Abstract
DNA ligase IV (Lig4) and the DNA-dependent protein kinase (DNA-PK) function in nonhomologous end joining (NHEJ). However, although Lig4 deficiency causes late embryonic lethality, deficiency in DNA-PK subunits (Ku70, Ku80, and DNA-PKcs) does not. Here we demonstrate that, similar to p53 deficiency, ataxia-telangiectasia-mutated (ATM) gene deficiency rescues the embryonic lethality and neuronal apoptosis, but not impaired lymphocyte development, associated with Lig4 deficiency. However, in contrast to p53 deficiency, ATM deficiency enhances deleterious effects of Lig4 deficiency on growth potential of embryonic fibroblasts (MEFs) and genomic instability in both MEFs and cultured progenitor lymphocytes, demonstrating significant differences in the interplay of p53 vs. ATM with respect to NHEJ. Finally, in dramatic contrast to effects on Lig4 deficiency, ATM deficiency causes early embryonic lethality in Ku- or DNA-PKcs-deficient mice, providing evidence for an NHEJ-independent role for the DNA-PK holoenzyme.
The stability of the mammalian genome relies on efficient repair of DNA double-strand breaks (DSBs), which can arise from normal metabolism, exogenous DNA-damaging agents, or endonuclease activity (1). In mammalian cells, the nonhomologous end-joining (NHEJ) and homologous recombination DSB-repair pathways serve as genomic caretakers (1, 2). Cellular gatekeepers regulate progression through the cell cycle in response to DNA damage and induce either apoptosis or cell-cycle arrest to provide sufficient time for repair by caretaker functions (3). The known NHEJ factors include Ku70, Ku80, and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which comprise the DNA PK holoenzyme, plus XRCC4 and Lig4, which function in ligation. These factors also are required for V(D)J recombination, the process by which antigen-receptor variable-region genes are assembled during lymphocyte development. Initially, the recombination-activating gene 1 and 2 (RAG1/2) endonuclease introduces DSBs at component V, D, and J recombination signal sequences; then, ubiquitously expressed NHEJ factors perform the subsequent joining steps (4).
Mice deficient in Lig4 (or XRCC4) exhibit a pleiotropic phenotype including late embryonic lethality, cellular growth defects and ionizing-radiation sensitivity, severely impaired lymphocyte development caused by inability to form V(D)J joins, and massive apoptosis of newly generated neurons (5–7). Ku-deficient mice display most of these defects and are smaller than littermates, but they are born in normal numbers and survive into adulthood; DNA-PKcs-deficient mice appear relatively normal except for some ionizing-radiation sensitivity and impaired lymphocyte development caused by defective V(D)J joining (8). The Ku, XRCC4, and Lig4 proteins are conserved in yeast, where they also function in NHEJ (9). To date, there is no compelling evidence for any in vivo function of the DNA-PK or Lig4–XRCC4 protein complexes beyond their common role in NHEJ.
The embryonic lethality and increased neuronal apoptosis associated with Lig4 and XRCC4 deficiency were rescued dramatically by p53 deficiency, indicating that these phenotypes result from a p53-dependent response to DNA damage (10, 11). In contrast, p53 deficiency did not rescue either the B or T cell developmental defects of Lig4- or XRCC4-deficient animals, consistent with inability of p53 deficiency to restore the V(D)J recombination/NHEJ defects (10, 11). Likewise, p53 deficiency did not rescue the V(D)J recombination defects associated with Ku80 or DNA-PKcs deficiency and also did not affect ability of these genotypes to generate normal numbers of viable progeny (12–16). However, in the p53-deficient background, absence of any NHEJ factor resulted in nearly universal development of aggressive pro-B cell lymphomas that harbored translocations between IgH and c-myc loci. NHEJ-factor deficiencies also led to increased spontaneous genomic instability in cultured embryonic fibroblasts (MEFs; refs. 10, 12, and 17–19), consistent with a critical role as a genomic caretaker.
The ATM protein, a serine/threonine protein kinase, is a member of a family of large proteins, including DNA-PK, which contains a phosphatidylinositol 3-kinase domain (20). ATM, like p53, functions as a cellular gatekeeper and is a key initiating factor in the cascade of events leading to activation of multiple DNA damage-responsive signaling pathways and cell-cycle checkpoints (2). In response to DSBs, ATM acts upstream of p53 and controls its activity through phosphorylation and stabilization of the protein (2). Cells deficient in ATM exhibit defective cell-cycle checkpoints at the G1/S transition, during S phase, and at the G2/M boundary; whereas p53 mutation predominantly effects the G1/S transition (2). In addition to impaired cell-cycle-checkpoint activation, ATM-deficient cells also possess distinct DNA-repair defects (20). This combination of defects may lead to the complex phenotypes observed in ataxia telangiectasia patients and cell lines, including ionizing-radiation sensitivity, genomic instability, neurological and vascular abnormalities, infertility, and predisposition to lymphoid malignancy (20).
