Abstract
Background
Sepsis and septic shock syndrome are the leading causes of death in critically ill patients. Lipopolysaccharide (LPS) released by the colonic microorganisms may translocate across a compromised lumen, leading to upregulated reactive oxidative stress, inflammation, and sepsis. The authors examined an enteral formula high in cysteine (antioxidant precursor), ω-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and prebiotic fructooligosaccharides (FOS) against systemic inflammatory syndrome.
Methods
Rats were allocated to (1) standard soy-based diet high in cysteine and crude fiber and devoid of EPA-DHA (CHOW); (2) whey-peptide-based liquid diet high in cysteine, EPA-DHA, and FOS (CYSPUFA); or (3) casein-based liquid isonitrogenous diet, low in cysteine and devoid of EPA-DHA-FOS (CASN). Liquid diets provided 25% and CHOW, 23% of calories as protein. After 6 days on diets, rats received an intraperitoneal injection of LPS or saline. Animals gained weight on their respective diets and lost weight after LPS administration. The CYSPUFA group lost considerably less weight (vs CASN or CHOW, P < .05). Inflammatory cytokines significantly increased by 4 hours and subsided 18 hours after assault. The CASN group showed elevated liver enzyme alanine aminotransferase release from damaged hepatocytes and developed severe hepatic pathology with low hematocrit. The CHOW group developed more severe hepatic lesions compared with those on liquid diets. Concentration of liver enzyme and pathology were improved in rats receiving CYSP-UFA.
Conclusions
Data indicate that CYSPUFA, a diet rich in EPA-DHA-FOS, protects against LPS-induced systemic inflammatory responses and warrants clinical studies in critically ill patients.
Keywords: sepsis, cysteine, eicosapentaenoic acid, docosahexaenoic acid, probiotic fructooligosaccharides, enteral formula
Severe sepsis and septic shock syndrome are the leading causes of death in critically ill patients, claiming approximately 225,000 deaths annually in the United States alone.1 The incidence of sepsis in intensive care patients has been increasing over the past 2 decades and is predicted to continue to rise over the next 20 years.2 Despite improvement in critical care management in recent years, sepsis still remains the major obstacle in managing these patients. Bacterial endotoxemia is considered to play a key role in sepsis, stemming from the release of lipopolysaccharide (LPS) by colonic microorganisms that translocate across a compromised lumen.3 In the lumen of the normal colon, there is a large population of microorganisms (approximately 1010–12/g contents). LPS, a cell wall constituent of gram-negative bacteria, is present in high concentrations in the normal colonic lumen. Critical illness has been associated with overgrowth of gut microbiota, increase in gut permeability, and uptake of lumenal toxins.4–7 This uptake is thought to lend itself to endotoxemia, increased reactive oxygen species (ROS), sepsis, multiple organ failure, and death.8–10
In patients with sepsis, the liver has 2 opposing roles: it is a source of inflammatory mediators and a target organ for the effects of the inflammatory mediators. The liver is pivotal in modulating the systemic response to severe infection because it contains the largest mass of macrophages in the body. Kupffer cells can clear the endotoxin and bacteria that initiate the systemic inflammatory response.11–13 There are binding sites for endotoxic lipopolysaccharide on the plasma membrane of hepatocytes,14 but the phagocytes (neutrophils and macrophages) provide the first line of defense against infections with bacteria and viruses as well as endotoxin.15,16 Lipopolysaccharide binding protein (LBP) and CD14 together may enhance the sensitivity to LPS, but neither protein is required for LPS responses. Toll-like receptor 4 (TLR4) and myeloid differentiation (MD)-2 are suggested to be required for mediating proinflammatory phagocytosis of gram-negative bacteria.17 This process induces proinflammatory cytokines, leukotrienes, and prostaglandins that localize the infection at the site of entry.18 Kupffer cells and neutrophils of the innate immune system provide the responses by expressing and secreting the cytokines.
Cytokines represent diverse groups of secreted proteins that together exert wide ranges of actions. Many individual cytokines are themselves pleiotropic, exerting multiple actions.19 Because cytokines are important targets in the management of inflammatory conditions, we decided to look at the levels of cytokines as markers of inflammation and/or repair in our experimental systemic inflammatory syndrome model that resembles sepsis. The responses of these cytokines may provide some insight into the role of nutrition components in sepsis.
Glutathione (GSH) is essential for both the functional and structural integrity of the gut.20 GSH protects against intracellular free radicals, ROS, and several endogenous and exogenous toxins.21–27 GSH is depleted during inflammatory illness,4 and GSH deficiency predisposes humans and animals to increased risk of organ failure and death.28–32
Knowing that cysteine is a conditionally essential amino acid and the rate-limiting precursor to GSH synthesis, it is reasonable to hypothesize that a diet rich in cysteine will increase the GSH deposits. In addition, the ω-3 long-chain polyunsaturated fatty acids (PUFA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been associated with a reduced production of proinflammatory eicosanoids in the modulation of the inflammatory response.33 Furthermore, probiotic short-chain frooctooligosaccharides (FOS) and inulin are considered to promote gut wall integrity, enhance colonic microbiota, and stimulate innate immune system.34
We hypothesized that an enteral nutrition formula high in cysteine, EPA-DHA, and FOS would protect against systemic inflammatory syndrome in a well-established rat model that resembles sepsis.
Materials and Methods
The test formula (CYSPUFA, Peptaman AF, Nestlé Healthcare Nutrition, Minneapolis, MN) contained 100% partially hydrolyzed whey protein as its source of nitrogen, providing a rich source of cysteine, the rate-limiting amino acid in glutathione synthesis used in this study (CYSPUFA 1800 mg/1000 mL). The test formula also provided the long-chain PUFAs EPA (900 mg/1000 mL) and DHA (1500 mg/1000 mL). EPA-DHA has been associated with a reduced production of proinflammatory eicosanoids. Additionally, the test formula contained a mixture of inulin and short-chain FOS blend fiber (5.24 g/1000 mL). The control diet was a commercially available casein-based liquid diet (CASN) that contained low levels of cysteine (100 mg/1000 mL) and was devoid of fiber and EPA-DHA, as well as a soy-based (plant) standard Harlan 1018 rat diet (CHOW). CHOW diet was rich in dietary fiber, trace elements, and cysteine (CHOW 3300 mg/1000 g). CYSPUFA, CASN, and CHOW diets each provided 25%, 25%, and 23% of calories as protein (Table 1), respectively. The chemicals, including LPS used in this model, were purchased from Sigma Chemical Co (St. Louis, MO) unless otherwise mentioned.
