Abstract
Background
Studies on artificial hybridization of different Anguilla species were conducted recently, i.e. female A. australis with male A. dieffenbachii, and female A. japonica with male A. anguilla. The existence of these artificial hybrids was however not demonstrated by independent genetic methods. Two species - A. anguilla and A. australis - that are phylogenetically close but have different sexual maturation times (12-25 weeks and 6-8 weeks, respectively), were expected to produce favourable hybrids for reproduction studies.
Results
A modification of the protocol for the reproduction of Anguilla japonica was used to produce eight-day Anguilla australis larvae, with a success rate of 71.4%. Thus ten out of 14 females produced eggs that could be fertilized, and three batches resulted in mass hatching. Hybrid larvae from female A. australis x male A. Anguilla survived for up to seven days post fertilization (dpf). The early development of the hybrid showed typical characteristics of A. anguilla tail pigmentation at 50 hours post fertilization (hpf), indicating expression of genes derived from the father.
Conclusions
In this paper we describe the first production of hybrid larvae from male A. anguilla and female A. australis and their survival for up to 7 dpf. A species-specific nucleotide difference in the 18 S rDNA gene confirmed that genes from both A. australis and A. anguilla were present in the hybrids. The developmental stages of the hybrid eel embryos and larvae are described using high resolution images. Video footage also indicated a heart beat in 5-dpf larva.
Background
A number of research groups have attempted artificial reproduction in various species of eel: A. japonica [1-5], A. anguilla [[6-9], Tomkiewicz, unpublished data], A. dieffenbachii [10], A. australis [[10], Kurwie, unpublished data], and A. rostrata [10]. Some Japanese scientists have also overcome major problems associated with developing artificial feeds for larvae and have successfully produced leptocephalus larvae [11] and glass eels [12,13]. Tanaka and his co-workers used a mix of shark egg powder, soya peptide, minerals, vitamins and krill paste [11] to develop a successful feed for A. japonica. Further research is, however, needed to develop suitable diets and rearing techniques for the production of larvae of other Anguilla species and their hybrids.
European eel (A. anguilla) females have a much slower, and widely-variable, response to hormonal stimulation [9] when compared to females of other freshwater eel species (e.g. A. japonica and A. australis). At the onset of the natural spawning migration, the gonadosomatic index (GSI) of A. anguilla females is close to 2% [A Palstra, unpublished data] and they are still in a previtellogenic state when they migrate to sea. However, females of A. australis have a higher GSI, of up to 4% [14], indicating that they are sexually more advanced than A. anguilla at the same stage The same holds true for A. japonica, which has a GSI of up to 4% at the commencement of its spawning migration [15]. Induction of vitellogenesis and final maturation in A. australis requires approximately six to eight weekly hormonal injections [[10], Kurwie, unpublished data] while 9-12 injections [4], or 6-15 weekly injections [11], are required for A. japonica and up to 12-25 weekly injections for A. anguilla [7-9].
There are several reasons for testing hybridization between European and New Zealand short finned eels. There are large differences in silver eel maturation states between these species. In contrast to the stage reached by A. australis, silver eels of A. anguilla have not yet commenced vitellogenesis. Shortening the artificial trajectory may overcome vitellogenic abnormalities, resulting in higher gamete quality and higher success rates of fertilization, hatching and larval development.
Anguilla anguilla is listed by the IUCN as critically endangered [16], which raises some problems in association with the culture of this species. Farming is reliant on the influx of wild glass eel, thereby pressurizing wild stocks. Breeding for aquaculture is, nevertheless, supposed to take pressure off wild stocks. Therefore, the hybridization of A. anguilla with a species such as A. australis, that has a short artificial trajectory, may be a suitable option for aquaculture. Since maturation levels at silvering are very different in the parent species, it is quite possible that the maturation level of the hybrid at the silver stage would be far more advanced than that of the European silver eel. Furthermore, since A. australis lives in the southern hemisphere, its migration is in January-June [17], in contrast to A. anguilla, which migrates in October-November. To gain insights into the combination of the properties of A. australis and A. anguilla present in hybrids, it is useful to study eel reproduction and to compare the early ontogeny of these species including their hybrids.
A number of studies on hybridization of various eel species have been previously conducted: for example, female A. australis with male A. dieffenbachii [10], and female A. japonica with male A. anguilla [18]. The existence of the artificial hybrids has not, however, been demonstrated by independent genetic methods. In contrast, genetic evidence for natural hybrids between the Atlantic species A. rostrata and A. anguilla has been demonstrated [19]. Since A. anguilla and A. australis are phylogenetically more closely related than some other hybrids (for example, A. anguilla and A. japonica [20]), we hypothesized that hybridization between the former two species would be possible. In this paper we describe experiments on the hybridization of A. anguilla and A. australis and post-fertilization survival levels. Investigations into the18 S rDNA gene -- for the purpose of genetic validation -- are also described.
Results
Reproduction
After 4 weeks of injections with hCG, the first A. australis male started to spermiate and all males had spermiated within 6 weeks. After 9 weeks the injections were stopped. Three of the 15 A. anguilla males produced sperm after 5 weekly injections and all males of A. anguilla had produced sperm after 6 weekly injections. Before use, the males received a booster hCG injection to reactivate spermiation.
