Abstract
Phosphoinositide 3-kinaseγ (PI3Kγ) is activated by G-protein coupled receptors (GPCRs). We show here that PI3Kγ inhibits protein phosphatase 2A (PP2A) at the β-adrenergic receptor (βAR, a GPCR) complex altering G-protein coupling. PI3Kγ inhibition results in significant increase of βAR-associated phosphatase activity leading to receptor dephosphorylation and resensitization preserving cardiac function. Mechanistically, PI3Kγ inhibits PP2A activity at the βAR complex by phosphorylating an intracellular inhibitor of PP2A (I2PP2A) on serine residues 9 & 93 resulting in enhanced binding to PP2A. Indeed, enhanced phosphorylation of β2ARs is observed with phosphomimetic I2PP2A mutant that was completely reversed with a mutant mimicking dephosphorylated state. siRNA depletion of endogenous I2PP2A augments PP2A activity despite active PI3K resulting in β2AR dephosphorylation and sustained signaling. Our study provides the underpinnings of a PI3Kγ mediated regulation of PP2A activity that has significant consequences on receptor function with broad implications in cellular signaling.
INTRODUCTION
G-protein-coupled receptors (GPCRs) are large family of seven transmembrane receptors regulating cellular responses to external stimuli(Heitzler et al., 2009). β-adrenergic receptor (βAR) is a representative GPCR regulating cardiovascular, respiratory, metabolic and reproductive functions(Rockman et al., 2002). Abnormalities in βAR function are well documented in many pathological conditions including heart failure(Rockman et al., 2002) and asthma(Penn, 2009). Agonist activation of β1 or β2 AR (ubiquitously expressed βARs) results in their phosphorylation by GPCR kinase 2 (GRK2) and protein kinase A (PKA) initiating desensitization(Rockman et al., 2002). Phosphorylation of βAR results in β-arrestin recruitment to the receptor complex(Rockman et al., 2002) that physically interdicts further coupling of the receptor to G-protein(Rockman et al., 2002) and targets the receptor for clathrin mediated internalization(Claing et al., 2002).
Internalized receptors are resensitized by dephosphorylation in the early endosomes by protein phosphatase 2A (PP2A)(Krueger et al., 1997) and are recycled back to the plasma membrane(Rockman et al., 2002). PP2A is a serine-threonine phosphatase, a holoenzyme containing heterodimers of catalytic and scaffolding subunits that associates with a combination of regulatory subunits conferring substrate selectivity, specificity and localization(Sontag, 2001; Virshup and Shenolikar, 2009). PP2A is also regulated by endogenously occurring inhibitor proteins called the inhibitors of PP2A (I1- and I2-PP2A)(Li and Damuni, 1998). Despite PP2A activity being tightly regulated, nothing is known about mechanisms regulating its activity at the βAR complex as dephosphorylation of βARs by PP2A is a prerequisite step in resensitization(Ferguson, 2001; Krueger et al., 1997) which has implications in pathology.
PI3Kγ (p110γ) belongs to the class IB family of PI3Ks that are activated upon GPCR stimulation(Vanhaesebroeck et al., 1997). All members of the PI3K family are dual specificity enzymes characterized by protein and lipid kinase activities(Dhand et al., 1994; Vanhaesebroeck et al., 1997). Previous studies have shown that protein and lipid kinase activities of PI3Kγ are critical for βAR internalization(Naga Prasad et al., 2005). Pharmacologic inhibition of PI3K and studies in PI3Kγ knock out (PI3Kγ KO) mice showed elevated βAR mediated intracellular Ca2+ transients and cAMP production with enhanced cardiac contractility(Crackower et al., 2002; Leblais et al., 2004). Studies in PI3Kγinact transgenic (Tg) showed marked improvement in cardiac function associated with significant preservation of βAR function(Nienaber et al., 2003) suggesting receptor resensitization. Since studies have shown that PI3Kγ activity is selectively augmented in heart failure(Perrino et al., 2007), we postulated that inhibition of PI3Kγ would result in βAR resensitization through an yet unknown mechanism.