Table 1.
Comparison of Major Nutrients in 2 Liquid Diet and CHOW Compositions
| Nutrients | CYSPUFA | CASN | CHOW 1018 Harlan |
|---|---|---|---|
| Volume, mL | 833 | 1000 | 1000g |
| Calories, kcal | 1000 | 1000 | — |
| Protein, g | 25% of kcal | 25% of kcal | 23% of kcal |
| Protein sources | Whey hydrolysate | Na and calcium caseinates | Plant (soy) |
| CHO, g | 36% of kcal | 52% of kcal | 61% of kcal |
| CHO sources | Maltodextrin, oligofructose | Corn maltodextrin, sugar | Corn, wheat |
| Dietary fiber, g | 4.3 | 0 | 3.5a |
| Fat, g | 39% of kcal | 23% of kcal | 16% of kcal |
| Fat sources | Soy, fish oil, MCT | High oleic safflower oil, canola oil, MCT | Soy, plant |
| ω6:ω3 ratio | 1.8:1 | 5.3:1 | 11:1 |
| Water, mL, % | 81 | 84 | 10 |
| Vitamin E, IU | 100 | 45 | 100 |
| Vitamin C, mg | 320 | 345 | — |
| Zinc, mg | 24 | 24 | 75 |
| Iron, mg | 12 | 18 | 200 |
| Copper, mg | 2 | 2 | 15 |
| Manganese, mg | 2.6 | 5 | 118 |
| Selenium, mcg | 133 | 70 | 200 |
CYSPUFA diet contains 7.8 g eicosapentaenoic acid + docosahexaenoic acid/1000 kcal. CHO, carbohydrate; MCT, medium-chain triglyceride.
Crude fiber.
Animals
Seventy 4- to 6-week-old (200 g) specific pathogen-free male Wistar rats were purchased from Sprague Dawley (Indianapolis, IN) and housed in micro-filter-top cages at the VA Medical Center Animal Research facility at Lexington, Kentucky. They were placed in a room maintained at 22°C with a 12:12-hour light:dark cycle and provided with rodent chow and water ad libitum. This experimental study was approved and performed in accordance with the guidelines for the Institutional Animal Care and Use Committee (IACUC/ACORP), the Veterans Administration Medical Center, and the University of Kentucky (UK) Research Resource Facility (Lexington, KY), certified by the American Association of Accreditation of Laboratory Animal Care (AAALAC). The experimental model was conducted in a manner consistent with the relevant ethical guidelines for animal research.
Experimental Design Methods
After an initial week of acclimatization, rats were randomized to receive the test diet, a whey-peptide-based liquid diet containing cysteine, EPA-DHA, and FOS (CYSPUFA); the control diets, a commercially available casein-based liquid diet devoid of EPA-DHA and fiber and low in cysteine (CASN); or a standard rat diet (CHOW), rich in crude fiber and cysteine but devoid of EPA-DHA.
Rats were provided with ad libitum fresh liquid diets twice daily or the dry chow. Food, water intake, and body weight were monitored daily. After 6 days on the respective diets, all rats consumed their designated diets and similarly gained weight with no statistical difference (Figure 1). Then the rats were randomly assigned to groups for intraperitoneal injection of 6 mg/kg LPS (Sigma Chemical) or saline for sham challenge. Subsequently, each rat was injected subcutaneously with 20 mL of ringer solution to prevent dehydration. Animals were euthanized at 0, 4, and 18 hours after the LPS administration, and blood and tissue samples were collected.
Figure 1.
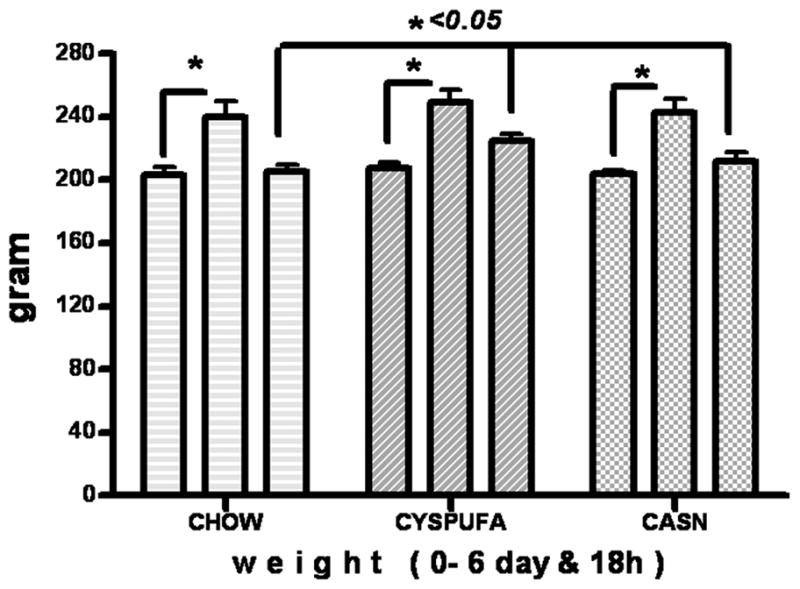
Rats gained weight (25%) after 6 days on designated diets and lost weight 18 hours after lipopolysaccharide assault. CYSPUFA diet provided partial but significant protection against weight loss after 18 hours (P < .05) (repeated ANOVA).
Whole Blood and Plasma Isolation
Immediately after euthanizing the rats with an overdose of halothane inhalation, blood was collected from the right ventricle of the heart into a syringe containing a minute amount of heparin and placed on ice. Plasma was separated by centrifugation at 5000 g for 5 minutes at 4°C. Samples were kept at −80°C until further analysis.
Tissue Collection
Liver was perfused with phosphate-buffered saline (PBS). Samples from liver and intestines were collected and fixed in formalin for histopathology, and the remaining tissue was immediately snap-frozen in liquid nitrogen and stored at −80°C for analysis of reduced GSH, oxidized GSH (GSSG), S-adenosylmethionine (SAMe) concentrations, and other analysis.
Blood and Tissue Preparation for Antioxidant Determination
Blood samples were collected in heparinized tubes, and a 20% homogenate in 5% metaphosphoric acid was prepared. After standing for 30 minutes on ice, the homogenate was centrifuged for 10 minutes (10,000 g) and the acid-soluble fraction was collected for measurement of sulfhydryl and disulfide compounds. Tissue homogenates (10%, w/v) were prepared in 5% (w/v) metaphosphoric acid, using all-glass Tenbroeck homogenizers, and kept on ice. After standing for 20–40 minutes, the homogenates were centrifuged for 1 minute (10,000 g) and the acid-soluble fractions collected for measurement of free thiol-disulfides.
