It is well known that atherosclerosis and restenosis are common cardiovascular diseases (CVDs) and major health care problems. Vascular remodeling along with migration and proliferation of vascular smooth muscle cells (VSMC) are key features of these pathologies. There has been enormous progress in drug development and clinical management of these disorders, with angioplasty and drug eluting stents being standard procedures to treat vascular obstruction. However, despite their benefits, these current treatment modalities are not always efficacious. Furthermore, they can also be associated with post operative complications and graft failures, some of which can be life threatening. Evaluation of newer mechanisms involved in VSMC proliferation aimed at uncovering additional therapeutic approaches to curb VSMC dysfunction in cardiovascular diseases is clearly warranted.
Accumulating evidence suggests that several common diseases, including cardiovascular disorders, diabetes and its vascular complications, are governed by a combination of genetic and environmental factors and that epigenetic mechanisms such as DNA methylation and histone modifications in chromatin form a key link between them (1–3). Epigenetics is the added layer of gene regulation that occurs in chromatin without changes in the actual underlying DNA sequence and plays a major role in dictating cell-specific gene expression patterns and transcriptional outcomes (4,5). Along with DNA methylation, key post-translational modifications (PTMs) of histone N-terminal tails can alter chromatin structure to form an added layer of gene regulation and modulate gene transcription (5). Therefore gene transcription depends upon chromatin structure that is very dynamic depending on a multitude of histone PTMs that allow for the conversion of inaccessible, compact or repressive heterochromatin to the accessible or active euchromatin state of DNA. PTMs that occur on histone tails include acetylation, methylation, phosphorylation and ubiquitylation (5,6). Since these epigenetic mechanisms can regulate the expression of genes involved in VSMC proliferation, migration, inflammation and matrix protein synthesis (3, 7–15), they present an exciting window of opportunity for therapeutic intervention.
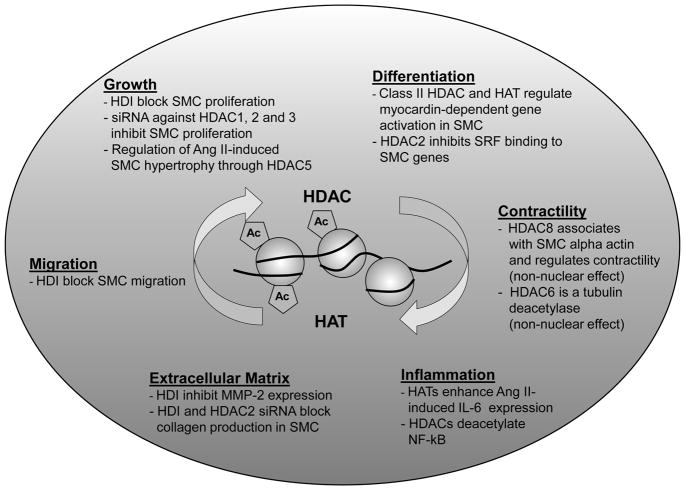
One of the best studied chromatin histone PTM is lysine acetylation mediated by histone acetyltransferases (HATs) and generally associated with gene activation. Histone deacetylases (HDACs), on the other hand, mediate the removal of lysine acetylation (8,16). While histone lysine acetylation enables a more relaxed or open chromatin structure allowing for transcription factor and RNA polymerase II recruitment permissible for transcription, HDACs are found to be components of repressor complexes or to be involved in various signaling pathways (5,16). Overall, histone acetylation can occur quite rapidly and is amenable to modulation by HDAC inhibitors. This is demonstrated by the dynamic balance between HATs and HDACs in regulating or fine-tuning cellular gene expression. This property of HDACs has been greatly exploited in the field of cancer where HDAC inhibitors have been successfully used to reactivate tumor suppressor genes and thereby inhibit the proliferation of cancer cells (8,17). This has triggered a flurry of research in other proliferative diseases such as atherosclerosis and restenosis (8). It is therefore important to determine how HATs and HDACs regulate the transcription of VSMC genes related to proliferation, migration and matrix deposition, which are the specific HDACs involved, and whether inhibitors of these HDACs can have protective effects. In this connection, there have been several reports demonstrating that key HATs and HDACs can regulate the expression of inflammatory and other genes involved in VSMC contractility, VSMC differentiation, migration, proliferation, inflammation, matrix deposition and Angiotensin II effects (3, 8–15) (Fig. 1). However, there is a clear paucity of in vivo data. Hence evaluation of HDAC inhibitors in models of VSMC injury is clearly needed.
Figure 1.
Major cellular processes in vascular smooth muscle cells that are regulated by histone acetylation/deacetylation.
SMC smooth muscle cells; HDAC Histone deacetylase; HAT Histone acetyltransferase; HDI Histone deacetylase inhibitor; Ang II Angiotensin II; MMP-2 Matrix metalloproteinase 2; IL-6 Interleukin 6
In this issue of ATVB, Findeisen et.al. (18) used a non-selective HDAC inhibitor, Scriptaid, to elegantly demonstrate its protective effects in vitro and in vivo in a mouse model of neointimal thickening. They demonstrate that mitogens induce the transcriptional upregulation of HDACs 1–3 in rat VSMC. Knockdown of these three HDACs with siRNAs or pharmacological inhibition of HDAC could prevent platelet-derived growth factor-induced VSMC proliferation. Additional mechanistic studies demonstrated that HDAC inhibition could lead to cell cycle arrest due to an inhibition of retinoblastoma protein phosphorylation, although the upregulation of the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip was relatively modest compared to the profound inhibition of VSMC proliferation. Importantly, they demonstrate that mitogen-induced cyclin D1 expression was downregulated by HDAC inhibition despite an increase in cyclin D1 promoter histone lysine-9 acetylation. In vivo relevance was obtained by demonstrating that HDAC inhibitors decrease neointimal thickening and cyclin D1 expression in a mouse model. These data confirm that HDAC is a critical component of the transcriptional machinery controlling the expression of key genes regulating VSMC proliferation (Fig. 1) and provide new insights into the cellular mechanisms involved . They also demonstrate the therapeutic efficacy of Scriptaid, a HDAC inhibitor that seems to work without toxicity at the doses used.
