Abstract
Children with classic galactosemia are at risk for motor speech disorders resulting from disruptions in motor planning and programming (childhood apraxia of speech or CAS) or motor execution (dysarthria). In the present study of 33 children with classic galactosemia, 21% were diagnosed with CAS, 3% with ataxic dysarthria, and 3% with mixed CAS-dysarthria. Voice disorders due to laryngeal insufficiency were common in children with dysarthria and co-occurred with CAS. Most (58%) of the children with classic galactosemia had decreased respiratory-phonatory support for speech, and 33% had disturbed vocal quality that was indicative of cerebellar dysfunction. Three children, two diagnosed with CAS and one not diagnosed with a motor speech disorder, had vocal tremors. Treatment of voice dysfunction in neurogenic speech disorders is discussed.
Introduction
Galactosemia and speech disorders
Classic galactosemia (shortened to galactosemia in the present paper) is a recessive inborn error of metabolism characterized by defective conversion of galactose to glucose, due to a near absence of the enzyme galactose-1-phosphate uridyl transferase. This enzyme deficiency leads to an accumulation of toxic metabolites. In many patients with galactosemia, abnormalities of myelinization are found in the cerebral hemispheres and cerebellum (Phelan et al. 2008). Despite early detection and adherence to lactose-restricted diets, speech disorders affect more than half of children with galactosemia (Nelson et al. 1991; Waggoner et al. 1990; Waisbren et al. 1983; Webb et al. 2003). Most studies have used the diagnostic term developmental verbal dyspraxia to refer to the type of speech disorder reported to occur in 35–77% of children with galactosemia (Hughes et al. 2009; Nelson et al. 1991; Robertson et al. 2000; Webb et al. 2003). The term developmental verbal dyspraxia has recently been replaced with childhood apraxia of speech (CAS; American Speech-Language-Hearing Association 2007) and will be used in this paper. CAS is a type of motor speech disorder (MSD). Motor speech disorders are a category of speech disorders caused by disruptions in higher-level motor commands, neuromuscular system impairments, or both (Duffy 2005). CAS is characterized by (1) inconsistent consonant and vowel errors, (2) difficulty transitioning between articulatory movements, and (3) inappropriate prosody during speech (American Speech-Language-Hearing Association 2007). In adults, apraxia may result from a disruption in higher-level motor commands primarily in the left hemisphere Broca’s area, supplementary motor area, or insula and may involve the cerebellum and basal ganglia leading to difficulty in planning and programming the sequence of speech movements. The neurobiological basis of CAS is not yet known (Terband and Maassen 2010). Currently, there is no accepted “gold standard” assessment instrument for diagnosing CAS, resulting in variability in diagnostic prevalence across studies.
CAS often is confused with, or co-presents with, another type of MSD, dysarthria, infrequently reported to occur in individuals with galactosemia (Koch et al. 1992; Lo et al. 1984). Dysarthria is characterized by consistent vowel and consonant errors and inappropriate prosody due to one or more of the following: (1) muscle weakness, (2) decreased range of motion, (3) decreased speed, or (4) impaired coordination (Duffy 2005). Impaired steadiness of movement may also be observed at rest, in a sustained posture, or during movement (e.g., tremors or fasciculations). Different types of dysarthria result from damage to the left hemisphere premotor and primary motor areas (spastic dysarthria), cerebellum (ataxic dysarthria), basal ganglia (hypokinetic or hyperkinetic dysarthria), peripheral nervous system, or speech musculature (flaccid dysarthria) leading to difficulty executing movement of the speech system. Dysarthria may co-occur with CAS. Determining the diagnostic boundaries among the types of dysarthria and between CAS and dysarthria is particularly challenging in children and adults when the onset of the MSD was congenital or acquired prior to speech acquisition because (1) CAS and the different types of dysarthria share several speech, prosody, and voice characteristics; (2) more than one area of the brain may be affected resulting in mixed types of dysarthria or mixed CAS-dysarthria; and (3) speech systems were not intact prior to the neurogenic insult (American Speech-Language-Hearing Association 2007). In galactosemia, the onset of an MSD likely occurred during prenatal development (Hughes et al. 2009).
Language and cognition
Speech disorders and language disorders frequently co-occur (American Speech-Language-Hearing Association 2007). As compared to children with speech disorders of unknown origin, children with MSDs have an increased risk of co-occurring persistent language disorders and low cognition. Multiple studies have found that approximately half of all children with galactosemia have intelligence quotient standard scores (IQ) in the borderline to low range (IQ scores below 85) and half have normal (IQ scores of 85–115) intelligence (Kaufman et al. 1995; Koch et al. 1992; Lo et al. 1984; Waggoner et al. 1990; Waisbren et al. 1983). Children with galactosemia and a speech disorder have a four- to sixfold greater risk for language disorders than children with speech disorders of unknown origin (Potter et al. 2008). The type of language disorder is associated with the individual’s level of cognitive function. In a study of the same sample of 33 children with galactosemia and speech disorder to be described in the present study, 88% of children (n=17) with IQ scores in the low and borderline range (standard scores below 85) have a language disorder, typically affecting both receptive and expressive language, while 56% of children (n=16) with average IQ (standard scores of 85–110) have a language disorder, typically affecting expressive language only.