Methods
Mice.
All mice were housed in a pathogen-free facility. The Lig4 (5), Ku70 (21), Ku80 (22), DNA-PKcs (23), and ATM (24–26) knockout mice used in this study were generated and characterized previously. The severe combined immunodeficient (scid) mice were obtained from The Jackson Laboratories. The P values for rescue of embryonic lethality were calculated based on the total number of Lig4−/−ATM+/+ (5, 11) and Lig4−/−ATM−/− or Lig4−/−ATM+/− pups born (this study) by using the Fisher's exact probability test. The P values for rescue of Lig4−/− embryonic lethality by ATM−/− and ATM+/− are P < 0.01 and P < 0.1, respectively.
Histological Analysis.
Whole embryos were fixed in either Bouins' solution or in 4% (wt/vol) paraformaldehyde (in PBS), processed, embedded in paraffin, sectioned serially (5 μm), and stained with hematoxylin/eosin. The terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay was performed on paraformaldehyde-fixed sections by using the DeadEnd colorimetric apoptosis detection kit (Promega).
Growth Assays.
Passage 3 MEFs (2 × 105) were plated in duplicate onto gelatinized 6-cm dishes. The cells were trypsinized, stained with trypan blue, and counted every 48 h. Cell number (×105) is plotted as a function of time (days). The results represent the average of three independent experiments by using MEFs derived from at least two different embryos. Senescence-associated β-galactosidase activity was measured by assaying for β-galactosidase activity as described (11).
Lymphocyte Development.
Fetal liver cultures were established as described (5). The cells were harvested at day 10 in culture and then stained with anti-IgM-phycoerythrin and anti-B220-FITC antibodies (PharMingen) and propidium iodide. Data were collected on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed by using FLOWJO software (TreeStar, San Carlos, CA). Single-cell suspensions of splenocytes from postnatal day-1 (P1) littermates were stained with anti-IgM and anti-B220 antibodies and analyzed as above. Single cell suspensions of thymocytes from P1 littermates were stained with anti-CD25, anti-CD4, and anti-CD8 fluorescence-conjugated antibodies (PharMingen and Southern Biotechnology Associates).
Spectral Karyotyping Analyses.
Passage 3 MEFs (1 × 106) were plated onto gelatinized 10-cm dishes and cultured for 16 h. Colcemid (GIBCO/BRL; KaryoMAX solution) was added (100 ng/ml), and the cultures were incubated for 3 h. Fetal liver cells (day 10 in culture) were incubated with colcemid (10 ng/ml) for 12 h. Chromosomal aberrations were quantified by using a Nikon Eclipse microscope equipped with an Applied Spectral Imaging interferometer (Carlsbad, CA) and ×40 and ×63 objectives.
Results
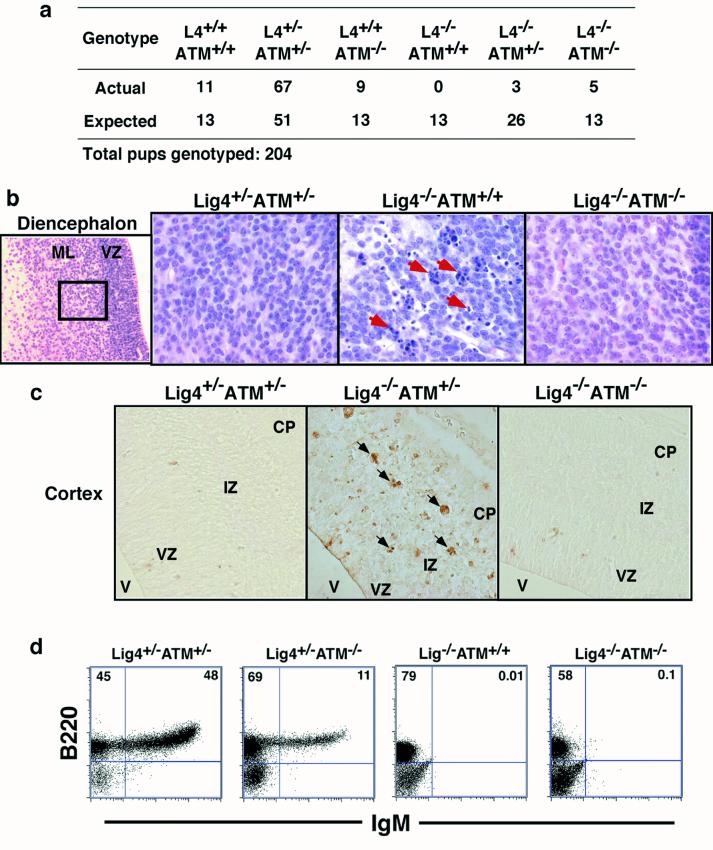
Given the role of ATM in regulating p53 activity, it seemed likely that ATM could modulate the cellular and organismal phenotypes associated with NHEJ deficiency in a manner similar to p53. Therefore, to elucidate potential genetic interactions between ATM and NHEJ, we bred the ATM-deficient background into various NHEJ-deficient backgrounds. First, we bred Lig4+/− mice (5) with ATM+/− mice (26), then bred Lig4+/−ATM+/− progeny to generate Lig4−/− mice with backgrounds of all ATM genotypes. Mendelian numbers of developing embryos of all genotypes were observed between embryonic days (E) 11.5 and 16 (Table 1). However, by E15 the Lig4−/−ATM−/− and Lig4−/−ATM+/− embryos were significantly smaller than littermate controls (20–25% of controls), although they were of similar size to Lig4−/−ATM+/+ embryos of the same age. As expected, no Lig4−/−ATM+/+ pups were born; however, we did observe live-born Lig4−/−ATM−/− pups (Fig. 1a), demonstrating that ATM deficiency can rescue Lig4−/− embryonic lethality, albeit at numbers below the predicted Mendelian frequency. ATM haplosufficiency also rescued Lig4−/− embryonic