In most females, hormone treatment resulted in a rapid increase in body weight after 9-13 injections with salmon pituitary extract (SPE). Ten of the 14 females (71.4%) ovulated once and seven females ovulated twice during this study. The second ovulation was induced 2 weeks after the first ovulation by a single injection of 20 mg SPE/kg dissolved in 1 mL 0.9% saline, one priming injection of SPE, and an injection with 17, 20β-dihydroxy-4-pregnen-3-one (DHP) one day later. Three females did not respond to the SPE treatment and one female died after the DHP injection, just before ovulation. In total, three out of the ten batches of eggs produced larvae (33.3%). The larvae of two of the three batches from A. australis x A. australis stayed alive for 5 dpf, and the larvae from one batch of the hybrid A. australis x A. anguilla stayed alive for 7 dpf. Some of the eggs from one batch of A. australis were reared at 25°C, which resulted in larvae that survived until 8 dpf.
Embryogenesis and early larval development of A. australis
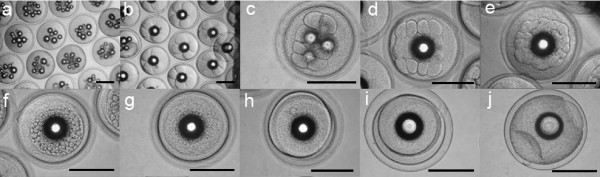
After fertilization (Figure 1a), developing eggs floated to a level just below the surface of the water, resulting in a clear separation from undeveloped eggs, which sank. Cell divisions occurred every 30-60 minutes. The 4-cell and 8-cell states were observed at the 1.5 hpf stage (Figure 1b) and the 2 hpf stage (Figure 1c), respectively. The 16-cell stage and 32-cell stage were observed at 2.5 hpf (Figure 1d) and 4 hpf (Figure 1e), respectively. The morula stage (Figure 1f) and the blastula stage (Figure 1g) were observed at 6 hpf and 7 hpf, respectively. An embryonic shield started to form between 9 and 13 hpf (Figure 1h and 1i), and the late gastrula stage was observed at about 20 hpf (Figure 1j). Somitogenesis started between 20 and 24 hpf (Figure 2). The Kuppfer's vesicle (Figure 2c, for description see [21]) was observed at about 26 hpf, and the first heartbeat occurred at about 43 hpf.
Figure 1.
Embryogenesis of A. australis. Early ontogeny until the late gastrula stage: a) Fertilized eggs, ~0.5 hours post fertilization (hpf); b) 4-cell stage, ~1.5 hpf; c) 8-cell stage, ~2 hpf; d) 16-cell stage, ~2.5 hpf; e) 32-cell stage, ~4 hpf; f) morula stage, ~6 hpf; g) blastula stage, ~7 hpf; h) and i) early gastrula stage (shield stage), 9-13 hpf; j) late gastrula stage, 20 hpf. (Scale bar = 1 mm).
Figure 2.
Somitogenesis in A. australis embryos. Stages of somitogenesis: a) 6-7 somites, ~24 hpf; b) 11 somites, ~26 hpf; c) 17 somites, ~30 hpf. (Scale bar = 0.5 mm; kv = Kuppfer's vesicle; sm = somites) Note: due to the limited depth of field of the microscope in relation to the size of the embryos, not all structures can be shown simultaneously.
Neutral buoyancy of the eggs was observed just before hatching. At approximately 43 hpf the embryos started to hatch and at that time had approximately 40 somites (Figure 3a; 45 hpf). After hatching (Figure 3b; 2.5 dpf) the larvae (at first C-shaped) were positioned upright in the water column, probably due to the position of the oil droplet. It appeared that the larvae were neutrally buoyant at 35 ppt. The larvae were immobile, except when disturbed by light or vibrations, which caused very fast and short horizontal movements. During sampling procedures (by pipette) the larvae avoided suction, and swam in the opposite direction at speeds of up to several body lengths per second. Sampling caused mechanical damage to larvae, followed by death within a short period. Discoloration of the brain and neural tube was observed within a few seconds, followed by cellular breakdown of the larvae.
Figure 3.
Hatching of A. australis. Structures in larvae: a) hatching larva, ~45 hpf; b) larva at 2.5 dpf. (Scale = 0.5 mm; ov = otic vesicle with otoliths).