Voice
Although voice disorders have not been reported in children with galactosemia, the high prevalence of MSDs in this population places children with galactosemia at risk for laryngeal insufficiency and resulting voice disorders. When examining voice disorders, respiration is assessed in tandem with phonation as disorders of one system affect the other. Neurological disturbances in voice production may be due to 1) vocal fold dysfunction, 2) incoordination between the timing of respiration and the onset of phonation, or 3) less commonly, inadequate respiration (Duffy 2005). Phonatory function is assessed during a motor speech examination using sustained vowel prolongation tasks, which can be analyzed for maximum phonation time and vocal quality. Production of a sustained vowel, typically /ah/, is produced at conversational voice volume (65–80 dB), recorded, and timed. Voice disorders, due to insufficient laryngeal function, are nearly universal in all types of dysarthria (Kent and Kim 2003). Laryngeal insufficiency is characterized by short maximum phonation times and disturbed vocal quality, and while these characteristics are primarily present in dysarthria, they also co-occur in CAS (American Speech-Language-Hearing Association 2007; Kent et al. 2003; Thoonen et al. 1999). Maximum phonation time is used to determine if an individual has adequate respiratory-phonatory capacity for conversational speech. In adults, a maximum phonation time of 8 s is thought to be adequate for speech (Duffy 2005).
As compared to maximum phonation time, vocal quality is more challenging to reliably assess. The inter-judge reliability of vocal quality has traditionally been poor because perceptual judgments of an individual’s voice require listeners to simultaneously attend to and judge multiple aspects including pitch, loudness, instability, and tremor (Cornwell et al. 2004). The Multi-Dimensional Voice Program™ (MDVP; Kay Elemetrics 2003) is a computer software program that objectively measures more than 30 different voice parameters. Nine of the 30 voice parameters are associated with abnormal voice production evidenced in different types of dysarthria in children and adults (Kent et al. 2003; Kent and Kim 2003).
Statement of purpose
Voice disorders are common in individuals with MSDs, and more than half of all children with galactosemia have an MSD. The goal of the present study was to examine phonatory function and voice quality in children with galactosemia and speech disorders.
Methods
Participants
Participants included 33 children with classic galactosemia and 130 typically developing children serving as healthy controls. The children with galactosemia, ages 4–16 years, were recruited through two support groups, Parents of Galactosemic Children and Galactosemic Families of Minnesota, and by email, postal, and website announcements to metabolic clinics; they were part of a larger study on CAS. All children with galactosemia were tested in their homes by a certified speech language pathologist (author). Inclusionary criteria were a diagnosis of classic galactosemia and a history of speech sound disorders, both by parental report. Exclusionary criteria included a first language other than English, significant hearing loss, and craniofacial anomalies, including cleft palate. All children had bilateral hearing within normal limits except for one child with a mild bilateral hearing impairment (20–35 dB). One child was tested, but excluded, due to a previously undetected severe-profound (70–90 dB) bilateral hearing loss. The most common genotype Q188R/Q188R was reported for 13/33 participants and Q188R/other was reported for 11/33 participants. Genotypes were not available for nine of the children. Adherence to a galactose-restricted diet was reported for all participants with galactosemia.
Control children, five females and five males of each age from 4 to 16 years, were recruited through teachers and speech language pathologists in preschools, elementary, middle, and high schools and tested by the same examiner (author). Inclusionary criteria for controls were normal hearing, articulation within normal limits on a standardized articulation test, no history of referral for special education services including speech-language, academic performance at grade level as reported on parent and teacher questionnaires, no craniofacial anomalies, and English as a first language. Results of the language assessments are reported in Potter et al. (2008) and speech assessments are available from Shriberg et al. (2010) for this group of children with galactosemia and the healthy controls.
The study was approved by the Institutional Review Boards of Washington State University and the University of Wisconsin-Madison. After explanation of the study, a parent of each participant provided informed written consent and all participants over age 11 years provided informed assent.
Procedures
All participants had their hearing screened at 25 dB for 1,000, 2,000, and 4,000 Hz. Speech and language were assessed through a battery of formal and informal tests (Potter et al. 2008; Shriberg et al., 2010). Expressive language was assessed with the Oral Expression subtest and receptive language was assessed with the Listening Comprehension subtest of the Oral and Written Language Scales (OWLS; Carrow-Woolfolk 1995), and IQ was assessed with the Kaufman Brief Intelligence Test, 2nd ed. (KBIT-2; Kaufman and Kaufman 2004).
Using video and audio recordings, Edythe Strand, PhD, a recognized expert in pediatric motor speech disorders from Mayo Clinic, classified the presence and type of motor speech using the Adapted Mayo Clinic Motor Speech Disorder Classification System (Table 1).
Table 1.