lethality, although to an even lesser extent (Fig. 1a). In all cases, the rescued pups were growth retarded, and most did not survive beyond postnatal day P1 or P2 (see Fig. 1a, legend). Thus, although clearly significant, neither the extent of postnatal rescue nor the duration of postnatal survival was as great as that observed in the context of rescue by p53 deficiency (11). Although differences in the genetic background of the ATM- vs. p53-deficient strains could contribute theoretically to these survival differences, we favor the possibility that more fundamental differences in the role of ATM vs. p53 in maintenance of genomic stability may be a major factor (see Discussion).
Table 1.
ATM-deficiency causes early embryonic lethality in mice deficient in the components of the DNA-PK holoenzyme
| Age | Genotype | ATM+/+ | ATM+/− | ATM−/− |
|---|---|---|---|---|
| Live-born pups | Ku70−/− | 16 (10) | 12 (20) | 0 (10) |
| Ku80−/− | 13 (13) | 24 (25) | 0 (13) | |
| scid | 21 (16) | 44 (33) | 0 (16) | |
| E13–16 | Lig4−/− | 9 (10) | 18 (20) | 16 (10) |
| E11.5–13.5 | Ku80−/− | 8 (11) | 23 (22) | 0 (11) |
The number of live-born pups and embryos at the indicated developmental stages of the relevant genotypes (as indicated) is shown with the expected Mendelian numbers in parentheses. No scid−/− ATM−/− pups were observed in over 360 pups born from scid+/− ATM+/− intercrosses (data not shown). In preliminary results, no live-born DNA-PKcs−/− ATM−/− pups were observed in breedings between DNA-PKcs+/− ATM+/− mice, and no scid−/− ATM−/− embryos (E12) from breedings between scid−/− ATM+/− and scid+/− ATM+/− mice were observed.
Figure 1.
Effects of ATM deficiency on Lig4−/− phenotypes. (a) ATM deficiency rescues embryonic lethality of Lig4 deficiency. The numbers of actual pups born and the Mendelian expected number of pups of the relevant genotypes are shown. The ATM-rescued pups, which were runted in comparison to littermates, did not appear to have nursed and died shortly after birth; however, the cause of the perinatal lethality is not known yet. (b) Histological analysis of hematoxylin/eosin-stained coronal sections of the diencephalon (E13.5) of the indicated genotypes. Arrows indicate pyknotic nuclei. VZ, ventricular zone; ML, mantle layer. Original magnification was ×400. (c) TUNEL staining of transverse sections of the developing cerebral cortex (E13.5) of the indicated genotypes. Arrows point to TUNEL-positive nuclei. V, ventricle; IZ, intermediate zone; CP, cortical plate. Original magnification was ×400. (d) Flow-cytometric analysis of lymphocytes from E15 fetal liver cells of the indicated genotypes showing B220 and IgM profile and the percentages of B220+ and IgM+ cells.
ATM has been suggested to play an important role during neuronal development as a survival checkpoint that eliminates neurons with excessive DNA damage (27, 28). We performed a histologic analysis of the developing central nervous system (CNS) at E13.5 in Lig4−/−ATM−/− embryos and found that ATM deficiency, like p53 deficiency (11), substantially alleviated the increased neuronal apoptosis in this NHEJ-deficient background (Fig. 1b). Similar conclusions were reached by a parallel study (29). However, levels of pyknosis remained substantial in Lig4−/−ATM+/− embryos (Fig. 1c, data not shown), which is in contrast to the significant level of rescue observed in the Lig4−/−p53+/− genotype (11). To confirm these observations, we assayed for cellular DNA fragmentation, a hallmark of apoptosis, by TUNEL. Significantly, we observed few TUNEL-positive cells in Lig4−/−ATM−/− embryos, as opposed to the high numbers in Lig4−/−ATM+/− embryos (Fig. 1c). These findings, together with our earlier findings of essentially complete rescue by p53 deficiency (11), suggest that most of the increased neuronal apoptosis in the developing Lig4−/− CNS is signaled by ATM via p53.