These studies are important for several reasons. Given that cyclin D1 overexpression is well known to promote VSMC proliferation, the observation that Scriptaid blocks its expression provides mechanistic information. While HDAC inhibitors intuitively are useful in cancer to reactivate protective tumor suppressor genes, a parallel mechanism in VSMC is not fully clear. Hence the fact that HDAC inhibitors decreased the expression of genes such as cyclin D1 in this study is interesting, given that an increase in cellular acetylation by HDAC inhibition would be expected to increase gene expression in general. However, as nicely discussed by the authors, it is now clear that HDACs have multiple cellular effects and also affect signal transduction parameters to interrupt the expression of specific genes. Moreover, HDACs exist in two different classes, with HDACs 1-3 and 8 belonging to Class I, and HDACs 4–7, 9 and 10 to Class II, and have subtle differences in cellular actions (8) due to their interactions with specific chromatin factors and repressor molecules. The authors used siRNAs to demonstrate that Class I HDACs 1–3 may be mediating VSMC proliferation, albeit via as yet unknown mechanisms. However they have not shown whether Scriptaid specifically blocks HDAC1-3 or also other HDACs, and whether Scriptaid actions can be attributed to more pleiotrophic effects. It should be noted that HATs/HDACs also have non-nuclear substrates. For example, HDAC8 has been shown to associate with smooth muscle alpha actin to regulate SMC contractility, and the class II HDAC, HDAC6, is a tubulin deacetylase (8, 12). HDACs can also deacetylate non-histone proteins such as p53 and NF-κB (8) and hence not all of their actions are at the level of chromatin. HDACs may also co-operate with other epigenetic chromatin factors like histone methylases and demethylases. In addition, since HATs have been shown to enhance inflammatory gene expression in VSMC (13), it is unclear if HDAC inhibitors may augment inflammation in vivo. Notwithstanding, the studies by Findeisen et al (18) provide additional impetus to explore the effect of HDAC inhibitors, including isoform specific ones, for CVDs associated with VSMC proliferation and migration, given the multiple functions of histone acetylation/de-acetylation in VSMC (Fig. 1). Specific HDAC inhibitors are already showing promise in the treatment of malignant disorders. Unlike the DNA code, the epigenetic code is reversible, and therefore amenable to therapeutic intervention. The hope is that such targeted epigenetic therapies can be used alone or in combination with other currently used drugs as more efficient treatment options for various CVDs.
Acknowledgments
Sources of Funding:
Dr. Natarajan’s laboratory is supported by funds from the National Institutes of Health (NHLBI and NIDDK) and the Juvenile Diabetes Research Foundation.
Footnotes
Disclosures: None
References
- 1.Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–2725. doi: 10.2337/db09-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portela A, Esteller M. Epigenetic Modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 3.Villeneuve LM, Natarajan R. The role of epigenetics in the pathology of diabetic complications. Am J Physiol. 2010;299:F14–F25. doi: 10.1152/ajprenal.00200.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23(7):781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 7.Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790:886–891. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Pons D, de Vries FR, van den Elsen PJ, Heijmans BT, Quax PH, Jukema JW. Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur Heart J. 2009;30:266–277. doi: 10.1093/eurheartj/ehn603. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, Jin ZG. Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27(11):2355–62. doi: 10.1161/ATVBAHA.107.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao D, Wang Z, Zhang CL, Oh J, Xing W, Li S, Richardson JA, Wang DZ, Olson EN. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol. 2005;25(1):364–76. doi: 10.1128/MCB.25.1.364-376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116(1):36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waltregny D, Glénisson W, Tran SL, North BJ, Verdin E, Colige A, Castronovo V. Histone deacetylase HDAC8 associates with smooth muscle alpha-actin and is essentialfor smooth muscle cell contractility. FASEB J. 2005;19(8):966–968. doi: 10.1096/fj.04-2303fje. [DOI] [PubMed] [Google Scholar]
- 13.Sahar S, Reddy MA, Wong C, Meng L, Wang M, Natarajan R. Cooperation of SRC-1 and p300 with NF-kappaB and CREB in angiotensin II-induced IL-6 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27(7):1528–1534. doi: 10.1161/ATVBAHA.107.145862. [DOI] [PubMed] [Google Scholar]
- 14.Vinh A, Gaspari TA, Liu HB, Dousha LF, Widdop RE, Dear AE. A novel histone deacetylase inhibitor reduces abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. J Vasc Res. 2008;45(2):143–152. doi: 10.1159/000110041. [DOI] [PubMed] [Google Scholar]
- 15.Yan ZQ, Yao QP, Zhang ML, Qi YX, Guo ZY, Shen BR, Jiang ZL. Histone deacetylases modulate vascular smooth muscle cell migration induced by cyclic mechanical strain. J Biomech. 2009;42(7):945–948. doi: 10.1016/j.jbiomech.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findeisen HM, Gizard F, Zhao Y, Qing H, Heywood EB, Jones KL, Cohn D, Bruemmer D. Epigenetic Regulation of Vascular Smooth muscle cell proliferation and neointimal formation by histone deacetylases inhibition. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.221952. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]