Abstract
Protein-polymer conjugates are of interest to researchers in diverse fields. Attachment of polymers to proteins results in improved pharmacokinetics, which is important in medicine. From an engineering standpoint, conjugates are exciting because they exhibit properties of both the biomolecules and synthetic polymers. This allows the activity of the protein to be altered or tuned, a key aspect in therapeutic design, anchoring conjugates to surfaces, and utilizing these materials for supramolecular self-assembly. Thus, there is broad interest in straightforward synthetic methods to make protein-polymer conjugates. Controlled radical polymerization (CRP) techniques have emerged as excellent strategies to make conjugates because the resulting polymers have narrow molecular weight distributions, targeted molecular weights, and attach to specific sites on proteins. Herein, recent advances in the synthesis and application of protein-polymer conjugates by CRP are highlighted.
Introduction
Conjugation of synthetic polymers to proteins yields macromolecular constructs that synergistically merge the properties of the individual components.[1,2] Covalent attachment of polymers to proteins is known to improve protein efficacy by increasing lifetimes in vivo and decreasing immunogenicity.[3–6] For patients, this translates to fewer doses of the drug. Depending on the properties of the polymer, directed self-assembly of the conjugate to higher ordered nanostructures can also occur.[7] Additionally, protein-polymer conjugates can exhibit higher biological activities compared to unmodified proteins when attached to surfaces.[8] As a result, synthesis and characterization of these materials is of interest to researchers in the fields of medicine, biotechnology, nanotechnology, mechanical engineering, polymer chemistry, and bioengineering. Examples of peptide-, oligonucleotide-, and side chain polymer-conjugates have been summarized elsewhere.[9–12] Conjugation of linear poly(ethylene glycol) (PEG) to biomolecules (PEGylation) has also been extensively reviewed.[13,14] Herein, we highlight representative examples of the application of controlled radical polymerization (CRP)[15,16] techniques in the synthesis of well-defined, protein-polymer conjugates. Since the point of attachment of the polymer is crucial to the resulting properties, such as bioactivity, we focus on site-specific conjugation strategies.[17] The CRP methods that will be discussed are reversible addition-fragmentation chain transfer (RAFT) polymerization[18,19] and atom transfer radical polymerization (ATRP).[20,21]
Controlled radical polymerizations
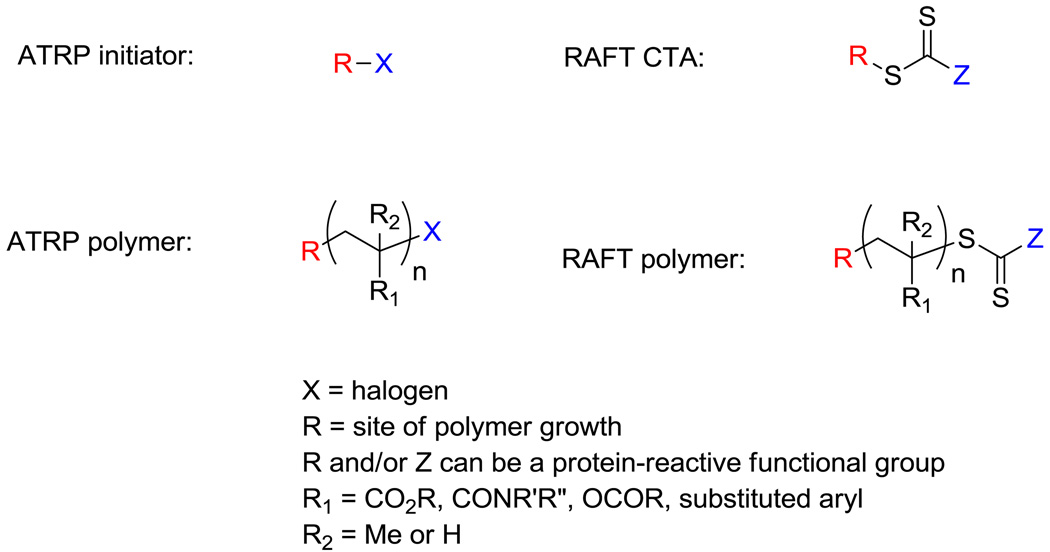
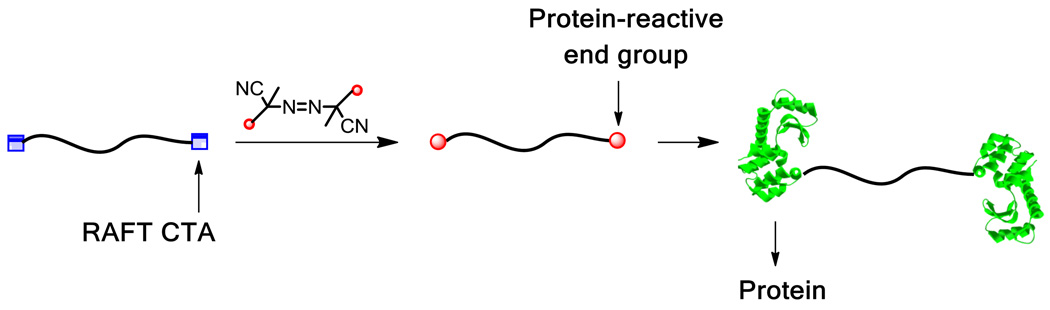
ATRP and RAFT polymerization are ideal methods to prepare bioreactive polymeric scaffolds. With ATRP, polymer growth occurs from an initiator. An ATRP initiator consists of a halogenated molecule capable of undergoing a reversible redox reaction with a transition metal catalyst to produce a reactive radical center. It is this reversible redox reaction that produces polymers with narrow molecular weight distributions (low polydispersity indices, PDIs) and pre-determined molecular weights. At the end of the reaction, ATRP polymers have the initiator fragment at the alpha chain end and a halogen at the omega chain end (Figure 1). RAFT polymerization is mediated by a reversible chain transfer agent (CTA), which typically contains a thiocarbonylthio group. The polymerization is often initiated with a small amount of a sacrificial initiator such as 2,2′-azobis(2-methylpropionitrile) (AIBN). Control during RAFT polymerization results from the chain transfer of the radical center to and from the thiocarbonylthio reactive group. Polymers produced by RAFT possess the thiocarbonylthio moiety covalently bonded to a radical stabilizing functional group (“Z” fragment) at the omega termini of the polymer. The alpha termini of the polymer contains the “R” fragment (site of polymer growth) of the CTA (Figure 1). Both techniques perform well in the presence of a wide range of solvents, functional groups, and reaction conditions. It is the high retention of the halogen and thiocarbonylthio groups at the omega termini postpolymerization for ATRP and RAFT, respectively, that allow further functionalization of these materials, as well as the ability to design protein-reactive initiators and CTAs to create bioreactive materials.