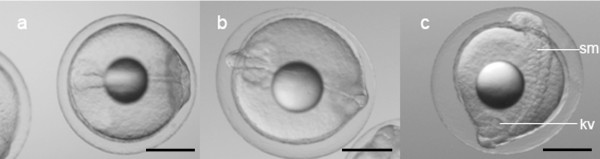
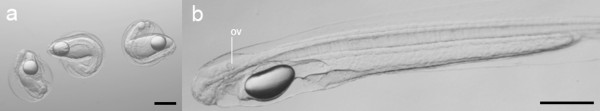
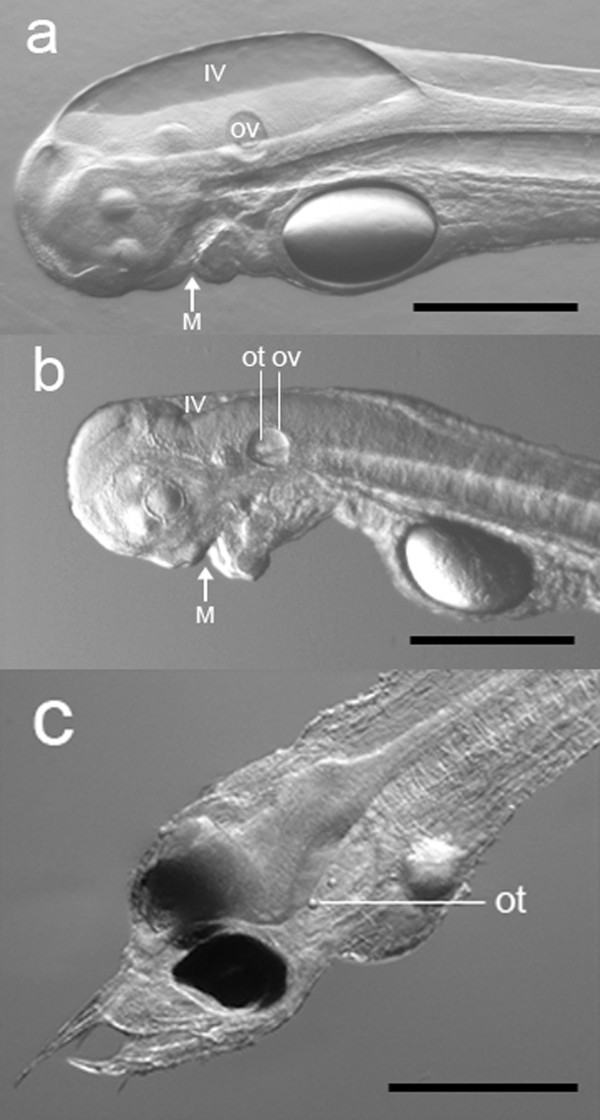
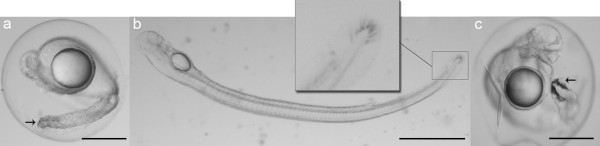
Larvae elongated during development and lateral neuromast cells on the flank were observed at 68 hpf. Head development showed remarkable changes over time, especially a decrease in volume of the 4th ventricle between 5 and 6.5 dpf (Figure 4a, b), the protrusion of the mouth and development of teeth between 5 and 8 dpf, and pigmentation of the eyes at 8 dpf (Figure 4c). The angle of the head also increased in such a way that the mouth protruded anteriorly. At 8 dpf the larvae showed well developed teeth and a straightened head, indicating that they had reached the feeding stage. No visual differences in development between larvae from A. australis and the hybrid species were observed, except for the development of tail pigment cells, which were already present at about 2 dpf in the hybrid. In contrast, the pigmented cells appeared much later (5-6 dpf) in A. australis (Figure 5a, b).
Figure 4.
Head development of A. australis. Head structures of A. australis: a) 5 dpf larva; b) 6.5 dpf larva; c) 8 dpf larva reared at 25°C (representing a stacking of two illustrations of the same larva). Note the remarkable differences between 5 dpf and 6.5 dpf larvae in the development of the 4th ventricle (IV) (with a decrease in volume) and the protrusion of the mouth (M). Between 6.5 dpf and 8 dpf, the formation of teeth commences, the angle of the head increases, and the eyes become pigmented. (Scale bar = 0.5 mm; ov = otic vesicle with otoliths; ot = otoliths).
Figure 5.
Timing of tail pigmentation. Differences in timing of tail pigmentation in A. australis and the A. australis x A. Anguilla hybrid: a) hybrid species at ~50 hpf; b) A. australis at 6 dpf; c) A. anguilla at 60 hpf. (Scale bar in a,c= 0.5 mm, in b= 1 mm; arrows indicate pigmentation of the tail).
Video recordings were made of several larvae, to observe the heart beat. The heart is bent in an S-shape, contracting regularly at a frequency of about 54 beats per minute in a 5 dpf larva (Additional file 1). There were no significant differences between the heart beat rates of 2 dpf and 5 dpf larvae, suggesting that the rate is based on the innate rhythm of heart muscle tissue.
