Abstract
Volatile monoterpenes such as 1,8-cineole inhibit the growth of Brassica campestris seedlings in a dose-dependent manner, and the growth-inhibitory effects are more severe for roots than hypocotyls. The preferential inhibition of root growth may be explained if the compounds inhibit cell proliferation more severely than cell elongation because root growth requires both elongation and proliferation of the constituent cells, whereas hypocotyl growth depends exclusively on elongation of existing cells. In order to examine this possibility, BY-2 suspension-cultured tobacco (Nicotiana tabacum) cells were treated with 1,8-cineole, and the inhibitory effects on cell proliferation and on cell elongation were assessed quantitatively. Treatment with 1,8-cineole lowered both the mitotic index and elongation of the cells in a dose-dependent manner, and the half-maximal inhibitory concentration (IC50) for cell elongation was lower than that for cell proliferation. Moreover, 1,8-cineole also inhibited starch synthesis, with IC50 lower than that for cell proliferation. Thus, the inhibitory effects of 1,8-cineole were not specific to cell proliferation; rather, 1,8-cineole seemed inhibitory to a variety of physiological activities when it was in direct contact with target cells. Based on these results, possible mechanisms for the mode of action of 1,8-cineole and for its preferential inhibition on root growth are discussed.
Key Words: BY-2; Cell elongation; Cell proliferation; 1,8-cineole; Monoterpene; Protoplast; Starch synthesis
Introduction
Monoterpenes are major components of essential oils, and are wide-spread secondary metabolites in the plant kingdom. Allelopathic interactions due to monoterpenes may be common in nature (e.g., Müller, 1970). They inhibit seed germination and plant growth (Tarayre et al., 1995; Abrahim et al., 2000), causing morphological and physiological changes in plant seedlings (Einhellig and Leather, 1988; Fischer, 1991; Dudai et al., 2000). They inhibit respiration in isolated mitochondria and mitosis, deteriorate membrane integrity of treated cells, interfere with cuticular waxes, enhance transpiration, cause lipid oxidation, and disrupt microtubules (Duke and Oliva, 2004; Dayan et al., 2000; Romagni et al., 2000; Schulz et al., 2007; Zunino and Zygadlo, 2004; Chaimovitsh et al., 2010). Nonetheless, the molecular mechanism (e.g., primary target point) for the allelopathic effects of monoterpenes is still obscure.
Koitabashi et al. (1997) and Nishida et al. (2005) examined the effects of five monoterpenes produced by S. leucophylla (camphor, 1,8-cineole, α-pinene, β-pinene, and camphene) on germination and seedling growth of a test plant, Brassica campestris. The monoterpenes inhibited growth of B. campestris seedlings in a dose-dependent manner, and their inhibitory effects appeared to affect root growth more severely than hypocotyl growth. The monoterpenes did not alter the sizes of matured cells in roots but effectively inhibited DNA synthesis and cell proliferation in the root apical meristem. These results suggested that monoterpenes preferentially inhibited some physiological processes involved in cell proliferation. Therefore, the observed preferential inhibition of root growth relative to hypocotyl growth might be explained by the preferential inhibition of cell proliferation, because root growth requires both cell proliferation and cell elongation whereas hypocotyl growth only requires elongation of existing cells (Obroucheva, 1999). Thus, the different sensitivities of hypocotyls and roots to monoterpenes could give us a clue towards understanding their mode of action.
In the present study, we examined the effects of a monoterpene, 1,8-cineole, on proliferation and elongation of plant cells by using BY-2 suspension-cultured tobacco (Nicotiana tabacum) cells as receiver cells. 1,8-Cineole was chosen because it is easy to handle (it is liquid at room temperature) and has strong inhibitory effects (Nishida et al., 2005). Tobacco BY-2 cells were chosen for the following reasons: (i) In liquid suspension culture, BY-2 cells form small cell clusters composed of several (2–16) cylindrical cells connected in tandem (Sakai et al., 2004). Since most of the surface area of individual cells is exposed directly to culture media, cells are expected to exhibit clear, synchronous, and homogeneous responses to any compound applied to the culture medium; (ii) BY-2 cells can exhibit either a high proliferation rate or drastic elongation, depending on culture conditions. Under conventional culture conditions, BY-2 cells rapidly proliferate and multiply up to 100-fold within a week (Nagata et al., 1992). In contrast, when cultured in an auxin-depleted, cytokinin-containing medium, they elongate drastically and accumulate starch without proliferation (Sakai et al., 1996). Thus, BY-2 cells should provide us with a suitable system for examining the effects of monoterpene on cell proliferation and cell elongation; (iii) A protoplast culture system, in which proliferation and elongation are induced separately depending on culture conditions (Hasezawa and Syono, 1983), is established. Because protoplasts are suspended individually without any protection from a cell wall or neighboring cells (at least initially, i.e., before regeneration of cell wall), a protoplast culture system may provide us with a more sensitive way to examine the effects of monoterpene.
We first examined the effects of 1,8-cineole on growth of tobacco seedlings to check whether the phenomena observed for B. campestris were also true for tobacco. Then, appropriate experimental conditions were determined by using the protoplast culture system. Finally, the effects of 1,8-cineole on cell proliferation and cell elongation were assessed quantitatively with BY-2 cell cultures. We also assessed the effects of 1,8-cineole on starch synthesis, as an example of a physiological activity other than cell proliferation and cell elongation. Based on the results, possible mechanisms for the differential inhibition of root growth and hypocotyl growth by monoterpenes as well as the mode of action of monoterpenes at cellular level are discussed.