Analysis of Glutathione (GSH) and Other Thiols (SH) and Disulfides (SS) by High-Performance Liquid Chromatography
GSH, GSSG, cysteine, and cystine were simultaneously quantified by high-performance liquid chromatography with dual electrochemical detection (HPLC-DEC).35 In brief, 20-μL samples were injected onto a 250 × 4.6-mm, 5-μm, C-18 column (Val-U-Pak HP, fully end-capped ODS, 5 μm, 250 × 4.6 mm; Chrom Tech, Inc, Apple Valley, MN). Samples (20 μL) were injected onto the column and eluted with a mobile phase consisting of 0.1 M monochloroacetic acid, 2 mM heptane sulfonic acid, and 2% acetonitrile at pH 2.8 and delivered at a flow rate of 1 mL/min. The compounds were detected in the eluant with a Bioanalytical Systems model LC4B dual electrochemical detector using 2 Au-Hg electrodes in series with potentials of −1.2 V and 0.15 V for the upstream and downstream electrodes, respectively. Current (nA) was measured at the downstream electrode. Analytes were quantified from peak area measurements using authentic external standards.
Intracellular SAMe assay by HPLC
Deproteinized tissue extracts (4% metaphosphoric acid) and blood were prepared, and SAMe was determined by an HPLC method using a 5-μm Hypersil C-18 column (250 × 4.6 mm). The mobile phase consisted of 40 mM ammonium phosphate, 8 mM heptane sulfonic acid (ion-pairing reagent, pH 5.0), and 6% acetonitrile, and was delivered at a flow rate of 1.0 mL/min. SAMe was detected using a Waters 740 UV detector at 254 nm. An internal standard, S-adenosylethionine (SAE), was added to all samples and standard solutions to a concentration of 100 nmol/mL. Protein concentrations were measured by a protein assay kit from Bio-Rad (Bio-Rad Laboratories, Hercules, California) in accordance with the manufacturer’s instructions.
Plasma Density Assay
Albumin density was measured using an optical densitometer.
Plasma Enzyme Assay
Plasma transaminase activities (alanine aminotransferase [ALT]) were measured by a spectrophotometer using a diagnostic kit (ALT/GPT, Stanbio labs, Boerne, TX) according to the instructions provided.
Histopathological Examination
A small portion of the right lobe from the liver, kidney, intestines, pancreas, and lungs was placed in cassettes and fixed with 10% neutral formalin. The specimens were dehydrated and embedded in paraffin, and tissue sections of 5 μm were stained by hematoxylin and eosin (H&E) and evaluated using light microscopy. Hepatic lesions were graded on a scale of 0 to 4+, as reported previously,24,26 based on degeneration, infiltration of inflammatory cells, and necrosis as follows:
Score 0: no degeneration, infiltration of inflammatory cells, or necrosis
Score 1+: 5%–30% of hepatocytes showing degeneration, infiltration of inflammatory cells, or necrosis
Score 2+: 31%–50% of hepatocytes showing degeneration, infiltration of inflammatory cells, or necrosis
Score 3+: 51%–70% of hepatocytes showing degeneration, infiltration of inflammatory cells, or necrosis
Score 4+: 71% or more of hepatocytes showing degeneration, infiltration of inflammatory cells, or necrosis
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analysis was performed on GraphPad Instat4 and Prism software using ordinary or repeated-measures analysis of variance (ANOVA). The data were further analyzed by post hoc test for statistical difference (Tukey-Kramer multiple comparison test). Differences between groups were considered to be statistically significant at P < .05.
Results
Seventy young Wistar rats (6 weeks of age, weighing approximately 200 g) consumed their diets for 6 consecutive days, gained weight (25%) on the respective diets, and, when only the saline (sham) injection was received, had normal results for all parameters measured in this study. As a whole, animals tolerated LPS administration, and all survived for at least 18 hours after assault but significantly lost weight (Figure 1).
Rats in the CYSPUFA group lost considerably less weight after the LPS injection (P < .05) compared with other dietary groups.
The LPS provoked release of inflammatory cytokines with no significant difference between the groups. Serum concentrations of inflammatory cytokines—tumor necrosis factor α (TNFα; 37-fold), interferon γ (IFNγ; 60-fold), and interleukin-6 (IL-6; 24-fold)—were significantly increased by 4 hours and subsided 18 hours after the LPS administration, regardless of the assigned diets (Table 2).
Table 2.
Levels of Hepatic rGSH, Its Disulfide (GSSG), Cysteine, and SAMe at Normal State (0h), 4 and 18 Hours After LPS Administration, Measured by HPLC as nmol/g of Tissue
| Parameters | CHOW | CYSPUFA | CASN |
|---|---|---|---|
| Normal hepatic rGSH, nmol/g | 5087 ± 269* | 5301 ± 416 | 5778 ± 132* |
| rGSH loss nmol/g, 4 hours | −952 ± 47** | −374 ± 85** | −650 ± 180* |
| Hepatic GSSG, nmol/g | 102 ± 7.8* | 82 ± 6.6* | 87.5 ± 10 |
| Cys nmol/g, 4 hours | 199 ± 41* | 246 ± 36* | 250 ± 27 |
| Cys nmol/g, 18 hours | 181 ± 13* | 255 ± 21* | 253 ± 16 |
| Hepatic SAMe, nmol/g, 18 hours | 56.9 ± 6** | 65.2 ± 2 | 79.1 ± 3** |
| HCT, 4 hours | 42.0 ± 2.5 | 43.7 ± 2.1* | 39.3 ± 1.2* |
| Serum albumin density, 0 hours | 1034 ± 1 | 1034 ± 1 | 1035 ± 1 |
| Serum albumin density, 4 hours | 1030 ± 1 | 1032 ± 2 | 1032 ± 1 |
| IL-6, pg/mL, 4 hours | 581 ± 5 | 579 ± 4 | 546 ± 24 |
| IL-6, pg/mL, 18 hours | 29 ± 2.6 | 35 ± 3.9 | 32 ± 3.8 |
| IFNγ, pg/mL, 4 hours | 2993 ± 51* | 2602 ± 247* | 2950 ± 54 |
| IFNγ, pg/mL, 18 hours | 209 ± 67 | 237 ± 81 | 275 ± 84 |
| TNFα, pg/mL, 4 hours | 258 ± 43 | 317 ± 85 | 275 ± 105 |
| TNFα, pg/mL, 18 hoursa | 7.4 ± 1.6 | 9.07 ± 2.2 | 5.3 ± 1.8 |
HCT and blood cytokines measured with the enzyme-linked immunosorbent assay (ELISA) technique. Data are expressed as mean ± SEM (ordinary and repeated-measures ANOVA followed by post hoc analysis). Cys, cysteine; GSSG, oxidized glutathione; HCT, hematocrit; HPLC, high-performance liquid chromatography; IL-6, interleukin-6; IFNγ, interferon γ; rGSH, reduced glutathione; SAMe, S-adenosylmethionine; TNFα, tumor necrosis factor α.