DNA analysis
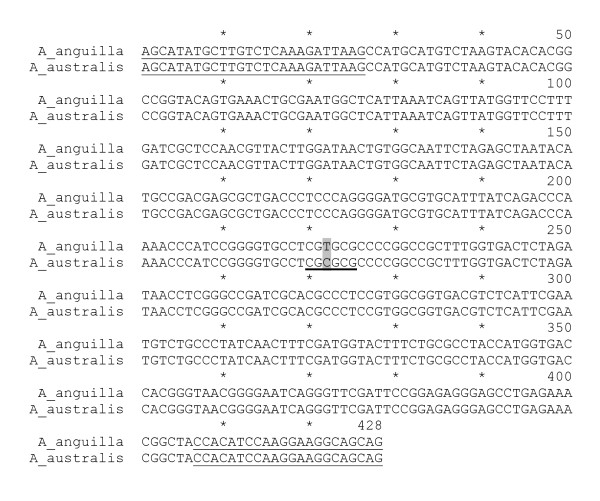
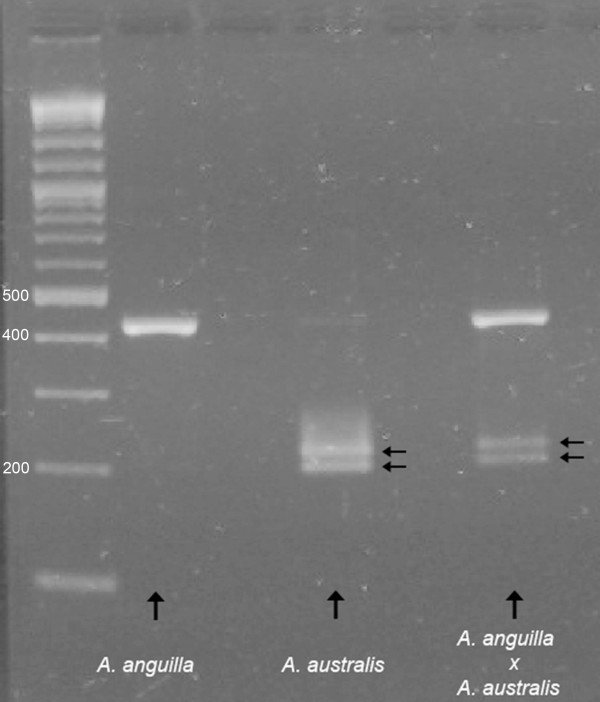
A species-specific nucleotide difference in the 18 S rDNA genes of A. australis and A. anguilla (Figure 6) allowed us to provide genetic evidence that we had produced hybrid offspring from the two species. The PCR product amplified from the 18 S rDNA gene is 428 bp long and has a single mismatch between A. anguilla and A. australis at position 222, resulting in a BssHII restriction site, specific for the A. australis product (Figure 6). BssHII digestion of the PCR product from A. australis therefore results in 207 bp and 221 bp fragments, whereas the 428 bp PCR product from A. anguilla is not digested by BssHII. As the hybrid species must contain both the A. anguilla and the A. australis 18 S rDNA genes, three fragments were expected. Figure 7 clearly shows that the parental species A. anguilla and A. australis, as well as their hybrid, can be identified using the 18 S rDNA gene.
Figure 6.
PCR fragments from the 18 S rDNA genes. Alignment of the PCR fragments amplified from the 18 S rDNA genes of A. anguilla and A. australis (location of forward and reverse PCR primers is single underlined; the restriction site for BssHII (position 220-225) is thick underlined; species-specific nucleotide difference at position 222 is highlighted in grey).
Figure 7.
PCR runs showing species-specific differences. Identification of both parental species, A. anguilla and A. australis, and their hybrid, A. anguilla × A. australis, based on the species-specific nucleotide difference in nuclear 18 S rDNA. Arrows indicate the two fragments after restriction with the enzyme BssHII; the first lane indicates the position of the DNA size marker.
Discussion
In this study, we succeeded in breeding Anguilla australis under artificial conditions and produced free swimming larvae of this species. In addition, hybrids of A. australis x A. anguilla were produced for the first time and genetic methods were used to confirm the existence of this hybrid. The hybrid larvae were kept alive for a maximum of 7 dpf and the larvae of A. australis for a maximum of 8 dpf.
Larvae of A. japonica reach the feeding stage at approximately 7 days after hatching [3]. This development is strongly temperature dependent and for A. japonica the optimal temperature was shown to be about 25°C [22-24]. It was noted that hatching also occurred at about 25°C [25] at the spawning site, suggesting that this may be the optimal temperature for early development. It was, however, noted that within a few days the larvae were distributed over an extremely large area at much lower temperatures [25], suggesting that early larvae are able to develop at a wide range of temperatures.
In our study, the larvae raised at 25°C (Figure 4c) were sufficiently developed to start feeding. In contrast, the 7 dpf larvae that were raised at 21°C had not yet reached the feeding stage and the head was still in a tilted position. The ten dpf larvae of A. australis -- collected by T. Kurwie at (illustrated in [26]) a prevailing temperature of 21°C -- were slightly more developed, although the 4th ventricle was still large and the eyes were not as fully pigmented, as in the 8 dpf larvae reared at 25°C (Figure 4d). This clearly indicated that the main differences occur in the development of the head. Compared to 8 dpf larvae reared at 25°C, the mouths of 10 dpf larvae reared at 21°C were not fully developed, the teeth were just starting to form, and the mouth angle was not fully protruded anteriorly. This shows that development is highly temperature dependent and that larval development cannot be indicated by age alone.
Compared to other species, such as zebrafish [27] and medaka [28], development of the Anguilla head seems relatively slow. On the other hand, the appearance of the lateral neuromast cells, which are part of the mechanosensory system, was already observed on the flanks at approximately 1 dpf. After hatching, the larvae were very sensitive to vibrations, indicating that mechanoreception is well developed at this stage. Similar results were also reported for A. australis [10] and A. japonica. [29].