Methods and Materials
Plant Materials, Chemicals, and Treatment of Seedlings with 1,8-Cineole
Seeds of N. tabacum (cv. Bright Yellow-2, from which a BY-2 cell line was established) were sown on two layers of filter paper (Whatman No. 3, diam 55 mm) soaked with 3 ml of water in transparent, airtight containers (5.5 cm diam × 9.6 cm height, 230 ml volume, 50 seeds per container). 1,8-Cineole, purchased from Aldrich (Milwaukee, WI, USA), was spotted onto a piece of filter paper (3 × 6 cm) hanging from the cap of the container and allowed to volatilize into the airspace within the container. The doses of 1,8-cineole were expressed as the theoretical concentrations in the airspace within the container, which were calculated assuming that the spotted compounds volatilize completely without adsorption or leakage. For example, spotting of 17 μl of 1,8-cineole solution (f.w. = 154, d = 0.921) to filter paper hanging within the 230-ml container gives final concentration of 440 μM; zero μM (control) indicates no application of 1,8-cineole liquid. (Note that actual concentrations in the airspace may be lower than those theoretical, calculated values as a result of absorption. See discussion.) The seeds/seedlings in the containers were incubated for several days at 23°C under a 14: 10 h, L:D photoperiod.
Measurement of Seedling and Cell Sizes
The lengths of hypocotyls and primary roots were measured with digital calipers. Only individuals that successfully germinated were used for measurements. For determination of cell size, seedlings were fixed, washed, and cleared according to the methods of Yadegari et al. (1994). The cortex cells at the root-hair-forming region (i.e., differentiation/elongation zone in the upper part of the root) of the primary roots were observed with a microscope (BX-60, Olympus, Tokyo, Japan) equipped with Nomarski optics and their sizes measured.
Determination of Mitotic Index in the Root Apical Meristem
Root tips (5 mm long) were excised, fixed with FAA solution (formalin : acetic acid : 50% ethanol = 1 : 1 : 18, v/v/v) for 12 h, rinsed with 0.1 M sodium phosphate buffer (pH 7.0), and dialyzed against 0.1 M sodium phosphate buffer containing increasing concentrations of sucrose (10, 20, and 30%, successively). The samples were immersed finally in O.C.T. compound (Sakura Finetech, Torrance, CA, USA), quickly frozen with liquid nitrogen, and the frozen, serial sections (7 μm thick) were prepared using a cryostat (OTE Cryostat, Bright, UK) at -26°C. Sections were mounted on gelatin-coated slides and air-dried, as described previously (Tamotsu et al., 1994). They were stained with 1 μg/ml 4’,6-diamidino-2-phenylindole (DAPI) without removal of the O.C.T. compound, observed with a fluorescence microscope (BX-60, Olympus) under UV irradiation, and the percentages of the cells in a mitotic phase were counted as described in Nishida et al. (2005).
Protoplast Culture and Treatment with 1,8-Cineole
Protoplasts were prepared and cultured according to the method of Hasezawa and Syono (1983) with slight modifications. BY-2 cells usually were propagated by inoculating 95 ml of fresh medium with 1.5–2 ml of suspension of stationary-phase cells at weekly intervals and incubating the culture at 26°C in the dark on a gyratory shaker (130 rpm), as described by Yasuda et al. (1988). Four-day-old BY-2 cells were converted to protoplasts by treating them with enzyme solution (0.4 M mannitol, 1% cellulase YC, 0.1% pectolyase Y23, pH 5.5) for 90 min at 30°C. The protoplasts were collected by centrifugation (260 g, 2 min), suspended in 0.4 M mannitol solution, centrifuged again, and suspended in either 2,4-D medium (LS medium containing 3% sucrose, 0.4 M mannitol, and 0.2 mg/l 2,4-D) or BA-NAA medium (LS medium containing 3% sucrose, 0.4 M mannitol, 1.0 mg/l BA, and 0.1 mg/l NAA) at a density of 0.5–1 × 105 cells/ml. The protoplast suspension was dispensed into 50-ml Erlenmeyer flasks (4.6 ml per flask), and cultured at 26°C in the dark without shaking. For treatment with 1,8-cineole, the protoplast-containing flasks were settled, one-by-one, in transparent, airtight containers (5.5 cm diam × 9.6 cm height, 230 ml volume). 1,8-Cineole was applied either by spotting appropriate volumes of 1,8-cineole liquid onto a piece of filter paper hanging from the cap of the container (volatilization method) or by adding it directly into the culture medium in the flask (direct-addition method). In both cases, the caps of the containers were closed immediately after the application of 1,8-cineole, and the compound was allowed to volatilize into the airspace within the container. The final concentrations of 1,8-cineole in the airspace were calculated assuming that the compound volatilized completely without adsorption or leakage, as described above.
Cell Culture and Treatment with 1,8-Cineole
Seven-day-old (stationary-phase) BY-2 cells were transferred to either fresh D-medium (modified LS medium containing 0.2 mg/l 2,4-D) or fresh B-medium (modified LS medium containing 1 mg/l BA) at a 1:20 dilution, and dispensed into 50-ml Erlenmeyer flasks (9.2 ml per flask). For treatment with 1,8-cineole, the cell suspension-containing flasks were settled, one-by-one, in transparent, airtight containers described above, and were fixed to prepare for shaking-culture. 1,8-Cineole was applied via the direct-addition method only. After the addition of 1,8-cineole, the caps of the containers were closed immediately, and the cells were cultured for 2 days at 26°C in the dark on a gyratory shaker (130 rpm).