Figure 1.
General structure of ATRP initiators, RAFT CTAs, ATRP polymers, and RAFT polymers.
General synthetic approaches to protein-polymer conjugates
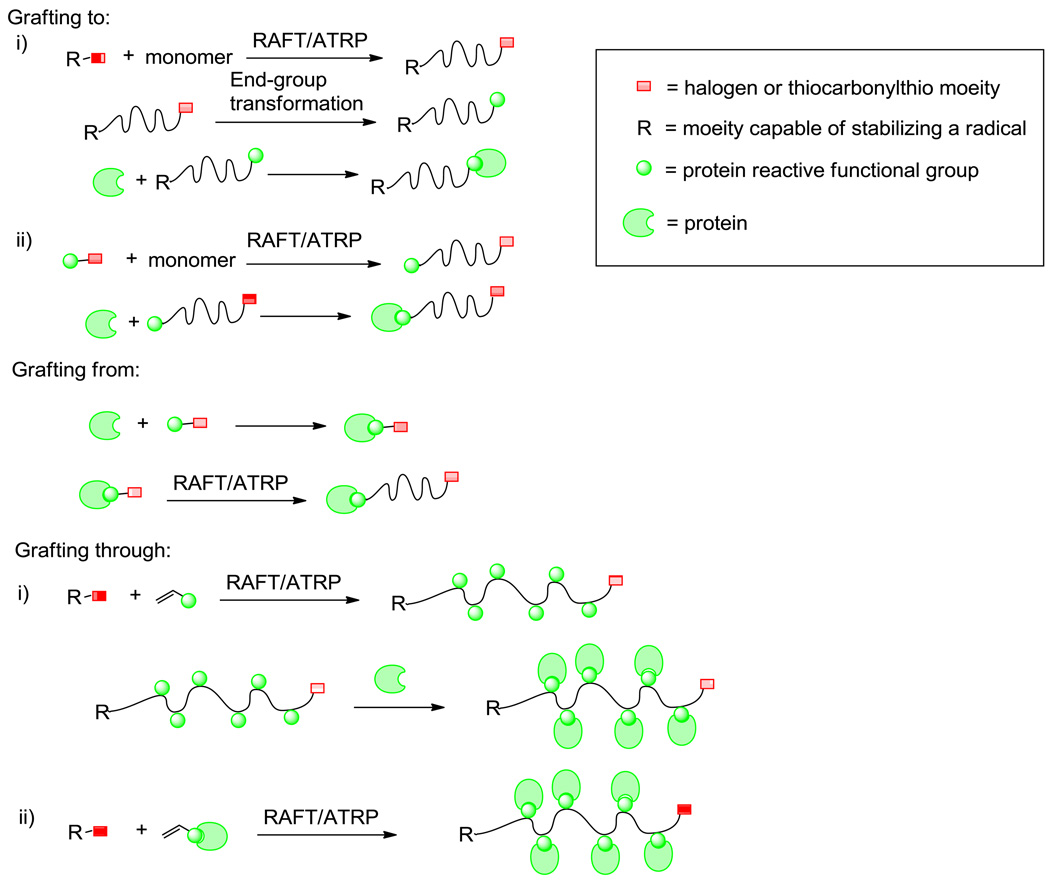
Synthesis of protein-polymer conjugates via CRP can be simplified into three distinct approaches (Figure 2).[22] Grafting to is the most common. The appropriate end-functionalized polymers are prepared either by modifying the chain postpolymerization to render it bioreactive or by growing the chain from a protein-reactive handle. Grafting from entails functionalization of the protein with a small molecule from which polymer growth can occur, such as an ATRP initiator or RAFT CTA, followed by polymerization. Grafting through involves biomolecular monomers and will not be discussed in this review.[12,22] The ultimate goal is to synthesize well-defined protein-polymer conjugates with high retentions of bioactivities relative to unmodified proteins. In order to achieve this, the targeted polymers have low PDIs (usually <1.3) and high retention of the end-groups. The sites of polymer conjugation to the protein or polymer growth from the protein are predetermined. The chemistry is mild, chemospecific, and efficient to maintain the tertiary and quaternary structure of the polypeptide, which is essential for activity. Therefore, three conjugation strategies are generally exploited for site-specific conjugations: 1) reaction with free cysteine residues, 2) modification of the N-terminal amine, or 3) use of protein-specific ligands.
Figure 2.
General approaches used to synthesize protein-polymer conjugates.