RESULTS
PI3K inhibits βAR resensitization
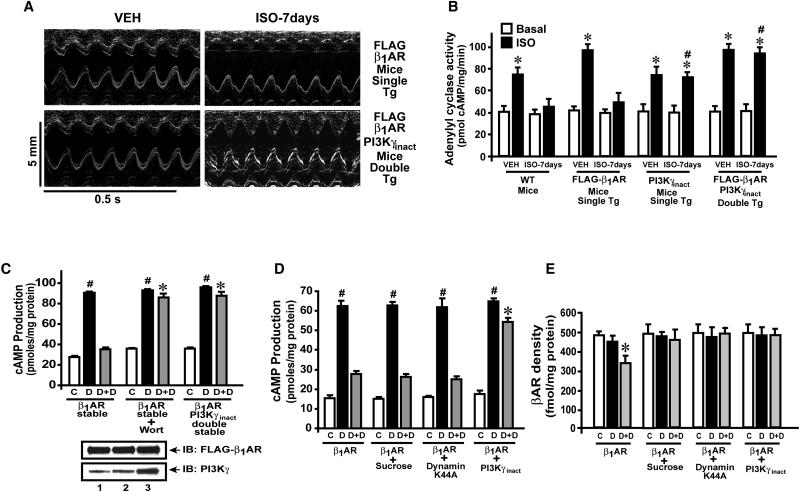
To investigate whether PI3K inhibits receptor resensitization in vivo, we generated double transgenic (Tg) mice with cardiac specific overexpression of FLAG-β1AR and PI3Kγinact. Wild type (WT), single or double Tg mice were administered vehicle (VEH) or isoproterenol (ISO) for seven days. M-mode echocardiography (Fig.1a) and fractional shortening (%FS) measurements (Fig. S1a) showed normalized cardiac function in double Tg mice compared to FLAG-β1AR single Tg mice. Consistent with previous studies(Nienaber et al., 2003), PI3Kγinact single Tg mice showed preservation of cardiac function (data not shown). Measurement of βAR function by adenylyl cyclase activity and receptor density by ligand binding βAR desensitization (Fig.1b) and internalization (Fig. S1b) in WT and FLAG-β1AR single Tg mice. Consistent with previous studies(Nienaber et al., 2003), ISO treated PI3Kγinact single Tg mice showed preserved βAR function (Fig. 1b). Furthermore, ISO treated double Tg mice showed significant βAR resensitization without internalization (Fig. 1b & Fig. S1b) establishing a role for PI3Kγ in receptor desensitization/resensitization.
Figure 1.
PI3Kγ inhibits β1AR resensitization. (a) Representative M-mode echocardiography of vehicle (VEH) and isoproterenol (ISO,30 mg/kg/day) (Casey et al., 2010)) treated FLAG-β1AR single and FLAG-β1AR & PI3Kγinact double transgenic (Tg) mice (n=6). (b) In vitro ISO-stimulated (black bar) cardiac adenylyl cyclase activity compared to VEH (Basal, white bars) in wild type (WT), single (FLAG-β1AR or PI3Kγinact) or double Tg mice administered with VEH or ISO chronically for 7 days. *p< 0.001 versus respective vehicle, #P<0.01 versus WT or FLAG-β1AR ISO-7days (ANova).(n=5-6). (c) cAMP measurement in cells with VEH (C, white) or Dobutamine (D, black, 10 μM, 5 min) or challenge (15 min) and rechallenge with D (5 min) (grey, D+D) following inhibition of PI3K by either wortmannin (Wort,100 nM, 30 min) or PI3Kγinact #p< 0.0001, versus C, *p< 0.0001, versus β1AR stable (D+D) (n=4-5). Lower panel, immunoblotting for FLAG-β1AR and PI3Kγ. (d) cAMP generation in β1AR cells co-expressing dynamin mutant or pre-treated with sucrose. #p< 0.0001, versus VEH (C), *p< 0.0001, versus other D+D samples (n=4-5). (e) Total βAR density in β1AR cells, pre-treated with sucrose or co-expressing Dynamin mutant or PI3Kγinact. VEH (C, White bars) or D (black, 5 min) or challenge (15 min) and re-challenge with D (5 min) (grey, D+D). *P < 0.001 D+D versus VEH (C) n=6. (See also Supplementary Figure S1 a-c).
To dissect the molecular mechanism of receptor resensitization we generated double (β1AR-PI3Kγinact) stable cells. Double and β1AR single stable cells were challenged and rechallenged (Rapacciuolo et al., 2003) with β1AR selective agonist dobutamine (Dob). Significant cAMP generation observed with Dob challenge in β1AR cells (Fig. 1c, black bar) was abolished following rechallenge with Dob (Fig.1c, grey bars) showing receptor desensitization. In contrast, inhibition of PI3K by PI3Kγinact or wortmannin (Wort, a selective PI3K inhibitor) resulted in significant cAMP generation (Fig. 1c, grey bars) showing β1AR resensitization. PI3K inhibition in neonatal ventricular myocytes also resulted in enhanced βAR function (Fig. S1c). Since PI3Kγinact attenuates βAR internalization(Naga Prasad et al., 2001), we tested whether βAR resensitization is due to reduced βAR internalization or independent of internalization. Cells expressing Dynamin K44A mutant, PI3Kγinact or treated with sucrose were challenged and rechallenged with Dob. Despite inhibition of receptor internalization by Dynamin K44A or sucrose (Fig. 1e), no β1AR resensitization was observed (Fig. 1d, grey bars). In contrast, significant resensitization was observed with PI3Kγinact (Fig. 1d, grey bars) demonstrating PI3Kγ specificity.