Returned to normal level.
P < .05.
P < .01.
The LPS caused significant increases (2-fold) in the concentration of liver enzyme ALT, a marker of hepatocyte injury, and those rats that received the CYSPUFA diet were partially but significantly protected against this effect (P ≤ .05; Figure 2). In comparison, rats on the CASN diet showed increased levels of ALT (2-fold) and developed severe hepatic pathology (H&E slides scored from 0 to 4; Figures 3–4) with low hematocrit (Table 2). In contrast, rats on the CHOW diet and injected with LPS developed more severe hepatic lesions with extensive multifocal necrosis and infiltration of inflammatory cells compared with those allocated to liquid diets (Figure 3). The CYSPUFA group was partially but significantly protected against low hematocrit, hepatic pathology, and the elevated levels of ALT after assault compared with those assigned to the other diets (P < .05). IL-10 (anti-inflammatory cytokine) levels were significantly increased 4 hours after LPS injection in rats allocated to the liquid diets (3-fold) and remained elevated (2-fold) 18 hours after in the CYSPUFA group (P < .05; Figure 5).
Figure 2.
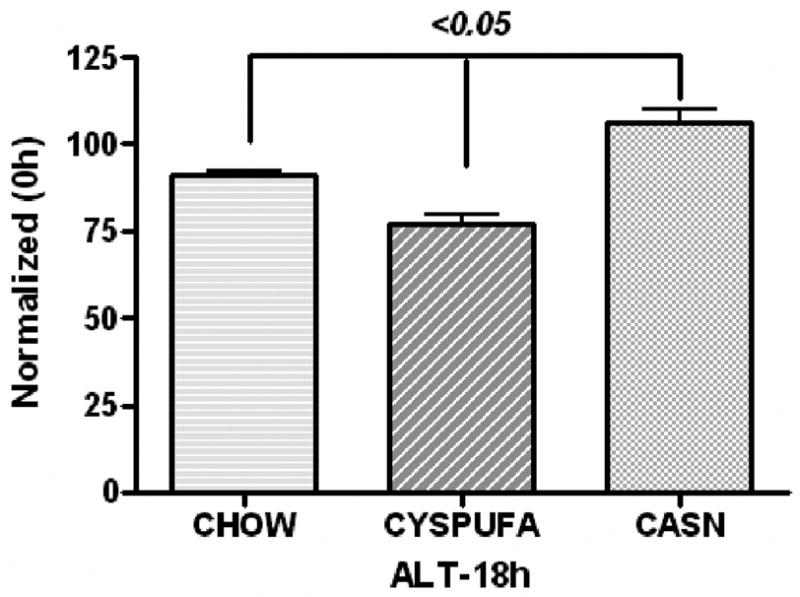
Liver enzyme (alanine aminotransferase [ALT], unit/mL) activity significantly increased due to hepatocyte injury after 18 hours from dietary groups. CYSPUFA diet partially protected against this injury (normalized with 0h).
Figure 3.
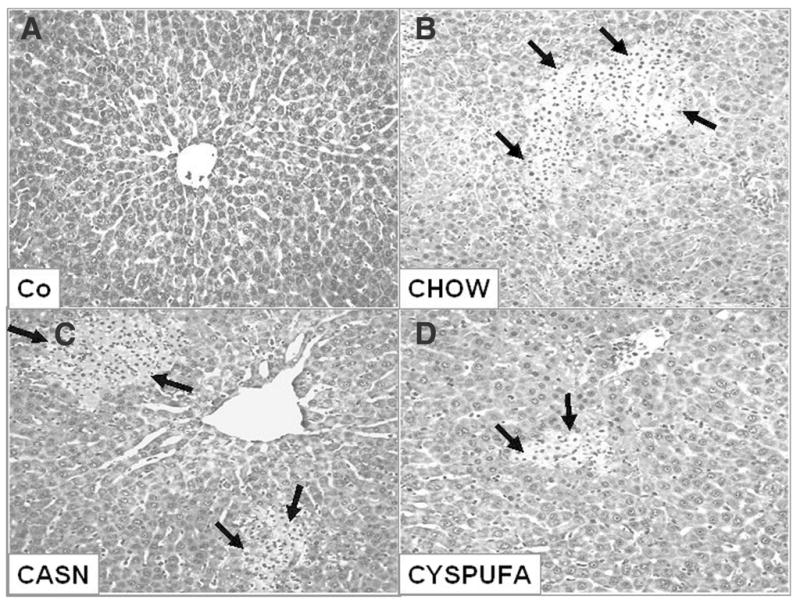
Representative hepatic sections stained with hematoxylin and eosin 18 hours after (A) saline and (B–D) lipopolysaccharide (LPS) administration. (A) Slide from representative rat injected with saline (Co) shows normal hepatic structure. (B–D) Hepatic section from representative rat that consumed CHOW (B) 18 hours after LPS injection shows severe multifocal necrosis, infiltration of inflammatory cells, and necrosis (arrows). (C) CASN diet and (D) CYSPUFA diet show mild focal necrosis with less severe lesions.
Figure 4.
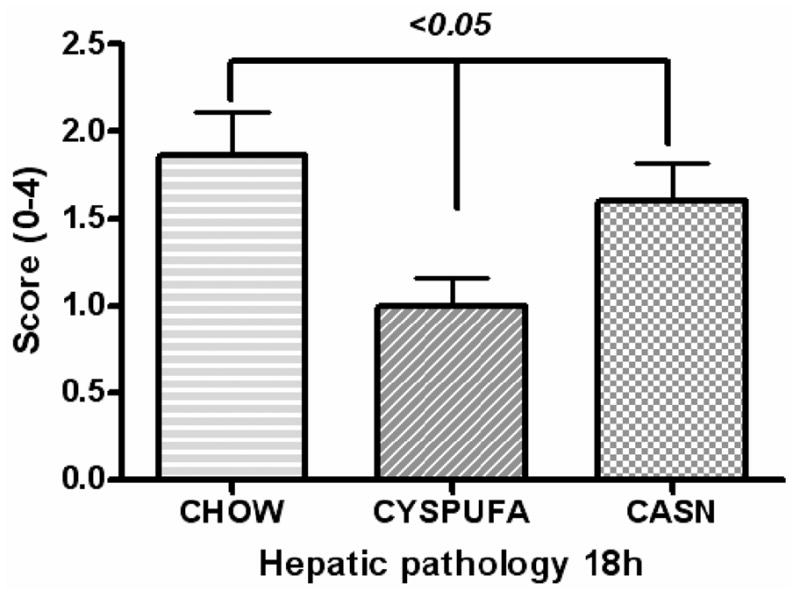
Hepatic pathology graded on a scale of 0 (representing no degeneration, necrosis, or inflammation) to 4+ (more than 70% of hepatocytes showing degeneration, necrosis, or infiltration of inflammatory cells). Rats on respective diets for 6 days.