Grafting polymers to proteins
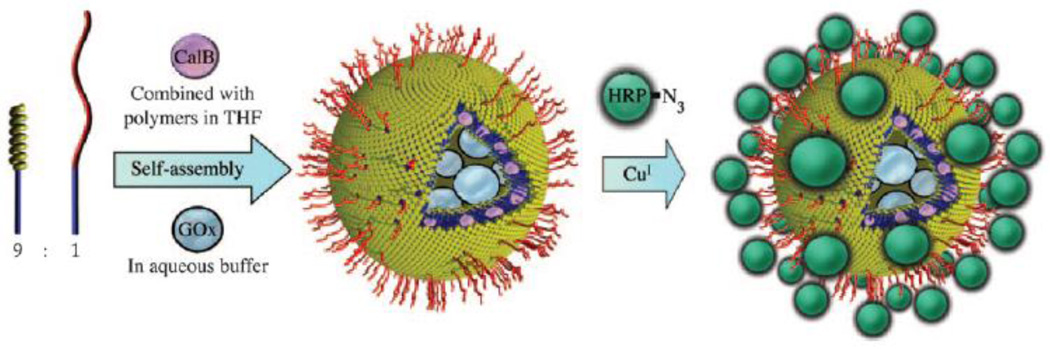
As mentioned above grafting to can be segregated into two approaches. The first entails postpolymerization bioactivation of the polymer by end-group transformation. ATRP polymers retain the halogen at the omega-termini postpolymerization. Therefore, the halogen can be displaced with an appropriate nucleophile to generate the semitelechelic polymer, and then reacted with a protein containing the complimentary functional group.[23,24] For example, van Hest and coworkers utilized nucleophilic displacement of the bromine on polystyrene (pS) with an azide.[24] This facilitated the synthesis of pS-b-PEG-acetylene polymer upon subsequent Huisgen cycloaddition with a bis-acetylene-PEG. These block copolymers where then self-assembled into polymersomes ranging from 90 to 180 nm in diameter with glucose oxidase (GOx) encapsulated in the lumen and lipase from Candida antarctica (Cal B) embedded in the bilayer. Diazo transfer was performed on horse radish peroxidase (HRP) to transform surface amines on the lysine residues into azides. HRP was conjugates onto the acetylene coated polymersome (Figure 3) by Huisgen cycloaddition. The three enzyme-cascade was initiated by incubation of the enzyme functionalized polymersomes with 2,3,4,6-tetra-O-benzyl–D-glucopyranose acetylated at the anomeric position and 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). The sugar was deacetylated by Cal B and then oxidized by GOx. This produced hydrogen peroxide, which in turn caused the oxidization of ABTS to ABTS•+ successfully demonstrating a three-enzyme cascade made possible by the polymersome architecture.
Figure 3.
Synthesis of a three enzyme functionalized nanoreactor.[24] Reprinted with permission from S.F.M. van Dongen, et. al, (2009), Chemistry-A European Journal, 15, 1107–1114. Copyright 2009 Wiley-VCH.
There are two approaches utilized for postpolymerization bioactivation of RAFT polymers, and both involve reaction with the thiocarbonylthiol group located at the chain end. Radical coupling with functionalized azoinitiators[25–28] and reduction of the thiocarbonylthio group to generate the free thiol, followed by further modification are both employed. Functionalization using azoinitiators will be discussed later in this review. Reduction of the thiocarbonylthio group is facilitated with reducing agents or good nucleophiles such as sodium borohydride and butylamine, respectively. The subsequent free thiol can then be adsorbed onto gold particles or surfaces,[29] trapped as an activated disulfide,[30] or modified using Michael acceptors.[31,32] For example, we synthesized Boc-protected α-aminooxy-poly N-isopropylacrylamide (pNIPAAm) ω-trithiocarbonate by RAFT. The Boc-aminooxy-pNIPAAm-trithiocarbonate was then reduced with butylamine and adsorbed onto a gold surface. After removal of the BOC group, oxidized heparin was immobilized on the surface via oxime bond formation. Bulmus, Davis and coworkers have recently demonstrated the ability to synthesize a variety of polymers by RAFT with both trithiocarbonate and dithioester CTAs that were then reduced with hexylamine.[30] The resulting thiols were trapped with 2,2’-dithiopyridine in order to produce activated disulfides. The versatility of the resulting semitelechelic polymers was demonstrated by reaction with a thiol containing peptide, oligonucleotide, and carbohydrate. We also recently demonstrated the in situ reduction of branched PEG polymers and reaction with divinylsulfone (Figure 4).[31] Conjugation of branched polymers to proteins is of interest because it has been shown that these types of scaffolds can result in conjugates with improved pharmacokinetic properties over linear polymer derivatives.[4–6,33] Specifically, PEG acrylate (Mn = 454 g/mole) (PEGA) was polymerized in the presence of a benzyl-dithiobenzoate CTA to generate a range of well-defined polymers.[31] The dithioester end-groups were reduced and incubated with divinyl sulfone, resulting in well-defined semitelechelic Michael acceptor pPEGAs. Conjugation of these polymers to bovine serum albumin (BSA), which contains a free cysteine residue (C34), resulted in conjugates with 92% esterase activity compared to the unmodified protein.
Figure 4.
Synthesis and conjugation of semitelechelic vinyl sulfone pPEGA to BSA (PDB # 1E7H).[31] Reproduced with permission from Macromolecules 2009, 42, 7657–7663. Copyright 2009 American Chemical Society.
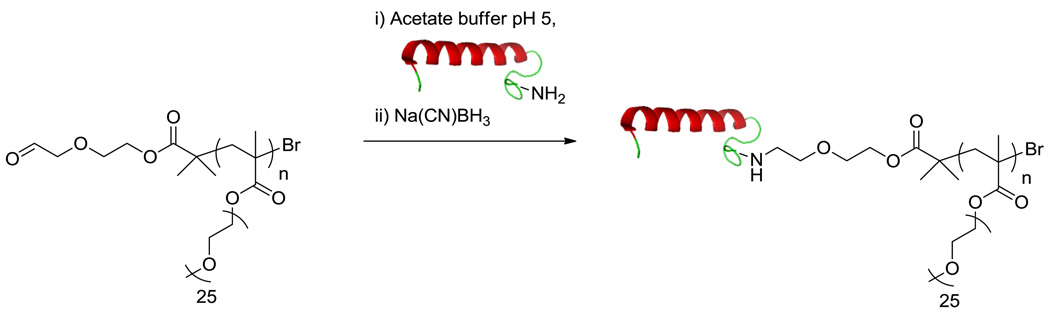
Polymers suitable for protein conjugation can also be synthesized in one step using reactive initiators or CTAs. We and others have published a number of papers that take this approach. For example, ATRP and RAFT were utilized to synthesize α-pyridyl disulfide-poly(N-acetyl-d-glucosamine)-ω-bromine and α-pyridyl disulfide-pNIPAAm-ω-trithiocarbonate, respectively.[34,35] Additionally, α-aminooxy polymers were synthesized by both ATRP and RAFT and conjugated to Nε-levulinyl lysine-modified BSA via oxime bonds.[29,36] PEG methacrylate (Mn = 1,100 g/mole) (PEGMA) was polymerized from an aldehyde initiator by ATRP to synthesize a range of well-defined poly(PEGMA)s (pPEGMAs).[37] polyPEGMA was then conjugated to salmon calcitonin (sCT) via Schiff base formation with the N-terminus and subsequent reduction with sodium cyanoborohydride (Figure 5). The sCT conjugate was able to elevate cyclic adenosine monophosphate (cAMP) 85% compared to the unmodified protein that demonstrated 92% elevation in T47D cells, a human ductal breast epithelial tumor cell line.