Measurements of Mitotic Index, Cell Length, and Starch Content of the Cells/Protoplasts
For measurements of mitotic index, protoplasts and cells were fixed with 1% formaldehyde, stained with 1 μg/ml DAPI, and examined with a fluorescence microscope (BX-60) under UV-irradiation. For measurements of the cell (or protoplast) length, samples (with or without fixation with 1% formaldehyde) were observed with a microscope, photographed, and the length measured. The starch content of BY-2 cells was measured according to the method of Sakai et al. (1996). In short, BY-2 cells present in 1 ml of cell suspension were collected by centrifugation, converted to protoplasts by incubation for 1 h at 30°C in enzyme solution (0.4 M mannitol, 1% cellulase YC, 0.1% pectolyase Y23, pH 5.5), and solubilized by adding 1/10 vols of 10% SDS and incubation for additional 30 min at 37°C. Insoluble materials, including starch granules, were collected by centrifugation, re-suspended in 1 ml of hot 80% ethanol, and collected again by centrifugation. The starch was solubilized in hot water, extracted with perchloric acid, and quantified by the phenol-sulfuric acid method (Dubois et al., 1956) using a dilution series of glucose solution as a standard.
Estimation of Half-maximal Inhibitory Concentrations (IC50)
We emploied IC50 value as the parameter to represent the sensitivities of the biological activities of interest to 1,8-cineole. The IC50 values were simply estimated from semi-log graphs of dose–response curves, without any mathematical model-fitting (Belz et al., 2005).
Results
Effects of 1, 8-Cineole on the Growth of Tobacco Seedlings
In the absence of 1,8-cineole, germination of tobacco seeds proceeded rapidly and synchronously under our experimental conditions. Almost 100% germination was achieved 5 days after sowing, and the length of hypocotyls and primary roots reached their maximum values by day 10 (data not shown). Thus, the effects of various concentrations of 1,8-cineole were examined on day 10.
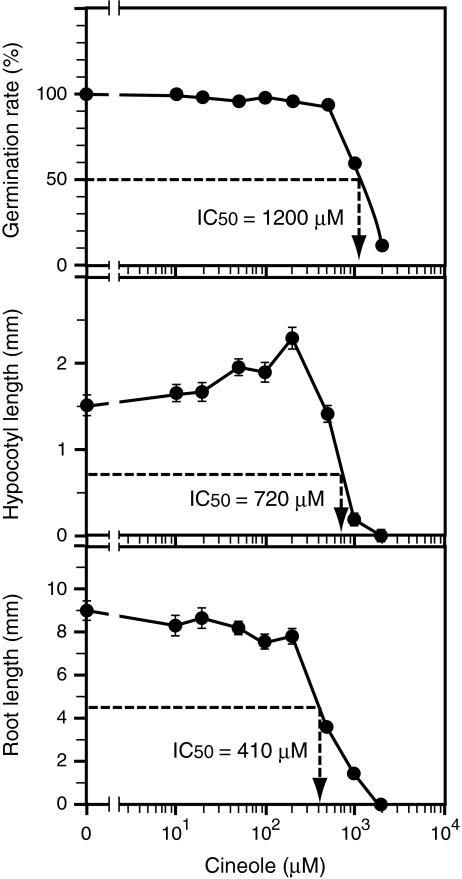
Germination of tobacco seeds (Fig. 1, top) was largely unaffected by 1,8-cineole concentrations of 500 μM or less. The germination rate suddenly dropped to 60% at 1,000 μM, and further dropped to 12% at 2,000 μM. The concentration of 1,8-cineole required to lower the germination rate to 50% of the control value (IC50) was about 1,200 μM. Hypocotyl growth (Fig. 1, middle) was not inhibited with 1,8-cineole concentrations up to 200 μM. 1,8-Cineole stimulated hypocotyl growth slightly in a dose-dependent manner within this low-concentration range (rs = 0.94, P < 0.05), indicating hormesis (Duke et al., 2006; Calabrese et al., 2009). At higher concentration ranges, 1,8-cineole inhibited hypocotyl growth in a dose-dependent manner. The IC50 for hypocotyl growth was about 720 μM. Root growth (Fig. 1, bottom) was not stimulated by any concentrations of 1,8-cineole tested; 1,8-cineole shortened root length even in the low concentration range (≤ 200 μM). The IC50 value for root growth was 410 μM, indicating that root growth was more sensitive to 1,8-cineole than was hypocotyl growth.
Fig. 1.
Effects of various concentrations of 1,8-cineole on Nicotiana tabacum seed germination and seedling growth. Germination rate (top), hypocotyl length (middle), and root length (bottom) were measured on day 10. Each data point for germination rate was calculated from the results of all 50 individuals contained in a single container, while each for hypocotyl and root length represents the average value from 30 individuals that successfully germinated. Vertical bars represent standard errors. IC50 values calculated from the data are shown in respective graphs
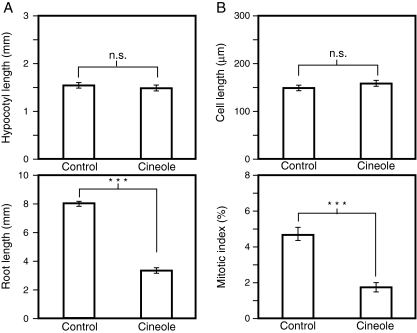
Next, relative contribution of cell elongation and cell proliferation to the root-growth inhibition by 1,8-cineole was examined (Fig. 2). The concentration of 1,8-cineole was set at 440 μM, where hypocotyl length was almost the same as the control value, while root length was reduced by approximately 50% (Fig. 2A). Treatment with 440-μM 1,8-cineole did not reduce the size of cells in the elongation/differentiation zone of tobacco roots, but it lowered the mitotic index in the root apical meristem to less than half the control value (Fig. 2B), indicating that cell proliferation in the root apical meristem was selectively inhibited, while cell expansion in the upper region of the root was largely unaffected with this concentration.