Figure 5.
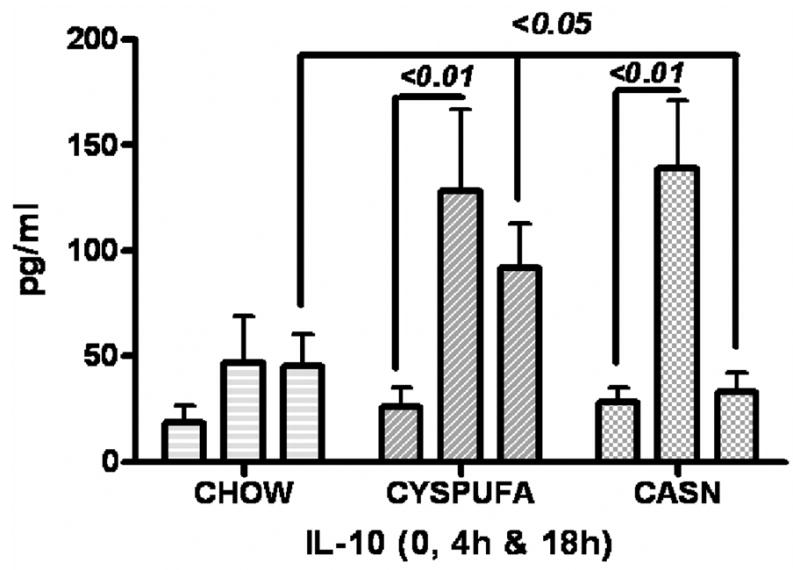
Anti-inflammatory cytokine interleukin-10 (IL-10) was upregulated 3-fold in blood of the rats that consumed liquid diets 4 hours after lipopolysaccharide (LPS) and remained high only in the CYSPUFA group 18 hours after.
The intrahepatic concentration of antioxidants, cysteine, GSH, and SAMe measured by HPLC increased significantly in rats on liquid diets compared with the CHOW diet (Table 2). The cysteine levels were drastically depleted (30%) in rats on CHOW 18 hours after the LPS administration and notably improved in those animals that had consumed the liquid diets (Table 2). Hepatic levels of adenosine assayed by HPLC (Figure 6) increased considerably in the CYSPUFA group during the normal state and also improved 4 hours after LPS administration compared with those rats that consumed other diets (P < .05). Concentration of intrahepatic-reduced GSH levels decreased in the LPS-injected rats. In contrast, this reduction was less prominent in the CYSPUFA group compared with other dietary groups (vs CHOW, P < .01; vs CASN, P < .05; Table 2). The LPS administration caused significant generation of GSSG in the intrahepatic tissues from rats on the CHOW diet compared with those rats on the liquid diets (P < .05; Table 2).
Figure 6.
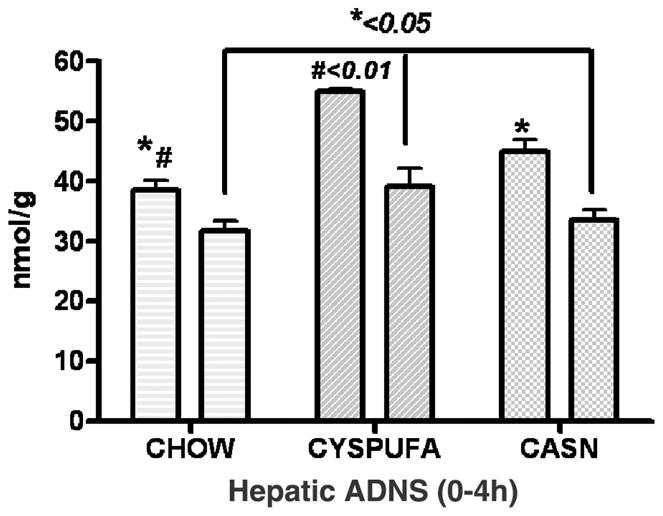
Concentration of hepatic adenosine (ADNS; nmol/g) assayed by high-performance liquid chromatography was significantly higher in the CYSPUFA group in the normal state and 4 hours after lipopolysaccharide administration.
Finally, rats developed pathological lesions, including congestion and mild to moderate inflammatory responses, in the cardiac and pulmonary tissues, with no significant differences noted between the groups (data not shown).
Discussion
Nutrition management of septic patients and those prone to sepsis (eg, critically ill) is often a double-edged sword, with mixed viewpoints as to route and timing of feeding, degrees of energy delivery, and choice and maintenance of specific nutrients. Data on “immunoenhancing” diets have not conclusively supported the use of any one or combination of nutrients and nutrient-like substances for septic patients, although some common themes are emerging. These include early use of enteral feeding, avoidance of excessive (yet undefined) feeding, and inclusion of ω-3 fatty acids in amounts greater than that consumed in a standard Western diet.33,36
In this animal trial, we evaluated the efficacy of 3 distinct diets: (1) whey-peptide-based formula defined as the source of nitrogen, thus providing a rich source of amino acid cysteine (1800 mg/1000 mL), the rate-limiting amino acid in GSH synthesis; (2) casein-based diet low in cysteine (100 mg/1000 mL); and (3) soy-based (plant) diet that is very rich in cysteine (CHOW 3300 mg/1000 g). Various components of the CYSPUFA diet, as compared with the other diets, could have contributed (synergistic effect) to the more positive response of animals to the LPS-induced inflammatory response.