Figure 5.
Conjugation and reduction of pPEGMA1100 to the N-terminus of salmon calcitonin (protein structure from PBD # 2GLH).[37]
Grafting polymers from proteins
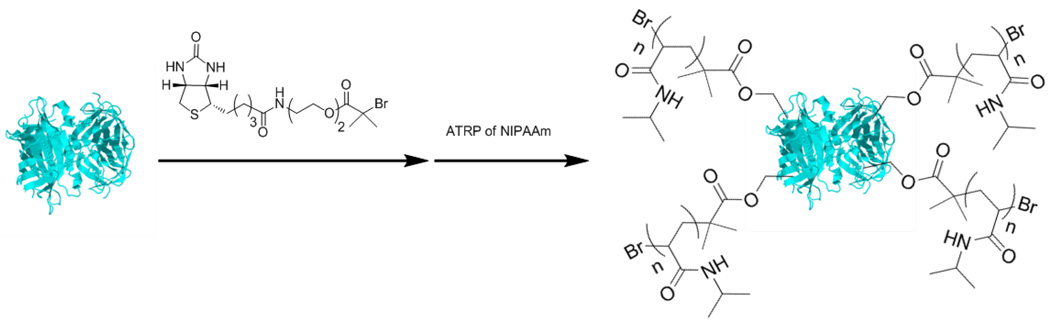
The grafting from strategy involves functionalization of a protein followed by polymerization. One advantage of this approach is that it does not require separation of unreacted polymer and protein from conjugate. Another advantage is that hydrophobic polymer conjugates are more readily synthesized. We pioneered this approach by demonstrating ATRP of thermoresponsive NIPAAm from streptavidin (SAv) and T4 lysozyme (T4L) V131C.[38,39] In the first example, a biotinylated ATRP initiator was synthesized and conjugated to SAv (Figure 6).[39] Polymer growth occurred only from the ATRP functionalized ligand bound by the protein, and the protein maintained activity postpolymerization. Thermoresponsiveness of the SAv-pNIPAAm conjugate imparted by the polymer was also demonstrated. In our second example, reversible and nonreversible ATRP macro-initiator of T4L V131C were synthesized upon conjugation of a pyridyl disulfide and maleimide ATRP initiator to the protein, respectively (Figure 7).[38] The ability of resulting T4L- pNIPAAm conjugates to lyse Micrococcus lysodeikticus was statistically the same as unmodified T4L. Both examples showed that this methodology is robust and able to synthesize protein-polymer conjugates with a high retention of bioactivity.
Figure 6.
Synthesis of streptavidin macroinitiator and polymerization of N-isopropylacryamide (protein structure from PBD # 3MG5).[39]
Figure 7.
Synthesis of reversible and nonreversible T4L-pNIPAAm conjugates (protein structure from PBD # 132L).[38]
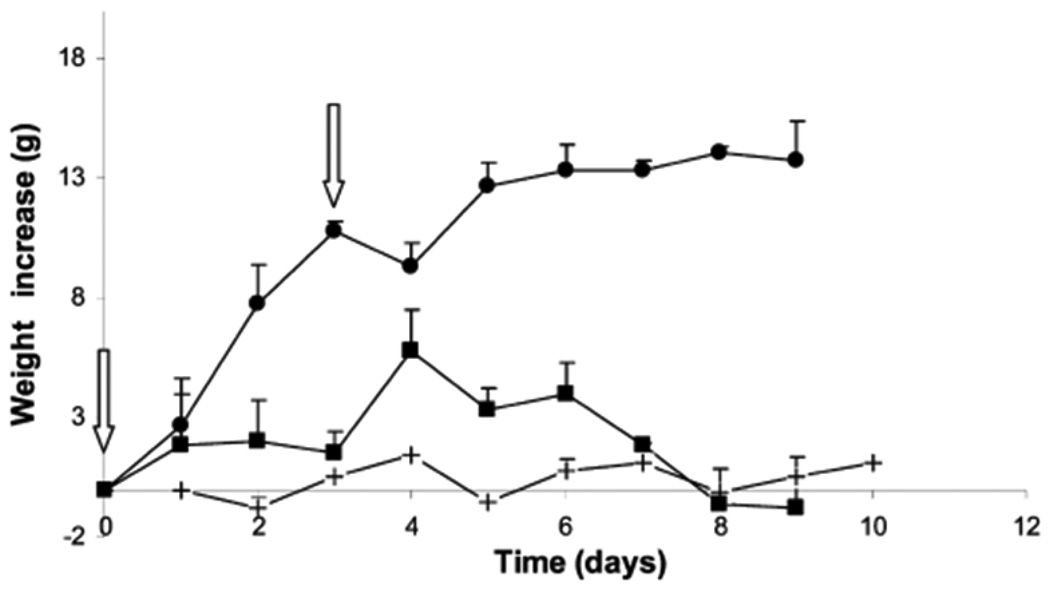
This technique of growing polymer chains directly from proteins has recently been applied to recombinant human growth hormone (rh-GH) by Caliceti and coworkers.[4] In this example N-hydroxysuccimide (NHS) bromoisobutyrate was coupled to rh-GH via the N-terminal amino acid and lysine residues and polymerization of PEGMA (Mn = 475 g/mol) was conducted. Conjugation of polymer to approximately nine out of ten available amine residues was confirmed by matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry. Maintenance of structural integrity of protein was verified by circular dichroism. Administration of 40 µg daily doses of the rh-GF-pPEGMA and unmodified rh-GF to female Sprague Dowley rats over six days resulted in similar weight growth profiles. When the animals were administered 120 µg dose of rh-GF or rh-GF-pPEGMA at day 0 and day 3 and monitored for nine days, only the group given rh-GF-pPEGMA showed increased mass gains over an extended period compared to rh-GF (Figure 8). This result nicely demonstrates that grafting from produces conjugates that are viable in vivo. This approach has also been applied by Chilkoti and coworkers for the polymerization of branched PEG monomers from the N-terminus of myoglobin and the C-terminus of green fluorescent protein (GFP).[5,6] Both conjugates demonstrated better pharmacokinetics than the unmodified proteins.