Mayo Clinic Motor Speech Disorder Classification System
| Mayo Clinic Motor Speech Disorder Classification System | |
|---|---|
| Apraxia of Speech | Dysarthria |
| 4/10 behaviors in three or more tasks | 3/10 behaviors in three or more tasks |
| Inconsistent articulation errors on vowels or consonants | Consistent imprecise articulation errors on vowels or consonants |
| Vowel distortions | Consonant and/or vowel distortions |
| Distorted substitutions | |
| Voicing errors | |
| Increased omissions or difficulty with sequencing multi-syllabic words | |
| Inappropriate prosody | Inappropriate prosody |
| Equal stress or lexical stress errors | Equal sentential stress |
| Slow rate | Slow rate |
| Slow diadochokinetic rates | Irregular diadochokinetic rates |
| Scanning speech | |
| Difficulty achieving initial articulatory configurations or transitionary movement gestures | Neuromuscular involvement |
| Intrusive schwa | Reduced range of articulatory motion |
| Syllable segregation | Reduced strength of articulatory contacts |
| Groping | Reduced respiratory support/reduced respiratory coordination |
| Strained or breathy phonatory quality | |
| Adventitious movement | |
Categorical features in shaded rows. Specific speech characteristics in non-shaded rows
A sustained maximum phonation task was used to obtain voice samples for analyses. Children were instructed to sustain the vowel /ah/ as long as possible on one breath using their normal pitch and loudness levels. The examiner provided an example prior to the children’s productions. Each child performed three trials with a 10–15 s rest between trials. Voice samples were recorded using a Sony DCR-DVD301 digital video recorder with a Shure WH30TOG cardioid microphone for 15 of the children with galactosemia (the first group of children tested) and a Marantz CDR 420 digital audio recorder with a Shure MX412 D/C microphone for 18 of the children with galactosemia and all control children. To reduce aerodynamic noise, the microphone was positioned at a fixed (15 cm) 30° off-axis position from the mouth. Prior to recording, the input signal was adjusted to a predetermined level to standardize input amplitude. Maximum phonation time was determined as the longest sustained phonation trial and was measured using Sound Forge 7.0 digital audio editing software (Sony Creative Software, Madison, WI). Vocal quality was analyzed using the MDVP program on the Computerized Speech Laboratory (CSL) model 4500 (Kay Elemetrics 2003). The MDVP analyzes 30 different voice parameters. Specific clusters of abnormal voice parameters, rather than abnormal values on individual voice parameters, are informative when diagnosing the presence of and type of dysarthria using the MDVP program. Elevated frequency parameters are associated with rough voices and laryngeal instability (Cornwell et al. 2004). Elevated amplitude parameters are associated with breathy voices. Elevated shimmer, jitter, and tremor parameters are associated with vocal tremor (Shao et al. 2010). Studies of adults with dysarthria typically average the values for each voice parameter across three trials (Kent et al. 2000). However, with young children variability due to behavior including attention span and distractibility is typical (Potter et al. 2009). To reduce the variability due to behavior in the present study, a single trial, determined to be the “best trial” with the steadiest amplitude at the child’s habitual volume and pitch was selected for analysis. The child’s best trial was selected for analysis by (1) visual inspection of the waveform for the most consistent amplitude across a 3 s segment and (2) longest sustained phonation time on a single breath. The first and last 50 ms of each sample were excluded for voice analysis as MDVP is highly sensitive to variability during phonation onset and offset (Kent et al. 2000).
Statistical analysis
For statistical analyses, children with galactosemia and a diagnosis of CAS and/or dysarthria were clustered into a single motor speech group due to the overlapping characteristics, diagnoses of mixed CAS-dysarthria, and the small cell size of children diagnosed with dysarthria only (n=1). Analysis of covariance (ANCOVA), with age and gender as the covariates, was used to assess between-group differences for the following three groups: (1) children with galactosemia diagnosed with MSDs (GAL-MSD), (2) children with galactosemia with a history of speech sound disorders but not diagnosed with an MSD (GAL), and (3) typically developing healthy control children. Tukey’s HSD was used post-hoc to adjust for unequal sample size. Pearson product moment correlation with age as a covariate was used to examine the relatio/nship among receptive and expressive language, IQ, and maximum phonation time. Significance was set at p<0.05.
Results
Motor speech disorders
Nine of the 33 children (27%) with galactosemia were diagnosed with an MSD (Shriberg et al., 2010). Of the children diagnosed with an MSD, eight (24%) were diagnosed with CAS including seven children (21%) who met the present criteria for a diagnosis of CAS only and one child (3%) who met the criteria for diagnosis of CAS and dysarthria (mixed CAS-dysarthria). One child (3%) met the criteria for a diagnosis of dysarthria only. All control children had normal speech.
Voice
All control children and 31 of the 33 children with galactosemia were able to sustain phonation for 3 s or longer, which is optimum for voice analyses. One 4 year old with galactosemia in the GAL group had a maximum phonation time of 2.5 s. The middle 1 s was used for voice analyses. One child with galactosemia, diagnosed with mixed CAS-dysarthria, had a maximum phonation time of 0.51 s and was included in the maximum phonation time calculations but was excluded from the vocal quality analyses as the sample was shorter than the required 1 s minimum. A single trial was analyzed rather than an average of the three trials, as is typical of studies with adults, as children with galactosemia and control children, 8 years of age and younger, showed a tendency to stop phonation during a trial due to distractibility or volitionally increase volume or pitch on one or more trials when asked to sustain /ah/, even after the examiner modeled the task.