The CYSPUFA formula contained the ω-3 long-chain PUFAs DHA (20:5 ω-3) and EPA (22:6 ω-3), which, when present in the diet, have been associated with a reduced production of proinflammatory eicosanoids, and thus an anti-inflammatory effect of this diet may have been anticipated. The soy diet was high in the ω-6 PUFA linoleic acid (C18:2ω-6 31.35 g/L kg), the precursor to arachidonic acid–derived eicosanoids, and other inflammatory mediators, found in plant seeds and oils. Some ω-3 PUFAs are also found in plant seeds and oil, but the main active compounds, DHA and EPA, originate in marine products and fish oils. The ω-3 and ω-6 PUFAs are incorporated into the cell membrane phospholipids that modify the membrane’s physical and biochemical signaling pathways.36 Mechanisms by which EPA and DHA limit inflammation in sepsis are not limited to their contribution to noninflammatory eicosanoids or their substitution for proinflammatory ω-6 fatty acids in the diet. Although the role of EPA and DHA in this study cannot be ascertained directly, it is anticipated that EPA and DHA contributed to the protective effect of the CYSPUFA.
EPA has been reported to downregulate the ubiquitin proteasome proteolysis pathway in cells. This pathway is a key modulator of protein catabolism in starvation, sepsis, and cancer.37 In sepsis, LPS binds directly to the α-subunit and β-subunit of the proteasome core, thus increasing ubiquitin proteasome proteolytic activity. Also, in pancreatic cancer patients, EPA supplementation has been shown to reduce weight loss, a common comorbidity and frequent cause of death.38 In the current study, it is conceivable that EPA and DHA (fish oil) present in the CYSPUFA diet have induced this downregulation and protected against weight loss.
In addition, the lipid amount and profiles of the diets are different, with almost 3 and 5 times as high in a ω-6:ω-3 ratio in the CASN and CHOW, respectively, compared with the CYSPUFA diet (Table 1). This ratio is recommended by the Institute of Medicine Guidelines for the general population.39,40
In a recent study, fish oil supplements given to normal participants for 3–4 weeks blunted the endocrine stress response to LPS and the elevated body temperature but had no impact on cytokine production (IL-6 and TNFα).41 This report is in accordance with our findings in rats and conflicts with the presumed anti-inflammatory cytokine effects of ω-3 alone on arachidonic acid42; therefore, ω-3 may not act primarily by downregulating cytokine release. Recently, DHA and EPA derivatives, resolvins, and protectins were found to act as anti-inflammatory mediators at the sites of inflammation and were generated during the spontaneous resolution phase. These recently discovered pathways and mediators contradict polymorphonuclear leukocyte (PMN) infiltration and promote resolution.43
When exposed to LPS, macrophage releases a variety of mediators of inflammation, of which TNFα is a prominent component that mediates disparate effects of LPS.44,45 In this study, LPS provoked release of inflammatory cytokines with no significant differences detected between dietary groups. Serum concentrations of inflammatory cytokines—TNFα (37-fold), IFNγ (60-fold), and IL-6 (24-fold)—were significantly increased by 4 hours and subsided 18 hours after LPS administration regardless of the assigned diets. This phenomenon is in concordance with the observation that intravenous administration of purified LPS (Salmonella abortus equi) to cancer patients induced high amounts of circulating TNFα and IL-6.46
The inflammatory process is downregulated hours or days later after an assault, triggered by IL-4, IL-10, and IL-13, resulting in the inflammatory and compensatory anti-inflammatory responses.3,47 These 2 opposing responses can induce a state of imbalance and the pattern of cytokine secretion that predominates in sepsis.
Conversely, overexpression of IL-10 in the gut is associated with suppression of the inflammatory response and protection of animals against sepsis.48 In the present study, we also report a significant increase in the IL-10 (anti-inflammatory cytokine) levels by 4 hours in rats allocated to the liquid diets (3-fold) and a persistence of elevated levels (2-fold) in the CYSPUFA group 18 hours after LPS administration (P < .05).
Some characteristics of whey protein that offer advantages over casein include its higher levels of branched chain amino acids and its tendency to more efficiently promote anabolism in both well and compromised states.49 Severe malnutrition is common in critically ill patients, sometimes despite adequate nutrition support.50 Weight loss due to muscular atrophy (lean tissue loss) occurs in sepsis patients, and this tissue loss is greater when the injury is more severe and persistent. Similarly, in our endotoxin-induced systemic inflammatory model, rats in all groups developed weight loss and muscular atrophy despite receiving different and adequate nutrition. In contrast, weight loss became significantly less prominent in the CYSPUFA group consuming the rich diet.
Limited muscle atrophy and weight loss were reported after the LPS injection in gastric fed rats with an enteral diet rich in arginine, taurine, ω-3 fatty acids (fish oil), and vitamin E compared with a standard polymeric formula diet. However, this effect was not associated with improved nitrogen balance, urinary 3-methylhistidine excretion, or the total tissue protein content.51 Furthermore, the diet increased ileal and hepatic blood flow, as well as blood glucose levels, suggesting enhanced nutrient absorption as an explanation for the increase in the body weight.51 Vary and Lynch52 showed that the amino acids (leucine, isoleucine, and valine), particularly leucine, not only stimulate a number of cell-signaling pathways important in the regulation of mRNA translation but also provide substrate for protein synthesis in striated muscles, an application to prevent wasting disease in human immunodeficiency virus (HIV) and sepsis patients. Recently, Fang et al53 investigated the role of nutrition in established sepsis. The authors concluded that intravenous nutrition including cysteine could support the basal requirement of amino acids and improve the immune function of patients with sepsis. The CYSP-UFA and CHOW diets were both high in cysteine content. However, our data indicated that only the CYSPUFA diet led to lower weight loss in rats injected with LPS.