There were no visual differences in development and appearance between the two species other than the difference in timing of tail pigmentation, which occurred a few days earlier in the hybrid species than in A. australis. This seems to be a trait of the European eel, as a similar appearance of tail pigmentation was observed in A. anguilla [A Palstra, unpublished data] (Figure 5c). The reason for tail pigmentation occurring at this early stage remains unknown.
In studies on the natural hybrid of A. anguilla with A. rostrata [19,30], hybridization was validated by an independent method. This was not the case for recent artificial Anguilla hybridizations performed by Okamura et al. [18] and Lokman and Young [10]. Our results (Figure 7), based on the species-specific nucleotide difference in nuclear 18 S rDNA, show that both A. anguilla and A. australis and their hybrid A. anguilla × A. australis can been identified according to the method of Frankowski & Bastrop [30], in which the following fragments were produced: a single 18 S rDNA fragment for A. anguilla, two fragments for A. australis, and three fragments for the hybrid species, indicating that two alleles from both parent species were present in the hybrid.
There are still problems with artificial reproduction and larval rearing of Anguilla species, partly due to high individual variability in response to hormone treatments, and partly due to selecting the correct feed for larvae. Tanaka et al. [11] developed a reasonably successful feed for Japanese eel larvae although almost all larvae died before, or shortly after, the first feeding stage, which may have been due to the unnatural feeding methods that were employed. Possibly due to negative phototaxis, the larvae swim downwards towards the shark egg paste, where they encounter the food [[11]; Yoshimatsu, personal communication].
It is assumed that the natural food sources for leptocephalus larvae of Anguilla spp. are the oikopleura larvacean shelters (or 'marine snow'), which have been found in larval digestive tracts of several Anguilloid species [31]. Recent analysis of A. anguilla larval gut contents indicated that the diet of the smallest larvae consisted of a variety of plankton organisms, with Hydrozoa and Polycystinea species occurring most frequently [32]. So it seems that eel larvae may take a variety of available food from their immediate environment.
Conclusions
The applied reproduction method resulted in healthy embryos and larvae of New Zealand short finned eels (A. australis) as well as hybrids from male European eels (A. anguilla) crossed with female New Zealand short finned eels. The developmental stages of eel embryos and larvae are described by means of high resolution images. In this paper we described the first production of hybrid larvae from male A. anguilla and female A. australis and their survival for up to 7 days post fertilization (dpf). Evidence for hybridization is based on a single nucleotide difference in the 18 S rDNA gene of both species. This is the first time that an artificial Anguilla hybrid has been validated by means of a genetic tool. Future work with this hybrid may provide further understanding of the reproductive mechanisms that affect breeding of the European eel, which is now on the CITES list of critically endangered species.
Methods
Eel collection
Silver females (n = 14; 80.1 ± 0.4 cm; 978.1 ± 19.5 g) and males (n = 8; 45.6 ± 1.4 cm; 172.5 ± 14.9 g) of New Zealand short-finned eels (A. australis) were caught in Lake Ellesmere in Christchurch, New Zealand, and transported to The Netherlands in aired plastic bags with a small amount of water, fitted into polystyrene boxes. Silver male European eels (A. anguilla) were purchased from the eel farm Royaal BV (Helmond, The Netherlands).
Anguilla australis females and males were kept together in a 1000 L tank filled with natural seawater, collected from Lake Grevelingen (30 ppt), and coupled to a 1500 L recirculation system (salinity 30 ppt, 21°C). The daily cycle was set with blue light (Philips special TLD Blue 36W/18) at 16:8 L:D. To compensate for the 11 h time difference between The Netherlands and New Zealand, the daily cycle was changed stepwise (1 h per week) to Central European Time (CET). Anguilla anguilla males (n = 15; 40.4 ± 0.6 cm; 118.8 ± 4.9 g) were kept in a 1500 L tank connected to a 2400 L recirculation system, in natural seawater (30 ppt, 21°C), under a complete dark regime. PVC pipes were introduced into both systems to provide refuges for animals. All animals were starved throughout the experiment, and treated on a weekly basis with Melafix (API aquarium pharmaceuticals, MARS Fishcare North America Inc., Chalfont, PA, USA) against infections. Prior to treatment, eels were anesthetized with 1-2 mL/L 10% clove oil (oil mixed 1:10 with absolute ethanol). At the start of this study all eels were tagged with passive transponders with unique identification numbers (Trovan, EID Aalten BV, Aalten, The Netherlands).
This experiment was approved by the animal ethical commission of the Leiden University (DEC# 08112).