Fig. 2.
Effects of 1,8-cineole on growth of Nicotiana tabacum seedlings. a Effects on hypocotyl length and root length. N. tabacum seedlings were grown in the absence (control) or presence (cineole) of 440 μM of 1,8-cineole for 10 days, and the hypocotyl length (top) and root length (bottom) were measured. Each data point represents the average of 20 individuals that successfully germinated. Vertical bars represent standard errors. b Effects on the cell elongation and cell proliferation in the affected roots. N. tabacum seedlings were grown in the absence (control) or presence (cineole) of 440 μM 1,8-cineole for 10 days, and the sizes of matured cells (top) and mitotic index in the root apical meristem (bottom) were measured. For cell length (top), each point represents average value of 60 cells derived from more than 4 individuals. Vertical bars represent standard errors. For mitotic index, each point represents the average value from 8 (control) or 7 (cineole) individuals. Vertical bars represent standard errors. n.s.; not significant (P > 0.05), ***; P < 0.001, Student’s t-test
Thus, the results obtained for tobacco seedlings (i.e., preferential inhibition of root growth over hypocotyl growth, relative insensitivity of cell elongation in the upper region of the root as compared to cell proliferation in the root apical meristem) were essentially the same as those obtained previously for B. campestris seedlings (Koitabashi et al., 1997; Nishida et al., 2005).
Effects of 1,8-Cineole on the Proliferation and Elongation of Protoplasts Prepared from BY-2 Cultured Tobacco Cells
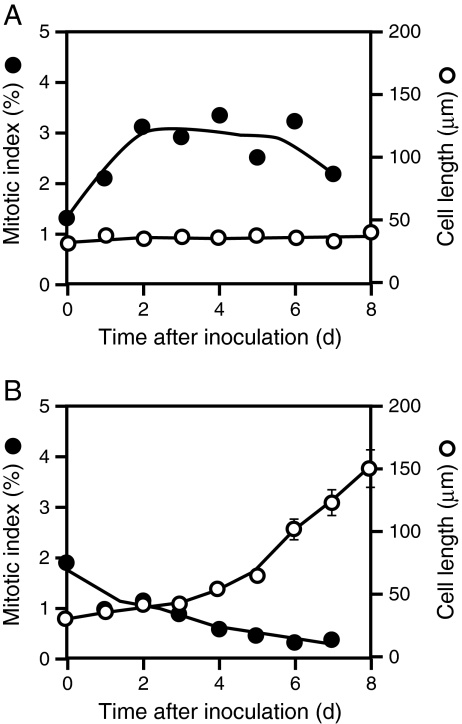
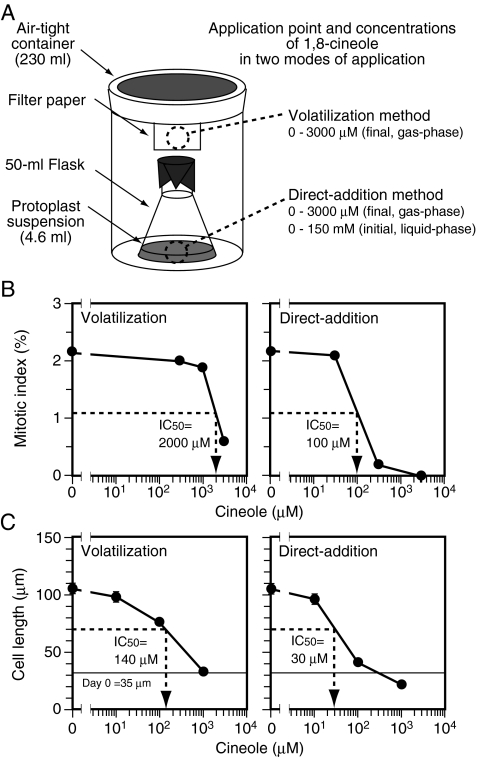
We examined the effects of 1,8-cineole on cell proliferation and cell elongation by using protoplasts prepared from BY-2 cultured tobacco cells. When cultured in 2,4-D medium, BY-2 protoplasts proliferated actively (with mitotic indices of about 3% from day 2 to day 6), but their sizes did not increase significantly during the culture period (Fig. 3A). In contrast, when cultured in BA-NAA medium, they ceased proliferation and elongated drastically by day 7 (Fig. 3B). Thus, BY-2 protoplasts cultured in 2,4-D medium and those cultured in BA-NAA medium were used to examine the effects of 1,8-cineole on cell proliferation and on cell elongation, respectively. In the experiments with protoplast cultures, 1,8-cineole was applied using the two different modes, i.e., the volatilization method and the direct-addition method (Fig. 4A).
Fig. 3.
Behavior of BY-2 protoplasts/cells during culture. Four-day-old BY-2 cells were converted to protoplasts and cultured in either 2,4-D medium A or BA-NAA medium B, and the changes in the mitotic index (●) and cell length (○) were followed during the culture. Each data point for mitotic index was determined by counting more than 1,000 cells/protoplasts. Each point for the length of cells/protoplasts represents the average value from 30 (for 2,4-D medium) or 60 (for BA-NAA medium) cells/protoplasts. Vertical bars represent standard errors
Fig. 4.