Figure 8.
Increase of rat weight (%) after administration of rh-GH and rh-GH-pPEGMA. Arrows represent injection 40 µg of rh-GH (black squares), rh-GH-pPEGMA (black circles), and buffer (crosses).[4] Reproduced with permission from Bioconjugate Chemistry 2010, 21, 671–678. Copyright 2010 American Chemical Society.
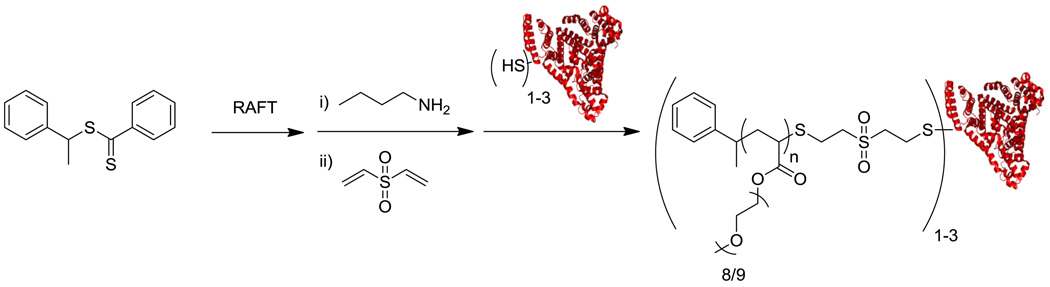
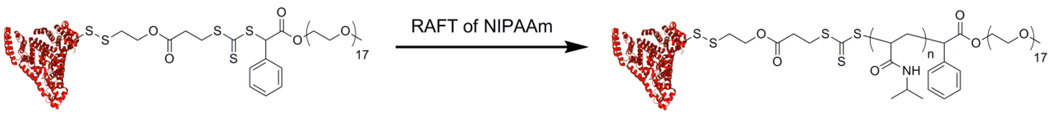
It was demonstrated that RAFT polymerization can also be used to graft polymers from proteins.[40–42] The Centre of Advanced Macromolecular Design (CAMD) group conjugated a pyridyl disulfide trithiocarbonate CTA to BSA (Figure 9). In their approach the protein was attached to the Z-group of the CTA followed by polymerization and subsequent capping by the protein (the Z-group approach). This BSA-macroCTA was utilized to prepare well-defined, pPEGA and pNIPAAm conjugates via gamma irradiation and thermal initiation with a sacrificial azoinitiator, respectively. The thermoresponsive nature of the pNIPAAm conjugates was confirmed, and 94% of esterase activity was maintained compared to unmodified BSA.[41] Sumerlin and coworkers synthesized a BSA-macroCTA in which the polymer chain was grown directly from the protein (R-group approach).[42] They demonstrated the ability of this BSA-macroCTA to mediate the polymerization of NIPAAm to generate well-defined protein-polymer conjugates that respond to changes in temperature. The conjugate exhibited 95% esterase activity of the native protein.
Figure 9.
Polymerization of NIPAAm from BSA (protein structure from PBD # 1E7H) with RAFT (Z-group approach).[41]
Besides improving conjugate pharmacokinetic properties, polymers can also transfer their properties to the protein. For example, as was described above, conjugates containing thermoresponsive pNIPAAm also respond to temperature. By tailoring the characteristics of the synthetic component it is possible for self-assembly of protein-polymer conjugates into higher order nanostructures to occur.[7] Nolte and coworkers synthesized the first giant amphiphile. Cal B was conjugated to maleimide poly(styrene) (pS).[43] The amphiphilic conjugate self-assembled into nanofibers in solution that maintained some of the activity of the protein. This approach has since been refined to generate the biopolymersome nanoreactors described above.[24] Le Droumaguet and Velonia recently utilized the grafting from approach to polymerize styrene from BSA by ATRP. The resulting conjugates self-assembled into spherical nanoparticles (NPs) 20 to 100 nm in diameter.[44] In situ NP formation was also demonstrated with human serum albumin (HSA) and reduced human calcitonin (rhCT). The authors were able to encapsulate fluorescently labeled papain and HRP into the NPs. Confocal fluorescence microscopy confirmed the presence of fluorescent papain. Incubation of the BSA-pS encapsulated with HRP NPs with hydrogen peroxide and 3,3’,5,5’-tetramethylbenzidine indicated that active HRP was present inside the NP. The above examples, demonstrate the versatility of CRP resulting in a range of materials with interesting properties.
Multimeric protein-polymer conjugates
Until recently most of the protein-polymer conjugates that have been reported were composed of one protein attached to one or more polymer chains. However, multimeric protein-polymer conjugates, where two or more proteins are attached to a single chain, are of interest. Linking multiple proteins can generate materials with higher affinity for a biological receptor, ability to target multiple biological processes or pathways, and/or provide a scaffold that contains a targeting group.[45] To obtain well-defined multimeric protein-polymer conjugates it is necessary to site-specifically link multiple proteins to a polymer scaffold.
Homodimeric and starred protein-polymer conjugates
We recently demonstrated a general approach for the construction of homodimeric, protein-polymer conjugates using CRP.[28] NIPAAm was polymerized in the presence of a two-armed CTA resulting in a well-defined polymer. Postpolymerization, the thiocarbonylthiol groups were exchanged for furan-protected maleimides via radical coupling with a functionalized azo-initiator (Figure 10). T4L V131C was then incubated with the deprotected, telechelic, maleimide-pNIPAAm resulting in the formation of dimeric T4L-polymer conjugates. This approach was expanded upon to produce protein star polymer conjugates.[27]
Figure 10.