In Lig4-deficient animals, B cell development is blocked at the B220+IgM− progenitor stage and T cell development is blocked at the CD4− CD8− progenitor stage because of inability to complete V(D)J recombination via the NHEJ pathway (11). Notably, p53 deficiency does not rescue either B or T cell development in the Lig4-deficient background because of its inability to rescue NHEJ (11). A parallel study to ours found that ATM deficiency also failed to restore Lig4-deficient T cell development (29). Surprisingly however, the latter study also reported that development of neonatal Lig4−/−ATM−/− splenic B cells was similar qualitatively to that of wild-type controls, with Lig4−/−ATM−/− B cells apparently progressing to the B220+IgM+ B cell stage (29). Such differentially “rescued” B cell development would imply substantial rescue of V(D)J recombination by ATM deficiency in Lig4-deficient B cells but not Lig4-deficient T cells. However, in preliminary studies, we found that both B and T cell development remained severely impaired at the progenitor stage in an analysis of thymocytes and spleen cells of a neonatal Lig4−/−ATM−/− mouse (postnatal-day P1; data not shown).
Because of poor survival and poor health, it is difficult to obtain large numbers of viable postnatal Lig4−/−ATM−/− animals for analyses of lymphocyte development. Moreover, it is impossible to obtain a Lig4−/− control mouse at this postnatal stage. Therefore, to assess the effects of ATM deficiency on Lig4 deficient B cell development more thoroughly, we used an in vitro differentiation system to determine the ability of fetal liver progenitor B cells from the various backgrounds to differentiate into IgM+ B cells (5). As a positive control, we observed progression of B220+IgM− progenitors to the B220+IgM+ B cell stage in all of 45 independent (each from a different embryo) fetal liver cultures from the various ATM genotypes on either a Lig4+/+ or Lig4+/− background (Fig. 1d, representative data are shown). However, we found no obvious rescue of B cell development beyond the IgM− pro-B stage in eight independent Lig4−/−ATM−/− cultures, which appeared similar to seven Lig4−/−ATM+/− and four Lig4−/−ATM+/+ cultures that served as negative controls (Fig. 1d, representative data are shown). We conclude that in the strains we have studied, ATM deficiency, similar to p53 deficiency, does not lead to significant rescue of Lig4-deficient B cell development beyond the IgM− pro-B stage. Potential reasons for this apparent discrepancy with the parallel study (29) will be considered in the Discussion.
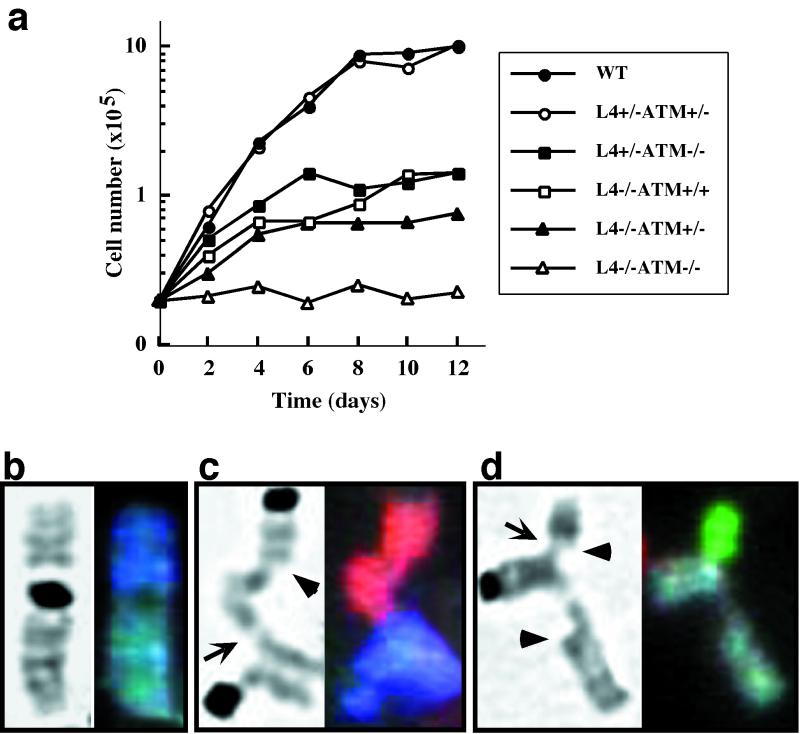
Increased doubling times and premature senescence are characteristics of Lig4-deficient MEFs, and p53 deficiency rescues these phenotypes (5, 11). In striking contrast, ATM deficiency further impaired the ability of Lig4−/− cells to proliferate in culture (Fig. 2a). The Lig4−/−ATM−/− MEFs showed nearly complete growth arrest under normal culture conditions, even at the earliest passage analyzed (passage 2–3). This severe growth defect correlates with a premature senescence phenotype as assayed by the senescence-associated β-galactosidase activity assay (data not shown). Under the same culture conditions, Lig4- and ATM-deficient MEFs exhibited significantly reduced growth rates but still retained measurable proliferative capacity (Fig. 2a; refs. 5 and 30). It seems likely that the dramatically impaired growth exhibited by Lig4−/−ATM−/− MEFs may result from overwhelming genomic instability in the absence of NHEJ factors and ATM (see below).