PI3Kγ regulates βAR resensitization by altering plasma membrane receptor dephosphorylation
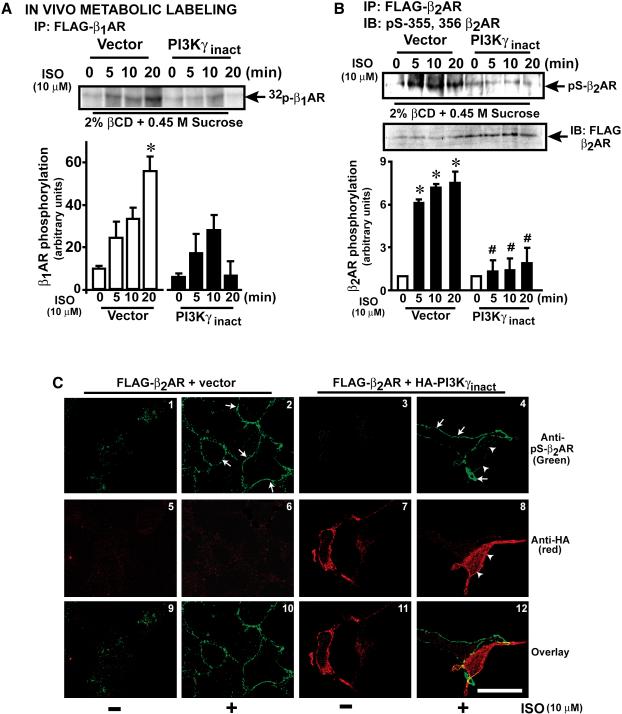
As agonist-mediated βAR phosphorylation is not altered with PI3Kγinact(Naga Prasad et al., 2001; Nienaber et al., 2003), preservation of βAR function may occur by regulating dephosphorylation. To test whether PI3Kγ regulates receptor dephosphorylation, metabolic labeling was carried out in β1AR cells pre-treated with endocytic inhibitors. Significant agonist-dependent β1AR phosphorylation observed on the plasma membranes (PM) with endogenous PI3Kγ (control cells i.e., vector) (Fig. 2a) was abolished in presence of PI3Kγinact (Fig. 2a). Since mechanisms of β1 and β2AR phosphorylation and desensitization (Rockman et al., 2002) are similar we assessed whether β2AR phosphorylation is altered with PI3Kγinact. Immunoblotting by anti-phospho-β2AR antibody(Tran et al., 2007) recognizing GRK phosphorylation sites showed significant receptor phosphorylation (Fig. 2b, vector) that was abolished with PI3Kγinact (Fig. 2b). This confirms that absence of active PI3Kγ results in βAR dephosphorylation at the plasma membrane (Fig. 2 a & b). This observation is contrary to the current paradigm which surmises that receptor resensitization occurs by PP2A-mediated dephosphorylation in the endosomes(Krueger et al., 1997). Consistently, confocal microscopy showed no β2AR phosphorylation (green) in the absence of ISO (Fig. 2c, panels 1, 3, 9 & 11). In contrast, ISO stimulation resulted in significant β2AR phosphorylation (green) (Fig. 2c, panels 2, 4, 10 &12, arrows) which was markedly reduced in the neighboring cells expressing PI3Kγinact (red) (Fig. 2c, panels 4, 8 & 12, arrow heads) indicating enhanced receptor dephosphorylation. These data demonstrate that absence of active PI3Kγ at the βAR complex results in receptor dephosphorylation leading to resensitization.
Figure 2.
PI3Kγ inhibits βAR dephosphorylation, (a) β1AR cells expressing vector or PI3Kγinact were metabolically labeled with [32]Pi, treated with endocytic inhibitors (2% β-cyclodextrin-0.45 M sucrose) followed by ISO, (n=4-5), *p< 0.001 versus other time points. (b) β2AR cells expressing vector or PI3Kγinact were labeled with non-radioactive phosphates, treated with endocytic inhibitors followed by ISO. β2AR were immunoprecipitated and immunoblotted with phospho-355/356-β2AR antibody (upper panel) and re-blotted with anti-FLAG antibody (middle panel). Summary data (lower panel, n=4-5). *p< 0.001 versus all samples. # p< 0.001 versus stimulated vector. (c) Confocal images of Flag-β2AR cells co-expressing vector or HA-PI3Kγinact, pre-treated with endocytic inhibitors followed by ISO depicting β2AR phosphorylation (green) and PI3Kγinact (red). Scale, 10 μm.