Effects of 1,8-cineole and its application methods on the proliferation and elongation of BY-2 protoplasts/cells. A Schematic representation of the experimental system. 1,8-Cineole was applied to either filter paper hanging from the cap of the air-tight container (volatilization method) or the culture medium in the flask (direct-addition method). The calculated final concentrations of 1,8-cineole in the airspace (gas-phase) were 0 to 3,000 μM. In direct-addition method, the initial concentrations of 1,8-cineole in the culture medium (liquid-phase) were 50 times higher than the calculated final concentrations in the airspace (gas-phase). (B) Effects of 1,8-cineole on the proliferation of BY-2 protoplasts grown in 2,4-D medium. Various doses of 1,8-cineole were applied through either the volatilization method (left) or the direct-addition method (right), and the mitotic index was examined on d 2. Each data point was determined by counting more than 1,000 cells/protoplasts. C Effects of 1,8-cineole on the elongation of BY-2 protoplasts grown in BA-NAA medium. Various doses of 1,8-cineole were applied through either the volatilization method (left) or the direct-addition method (right), and cell length was examined on day 0 and day 7. Each point represents the average value from 200–600 cells/protoplasts. Vertical bars represent standard errors. In B and C, IC50 values estimated from the data are shown in the respective graphs
Figure 4B shows the mitotic indices of BY-2 protoplasts cultured in the presence of various concentrations of 1,8-cineole in 2,4-D medium for 2 days. In either of the two application modes, relatively high concentrations of 1,8-cineole were necessary to lower the mitotic index. With the protoplast culture system, IC50 values of 1,8-cineole for proliferation were estimated to be about 2,000 μM for the volatilization method and 100 μM for the direct-addition method. Figure 4C shows the length of BY-2 cells cultured in the presence of various concentrations of 1,8-cineole in BA-NAA medium for 7 days. Normally, the cell wall should have regenerated by day 7. In either of the two application modes, 1,8-cineole lowered cell length in a dose-dependent manner and completely inhibited cell elongation at the highest concentration tested (1,000 μM). IC50 values for cell elongation were about 140 μM for the volatilization method and 30 μM for the direct-addition method. These results demonstrate that (i) the direct addition method was more effective than the volatilization method, and (ii) in a BY-2 protoplast culture system, cell elongation was more sensitive to 1,8-cineole than cell proliferation.
Effects of 1,8-Cineole on Proliferation, Elongation, and Starch Accumulation of BY-2 Cells
We examined the effects of 1,8-cineole on BY-2 cells that retained intact cell walls. BY-2 cells proliferate actively without significant increase of cell volume when cultured in D-medium that contains 0.2 mg/l 2,4-D, whereas they increase in cell volume (mostly due to cell elongation) and accumulate a large amount of starch without proliferation when cultured in B-medium that contains 1 mg/l BA (Sakai et al., 1996). Accordingly, BY-2 cells cultured in D-medium were used to examine the effects of 1,8-cineole on cell proliferation, while those cultured in B-medium were used to examine the effects on cell elongation and starch accumulation. As the differentiation of cells in the two culture media becomes clear 2 days after transfer (Sakai et al., 1996), measurements were done on d 2. Based on the results of the experiments with the protoplast culture system, only the direct-addition method was used to apply 1,8-cineole.
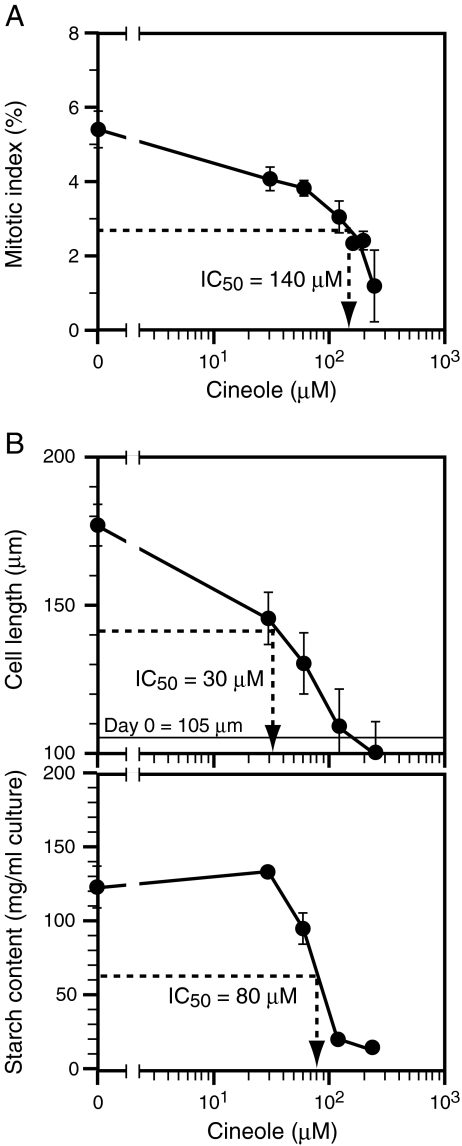
1,8-Cineole lowered the mitotic index in a dose-dependent manner, and the IC50 value for proliferation was about 140 μM (Fig. 5A). 1,8-Cineole also decreased cell length in a dose-dependent manner (Fig. 5B), and at high concentrations (120 or 240 μM), the cell size did not increase as compared to the value at the start of the culture (105 μm). The IC50 value for cell elongation was about 30 μM, and was lower than the IC50 value for cell proliferation. 1,8-Cineole also lowered starch content in a dose-dependent manner. IC50 value for starch accumulation was about 80 μM and was also lower than the IC50 value for cell proliferation. These results demonstrate that the inhibition by 1,8-cineole is not specific to cell proliferation.
Fig. 5.