Figure 2.
Growth analysis and cytogenetic studies of MEFs. (a) Growth curves of Lig4−/−ATM−/− MEFs with controls. Genotypes are as indicated in the graph. (b–d) Metaphase chromosomes from primary MEF cultures. Arrows indicate single chromatid fusions; arrowheads indicate single chromatid breaks. (b) Robertsonian translocation (chromosome fusion) in an ATM−/− metaphase involving chromosomes 4 (dark blue) and 1 (gray). (c and d) Partial translocations in Lig4−/−ATM−/− metaphases. (c) Chromosome 2 (red) contains a broken chromatid in which one end remains free and the other is fused to a chromatid on chromosome 13 (bright blue). (d) Chromosome 1 (gray) contains a broken chromatid with two free ends, whereas the other chromatid is fused to a single chromatid of chromosome 8 (green).
We used spectral karyotyping (SKY), a chromosome-painting technique, to assay for chromosomal abnormalities in Lig4−/−ATM−/− MEFs and developing lymphocytes. Lig4 or ATM deficiency alone significantly increased the frequency of chromosomal aberrations in early-passage MEFs (19). However, p53 deficiency, either alone or in combination with Lig4 deficiency, had no observable effect on genomic stability as assayed by SKY (19). In contrast, the combined Lig4 and ATM deficiencies resulted in an additive phenotype in which 100% of the metaphases examined contained at least one chromosomal abnormality (Table 2). In addition to the quantitative effect, the combined Lig4 and ATM deficiencies led to frequent appearance of complex aberrations in which single chromatids from different chromosomes were fused, whereas the sister chromatids of each either remained intact or were broken (Fig. 2 b–d). These unusual interchromosomal structures may represent partial translocations in which intermediates are trapped because of impaired ability of cells to complete the generation of translocations in the absence of both Lig4 and ATM functions. SKY analysis of fetal liver progenitor B cell cultures revealed further differences between the effects of p53 and ATM deficiencies (Table 2). Surprisingly, of the metaphases examined, neither Lig4 nor p53 deficiency, either as single or double mutants, led to genomic instability in developing B lineage cells. However, ATM deficiency caused observable levels of genomic instability in the developing B cell cultures, and in Lig4−/−ATM−/− populations instability was further increased. Overall, our findings indicate that ATM and the NHEJ pathway play separate but complementary roles in maintaining genomic stability and support the notion that ATM plays a more significant role than p53 in suppressing chromosomal aberrations.
Table 2.
Combined effects of ATM and Lig4 deficiencies result in increased genomic stability
| Genomic instability in MEFs
| ||||
|---|---|---|---|---|
| Lig4+/−ATM+/− | Lig4−/− | ATM−/− | Lig4−/−ATM−/− | |
| Metaphases karyotyped | 24 | 22 | 22 | 21 |
| Fragmented chromatids and chromosomes | 1 | 26 | 21 | 34 |
| Translocations | 0 | 1 | 1 | 0 |
| Partial translocations | 0 | 0 | 1 | 7 |
| Robertsonian translocations | 0 | 1 | 2 | 4 |
| Metaphases with structural abnormality | 4% | 54% | 61% | 100% |
| Genomic instability in developing
lymphocytes
| ||||||
|---|---|---|---|---|---|---|
| Wild type | Lig4−/− | p53−/− | Lig4−/−p53−/− | ATM−/− | Lig4−/−ATM−/− | |
| Metaphases karyotyped | 34 | 26 | 29 | 16 | 38 | 35 |
| Fragmented chromatids and chromosomes | 0 | 0 | 0 | 0 | 8 | 12 |
| Robertsonian translocations | 0 | 0 | 0 | 0 | 0 | 2 |
| Metaphases with structural abnormality | 0% | 0% | 0% | 0% | 16% | 42% |
The number of events observed in each of the categories of chromosomal aberrations was scored for metaphases from MEFs (Upper) and developing lymphocytes from fetal liver cultures (Lower) of the indicated genotypes. The percentage of metaphases with any structural abnormalities is shown on the bottom row. Some metaphases contain multiple anomalies.