Hormone treatments
Female eels were distributed into four groups, with the starting point of hormonal treatment for each group being shifted one week forward, on a weekly basis. Treatment followed a modified version of Ohta's protocol [4,5]. On the first day of the week, females were weighed and injected intramuscularly (IM), at a point approximately 1 cm below the rostral attachment of the dorsal fin, with 20 mg salmon pituitary extract (SPE; Argent Labs, Redmond, WA, USA) per kg dissolved in 0.9% saline. When a 5% increase in body weight (BW) -- with respect to initial BW -- was reached, females were transferred to a separate 400 L tank, connected to the same system. The BW was measured the day after the transfer and/or 2 days later. When a 10% increase in BW was reached, an oocyte sample was collected by means of inserting a cannula (polyethylene tube, inner diameter 1.4 mm) through the oviduct. Oocytes were checked under a microscope to ascertain developmental stages. When migration of the germinal vesicle -- still with many oil droplets in the oocytes (stage 3/4 in European eel according to [9]) -- was observed, the female was primed with a single injection of SPE (20 mg/kg dissolved in 1 mL 0.9% saline). After 24 h, ovulation was induced by means of intraperitoneal injections of 17, 20β-dihydroxy-4-pregnen-3-one (DHP 2 mg/kg, Sigma-Aldrich BV, Zwijndrecht, The Netherlands) dissolved in DMSO, administered at six to eight locations.
Male New-Zealand short-finned eels were treated according to a modified version of Ohta's protocol [4,5]. They were subjected to a weekly IM injection procedure (at a site approximately 0.5-1 cm below the rostral attachment of the dorsal fin) with 250 IU human chorionic gonadotropin (hCG, Sigma-Aldrich BV, Zwijndrecht, The Netherlands) dissolved in 0.1 mL 0.9% saline. Males were injected every week for up to 9 weeks and checked for spermiation by hand stripping. After 9 weeks, all males produced milt and injections were stopped until a female was ready to spawn. (This reduced handling stress; it was also noted that the sperm quality did not decline much during the "holding" period). On the day before the eggs were to be stripped, two to three males that demonstrated high sperm motility were selected and stimulated with a single booster dose of 500 IU dissolved in 0.1 mL saline. Sperm motility was determined visually under a microscope, after mixing a drop of sperm with a drop of seawater. Only sperm with at least 50% motility (continuous activity of > 50% of spermatozoa) was used for fertilization.
For the production of a hybrid between A. australis and A. anguilla, 15 farmed male European eels received weekly intraperitoneal (IP) hCG injections, according to the protocol for European eel [9], at a dose of 200 IU/male, followed by a booster dose of 1,000 IU hCG (in 0.2 ml 0.9% saline) 24 h before a fertilization trial.
Artificial fertilization and larval rearing
Two to three males per species were hand stripped 24 h after the hCG booster injection. Milt was collected in a syringe (10 mL) and kept on ice or in the refrigerator for a maximum of 48 h. Sperm motility was checked prior to fertilization by means of microscopic examination. Females were expected to ovulate between 11 and 15 h after the final injection with DHP. The artificial fertilization programme was terminated in cases when female spawning only commenced after more than 18 h after the final injection, which is indicative of low fertility and hatchability [5]. After the final injection, females were checked hourly for egg release, by gently pressing on the abdomen near the vent. When a female showed an ovarian plug, the plug was gently removed. Eggs were collected in plastic, pre-weighed, sterilized bowls. The first flow of eggs (~50 g) was not used for fertilization. The combined egg weight was determined after all eggs had been stripped.
The collected sperm was added to dry eggs in bowls and mixed. Fresh seawater (35 ppt, 20°C) was added, and after approximately 3-4 min the eggs were transferred into buckets with fresh (sterile) seawater (~20 L). A net (of mesh size 600 μm) was used to separate floating eggs from sinking eggs. The former were transferred, after 30-45 min, to another bucket containing fresh seawater. Finally, the eggs were transferred to 1 L glass beakers and/or 200 mL Petri dishes, for observation. At this stage they were kept in complete darkness at 21°C and a salinity of 35 ppt. Approximately 24 hours after incubation, the water was refreshed by transferring the still-floating eggs into new glass beakers or Petri dishes. During the trial, all white or sunken eggs were removed. A portion of eggs from one batch were also reared at 25°C.
DNA analysis
As described by Frankowski & Bastrop [30], parental species and their hybrids can be identified by means of polymorphism in nuclear 18 S rDNA. For our study we used a slightly different protocol, as described below.
Total DNA was isolated and purified from ten whole hybrid larvae and fin-clips of parental specimens, using a DNeasy Blood & Tissue Kit (Qiagen). The polymerase chain reaction (PCR) was performed using the FastStart High Fidelity PCR System protocol (Roche) and an amplification profile consisting of denaturation for 3 min at 94°C, 35 cycles of 30 s at 94°C, 30 s at 60°C and 1 min at 72°C, followed by 5 min at 72°C for final extension. Amplification was carried out according to the manufacturer's instructions in 50 μL 1 × FastStart High Fidelity Reaction Buffer containing 1.8 mM MgCl2, 2.5 U FastStart High Fidelity Enzyme Blend, 1 μg chromosomal DNA, 0.4 μM of the 18 S rDNA forward and reverse primers, and 0.2 mM dNTPs.