Effects of 1,8-cineole on the proliferation and elongation of BY-2 cells. A The effects of a range of doses of 1,8-cineole on the proliferation of BY-2 cells cultured in D-medium are shown. The mitotic index was examined on d 2. The average values from three independent experiments are shown. Vertical bars represent standard errors. B The effects of a range of doses of 1,8-cineole on elongation (top) and starch accumulation (bottom) in BY-2 cells cultured in B-medium. Cell length and starch content were examined on d 2. The average values from three independent experiments are shown. Vertical bars represent standard errors. In A and B, 1,8-cineole was applied through the direct-addition method only. The estimated final concentrations of 1,8-cineole in the airspace (gas phase) were 0 to 240 μM, while the initial concentrations in the culture medium (liquid phase) immediately after application were 25 times higher. IC50 values estimated from the data are shown in respective graphs
Discussion
We found here that the effects of 1,8-cineole on N. tabacum seedlings were essentially the same as those on B. campestris seedlings; 1,8-Cineole preferentially inhibited root growth over hypocotyl growth (Fig. 1). In the affected roots, cell size in the elongation zone (upper region of the root) was largely unaffected, whereas cell proliferation in the root apical region was severely inhibited (Fig. 2). These results indicate that the mode of action of growth inhibition by 1,8-cineole is common within the two plant species. Thus, we consider that tobacco, especially cultured tobacco cells, can be used as experimental material to examine the mode of action of 1,8-cineole at the cellular level.
We compared the effectiveness of two modes of application of 1,8-cineole, i.e., the volatilization method and the direct-addition method, by using a protoplast culture system (Fig. 4), and we found that the direct-addition method was more effective. This result is quite reasonable when the predicted behavior of the applied 1,8-cineole is considered. In the volatilization method, applied 1,8-cineole first volatilizes into air and then dissolves into a liquid phase. Thus, the concentrations of 1,8-cineole that the cells experience should gradually increase and finally reach the highest values determined by equilibrium between the liquid and gas phase. In the direct-addition method, the cells should experience the highest concentrations of 1,8-cineole immediately after application of the compound. Then, concentrations should decline to the lower values determined by the equilibrium. Thus, in the latter method, the actual concentration that the cells experience should be higher and the time at which the cells experience the highest concentration should be earlier.
Because protoplasts lose their cell wall and are completely separated from neighboring cells, we expected that they might exhibit higher sensitivity to externally added compounds as compared to cultured cells that have intact cell walls and neighboring cells. For this reason, initially we examined the effects of 1,8-cineole on BY-2 cells using protoplasts (Figs. 3 and 4). However, IC50 values obtained for protoplasts and intact cells were essentially the same (compare Figs. 4 and 5), indicating that cell wall and neighboring cells did not function as a barrier to the permeation of 1,8-cineole into the cells.
In contrast to our initial expectation, 1,8-cineole inhibited cell elongation more efficiently than cell proliferation; the IC50 value for cell proliferation was always higher than that for cell elongation, irrespective of the application methods (Fig. 4) or the condition (i.e., presence or absence of cell wall) of receiver cells (Figs. 4 and 5). Moreover, 1,8-cineole also inhibited starch accumulation with an IC50 value lower than that for cell proliferation (Fig. 5). These results demonstrated clearly that the inhibitory effect of 1,8-cineole was not specific to cell proliferation; rather, 1,8-cineole appeared to affect a wide spectrum of cellular activities in an almost non-specific manner when target cells were efficiently exposed to the compound.
Despite numerous reports on inhibitory effects of monoterpenes, their mechanisms of action remain obscure. Recently, Chaimovitsh et al. (2010) reported that microtubules are an intracellular target of a monoterpene, citral. However, they also reported that the effects of citral and microtubule inhibitor oryzalin on plant seedlings are different from each other, suggesting that there should be additional target(s) of the monoterpene. Our observation that starch accumulation was highly sensitive to 1,8-cineole (Fig. 5) also suggests the presence of additional target(s), as starch accumulation itself does not seem so tightly associated with microtubule function. Then, what is the plausible target of monoterpenes?
A reduction in respiratory oxygen consumption resulting from monoterpene treatment has been reported in a number of studies (e.g., Müller et al., 1968; Peñuelas et al., 1996). Moreover, monoterpenes affect the respiratory activity of isolated mitochondria (Müller et al., 1969; Abrahim et al., 2000, 2003a) via uncoupling of oxidative phosphorylation and inhibition of electron transfer (Abrahim et al., 2003b). The lipophilic property of monoterpenes (Weidenhamer et al., 1993), as well as lipid oxidation and deterioration of membrane integrity in plant cells exposed to monoterpenes (Lorber and Müller, 1976; Fischer, 1986; Zunino and Zygadlo, 2004) suggests that biological membranes, including mitochondrial membranes, are the primary target of monoterpenes. Among all the possible effects on biological membranes, the deleterious effects on mitochondrial membranes should cause inhibition of mitochondrial energy metabolism and result in disturbances in a wide range of physiological and biochemical processes within the cell.
Our results rule out the possibility that preferential inhibition by 1,8-cineole of root growth is explained by preferential inhibition of cell proliferation; 1,8-cineole inhibited multiple distinct biological processes (cell proliferation, cell elongation, and starch synthesis) in cultured cells, and the sensitivity of cell proliferation to 1,8-cineole was, contrary to our initial expectation, relatively low (Figs. 4 and 5). Then, in plant seedlings, why is root growth more sensitive than hypocotyl growth to 1,8-cineole?