To explore the interplay between ATM and the NHEJ pathway further, Ku70+/− (21), Ku80+/− (22), and mice homozygous for the scid mutation (which inactivates DNA-PKcs) were bred with ATM+/− mice. The resulting Ku70+/−ATM+/−, Ku80+/−ATM+/−, and scid−/−ATM+/− progeny were intercrossed to generate NHEJ−/− offspring in ATM+/+, ATM+/−, and ATM−/− backgrounds. We note that Ku70−/−, Ku80−/−, and scid−/− mice are born in expected Mendelian ratios after crosses of their respective heterozygous parents (8). In addition, these ratios were not influenced significantly by p53 deficiency (12–16). Surprisingly however, we observed no live-born Ku70−/−ATM−/−, Ku80−/−ATM−/−, or scid−/−ATM−/− pups from these intercrosses, indicating that ATM deficiency induces embryonic lethality in a DNA-PK-deficient background (P < 0.001 for each intercross; Table 1). Moreover, Ku80/ATM double-deficient embryos were not represented at E11.5, indicating that death occurred early in development (P < 0.001; Table 1). This embryonic lethality was not associated with a specific ATM−/− background, because we tested different ATM-deficient strains in the context of the Ku70 (26), Ku80 (25), and scid (24) breedings (Table 1). Therefore, these observations coupled with the contrasting results of ATM deficiency in rescuing Lig4−/− embryonic lethality point to a unique and vital role for the DNA-PK holoenzyme during embryonic development, which is distinct from its role in Lig4-dependent NHEJ.
Discussion
ATM deficiency significantly rescues embryonic lethality and increased neuronal apoptosis associated with Lig4 deficiency. On the basis of our previous findings of similar rescue by p53 deficiency (10, 11), our current findings indicate that most neuronal apoptosis results from an ATM-signaled p53 response to unrepaired DSBs in the NHEJ deficient background. This interpretation is consistent with the observation that ionizing-radiation-induced apoptosis of neuronal cells is an ATM-dependent response (27). Likewise, our findings indicate that the ATM pathway is a major contributor to the embryonic lethality associated with Lig4 deficiency. Because the ATM-dependent cellular response is specific to DSBs, these findings further support the notion that DSBs, as opposed to other types of damage, are the major offending lesions in developing NHEJ-deficient neurons. Finally, we note that ATM deficiency, like p53 deficiency, failed to substantially rescue impaired B lymphocyte development that occurs in a Lig4−/− background. Our preliminary analyses also indicate that ATM deficiency similarly failed to rescue T cell development. The latter observations further suggest that absence of ATM, similar to absence of p53 (10, 11), does not rescue Lig4-deficient lymphocyte development, likely because of failure to restore NHEJ required for normal joining of RAG-liberated V, D, and J ends.
Our findings of severely impaired development of ATM−/−Lig4−/− B lineage cells beyond the IgM− progenitor stage contrasts with those of a parallel study that reports development of ATM−/−Lig4−/− B cells to the IgM+ stage (29), which would require V(D)J recombination to allow expression of both Ig heavy- and light-chain genes. One possibility for this apparent discrepancy would be strain differences. In this regard, there are no reports of the effects of the homozygous Lig4 mutation (7) used in the parallel study on lymphocyte development in the absence of ATM deficiency (29); conceivably, this mutation could be leaky. However, more detailed molecular analyses still may reveal subtle differences, perhaps strain dependent, in the repair of DSBs between the p53- and ATM-deficient backgrounds; for example, because of the absence of additional cell-cycle checkpoints in the latter that theoretically lead to ability to generate functional V(D)J ends. Finally, we note that the apparent rescue of B cell development observed in the parallel study may have resulted from an overestimate of the numbers of IgM+ cells in all samples. In this regard, essentially no B220+IgM− B lineage cells (that should be resident even in wild-type neonatal spleen) were noted in any reported sample (29). In any case, we conclude that in our lines ATM deficiency does not result in a substantial rescue of Lig4-deficient B cell development.
Despite the qualitatively similar effects of p53 and ATM deficiencies on the rescue of Lig4−/− embryonic lethality and neuronal apoptosis, our studies demonstrate significant differences in the function of these cellular gatekeepers. Thus, ATM deficiency significantly augments the premature senescence and growth defects exhibited by Lig4-deficient MEFs and enhances the spontaneous genomic instability in these cells. In contrast, p53 deficiency has been shown to rescue the growth defects of Lig4−/− MEFs and has no significant impact on genomic instability. These differences likely result from loss of the more pleiotropic functions of ATM in comparison to those of p53. ATM-deficient cells exhibit multiple defects in cell-cycle checkpoints (G1/S transition as well as S phase and G2/M boundary), which may decrease the potential for accumulated DSBs to be repaired efficiently by NHEJ-independent repair pathways in the Lig4−/−ATM−/− background. In addition, ATM plays a distinct role in controlling DSB repair (20). This role may be mediated through the regulation of DNA-repair proteins such as NBS1 (31–34) and BRCA1 (35, 36). The combined effects of loss of these ATM-dependent functions may contribute to the early postnatal death of Lig4−/−ATM−/− mice, which contrasts with the survival of Lig4−/−p53−/− mice into young adulthood (11). In this regard, p53 deficiency allows cells normally arrested at G1 to progress into other phases of the cell cycle in which functional checkpoints may permit damage repair by alternative pathways (i.e., homologous recombination; ref. 37).