Synthesis of homodimeric protein-polymer conjugates (protein structure from PBD # 1P56).[28] Tao, et al., Chem. Commun. 2009, 2148–2150 – Reproduced by permission of The Royal Society of Chemistry.
Heterodimeric protein-polymer conjugates
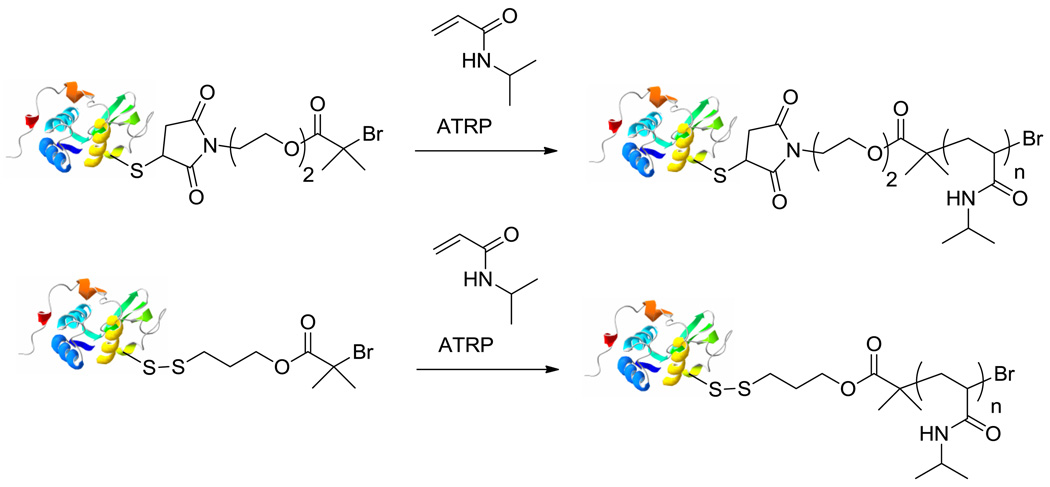
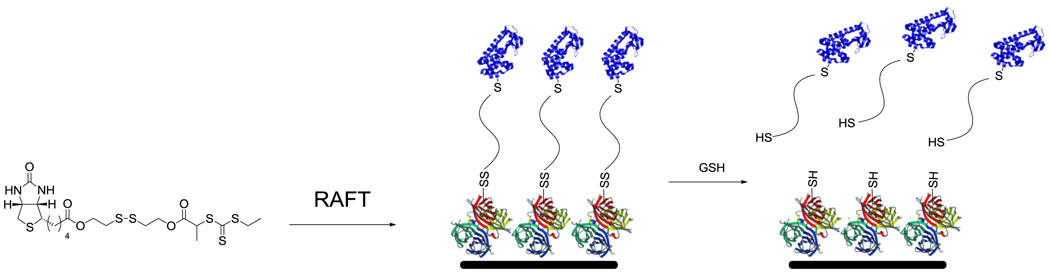
Multiple methodologies have also been developed for the synthesis of heterotelechelic polymers for bioconjugation.[25,26,46–48] The CAMD group performed polymerization of a series of monomers via RAFT in the presence of a CTA with an azide on the R-group and pyridyl disulfide on the Z-group.[46] Postpolymerization, a biotinylated alkyne was clicked to the well-defined polymers. Incubation of these polymers with SAv and BSA resulted in starred BSA conjugates with a SAv core. Theato and coworkers mediated the RAFT polymerization of diethyleneglycol monomethylether methacrylate (DEGMA) with an activated ester CTA. Thyroxin (a ligand for transthyretin) was then conjugated to the polymer via the activated ester. The thiocarbonylthiol group was then reduced with butylamine and the free thiol trapped with N-biotinylaminoethyl methanethiosulfonate resulting in heterotelechelic α-thyroxin, ω-biotinpNIPAAm. Heterodimer formation was demonstrated upon incubation with SAv and transthyretin as well as conjugation to SAv coated surfaces. We used the radical coupling approach described above for homodimers/stars in the synthesis of heterodimeric protein-polymer conjugates.[25,26] RAFT polymerization of NIPAAm was performed in the presence of biotinylated CTAs with or without a cleavable disulfide bond in the “R” group. Postpolymerization, the thiocarbonylthiol group was exchanged, and deprotection of the end-group afforded the α-biotin, ω-maleimide-pNIPAAm. Heterodimer formation with T4L V131C or BSA and SAv was demonstrated. Both maleimide-pNIPAAm-biotin and maleimide-pNIPAAm- disulfide-biotin (Figure 11) were used to tether proteins to surfaces. Enzyme linked immunosorbant assays (ELISAs) on SAv-coated surfaces demonstrated the reversibility of the disulfide containing conjugate indicating that the polymer will be useful for capture and release-type applications.
Figure 11.
Synthesis of heterotelechelic polymers for reversible surface immobilization of protein-polymer conjugates (protein structure from PBD # 1P56 and 1SWA).[26] Heredia, et al., Polym. Chem. 2010, 1, 168–170 – Reproduced by permission of The Royal Society of Chemistry.
Conclusions
Controlled radical polymerizations enable the synthesis of protein-polymer conjugates with interesting macromolecular structures and tailored properties. The ability to finely tune the chemical composition and conjugation site of the polymers provides access to new properties. PEG-graft polymer conjugates are promising as longer circulating therapeutics. Multi-protein encapsulated polymersomes have the potential for drug delivery and as cell/organelle mimics. Multimeric protein-polymer conjugates should result in more effective therapeutics and better biosensors. These materials, made possible by controlled radical polymerization techniques, stand to revolutionize the fields of biotechnology, nanotechnology, and medicine.