The sense (5'-AGC ATA TGC TTG TCT CAA AGA TTA AG-3') and antisense (5'-CTG CTG CCT TCC TTG GAT GTG G-3") primers were based on NCBI accession numbers FM946133 (A. australis) and FM946070 (A. anguilla) [23]. The PCR product was purified using a QIAquick PCR Purification Kit (Qiagen) and 0.5 μg of the purified fragments were digested with two units of the restricting enzyme BssHII (New England Biolabs Inc) according to the manufacturer's instructions. Restriction enzyme digestion was conducted in 10 μL reaction buffer for 1 h at 37°C. DNA fragments were made visible using a 2% agarose gel.
Authors' contributions
EB is a PhD student working on early development of Anguilla species. He carried out reproduction experiments, DNA-assays, developmental analyses and writing of the manuscript. SB is a biologist, who carried out reproduction experiments (with EB). TK is senior scientist at MTI; she designed the reproduction experiments. RD is a senior scientist at ZF; he designed the PCR-probe and assay and corrected the manuscript. PD is director of MTI; he planned transport of NZ eels and was involved in the reproduction design. At the time when these experiments were undertaken AP was a postdoctoral student involved in the reproduction design. HP is head of the Molecular Cell Biology Section and was involved in producing high resolution pictures and videos of embryos and larvae. GvdT is associate professor, supervisor of EB, and project leader. All authors read and approved the final manuscript.
Supplementary Material
Video clip of a 5 dpf embryo of A. australis with a heart rate of 54 beats per minute. The heart is S-shaped and beats regularly.
Contributor Information
Erik Burgerhout, Email: E.Burgerhout@biology.leidenuniv.nl.
Sebastiaan A Brittijn, Email: basbrittijn@gmail.com.
Tagried Kurwie, Email: tagried@mti.net.nz.
Paul Decker, Email: paul@mti.net.nz.
Ron P Dirks, Email: dirks@zfscreens.com.
Arjan P Palstra, Email: arjanpalstra@gmail.com.
Herman P Spaink, Email: h.p.spaink@biology.leidenuniv.nl.
Guido EEJM Van den Thillart, Email: g.van.den.thillart@biology.leidenuniv.nl.
Acknowledgements
We would like to thank D de Wijze and H Jansen for helping with the DNA analysis and P Niemantsverdriet for his practical assistance. We also thank G Lamers for her help with microscopic photography and K Bosma for editing the figures. This study was financed by a grant from the Dutch Ministry of Agriculture, Nature and Food Quality (#VISS/1447/2408008).
References
- Yamamoto K, Yamauchi K. Sexual-maturation of Japanese eel and production of eel larvae in aquarium. Nature. 1974;251:220–221. doi: 10.1038/251220a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Morioka T, Hiroi O, Omori M. Artificial maturation of female Japanese eels by injection of salmonid pituitary. Bulletin of the Japanese Society of Scientific Fisheries. 1976;40:1–7. [Google Scholar]
- Tanaka H, Kawaga H, Ohta H, Okuzawa K, Hirose K. The first report of eel larvae ingesting rotifers. Fish Science. 1995;61:171–172. [Google Scholar]
- Ohta H, Kagawa H, Tanaka H, Okuzawa K, Hirose K. Changes in fertilization and hatching rates with time after ovulation induced by 17,20β-dihydroxy-4-pregnen-3-one in the Japanese eel, Anguilla japonica. Aquaculture. 1996;139:291–301. doi: 10.1016/0044-8486(95)01167-6. [DOI] [Google Scholar]
- Ohta H, Kagawa H, Tanaka H, Okuzawa K, Hirose K. Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiology and Biochemistry. 1997;17:163–169. doi: 10.1023/A:1007720600588. [DOI] [Google Scholar]
- Bezdenezhnykh VA, Prokhorcik GA, Petrikov AM, Petukov VB, Plyuta MV. Obtaining the larvae of European eel Anguilla anguilla L. (Pisces, Anguillidae), under experimental conditions. Dokl Akad Nauk SSSR. 1983;268:1264–1266. (In Russian) [Google Scholar]
- Pedersen BH. Induced sexual maturation of the European eel Anguilla anguilla and fertilization of the eggs. Aquaculture. 2003;224:323–338. doi: 10.1016/S0044-8486(03)00242-4. [DOI] [Google Scholar]
- Pedersen BH. Fertilization of eggs, rate of embryonic development and hatching following induced maturation of the European eel Anguilla anguilla. Aquaculture. 2004;237:461–473. doi: 10.1016/j.aquaculture.2004.04.019. [DOI] [Google Scholar]
- Palstra AP, Cohen EGH, Niemantsverdriet PRW, van Ginneken VJT, van den Thillart GEEJM. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture. 2005;249:533–547. doi: 10.1016/j.aquaculture.2005.04.031. [DOI] [Google Scholar]
- Lokman PM, Young G. Induced spawning and early ontogeny of New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) New Zealand Journal of Marine and Freshwater Research. 2000;34:135–145. doi: 10.1080/00288330.2000.9516921. [DOI] [Google Scholar]
- Tanaka H, Kagawa H, Ohta H. Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture. 2001;201:51–60. doi: 10.1016/S0044-8486(01)00553-1. [DOI] [Google Scholar]
- Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K. The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiology and Biochemistry. 2003;28:493–497. doi: 10.1023/B:FISH.0000030638.56031.ed. [DOI] [Google Scholar]
- Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K. The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiology and Biochemistry. 2005;31:193–199. doi: 10.1007/s10695-006-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokman PM, Vermeulen GJ, Lambert JGD, Young G. Gonad histology and plasma steroid profiles in wild New Zealand freshwater eels (Anguilla dieffenbachii and A. australis) before and at the onset of the natural spawning migration. I. Females. Fish Physiology and Biochemistry. 1998;19:325–338. doi: 10.1023/A:1007719414295. [DOI] [Google Scholar]
- Okamura A, Yamada Y, Yokouchi K, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K. A silvering index for the Japanese eel Anguilla japonica. Environmental Biology of Fishes. 2007;80:77–89. doi: 10.1007/s10641-006-9121-5. [DOI] [Google Scholar]
- Freyhof J, Kottelat M. Anguilla anguilla. IUCN 2010. IUCN Red List of Threatened Species. 2008. Version 2010.4.