Interestingly, in a double-blind protocol, the recreational bodybuilders’ normal diets were supplemented with hydrolyzed whey protein or casein for the duration of 10 weeks. The whey protein group achieved a significantly greater (P < .01) gain in lean mass than the casein group and a significant (P < .05) reduction in fat mass compared with the casein group. The whey protein group also achieved significantly greater (P < .05) improvements in strength assessment compared with the casein group.54 One explanation of this effect may be the difference in speed of absorption of dietary amino acids by different proteins. Absorption of amino acids is significantly faster with whey protein compared with the slow adsorption of casein proteins measured using intrinsically 13C-leucine-labeled milk proteins ingested as one single meal by healthy adults.55
Furthermore, rats fed the whey-based CYSPUFA formula for 6 days prior to the LPS exposure showed a significant reduction in histological hepatic damage compared with those fed the casein-based (CASN) diet or the normal CHOW. The presence of whey in the CYSPUFA study diet may supply a better source and exact amount of cysteine needed as the rate-limiting precursor to GSH synthesis. Because the CHOW diet contained about 2 times more cysteine (in the form of cystine) compared with the CYSPUFA diet, the intrahepatic levels of reduced GSH remained significantly higher in the CYSPUFA group than in the CHOW (P < .01) or CASN (P < .05) groups. In addition, whey accounts for a greater protein quality (protein efficiency ratio, amino acid score, and biologic value) than casein54,55 and plant protein. It is conceivable that in stressed states, peptides are better absorbed than the intact proteins. Similarly, as administration of the LPS provokes muscle mass loss, the lesser weight loss in the CYSPUFA group may be attributed to the multiple factors, including the better absorbed protein quality and form of this diet. The potential role of the prebiotic fructans (FAS) in improving outcomes in sepsis is also variable, ranging from the promotion of gut wall integrity, enhancement of colonic microbiota, and indirect stimulation of the innate immune system.34 It is also possible that FOS elements present in this diet may have provided some support to the gut wall and thus potentially protected against gut leakage and bacterial translocation. These mechanisms of support need to be investigated in future studies.
ROS promotes oxidative damage and contributes to tissue destruction in a wide variety of diseases,24,26,30,56–58 including sepsis. Antibacterial defense PMNs can cause substantial tissue injury. Adenosine, a retaliatory metabolite, is produced in response to metabolically unfavorable conditions such as inflammation. Adenosine or A2A agonists have been reported to selectively inhibit the potentially tissue-toxic H2O2 production elicited by soluble inflammatory mediators in patients with septic shock. As compared with healthy volunteers, PMNs from septic shock patients showed strongly enhanced tissue-toxic H2O2 production elicited by TNFα/N-formyl-methionyl-leucyl-phenylalanine (fMLP). Increasing concentrations of adenosine dose-dependently reduced this tissue-toxic H2O2 production in both groups with half-maximal inhibitory concentrations of 25 nmol/L and 114 nmol/L in healthy volunteers and septic shock patients, respectively.59 Similarly, we report elevated hepatic levels of adenosine in the CYSPUFA group during the normal state (before LPS injection) and also a significant improvement 4 hours after LPS assault compared with those rats that consumed other diets (P < .05).
This study indicates a possible approach against systemic inflammatory diseases with an enteral formula supplemented with specific nutritional-ingredient properties. The physiologic importance lies in the potential ability of amino acids as specific nutrients that are designed to counteract the accelerated host protein wasting associated with a number of disease entities, including sepsis.
Conclusions
These data indicate that the whey-peptide-based diet rich in EPA-DHA, cysteine, and FOS protected rats against some specific and general effects of systemic inflammatory syndrome and warrant application in a clinical trial in critically ill patients.
Acknowledgments
We appreciate the nutrition input from Heidi Storm, Research and Initiatives, Nestlé Nutrition. Marcia C. Liu provided technical assistance.
Footnotes
This study was presented in part at the Clinical Nutrition Week 2007 in Phoenix, Arizona.
Financial disclosure: This study was supported by grants from Nestlé Healthcare Nutrition Inc, NIH-NCCM AT1490, and NCRR P20RR020145, COHR-Pilot Project (HO).
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Balk RA. Optimum treatment of severe sepsis and septic shock: evidence in support of the recommendations. Dis Mon. 2004;50:168–213. doi: 10.1016/j.disamonth.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 4.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and nonalcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 5.Hanck C, Rossol S, Bocker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol. 1998;33:606–608. doi: 10.1093/alcalc/33.6.606. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler TR, Smith RJ, O’Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123:1313–1319. doi: 10.1001/archsurg.1988.01400350027003. [DOI] [PubMed] [Google Scholar]
- 7.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 8.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albillos A, de la Hera A, Gonzalez M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 10.Kaser A, Ludwiczek O, Waldenberger P, Jaschke W, Vogel W, Tilg H. Endotoxin and its binding proteins in chronic liver disease: the effect of transjugular intrahepatic portosystemic shunting. Liver. 2002;22:380–387. doi: 10.1034/j.1600-0676.2002.01666.x. [DOI] [PubMed] [Google Scholar]
- 11.Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304–1309. [PubMed] [Google Scholar]
- 12.Scapa E, Orda R, Neuman M, Sayfan J, Abramovici J, Eshchar J. B-N-acetyl hexosaminidase (B-NAH) serum levels after endotoxin administration into the portal vein in the rat. Liver. 1991;11:3941. doi: 10.1111/j.1600-0676.1991.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 13.Szabo G, Romics L, Jr, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis. 2002;6:1045–1066. doi: 10.1016/s1089-3261(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 14.Ramadori G, Hopf U, Meyer zum Buschenfelde KH. Binding sites for endotoxic lipopolysaccharide on the plasma membrane of isolated rabbit hepatocytes. Acta Hepatogastroenterol (Stuttg) 1979;26:368–374. [PubMed] [Google Scholar]
- 15.Neuman MG, Benhamou JP, Marcellin P, et al. Cytokine-chemokine and apoptotic signatures in patients with chronic hepatitis C. Transl Res. 2007;149:126–136. doi: 10.1016/j.trsl.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Schafer C, Schips I, Landig J, Bode JC, Bode C. Tumor-necrosis-factor and interleukin-6 response of peripheral blood monocytes to low concentrations of lipopolysaccharide in patients with alcoholic liver disease. Z Gastroenterol. 1995;33:503–508. [PubMed] [Google Scholar]