Acknowledgements
The authors are grateful to the National Science Foundation (CHE-0809832) for funding. GNG thanks the Christopher S. Foote Graduate Research Fellowship in Organic Chemistry and the NIH Biotechnology Training Grant. HDM appreciates the Alfred P. Sloan Foundation for additional funding.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Duncan R. The dawning era of polymer therapeutics. Nature Reviews Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 2.Abuchowski A, McCoy JR, Palczuk NC, Vanes T, Davis FF. Effect of covalent attachment of polyethylene-glycol on immunogenicity and circulating life of bovine liver catalase. Journal of Biological Chemistry. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 3.Glue P, Fang JWS, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S. Hepatitis CITG: Pegylated interferon-alpha 2b: Pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clinical Pharmacology & Therapeutics. 2000;68:556–567. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 4.Magnusson JP, Bersani S, Salmaso S, Alexander C, Caliceti P. In situ growth of side-chain PEG polymers from functionalized human growth hormone-a new technique for preparation of enhanced protein-polymer conjugates. Bioconjugate Chemistry. 2010;21:671–678. doi: 10.1021/bc900468v. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, Liu W, Christensen T, Zalutsky MR, Chilkoti A. In situ growth of a PEG-like polymer from the C terminus of an intein fusion protein improves pharmacokinetics and tumor accumulation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16432–16437. doi: 10.1073/pnas.1006044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao WP, Liu WG, Mackay JA, Zalutsky MR, Toone EJ, Chilkoti A. In situ growth of a stoichiometric PEG-like conjugate at a protein's N-terminus with significantly improved pharmacokinetics. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15231–15236. doi: 10.1073/pnas.0904378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirks AJ, Nolte RJM, Cornelissen J. Protein-Polymer Hybrid Amphiphiles. Advanced Materials. 2008;20:3953–3957. [Google Scholar]
- 8.Lin PC, Weinrich D, Waldmann H. Protein Biochips: Oriented Surface Immobilization of Proteins. Macromolecular Chemistry and Physics. 2010;211:136–144. [Google Scholar]
- 9.Apostolovic B, Deacon SPE, Duncan R, Klok HA. Hybrid Polymer Therapeutics Incorporating Bioresponsive, Coiled Coil Peptide Linkers. Biomacromolecules. 2010;11:1187–1195. doi: 10.1021/bm901313c. [DOI] [PubMed] [Google Scholar]
- 10.Osada K, Kataoka K. Drug and gene delivery based on supramolecular assembly of PEG-polypeptide hybrid block copolymers. In: Klok HA, Schlaad H, editors. Peptide Hybrid Polymers. Springer: 2006. pp. 113–153. Advances in Polymer Science, vol 202. [Google Scholar]
- 11.Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu JQ, Perrier S. Bioapplications of RAFT Polymerization. Chemical Reviews. 2009;109:5402–5436. doi: 10.1021/cr9001403. [DOI] [PubMed] [Google Scholar]
- 12.Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Applications of Orthogonal "Click" Chemistries in the Synthesis of Functional Soft Materials. Chemical Reviews. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joralemon MJ, McRae S, Emrick T. PEGylated polymers for medicine: from conjugation to self-assembled systems. Chemical Communications. 2010;46:1377–1393. doi: 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- 14.Pasut G, Veronese FM. PEGylation of proteins as tailored chemistry for optimized bioconjugates. Polymer Therapeutics I: Polymers as Drugs, Conjugates and Gene Delivery Systems. 2006;192:95–134. [Google Scholar]
- 15.Otsu T, Yoshida M. Role of initiator-transfer agent-terminator (iniferter) in radical polymerizations-polymer design by organic disulfides as iniferters. Makromolekulare Chemie-Rapid Communications. 1982;3:127–132. [Google Scholar]
- 16.Otsu T, Yoshida M, Tazaki T. A model for living radical polymerization. Makromolekulare Chemie-Rapid Communications. 1982;3:133–140. [Google Scholar]
- 17.Kochendoerfer GG. Site-specific polymer modification of therapeutic proteins. Current Opinion in Chemical Biology. 2005;9:555–560. doi: 10.1016/j.cbpa.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules. 1998;31:5559–5562. [Google Scholar]
- 19.Charmot D, Corpart P, Adam H, Zard SZ, Biadatti T, Bouhadir G. Controlled radical polymerization in dispersed media. Macromolecular Symposia. 2000;150:23–32. [Google Scholar]
- 20.Kato M, Kamigaito M, Sawamoto M, Higashimura T. Polymerization of methyl-methacrylate with the carbon-tetrachloride dichlorotris(triphenylphosphine) ruthenium(II) methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system-possibility of living radical polymerization. Macromolecules. 1995;28:1721–1723. [Google Scholar]
- 21.Wang JS, Matyjaszewski K. Controlled living radical polymerization-atom-transfer radical polymerization in the presence of transition-metal complexes. Journal of the American Chemical Society. 1995;117:5614–5615. [Google Scholar]
- 22.Canalle LA, Lowik D, van Hest JCM. Polypeptide-polymer bioconjugates. Chemical Society Reviews. 2010;39:329–353. doi: 10.1039/b807871h. [DOI] [PubMed] [Google Scholar]
- 23.Xu FJ, Neoh KG, Kang ET. Bioactive surfaces and biomaterials via atom transfer radical polymerization. Progress in Polymer Science. 2009;34:719–761. [Google Scholar]
- 24.van Dongen SFM, Nallani M, Cornelissen J, Nolte RJM, van Hest JCM. A Three-Enzyme Cascade Reaction through Positional Assembly of Enzymes in a Polymersome Nanoreactor. Chemistry-a European Journal. 2009;15:1107–1114. doi: 10.1002/chem.200802114. [DOI] [PubMed] [Google Scholar]
