (blue line) and female (red line) Daphnia at developmental points after ovulation. Copy numbers were estimated by quantification of plasmid DNA for each DM-domain gene and divided by the number of individuals examined. Bars indicate S.E.M.
(3.46 MB TIF)
Nucleotide and deduced amino acid sequences of dsx1 cDNA. Nucleotide sequences of UTR-α (A), UTR-β (B) are indicated. Nucleotide sequences of the common region including ORF and 3'UTR (C) are indicated with deduced amino acid sequence. Sequences corresponding to the #1-dsRNA and #2-dsRNA are highlighted in red and green. Squares indicate polyadenylation signals identified by 3'RACE.
(5.44 MB TIF)
Nucleotide and deduced amino acid sequences of dsx2 cDNA. The deduced amino acid sequence is shown below the nucleotide sequence. Sequences corresponding to the #1-dsRNA and #2-dsRNA are highlighted in red and green. The square indicates the putative polyadenylation signal.
(3.15 MB TIF)
Phylogenetic tree connecting the dsx genes. Branch lengths are proportional to the evolutionary distances. Numbers in each branch represent the bootstrap values obtained by Neighbor-Joining.
(8.92 MB TIF)
Shortened first antenna in males induced by RNAi using #2-dsRNAs of dsx1 and dsx2, respectively. The phenotype was examined at the third instar. Arrowheads indicate the first antenna. Bar indicates 200 µm.
(1.48 MB TIF)
Primer sequences for 5'RACE and 3'RACE.
(0.03 MB DOC)
Accession numbers of insect Dsx genes used for phylogenetic analysis.
(0.03 MB DOC)
Primer sequences for quantitative real-time PCR.
(0.03 MB DOC)
Primer sequences for probe preparation.
(0.03 MB DOC)
Primer sequences for probe preparation.
(0.03 MB DOC)