Maximum phonation time
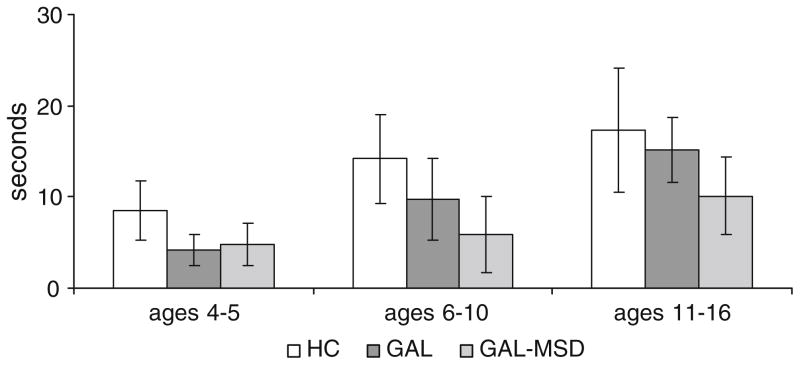
The GAL and the GAL-MSD groups had significantly shorter maximum phonation times than the control group (F2,160 =12.25, P <0.001). Maximum phonation times were reduced by an average of 32% in the GAL and by 62% in the GAL-MSD groups as compared to their age-matched controls (Fig. 1). Maximum phonation times for the GAL-MSD group did not significantly differ from the GAL group. The data for participants ages 4–6, 7–10, and 11–16 years are shown in separate columns for visual inspection across development in Fig. 1. The number and percentage of children in each group able to sustain phonation for a minimum of 8 s is shown in Table 2. Maximum phonation times were not related to expressive language, receptive language, or IQ standard scores.
Fig. 1.
Comparison of maximum phonation duration in healthy controls (HC), galactosemia without motor speech disorders (GAL), and galactosemia with motor speech disorders (GAL-MSD)
Table 2.
Number and percentage of children able to sustain phonation for 8 s or longer by age and group
| Group | Age range in years |
||
|---|---|---|---|
| 4–6 | 7–10 | 11–16 | |
| Healthy controls | 19/30 (63%) | 40/40 (100%) | 59/60 (98%) |
| GAL | 1/5 (20%) | 9/14 (64%) | 5/5 (100%) |
| GAL-MSD | 0/3 (0%) | 1/4 (25%) | 1/2 (50%) |
GAL Children with galactosemia not diagnosed with a motor speech disorder, GAL-MSD children with galactosemia diagnosed with a motor speech disorder
Vocal quality
For vocal quality analyses, the mean score ±2 standard deviations for each of the nine voice parameters associated with dysarthria was used as a conservative estimate of abnormality (Kent et al. 2000). Eight (6%) children in the control group, eight (33%) children in the GAL group, and three (33%) children in the GAL-MSD group had two or more of the nine voice parameters that were greater than two standard deviations above the mean. Abnormal voice parameters were not associated with reduced maximum phonation times. Although MDVP results differed by voice parameter across individuals, group patterns emerged to characterize laryngeal insufficiency in GAL and GAL-MSD as compared to the control children. Table 3 lists the nine MDVP voice parameters associated with different types of dysarthria, including ataxic dysarthria in adults (Kent et al. 2000) and children (Cornwell et al. 2004) and Parkinson’s disease and vocal polyps in adults (Shao et al. 2010) and shows which voice parameters differed across GAL or GAL-MSD groups as compared to the controls. The largest and most frequent vocal abnormalities were variations in parameters measuring fundamental frequency (Fo; the lowest frequency produced by the vocal folds) and amplitude (volume), which are indicative of neurogenic laryngeal instability and perceptually rough and breathy voices. The GAL and GAL-MSD groups did not significantly differ from the control group on parameters indicative of vocal tremor. However three individuals, one (4%) from the GAL group and two (22%) from the GAL-MSD group, had values greater than two standard deviations from the mean of the control group on at least three of the four parameters indicative of vocal tremors: jitter percent, shimmer percent, fundamental frequency tremor frequency, and fundamental frequency tremor intensity index.
Table 3.