In contrast to Lig4 (or XRCC4) deficiency, which causes late embryonic lethality, mice deficient in Ku- or DNA-PKcs survive into adulthood and have a relatively normal lifespan. Although p53 deficiency dramatically rescued the embryonic lethality of Lig4 (or XRCC4) deficiency, it had no impact on development in Ku- or DNA-PKcs-deficient mice. Strikingly, despite the ability of ATM deficiency to rescue embryonic lethality of Lig4 deficiency, it caused early embryonic lethality in Ku- and DNA-PKcs-deficient backgrounds. This synthetic lethal effect is distinct from the embryonic lethality caused by mutation in Lig4 or XRCC4, which leads to death of the mutant embryos at a significantly later stage of development. On the basis of our findings, we conclude that the DNA-PK holoenzyme must have a function that is distinct from its role in the NHEJ pathway of DNA repair. Based on the known function of the evolutionarily conserved Ku heterodimer as a DNA end-binding factor, this additional DNA-PK function likely involves DSB recognition. Thus, it is possible that such a function may overlap with an essential ATM function involved in recognition of DNA lesions and initiation of subsequent cellular responses,leading to early death of embryos deficient for both ATM and DNA-PK activities. Alternatively, this DNA-PK function may be distinct from that of ATM but additive in the impact of its loss. For example, the two proteins may have different functions in maintenance of telomere length (18, 38, 39). Further resolution of such issues may be provided by analyses of cells and mice containing conditional mutations that inactivate ATM and/or DNA-PK function.
Acknowledgments
We thank Dr. A. Wynshaw-Boris for providing the ATM+/− mice used in the Ku80 breedings. We also thank Dr. Ronald DePinho for critically reading this manuscript. This work was supported by National Institutes of Health Grants AI35714 and AI20047 (F.W.A.), CA77563 (Y.X.), and AI01428 (K.M.F.). J.S. is the Richard D. Frisbee III Foundation Fellow of the Leukemia and Lymphoma Society, and Y.G. was an associate and F.W.A is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- DSB
double-strand break
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- NHEJ
nonhomologous end-joining
- PK
protein kinase
- MEF
embryonic fibroblast
- scid
severe combined immunodeficient
- En
embryonic day n
References
- 1.Pfeiffer P, Goedecke W, Obe G. Mutagenesis. 2000;15:289–302. doi: 10.1093/mutage/15.4.289. [DOI] [PubMed] [Google Scholar]
- 2.Dasika G K, Lin S C, Zhao S, Sung P, Tomkinson A, Lee E Y. Oncogene. 1999;18:7883–7899. doi: 10.1038/sj.onc.1203283. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler K W, Vogelstein B. Nature (London) 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 4.Jeggo P A. Radiat Res. 1998;150:S80–S91. [PubMed] [Google Scholar]
- 5.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H L, Davidson L, Kangaloo L, Alt F W. Nature (London) 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Sun Y, Frank K M, Dikkes P, Fujiwara Y, Seidl K J, Sekiguchi J M, Rathbun G A, Swat W, Wang J, et al. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 7.Barnes D E, Stamp G, Rosewell I, Denzel A, Lindahl T. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 8.Sekiguchi J M, Gao Y, Gu Y, Frank K, Sun Y, Chaudhuri J, Zhu C, Cheng H-L, Manis J, Ferguson D, et al. Cold Spring Harbor Symp Quant Biol. 1999;64:169–181. doi: 10.1101/sqb.1999.64.169. [DOI] [PubMed] [Google Scholar]
- 9.Lewis L K, Resnick M A. Mutat Res. 2000;451:71–89. doi: 10.1016/s0027-5107(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Ferguson D O, Xie W, Manis J, Sekiguchi J, Frank K M, Chaudhuri J, J, H, DePinho R A, Alt F W. Nature (London) 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 11.Frank K M, Sharpless N E, Gao Y, Sekiguchi J M, Ferguson D O, Zhu C, Manis J P, Horner J, DePinho R A, Alt F W. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 12.Difilippantonio M J, Zhu J, Chen H T, Meffre E, Nussenzweig M C, Max E E, Ried T, Nussenzweig A. Nature (London) 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanasse G J, Halbrook J, Thomas S, Burgess A, Hoekstra M F, Disteche C M, Willerford D M. J Clin Invest. 1999;103:1669–1675. doi: 10.1172/JCI6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nacht M, Strasser A, Chan Y R, Harris A W, Schlissel M, Bronson R T, Jacks T. Genes Dev. 1996;10:2055–2066. doi: 10.1101/gad.10.16.2055. [DOI] [PubMed] [Google Scholar]