- Todd PR. Timing and periodicity of migrating New Zealand freshwater eels (Anguilla spp,) New Zealand Journal of Marine and Freshwater Research. 1981;15:225–235. doi: 10.1080/00288330.1981.9515915. [DOI] [Google Scholar]
- Okamura A, Zhang H, Utoh T, Akazawa A, Yamada Y, Horie N, Mikawa N, Tanaka S, Oka HP. Artificial hybrid between Anguilla anguilla and A. japonica. Journal of Fish Biology. 2004;64:1450–1454. doi: 10.1111/j.0022-1112.2004.00409.x. [DOI] [Google Scholar]
- Albert V, Jonsson B, Bernatchez L. Natural hybrids in Atlantic eels (Anguilla anguilla, A. rostrata): evidence for successful reproduction and fluctuating abundance in space and time. Molecular Ecology. 2006;15:1903–1916. doi: 10.1111/j.1365-294X.2006.02917.x. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Aoyama J, Inoue JG, Miya M, Nishida M, Tsukamoto K. Molecular phylogeny and evolution of freshwater eels genus Anguilla based on the whole mitochondrial genome sequences. Molecular Phylogenetics and Evolution. 2005;34:134–146. doi: 10.1016/j.ympev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost J. Kuppfer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Chang SL, Kou GH, Liao IC. Temperature adaptation of the Japanese eel (Anguilla japonica) in its early stages. Zoological Studies. 2004;43:571–579. [Google Scholar]
- Okamura A, Yamada Y, Horie N, Utoh T, Mikawa N, Tanaka S, Tsukamoto K. Effects of water temperature on early development of Japanese eel Anguilla japonica. Fisheries Science. 2007;73:1241–1248. [Google Scholar]
- Kurokawa T, Okamoto T, Gen K, Uji S, Murashita K, Unuma T, Nomura K, Matsubara H, Kim S, Ohta H, Tanaka H. Influence of water temperature on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. Journal of the World Aquaculture Society. 2008;39:726–735. doi: 10.1111/j.1749-7345.2008.00208.x. [DOI] [Google Scholar]
- Tsukamoto K, Yamada Y, Okamura A, Kaneko T, Tanaka H, Miller MJ, Horie N, Mikawa N, Utoh T, Tanaka S. Positive buoyancy in eel leptocephali: an adaptation for life in the ocean surface layer. Marine Biology. 2009;156:835–846. doi: 10.1007/s00227-008-1123-8. [DOI] [Google Scholar]
- Dufour S, van den Thillart G. In: Spawning migration of the European eel, reproduction index, a useful tool for conservation management. van den Thillart G, Dufour S, Rankin JC, editor. Vol. 30. Springer. Fish & Fisheries 2009, Series; Reproduction capacity of European eels; pp. 465–475. full_text. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mechanisms of Development. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Okamura A, Oka HP, Yamada Y, Utoh T, Mikawa N, Horie N, Tanaka S. Development of lateral line organs in leptocephali of the freshwater eel Anguilla japonica (Teleostei, Anguilliformes) Journal of Morphology. 2002;254:81–91. doi: 10.1002/jmor.10021. [DOI] [PubMed] [Google Scholar]
- Frankowski J, Bastrop R. Identification of Anguilla anguilla (L.) and Anguilla rostrata (Le Sueur) and their hybrids based on a diagnostic single nucleotide polymorphism in nuclear 18 S rDNA. Molecular Ecology Resources. 2009;10:173–176. doi: 10.1111/j.1755-0998.2009.02698.x. [DOI] [PubMed] [Google Scholar]
- Mochioka M, Iwamizu M. Diet of anguilloid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Marine Biology. 1996;125:447–452. [Google Scholar]
- Riemann L, Alfredsson H, Hansen MM, Als TD, Nielsen TG, Munk P, Aarestrup K, Maes GE, Sparholt H, Petersen MI, Bachler M, Castonguay M. Qualitative assessment of the diet of European eel larvae in the Sargasso Sea resolved by DNA barcoding. Biology Letters. 2010;6:1–4. doi: 10.1098/rsbl.2010.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video clip of a 5 dpf embryo of A. australis with a heart rate of 54 beats per minute. The heart is S-shaped and beats regularly.