Comparison across groups on the nine MDVP parameters indicative of laryngeal insufficiency observed in ataxic dysarthria due to cerebellar lesions or tumors (Cornwell et al. 2004; Kent et al. 2000) or in laryngeal hypo-function or tremor in hypokinetic dysarthria due to basal ganglia dysfunction in Parkinson’s disease or vocal polyps (Shao et al. 2010)
| Parameter | Task | Abbreviation | GAL vs. controls | GAL-MSD vs. controls | Indicative of: |
|---|---|---|---|---|---|
| F1,154 | F1,138 | Observed in: | |||
| Fundamental frequency (Fo) | Standard deviation of fundamental frequency | STD | 8.75** | 5.57* | Laryngeal insufficiency Ataxic dysarthria in adults |
| Phonatory Fo range in semi-tones | PFR | 8.07** | 5.62* | Laryngeal insufficiency Cerebellar tumor in children |
|
| Fundamental frequency variation | Vfo | 6.76* | ns | Laryngeal insufficiency Ataxic dysarthria in adults Cerebellar tumor in children |
|
| Amplitude | Smoothed amplitude perturbation quotient | sAPQ | 18.21*** | 10.83** | Laryngeal insufficiency Cerebellar tumor in children |
| Peak-amplitude variation | vAm | 12.25*** | 6.15* | Laryngeal insufficiency Laryngeal hypo-function Incomplete closure of vocal folds Ataxic dysarthria in adults Cerebellar tumor in children |
|
| Tremor | Jitter percent | Jitt | ns | ns | Voice tremor Parkinson’ s disease Vocal polyps |
| Shimmer percent | Shim | ns | ns | Voice tremor Parkinson’ s disease Vocal polyps |
|
| Fundamental frequency tremor frequency | Fftr | ns | ns | Voice tremor Parkinson’ s disease Vocal polyps |
|
| Fundamental frequency tremor intensity index | FTRI | ns | ns | Voice tremor Parkinson’ s disease Vocal polyps |
|
P < 0.05,
P < 0.01,
P < 0.001
Discussion
Motor speech disorders
Children with speech disorders secondary to galactosemia are conservatively at a six- to eight fold greater risk for CAS as compared to children with speech disorders of unknown origin. In a recent study of an estimated 12,000–15,000 children evaluated for speech disorders at a large metropolitan children’s hospital, 516 (3–4%) were diagnosed with CAS (Delaney and Kent 2004). In the present study, 21% of the children with galactosemia and a history of speech disorders were diagnosed with CAS only, 3% with mixed CAS-dysarthria, and 0.3% with ataxic dysarthria only. The actual prevalence of CAS in children with galactosemia is expected to be less than the total 24% reported in the present study as a history of speech disorders was an inclusionary criterion. The lower prevalence of CAS reported in the present study with its speech disorder bias, as compared to 35–77% reported in previous studies (Hughes et al. 2009; Nelson et al. 1991; Robertson et al. 2000; Webb et al. 2003), is likely due to stricter criteria for the diagnosis of CAS. Prevalence estimates for dysarthria in children with speech disorders are not known. The difference in prevalence of CAS across studies of children with galactosemia speaks to the pressing need for accepted standardized assessments that allow for reliable and consistent reporting of developmental MSDs including the differential diagnosis of CAS and dysarthria. A more detailed description of the diagnostic procedure for classifying speech disorders in the children participating in the present study can be acquired from Shriberg et al. (2010).
Voice
As a group, children with galactosemia, with or without diagnosed MSDs, had increased laryngeal insufficiency characterized by short maximum phonation times and disturbed vocal quality as compared to the control children. Disturbed vocal quality was perceptually evident in children with galactosemia. For example, a parent of a child with galactosemia reported that after years of speech therapy the child’s speech was intelligible to everyday listeners, but in public people turned to see who was speaking because of the child’s unusual voice quality.
Maximum phonation time
While severity of laryngeal insufficiency differed across individuals, one-third of the children with galactosemia not diagnosed with an MSD and two-thirds of the children with galactosemia and an MSD had reduced respiratory-phonatory support for speech when compared to the controls. Younger children with galactosemia (ages 4–6 years) and children diagnosed with an MSD and galactosemia were at greatest risk for reduced respiratory-phonatory function. For normal adult-length conversation, individuals should be able to sustain phonation for 8 s or longer (Duffy 2005). Most control children (60%) ages 4–6 years and nearly all (98%) age 7 years and older were able to sustain phonation for a minimum of 8 s. In contrast, in children with galactosemia not diagnosed with an MSD, 28% of children age 7 and older and 80% of children ages 4–6 years were unable to sustain phonation for a minimum of 8 s. Of the children with galactosemia and an MSD, 66% of those age 7 years and older and all children aged 4–6 years were unable to sustain phonation for 8 s.
Decreased ability to sustain phonation may be due to incoordination in the timing of the onset of respiration and the onset of phonation resulting in air wastage or in decreased strength and endurance of the respiratory-phonatory system resulting in difficulty sustaining adequate volume levels (Duffy 2005). Most children with galactosemia were able to carry on conversation at a normal volume; however one child consistently spoke in a soft, barely audible voice and one child spoke with a markedly breathy voice with intermittent periods of aphonia (no voice). Three children had noticeably abrupt voice onsets. The parents reported that these vocal behaviors were typical of their children in all situations. The short maximum phonation times, breathy voices, and abrupt voice onsets were likely due to respiratory-phonatory incoordination as most children with galactosemia were able to maintain adequate voice volume without a progressive decay in volume across the maximum phonation task. Ideally, stroboscopic examinations should be completed on individuals with laryngeal insufficiency to rule out other vocal pathologies; however, this was not feasible in the present study as the children were tested in their homes and the equipment, at this time, is not portable.