One possibility is that the actual concentration of 1,8-cineole may be higher around roots (liquid/solid phase) than around hypocotyls (gas-phase). In nature, soil colloids adsorb volatile monoterpenes in the atomosphere and exhibit toxicity for several months (Müller and Moral, 1966). Under our experimental conditions for the seedling assay, monoterpenes within an air-tight container might absorb to the wet filter paper wad used as supporting substance, resulting in higher concentrations of the compound around the roots as compared to the concentrations around the aerial tissues. (This also suggests that the actual concentrations of 1,8-cineole in the gas-phase should be lower than the calculated values.) However, root growth was more sensitive to 1,8-cineole than hypocotyl growth even when the seedlings were grown under conditions in which both hypocotyls and roots were in equal contact with the filter paper, and therefore, the concentration of 1,8-cineole surrounding the two organs should have been equal (Nishida et al., 2005). Thus, differences in the actual concentrations around roots and hypocotyls do not seem to play a critical role under our experimental conditions.
Another possible explanation is that root surfaces may have higher permeability to 1,8-cineole as compared to hypocotyl surfaces, resulting in higher doses of the compound within root tissues as compared to those within hypocotyl tissues. The surface of aerial parts of the plant body is covered with a well-developed cuticle layer, while that of roots is not (Bessire et al., 2007). In addition, a permeability assay, based on staining with toluidine blue dye (Bessire et al., 2007), demonstrated differential permeability (and thus differential development of a cuticle layer) within a root; in various plant species, the root tip region that includes the root apical meristem exhibited higher permeability than that of the upper region of the same root, which corresponded to elongation/differentiation zone (unpublished data). Thus, differential permeability due to differential development of a cuticle layer might be involved in not only preferential inhibition of root growth over hypocotyl growth but also preferential inhibition of cell proliferation in the root apical region over cell elongation in the upper region of the same root. The relationship between development of the surface barriers, such as cuticle layer, and sensitivity to monoterpenes is now under further investigation.
Acknowledgements
The authors thank Miss M. Takusagawa for critical reading of the manuscript. This work was partly supported by Grants-in-Aid for Scientific Research on Priority Areas (No. 16085208 to A.S.) from the Japan Society for the Promotion of Science.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- ABRAHIM D, BURAGUINI WL, KELMER-BRACHT AM, ISHII-IWAMOTO EL. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000;26:611–624. doi: 10.1023/A:1005467903297. [DOI] [Google Scholar]
- ABRAHIM D, TAKAHASHI L, KELMER-BRACHT AM, ISHII-IWAMOTO EL. Effects of phenolic acids and monoterpenes on the mitochondrial respiration of soybean hypocotyl axes. Allelopathy J. 2003;11:21–30. [Google Scholar]
- ABRAHIM D, FRANCISCHINI AC, PERGO EM, KELMER-BRACHT AM, ISHII-IWAMOTO EL. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol. Biochem. 2003;41:985–991. doi: 10.1016/j.plaphy.2003.07.003. [DOI] [Google Scholar]
- BELZ RG, HURLE K, DUKE SO. Dose-response – A challenge for allelopathy? Nonlinearity Biol. Toxicol. Med. 2005;3:173–211. doi: 10.2201/nonlin.003.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESSIRE M, CHASSOT C, JACQUAT A-C, HUMPHRY M, BOREL S, PETÉTOT JM-C, MÉTRAUX J-P, NAWRATH C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007;26:2158–2168. doi: 10.1038/sj.emboj.7601658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIMOVITSH D, ABU-ABIED M, BELAUSOV E, RUBIN B, DUDAI N, SADOT E. Microtubules are an intracellular target of the plant terpene ctral. Plant J. 2010;61:399–408. doi: 10.1111/j.1365-313X.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- CALABRESE EJ, BLAIN RB. Hormesis and plant biology. Environmental Pollution. 2009;157:42–48. doi: 10.1016/j.envpol.2008.07.028. [DOI] [PubMed] [Google Scholar]
- DAYAN FE, ROMAGNI JG, DUKE SO. Investigation the mode of action of natural phytotoxins. J. Chem. Ecol. 2000;26:2079–2094. doi: 10.1023/A:1005512331061. [DOI] [Google Scholar]
- DUBOIS M, GILLES KA, HAMILTON JK, REBERS PA, SMITH F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- DUDAI N, LARKOV O, MAYER AM, POLJAKOFF-MAYBER A, PUTIEVSKY E, LERNER HR. Metabolism of essential oils during inhibition of wheat seed germination. In: Black M, Bradford KJ, Vázquez-Ramos J, editors. Seed Biology: Advances and Applications. Wallingford, UK: CABI; 2000. p. 315. [Google Scholar]
- DUKE SO, CEDERGREEN N, VELINI ED, BELZ RG. Hormesis: Is it an important factor in herbicide use and allelopathy? Outlooks Pest Management. 2006;2:29–33. [Google Scholar]
- DUKE SO, OLIVA A. Mode of action of phytotoxic terpenoids. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG, editors. Allelopathy, Chemistry and Mode of Action of Allelochemicals. Boca Raton: CRC Press; 2004. [Google Scholar]
- EINHELLIG FA, LEATHER GR. Potentials for exploiting allelopathy to enhance crop production. J. Chem. Ecol. 1988;14:1829–1844. doi: 10.1007/BF01013480. [DOI] [PubMed] [Google Scholar]
- FISCHER, N. H. 1986. The function of mono and sesquiterpenes as plant germination and growth regulators. in: Putnam, A. R., and Tang, C-S. (eds) The Science of allelopathy. John Wiley and Sons, New York.
- FISCHER, N. H. 1991. Plant terpenoids as allelopathic agents. p 387 in: Harborne, J. B., Tomas-Barberan, F. A. (eds) Ecological Chemistry and Biochemistry of Plant Terpenoids. Clarenden, Oxford.