- 25.Heredia KL, Grover GN, Tao L, Maynard HD. Synthesis of Heterotelechelic Polymers for Conjugation of Two Different Proteins. Macromolecules. 2009;42:2360–2367. doi: 10.1021/ma8022712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heredia KL, Tao L, Grover GN, Maynard HD. Heterotelechelic Polymers for Capture and Release of Protein-Polymer Conjugates. Polymer Chemistry. 2010;1:168–170. doi: 10.1039/B9PY00369J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao L, Kaddis CS, Loo RRO, Grover GN, Loo JA, Maynard HD. Synthesis of Maleimide-End-Functionalized Star Polymers and Multimeric Protein-Polymer Conjugates. Macromolecules. 2009;42:8028–8033. doi: 10.1021/ma901540p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Kaddis CS, Loo RRO, Grover GN, Loo JA, Maynard HD. Synthetic approach to homodimeric protein-polymer conjugates. Chemical Communications. 2009:2148–2150. doi: 10.1039/b822799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vazquez-Dorbatt V, Tolstyka ZP, Maynard HD. Synthesis of Aminooxy End-Functionalized pNIPAAm by RAFT Polymerization for Protein and Polysaccharide Conjugation. Macromolecules. 2009;42:7650–7656. doi: 10.1021/ma9013803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyer C, Liu JQ, Bulmus V, Davis TP. RAFT Polymer End-Group Modification and Chain Coupling/Conjugation Via Disulfide Bonds. Australian Journal of Chemistry. 2009;62:830–847. [Google Scholar]
- 31.Grover GN, Alconcel SNS, Matsumoto NM, Maynard HD. Trapping of Thiol-Terminated Acrylate Polymers with Divinyl Sulfone To Generate Well-Defined Semitelechelic Michael Acceptor Polymers. Macromolecules. 2009;42:7657–7663. doi: 10.1021/ma901036x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spruell JM, Levy BA, Sutherland A, Dichtel WR, Cheng JY, Stoddart JF, Nelson A. Facile Postpolymerization End-Modification of RAFT Polymers. Journal of Polymer Science Part a-Polymer Chemistry. 2009;47:346–356. [Google Scholar]
- 33.Luxon BA, Grace M, Brassard D, Bordens R. Pegylated interferons for the treatment of chronic hepatitis C infection. Clinical Therapeutics. 2002;24:1363–1383. doi: 10.1016/s0149-2918(02)80042-x. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Dorbatt V, Tolstyka ZP, Chang CW, Maynard HD. Synthesis of a Pyridyl Disulfide End-Functionalized Glycopolymer for Conjugation to Biomolecules and Patterning on Gold Surfaces. Biomacromolecules. 2009;10:2207–2212. doi: 10.1021/bm900395h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heredia KL, Nguyen TH, Chang CW, Bulmus V, Davis TP, Maynard HD. Reversible siRNA-polymer conjugates by RAFT polymerization. Chemical Communications. 2008:3245–3247. doi: 10.1039/b804812f. [DOI] [PubMed] [Google Scholar]
- 36.Heredia KL, Tolstyka ZP, Maynard HD. Aminooxy end-functionalized polymers synthesized by ATRP for chemoselective conjugation to proteins. Macromolecules. 2007;40:4772–4779. [Google Scholar]
- 37.Sayers CT, Mantovani G, Ryan SM, Randev RK, Keiper O, Leszczyszyn OI, Blindauer C, Brayden DJ, Haddleton DM. Site-specific N-terminus conjugation of poly(mPEG(1100)) methacrylates to salmon calcitonin: synthesis and preliminary biological evaluation. Soft Matter. 2009;5:3038–3046. [Google Scholar]
- 38.Heredia KL, Bontempo D, Ly T, Byers JT, Halstenberg S, Maynard HD. In situ preparation of protein - "Smart" polymer conjugates with retention of bioactivity. Journal of the American Chemical Society. 2005;127:16955–16960. doi: 10.1021/ja054482w. [DOI] [PubMed] [Google Scholar]
- 39.Bontempo D, Maynard HD. Streptavidin as a macroinitiator for polymerization: In situ protein-polymer conjugate formation. Journal of the American Chemical Society. 2005;127:6508–6509. doi: 10.1021/ja042230+. [DOI] [PubMed] [Google Scholar]
- 40.Liu JQ, Bulmus V, Herlambang DL, Barner-Kowollik C, Stenzel MH, Davis TP. In situ formation of protein-polymer conjugates through reversible addition fragmentation chain transfer polymerization. Angewandte Chemie-International Edition. 2007;46:3099–3103. doi: 10.1002/anie.200604922. [DOI] [PubMed] [Google Scholar]
- 41.Boyer C, Bulmus V, Liu JQ, Davis TP, Stenzel MH, Barner-Kowollik C. Well-defined protein-polymer conjugates via in situ RAFT polymerization. Journal of the American Chemical Society. 2007;129:7145–7154. doi: 10.1021/ja070956a. [DOI] [PubMed] [Google Scholar]
- 42.De P, Li M, Gondi SR, Sumerlin BS. Temperature-regulated activity of responsive polymer-protein conjugates prepared by grafting-from via RAFT polymerization. Journal of the American Chemical Society. 2008;130:11288–11289. doi: 10.1021/ja804495v. [DOI] [PubMed] [Google Scholar]
- 43.Velonia K, Rowan AE, Nolte RJM. Lipase polystyrene giant amphiphiles. Journal of the American Chemical Society. 2002;124:4224–4225. doi: 10.1021/ja017809b. [DOI] [PubMed] [Google Scholar]
- 44.Le Droumaguet B, Velonia K. In situ ATRP-Mediated hierarchical formation of giant amphiphile bionanoreactors. Angewandte Chemie-International Edition. 2008;47:6263–6266. doi: 10.1002/anie.200801007. [DOI] [PubMed] [Google Scholar]
- 45.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angewandte Chemie-International Edition. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Boyer C, Liu J, Bulmus V, Davis TP, Barner-Kowollik C, Stenzel MH. Direct synthesis of well-defined heterotelechelic polymers for bioconjugations. Macromolecules. 2008;41:5641–5650. [Google Scholar]
- 47.Liu JQ, Liu HY, Bulmus V, Tao L, Boyer C, Davis TP. A simple methodology for the synthesis of heterotelechelic protein-polymer-biomolecule conjugates. Journal of Polymer Science Part a-Polymer Chemistry. 2010;48:1399–1405. [Google Scholar]
- 48.Roth PJ, Jochum FD, Zentel R, Theato P. Synthesis of hetero-telechelic alpha,omega bio-functionalized polymers. Biomacromolecules. 2010;11:238–244. doi: 10.1021/bm901095j. [DOI] [PubMed] [Google Scholar]