Although children with galactosemia were typically able to maintain adequate voice volume, they compensated for decreased respiratory-phonatory support by using shorter utterances. Half (47%) of the children with galactosemia had average utterance lengths that were 1.5 SD or more below their typically developing peers (Potter et al. 2006). Laryngeal insufficiency has been reported to contribute to decreased utterance length in other neurologic diseases including Parkinson’s disease and ataxic dysarthria (Sapir et al. 2003, 2007). The effect of decreased respiratory-phonatory support for speech is evident in the following example. The child with galactosemia and mixed CAS-dysarthria had a speech therapy goal of increasing average utterance length to three to four words in conversational speech. This child had a maximum phonation time of 0.51 s. The average speaking rate for a child is 3.3 syllables per second (Pindzola et al. 1989). Without consideration of the influence of CAS and dysarthria, this child could not be expected to produce more than two to three words on one breath, physically limiting the production of long sentences, yet increasing respiration and phonation were not included in the speech goals. Laryngeal insufficiency is not the only contributing factor to decreased utterance length in children with galactosemia, but rather compounds other problems including motor planning and articulation skills (Nelson et al. 1991), and short-term memory (Widhalm et al. 2002; Waisbren et al. 1983) and language disorders (Potter et al. 2008).
Vocal quality
Disturbed vocal quality, as measured by MDVP, was evident in one-third of the children with galactosemia as compared to 6% of the control children and was not associated with short phonation times or an MSD diagnosis. The control children typically had one or two elevated voice parameters while the children with galactosemia and disturbed vocal quality typically had multiple elevated parameters. Unlike the control children, the children with galactosemia and disturbed vocal quality had voice profiles that were typical of ataxic dysarthria present in children with cerebellar tumors and adults with cerebellar lesions (Cornwell et al. 2004; Kent et al. 2000). Perceptually, ataxic voice disorders are characterized by roughness, instability, breathiness, weakness, and abrupt voice onsets, which were evident in some children with galactosemia. Cerebellar dysfunction has been reported in multiple studies of individuals with galactosemia. Kaufman et al. (1995) reported that 25% of individuals with galactosemia showed evidence of cerebellar dysfunction including ataxia, tremors, and dysmetria. Phelan et al. (2008) found focal white matter abnormalities in the cerebellum and cerebrum, and in a recent study of cerebral function using [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) scans in adults with classic galactosemia, Dubroff and colleagues (2008) found significant widespread decreases in glucose metabolism in the cerebrum and in multiple areas of the cerebellum. They proposed that decreased cerebellar glucose metabolism was associated with the overt cerebellar signs observed in many individuals with galactosemia. The voice profiles in the present study implicate the cerebellum as a likely contributor to galactosemic voice disorders and suggest the presence of an underlying or subclinical ataxic dysarthria in some children with galactosemia and CAS and children with galactosemia not diagnosed with an MSD.
As a group, children with galactosemia did not evidence MDVP profiles typical of adults with vocal tremors; however, three children with galactosemia, two diagnosed with CAS and one not diagnosed with an MSD, had perceptually evident vocal tremors during sustained phonation. All three children’s MDVP voice profiles had elevated vocal tremor parameters (Jitt, Shim, Fftr, and FTRI) in addition to elevated voice parameters associated with cerebellar dysfunction. The three children with vocal tremors also had overt upper extremity tremors that were most evident during extension. Vocal tremors are not always evident in children with limb tremors. Two children with galactosemia, not diagnosed with an MSD, had obvious upper extremity tremors but did not have elevated vocal tremor parameters. The child diagnosed with ataxic dysarthria only had ataxic speech, gait, and upper extremity movements but did not have elevated vocal tremor parameters. Tremors, primarily affecting upper extremities, have been reported to affect approximately 10–20% of individuals with galactosemia and frequently occur with signs of ataxia (Reidel et al. 2005). While cerebellar disease may infrequently result in tremor of the laryngeal and respiratory muscles (Duffy 2005), the cause or causes of vocal tremor could not be determined by the analyses used in the present study. MDVP tremor parameter profiles do not distinguish among types of tremor or site of lesion. For example, individuals with tremors due to central nervous system (Parkinson’s disease) and peripheral (vocal fold polyps) pathologies have similar MDVP tremor profiles (Shao et al. 2010). Vocal tremors observed in the three children with galactosemia may be the result of dysfunction in the cerebellar or subcortical structures. Dubroff et al. (2008) suggested that tremors in individuals with galactosemia may be associated with decreased glucose metabolism in the cerebellum and increased glucose metabolism in the basal ganglia.
Diversity of effects across patients
The late Dr. Stanton Segal described galactosemia as “an enigmatic disorder that presents a challenge in unraveling the basis of long-term complications” (Segal 1995, pg. S97). Fifteen years later, galactosemia remains an enigmatic disorder in part due to its diverse effects. Statements about the disorders associated with galactosemia may be characteristic of the group as a whole, but the effects of galactosemia differ widely across individuals.