- HASEZAWA S, SYONO K. Hormonal control of elongation of tobacco cells derived from protoplasts. Plant Cell Physiol. 1983;24:127–132. [Google Scholar]
- KOITABASHI R, SUZUKI T, KAWAZU T, SAKAI A, KUROIWA H, KUROIWA T. 1, 8-Cineole inhibits root growth and DNA synthesis in the root apical meristem of Brassica campestris L. J. Plant Res. 1997;110:1–6. doi: 10.1007/BF02506836. [DOI] [PubMed] [Google Scholar]
- LORBER P, MÜLLER WH. Volatile growth inhibitors produced by Salvia leucophylla: effects on seedling root tip ultrastructure. Am. J. Bot. 1976;63:196–200. doi: 10.2307/2441700. [DOI] [Google Scholar]
- MÜLLER CH. Phytotoxins as plant habitat variables. Recent Adv. Phytochem. 1970;3:106–121. [Google Scholar]
- MÜLLER CH, MORAL RD. Soil toxicity induced by terpenes from Salvia leucophylla. Bull. Torr. Bot. Club. 1966;93:130–137. doi: 10.2307/2483755. [DOI] [Google Scholar]
- MÜLLER WH, LORBER P, HALEY B. Volatile growth inhibitors produced by Salvia leucophylla: effect on seedling growth and respiration. Bull. Torr. Bot. Club. 1968;95:415–422. doi: 10.2307/2483472. [DOI] [Google Scholar]
- MÜLLER WH, LORBER P, HALEY B, JOHNSON K. Volatile growth inhibitors produced by Salvia leucophylla: effect on oxygen uptake by mitochondrial suspensions. Bull. Torr. Bot. Club. 1969;96:89–95. doi: 10.2307/2484011. [DOI] [Google Scholar]
- NAGATA T, NEMOTO Y, HASEZAWA S. Tobacco BY-2 cell line as the “Hela” cell in the cell biology of higher plants. Int. Rev. Cytol. 1992;132:1–30. doi: 10.1016/S0074-7696(08)62452-3. [DOI] [Google Scholar]
- NISHIDA N, TAMOTSU S, NAGATA N, SAITO C, SAKAI A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005;31:1187–1203. doi: 10.1007/s10886-005-4256-y. [DOI] [PubMed] [Google Scholar]
- OBROUCHEVA NV. Seed Germination: A Guide to the Early Stages. Leiden: Buckhuys Publischers; 1999. [Google Scholar]
- PEÑUELAS J, RIBAS-CARBO M, GILES L. Effects ofallelochemicals on plant respiration and oxygen isotope fractionation by the alternative oxidase. J. Chem. Ecol. 1996;22:801–805. doi: 10.1007/BF02033587. [DOI] [PubMed] [Google Scholar]
- ROMAGNI JG, ALLEN SN, DAYAN FE. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000;26:303–313. doi: 10.1023/A:1005414216848. [DOI] [Google Scholar]
- SAKAI A, YASHIRO K, KAWANO S, KUROIWA T. Amyloplast formation in cultured tobacco cells; effects of plant hormones on multiplication, size, and starch content. Plant Cell Reports. 1996;15:601–605. doi: 10.1007/BF00232461. [DOI] [PubMed] [Google Scholar]
- SAKAI, A., MIYAZAWA, Y., and KUROIWA, T. 2004. Studies on dynamic changes of organelles using tobacco BY-2 as the model plant cell line. in: Nagata, T., Hasezawa, S., and Inzé, D. (eds) Biotechnology in Agriculture and Forestry, Vol.53, Tobacco BY-2 cells. Springer-Verlag, Berlin, Heidelberg.
- SCHULZ M, KUSSMANN P, KNOP M, KRIEGS B, GRESENS F, EICHERT T, ULBRICH A, MARX F, FABRICIUS H, GOLDBACH H, NOGA G. Allelopathic monoterpenes interfere with Arabidopsis thaliana cuticular waxes and enhance transpiration. Plant Signal. Behav. 2007;2:231–239. doi: 10.4161/psb.2.4.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMOTSU S, OISHI T, NAKAO K, FUKADA Y, SHICHIDA Y, YOSHIZAWA T, MORITA Y. Localization of iodopsin and rodopsin immunoreactivity in the retina and pineal complex of the river lamprey, Lampetra japonica. Cell Tissue Res. 1994;278:1–10. doi: 10.1007/BF00305772. [DOI] [Google Scholar]
- TARAYRE M, THOMPSON JD, ESCARRÉ J, LINHART YB. Intra-specific variation in the inhibitory effects of Thymus vulgaris (Labiatae) monoterpenes on seed germination. Oecologia. 1995;101:110–118. doi: 10.1007/BF00328907. [DOI] [PubMed] [Google Scholar]
- WEIDENHAMER JD, MACIAS FA, FISCHER NH, WILLIAMSON GB. Just how insoluble are monoterpenes? J. Chem. Ecol. 1993;19:1827–1835. doi: 10.1007/BF00982309. [DOI] [PubMed] [Google Scholar]
- YADEGARI R, PAIVA G, LAUX T, KOLTUNOW A, APUYA N, ZIMMERMAN J, FISCHER R, HARADA J, GOLDBERG R. Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell. 1994;6:1713–1729. doi: 10.1105/tpc.6.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASUDA T, KUROIWA T, NAGATA T. Preferential synthesis of plastid DNA and increased replication of plastids in cultured tobacco cells following medium renewal. Planta. 1988;174:235–241. doi: 10.1007/BF00394776. [DOI] [PubMed] [Google Scholar]
- ZUNINO MP, ZYGADLO JA. Effects of monoterpenes on lipid oxidation in maize. Planta. 2004;219:303–309. doi: 10.1007/s00425-004-1216-7. [DOI] [PubMed] [Google Scholar]