- 17.Jain V, Halle A, Halmen KA, et al. Phagocytosis and intracellular killing of MD-2 opsonized gram-negative bacteria depend on TLR4 signaling. Blood. 2008;111:4637–4645. doi: 10.1182/blood-2007-11-126862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbaschek R, McCuskey RS, Rudi V, et al. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- 19.Neuman M. Cytokines: central factors in alcoholic liver disease. Alcohol Res Health. 2003;27:307–316. [PMC free article] [PubMed] [Google Scholar]
- 20.Shi ZZ, Osei-Frimpong J, Kala G, et al. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci USA. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotgreave IA, Grafström RC, Moldéus P. Modulation of pneumotoxicity by cellular glutathione and precursors. Bull Eur Physiopathol Respir. 1986;22:263s–266s. [PubMed] [Google Scholar]
- 22.Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta. 1997;257:59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- 23.Oz HS, Chen TS, McClain CJ, de Villiers WJS. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Oz HS, McClain C, Nagasawa H, Ray M, Chen T. Diverse antioxidants protect against acetaminophen hepatotoxicity. J Biochem Mol Tox. 2004;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- 25.Oz HS, Chen T, Nagasawa H. Comparative efficacies of two cysteine prodrugs and a glutathione delivery agent in a colitis model. Trans Res. 2007;150:122–129. doi: 10.1016/j.trsl.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oz HS, Im H, Chen T, McClain C, de Villiers WJS. Glutathione–enhancing agents protect against steatohepatitis in a dietary model. J Biochem Mol Toxicol. 2006;20:39–47. doi: 10.1002/jbt.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oz HS, Chen T, de Villiers WJ, McClain CJ. Metallothionein overexpression does not protect against inflammatory bowel disease in a murine colitis model. Med Sci Monit. 2005;11:BR69–BR73. [PubMed] [Google Scholar]
- 28.Robinson MK, Rounds JD, Hong RW, Jacobs DO, Wilmore DW. Glutathione deficiency increases organ dysfunction after hemorrhagic shock. Surgery. 1992;112:140–147. [PubMed] [Google Scholar]
- 29.Mårtensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci USA. 1990;87:1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brun P, Castagliuolo I, Di Leo V, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 31.Kaplowitz N, Fernández-Checa JC, Kannan R, García-Ruiz C, Ookhtens M, Yi JR. GSH transporters: molecular characterization and role in GSH homeostasis. Biol Chem Hoppe Seyler. 1996;377:267–273. doi: 10.1515/bchm3.1996.377.5.267. [DOI] [PubMed] [Google Scholar]
- 32.Kaplowitz N, Tsukamoto H. Oxidative stress and liver disease. Prog Liver Dis. 1996;14:131–159. [PubMed] [Google Scholar]
- 33.Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008;32:127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Oz HS, Ray M, Chen T, McClain C. Efficacy of a TGF-β2 containing nutritional support formula in a murine model of IBD. J Am Coll Nutr. 2004;23:220–226. doi: 10.1080/07315724.2004.10719364. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC. n-3 fatty acids, inflammation, and immunity: relevance to postsurgical and critically ill patients. Lipids. 2004;39:1147–1161. doi: 10.1007/s11745-004-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breen HB, Espat NJ. The ubiquitin-proteasome proteolysis pathway: potential target for disease intervention. JPEN J Parenter Enteral Nutr. 2004;28:272–277. doi: 10.1177/0148607104028004272. [DOI] [PubMed] [Google Scholar]
- 38.Barber MD. Cancer cachexia and its treatment with fish oil enriched nutritional supplementation. Nutrition. 2001;17:751–755. doi: 10.1016/s0899-9007(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 39.Covington MB. Omega-3 fatty acids. Am Fam Physician. 2004;70:133–140. [PubMed] [Google Scholar]
- 40.Mead A, Atkinson G, Albin D, et al. Dietetic guidelines on food and nutrition in the secondary prevention of cardiovascular disease: evidence from systematic reviews of randomized controlled trials (second update, January 2006) J Hum Nutr Diet. 2006;19:401–419. doi: 10.1111/j.1365-277X.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 41.Michaeli B, Berger MM, Revelly JP, Tappy L, Chiolero R. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr. 2007;26:70–77. doi: 10.1016/j.clnu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Cooper AL, Gibbons L, Horan MA, Little RA, Rothwell NJ. Effect of dietary fish oil supplementation on fever and cytokine production in human volunteers. Clin Nutr. 1993;12:321–328. doi: 10.1016/0261-5614(93)90027-2. [DOI] [PubMed] [Google Scholar]
- 43.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 45.Beutler B, Kruys V. Lipopolysaccharide signal transduction, regulation of tumor necrosis factor biosynthesis, and signaling by tumor necrosis factor itself. J Cardiovasc Pharmacol. 1995;25(suppl 2):S1–S8. doi: 10.1097/00005344-199500252-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mackensen A, Galanos C, Wehr U, Engelhardt R. Endotoxin tolerance: regulation of cytokine production and cellular changes in response to endotoxin application in cancer patients. Eur Cytokine Netw. 1992;3:571–579. [PubMed] [Google Scholar]
- 47.Shikora SA, Martindale RG, Schwarzberg SD, editors. Nutritional Considerations in the Intensive Care Unit. Dubuque, IA: Kendall/Hunt; 2002. [Google Scholar]
- 48.Rajan S, Buchman TG, Coopersmith CM, et al. Intestine specific overexpression of IL-10 improves survival in a polymicrobial model of sepsis. Crit Care Med. 2005;33(suppl):A133. doi: 10.1097/shk.0b013e31815bbb26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tessari P, Kiwanuka E, Cristini M, et al. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes Metab Res Rev. 2007;23:378–385. doi: 10.1002/dmrr.698. [DOI] [PubMed] [Google Scholar]
- 50.Ledesma Castano F, Echevarria Vierna S, Lozano Polo JL, Oloriz Rivas R, Alvarez Moreno C, Pons Romero F. Interleukin-1 in alcoholic cirrhosis of the liver: the influence of nutrition. Eur J Clin Nutr. 1992;46:527–533. [PubMed] [Google Scholar]
- 51.Loi C, Osowska S, Neveux N, Darquy S, Cynober L, Moinard C. Effects of an immune-enhancing diet in endotoxemic rats. Nutrition. 2004;21:255–263. doi: 10.1016/j.nut.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137:1835–1843. doi: 10.1093/jn/137.8.1835. [DOI] [PubMed] [Google Scholar]
- 53.Fang XL, Zhang YT, Fang Q. Impact of intravenous nutrition on plasma free amino acid spectrum and immune function for patients with sepsis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007;36:298–302. doi: 10.3785/j.issn.1008-9292.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Cribb PJ, Williams AD, Carey MF, Hayes A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab. 2006;16:494–509. doi: 10.1123/ijsnem.16.5.494. [DOI] [PubMed] [Google Scholar]
- 55.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yermolaieva O, Xu R, Schinstock C, et al. Methionine sulfoxide reductase A protects neuronal cells against brief hypoxia/reoxygenation. Proc Natl Acad Sci USA. 2004;101:1159–1164. doi: 10.1073/pnas.0308215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oz HS, Ebersole JL. Application of prodrugs to inflammatory diseases of the gut [review] Molecules. 2008;13:452–474. doi: 10.3390/molecules13020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmonds NJ, Allen RE, Stevens TR, Van Someren RN, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann I, Hoelzl A, Schliephake F, et al. Effects of adenosine on functions of polymorphonuclear leukocytes from patients with septic shock. Shock. 2007;27:25–31. doi: 10.1097/01.shk.0000238066.00074.90. [DOI] [PubMed] [Google Scholar]