Directions in treatment
Much has been written about the challenges associated with galactosemia, but little has addressed treatment strategies. Therapy programs effective in increasing respiratory-phonatory support for speech in other neurologic populations may improve long-term outcomes in galactosemia. Approaches such as the Lee Silverman Voice Treatment program (LSVT) have been successful in increasing respiratory-phonatory strength and coordination in patients with Parkinson’s disease and in patients with ataxic dysarthria (Ramig et al. 2001; Sapir et al. 2007). LSVT, conducted under the direction of a speech-language pathologist certified in this approach, involves 16 therapy sessions over 4 weeks focusing on increasing respiratory-phonatory intensity and duration. Brain imaging studies have shown an increase in recruitment in the cerebellum and right hemisphere motor cortex after 4 weeks, with long-term (2 year) increases in respiratory-phonatory strength and coordination and improved speech intelligibility (Narayana et al. 2009; Ramig et al. 2001). Appropriate candidates for LSVT are individuals with short maximum phonation times or disturbed vocal quality due to laryngeal insufficiency, who are able to follow directions and practice independently. Treatment research using systematic therapy approaches, such as LSVT, is needed to examine if voice disorders are remediable in individuals with galactosemia.
In summary, more than half (58%) of the children with galactosemia and speech disorders have reduced respiratory-phonatory support for speech, and one-third have disturbed vocal quality to laryngeal insufficiency, likely associated with cerebellar dysfunction. In addition vocal tremors of unknown origin are present in a small number of children with galactosemia (9% in the present study). Treatment approaches successful in patients with Parkinson’s disease and ataxic dysarthria may increase respiratory-phonatory coordination and contribute to improved speech and voice in individuals with galactosemia.
Acknowledgments
The research was supported by the National Institutes on Deafness and Other Communication Disorders (Grant DC000496, Lawrence D. Shriberg, PI), National Institute of Child Heath and Development (Grant HD03352, core grant to the University of Wisconsin-Madison Waisman Center), and Washington State University Spokane (Faculty seed grant, Nancy L. Potter, PI). I thank the following colleagues for their contributions to this study: Lawrence Shriberg, Edythe Strand, Karen Babson, Terrin Cox, Tonia Duggins, LaTrisha Griffiths, Erin Hawkins, Sandhip Minhas, Lola Rickey, Sue Siemsen, and the children and their families who participated in this study.
Abbreviations
- CAS
Childhood apraxia of speech
- GAL-MSD
Individuals with classic galactosemia diagnosed with a motor speech disorder
- GAL
Individuals with classic galactosemia not diagnosed with a motor speech disorder
- LSVT
Lee Silverman voice treatment program
- OWLS
Oral and written language scale
- MSD
Motor speech disorder
Footnotes
Competing interest: None declared
References
- American Speech-Language-Hearing Association. Childhood apraxia of speech (technical report) 2007 www.asha.org/policy.
- Carrow-Woolfolk E. OWLS: listening comprehension scale and oral expression scale. AGS; Circle Pines, MN: 1995. [Google Scholar]
- Cornwell PL, Murdoch BE, Ward EC, Kellie S. Acoustic investigation of vocal quality following treatment for childhood cerebellar tumor. Folia Phoniatr Logop. 2004;56:93–107. doi: 10.1159/000076061. [DOI] [PubMed] [Google Scholar]
- Delaney AL, Kent RD. Developmental profiles of children diagnosed with apraxia of speech. Poster session presented at the annual convention of the American Speech-Language-Hearing Association; Philadelphia, PA. 2004. [Google Scholar]
- Duffy JR. Motor speech disorders: substrates, differential diagnosis, and management. 2. Elsevier Science; St. Louis: 2005. [Google Scholar]
- Dubroff JG, Ficicioglu C, Segal S, Wintering NA, Alvi A, Newberg AB. FDG-PET findings in patients with galactosaemia. J Inherit Metab Dis. 2008;31:533–559. doi: 10.1007/s10545-008-0806-0. [DOI] [PubMed] [Google Scholar]
- Hughes J, Ryan S, Lambert D, Geoghegan O, Clark A, Rogers Y, Hendroff U, Monvavari A, Twomey E, Treacy EP. Outcomes of siblings with galactosemia. J Pediatr. 2009;154:721–726. doi: 10.1016/j.jpeds.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test. 2. AGS; Circle Pines, MN: 2004. [Google Scholar]
- Kaufman FR, McBride-Chang C, Manis FR, Wolff JA, Nelson MD. Cognitive functioning, neurologic status and brain imaging in classical galactosemia. Eur J Pediatr Suppl. 1995;154:S2–S5. doi: 10.1007/BF02143794. [DOI] [PubMed] [Google Scholar]
- Kay Elemetrics. Multi-Dimensional Voice Program: software instruction manual. Pine Brook, Kay Elemetrics; 2003. [Google Scholar]
- Kent RD, Kim YJ. Toward an acoustic typology of motor speech disorders. Clin Linguist Phon. 2003;17:427–445. doi: 10.1080/0269920031000086248. [DOI] [PubMed] [Google Scholar]