- 15.Guidos C J, Williams C J, Grandal I, Knowles G, Huang M T, Danska J S. Genes Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 16.Lim D S, Vogel H, Willerford D M, Sands A T, Platt K A, Hasty P. Mol Cell Biol. 2000;20:3772–3780. doi: 10.1128/mcb.20.11.3772-3780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karanjawala Z E, Grawunder U, Hsieh C L, Lieber M R. Curr Biol. 1999;9:1501–1504. doi: 10.1016/s0960-9822(00)80123-2. [DOI] [PubMed] [Google Scholar]
- 18.Bailey S M, Meyne J, Chen D J, Kurimasa A, Li G C, Lehnert B E, Goodwin E H. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson D O, Sekiguchi J M, Chang S, Frank K M, Gao Y, DePinho R A, Alt F W. Proc Natl Acad Sci USA. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. . (First Published May 23, 2000; 10.1073/pnas.110152897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotman G, Shiloh Y. Oncogene. 1999;18:6135–6144. doi: 10.1038/sj.onc.1203124. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Seidl K J, Rathbun G A, Zhu C, Manis J P, van der Stoep N, Davidson L, Cheng H L, Sekiguchi J M, Frank K, et al. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 22.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Nature (London) 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver D T, Alt F W. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Ashley T, Brainerd E E, Bronson R T, Meyn M S, Baltimore D. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 25.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-Boris A. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 26.Borghesani P R, Alt F W, Bottaro A, Davidson L, Aksoy S, Rathbun G A, Roberts T M, Swat W, Segal R A, Gu Y. Proc Natl Acad Sci USA. 2000;97:3336–3341. doi: 10.1073/pnas.050584897. . (First Published March 14, 2000; 10.1073/pnas.050584897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog K H, Chong M J, Kapsetaki M, Morgan J I, McKinnon P J. Science. 1998;280:1089–1091. doi: 10.1126/science.280.5366.1089. [DOI] [PubMed] [Google Scholar]
- 28.Chong M J, Murray M R, Gosink E C, Russell H R, Srinivasan A, Kapsetaki M, Korsmeyer S J, McKinnon P J. Proc Natl Acad Sci USA. 2000;97:889–894. doi: 10.1073/pnas.97.2.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y, Barnes D E, Lindahl T, McKinnon P J. Genes Dev. 2000;14:2576–2580. doi: 10.1101/gad.837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown K D, Barlow C, Wynshaw-Boris A. Am J Hum Genet. 1999;64:46–50. doi: 10.1086/302223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Ranganathan V, Weisman D S, Heine W F, Ciccone D N, O'Neill T B, Crick K E, Pierce K A, Lane W S, Rathbun G, et al. Nature (London) 2000;405:477–482. doi: 10.1038/35013089. [DOI] [PubMed] [Google Scholar]
- 32.Zhao S, Weng Y C, Yuan S S, Lin Y T, Hsu H C, Lin S C, Gerbino E, Song M H, Zdzienicka M Z, Gatti R A, et al. Nature (London) 2000;405:473–477. doi: 10.1038/35013083. [DOI] [PubMed] [Google Scholar]
- 33.Gatei M, Young D, Cerosaletti K M, Desai-Mehta A, Spring K, Kozlov S, Lavin M F, Gatti R A, Concannon P, Khanna K. Nat Genet. 2000;25:115–119. doi: 10.1038/75508. [DOI] [PubMed] [Google Scholar]
- 34.Lim D S, Kim S T, Xu B, Maser R S, Lin J, Petrini J H, Kastan M B. Nature (London) 2000;404:613–617. doi: 10.1038/35007091. [DOI] [PubMed] [Google Scholar]
- 35.Cortez D, Wang Y, Qin J, Elledge S J. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 36.Gatei M, Scott S P, Filippovitch I, Soronika N, Lavin M F, Weber B, Khanna K K. Cancer Res. 2000;60:3299–3304. [PubMed] [Google Scholar]
- 37.Morrison C, Sonoda E, Takao N, Shinohara A, Yamamoto K, Takeda S. EMBO J. 2000;19:463–471. doi: 10.1093/emboj/19.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hande P, Slijepcevic P, Silver A, Bouffler S, van Buul P, Bryant P, Lansdorp P. Genomics. 1999;56:221–223. doi: 10.1006/geno.1998.5668. [DOI] [PubMed] [Google Scholar]
- 39.Vaziri H. Biochemistry (Moscow) 1997;62:1306–1310. [PubMed] [Google Scholar]