- Kent RD, Kent JF, Duffy JR, Thomas JE, Weismer G, Stuntebeck S. Ataxic dysarthria. J Speech Lang Hear Res. 2000;43:1275–1289. doi: 10.1044/jslhr.4305.1275. [DOI] [PubMed] [Google Scholar]
- Kent RD, Vorperian HK, Kent JF, Duffy JR. Voice dysfunction in dysarthria: application of the Multi-Dimensional Voice Program™. J Commun Disord. 2003;36:281–306. doi: 10.1016/s0021-9924(03)00016-9. [DOI] [PubMed] [Google Scholar]
- Koch TK, Schmidt KA, Wagstoff JE, Ng WG, Packman S. Neurologic complications in galactosemia. Pediatr Neurol. 1992;8:217–220. doi: 10.1016/0887-8994(92)90072-7. [DOI] [PubMed] [Google Scholar]
- Lo W, Packman S, Nash S, et al. Curious neurological sequelae in galactosemia. Pediatrics. 1984;73:309–312. [PubMed] [Google Scholar]
- Narayana S, Fox PT, Zhang W, Franklin C, Robin DA, Vogel D, Ramig LO. Neural correlates of efficacy of voice therapy in Parkinson’s disease identified by performance-correlation analysis. Hum Brain Mapp. 2009;31:222–236. doi: 10.1002/hbm.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CD, Waggoner DD, Donnell GN, Tuerck JM, Buist NR. Verbal dyspraxia in treated galactosemia. Pediatrics. 1991;88:346–350. [PubMed] [Google Scholar]
- Phelan JA, Lowe LH, Glasier CM. Pediatric neurodegenerative white matter processes: leukodystrophies and beyond. Pediatr Radiol. 2008;38:729–749. doi: 10.1007/s00247-008-0817-x. [DOI] [PubMed] [Google Scholar]
- Pindzola RH, Jenkins MM, Lokken KJ. Speaking rates of young children. Lang Speech Hear Serv Sch. 1989;20:133–138. [Google Scholar]
- Potter NL, Johnson JM, Shriberg LD. Speech and language characteristics of children with galactosemia. Poster session presented at the annual convention of the Symposium for Research in Child Language Disorders; Madison, WI. 2006. [Google Scholar]
- Potter NL, Lazarus J-AC, Johnson JM, Steiner RD, Shriberg LD. Correlates of language impairment in children with galactosaemia. J Inherit Metab Dis. 2008;31:524–532. doi: 10.1007/s10545-008-0877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter NL, Kent RD, Lazarus JA. Oral and manual force control in children: is there evidence for common control? J Motor Behav. 2009;41:66–82. doi: 10.1080/00222895.2009.10125919. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, Thompson LL. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel KR, Leslie ND, Gilbert DL. An updated review of the long-term neurological effects of galactosemia. Pediatr Neurol. 2005;33:153–161. doi: 10.1016/j.pediatrneurol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Robertson A, Singh RH, Guerrero NV, Hundley M, Elsas LJ. Outcomes analysis of verbal dyspraxia in classic galactosemia. Genet Med. 2000;2:142–148. doi: 10.1097/00125817-200003000-00005. [DOI] [PubMed] [Google Scholar]
- Sapir S, Spielman J, Countryman S, Ramig L, Hinds S, Fox C, Story B. Phonatory and articulatory changes in ataxic dysarthria following intensive voice therapy with the LSVT: a single subject study. Am J Speech Lang Pathol. 2003;12:387–399. doi: 10.1044/1058-0360(2003/085). [DOI] [PubMed] [Google Scholar]
- Sapir S, Spielman JL, Ramig LO, Story BH, Fox C. Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on vowel articulation in dysarthric individuals with idiopathic Parkinson disease: acoustic and perceptual findings. J Speech Lang Hear Res. 2007;50:899–912. doi: 10.1044/1092-4388(2007/064). [DOI] [PubMed] [Google Scholar]
- Segal S. Galactosemia unsolved. Eur J Pediatr. 1995;154:S97–S102. doi: 10.1007/BF02143813. [DOI] [PubMed] [Google Scholar]
- Shao J, MacCallum JK, Zhang Y, Sprecher A, Jiang JJ. Acoustic analysis of the tremulous voice: assessing the utility of the correlation dimension and perturbation parameters. J Commun Disord. 2010;43:35–44. doi: 10.1016/j.jcomdis.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Potter NL, Strand EA. Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. J Speech Lang Hear R. 2010 doi: 10.1044/1092-4388(2010/10-0068). (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terband H, Maassen B. Speech motor development in childhood apraxia of speech: generating testable hypotheses by neurocomputational modeling. Folia Phoniatr Logop. 2010;62:134–142. doi: 10.1159/000287212. [DOI] [PubMed] [Google Scholar]
- Thoonen F, Maassen B, Gabreels F, Schreuder R. Validity of maximum performance tasks to diagnose motor speech disorders in children. Clin Linguist Phon. 1999;13:1–23. [Google Scholar]
- Waggoner DD, Buist NR, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- Waisbren SE, Norman TR, Schnell RR, Levy HL. Speech and language deficits in early-treated children with galactosemia. J Pediatr. 1983;102:75–77. doi: 10.1016/s0022-3476(83)80292-3. [DOI] [PubMed] [Google Scholar]
- Webb AL, Singh RH, Kennedy MJ, Elsas LJ. Verbal dyspraxia and galactosemia. Pediatr Res. 2003;53:396–402. doi: 10.1203/01.PDR.0000049666.19532.1B. [DOI] [PubMed] [Google Scholar]
- Widhalm K, Miranda-da-Cruz BM, de Sonneville LMJ. Information processing characteristics in children with classical galactosemia. Nutr Res. 2002;22:257–270. [Google Scholar]