PI3Kγ regulates receptor resensitization by inhibiting PP2A activity at the βAR complex
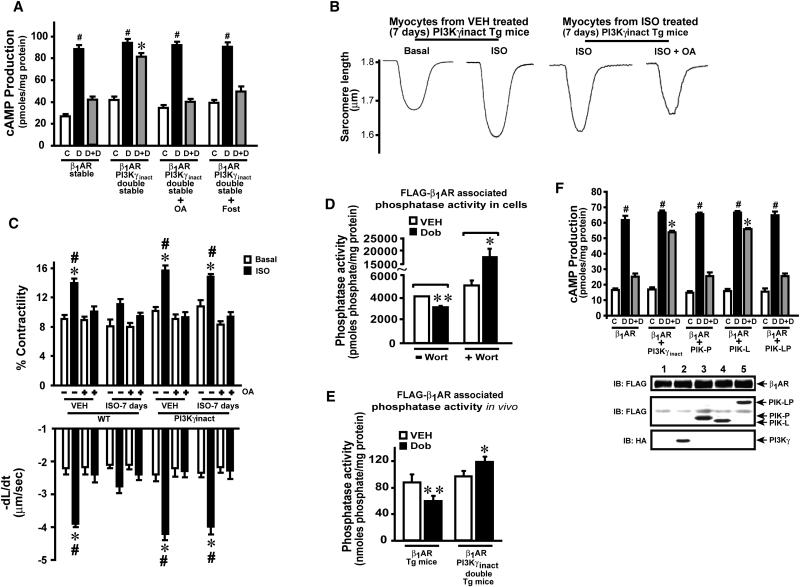
Studies have shown that dephosphorylation of βARs is mediated by PP2A(Ferguson, 2001). To test whether PP2A is a target of PI3Kγ in βAR resensitization, PP2A was inhibited in β1AR-PI3Kγinact cells. Significant cAMP generation was observed in β1AR-PI3Kγinact cells following rechallenge with Dob (Fig. 3a, grey bars). In contrast, inhibition of endogenous PP2A by okadaic acid (OA) or fostriecin (Fost)(Swingle et al., 2007; Weinbrenner et al., 1998) resulted in loss of β1AR resensitization conferred by PI3Kγinact (Fig. 3a, grey bars) showing that PP2A is a downstream target of PI3Kγ. Consistent with these observations, significant inhibition of plasma membrane Serine-Threonine phosphatase activity was observed in cells expressing WT PI3Kγ which was reversed with PI3Kγinact following agonist (Fig. S3a).
Figure 3.
PI3Kγ inhibits β1AR resensitization through PP2A, (a) cAMP generation in cells pre-treated with PP2A inhibitors okadaic acid (OA, 5 nM, 30 min) or Fostriecin (Fost, 1 μM, 15 min), #p< 0.0001, versus VEH (C), *p< 0.0001, versus other D+D samples (n=3-5). (b) Representative tracings of isolated myocytes upon ISO from PI3Kγinact Tg mice treated with VEH or ISO for 7 days, or ISO+OA (okadaic acid) following 7 days of ISO treatment in PI3Kγinact Tg mice. (c) Cell shortening measurements upon ISO or ISO+OA in myocytes from WT and PI3Kγinact mice treated with VEH or ISO for 7 days. *p<0.01 versus ISO-administered WT myocytes treated with ISO; #p<0.01 versus respective okadaic treated samples (n=3-4, 15-20 cells/experiment). (d) Plasma membrane β1AR-associated serine-threonine phosphatase activity in VEH or Wort (100 nM, 30 min) treated cells (n=5) **p< 0.001 or *p<0.0001, versus VEH. (e) Cardiac plasma membrane β1AR-associated Serine-Threonine phosphatase activity in PI3Kγinact Tg following Dob bolus (1mg/kg) (n=6), *p< 0.001 or **p< 0.001 versus VEH. (f) cAMP generation in β1AR cells co-expressing PI3Kγ mutants (n=3-4), #p< 0.0001, versus C, *p< 0.0001, versus other D+D samples. Lower panel shows β1AR, PI3K mutants and PI3Kγinact immunoblotting. (See also Supplementary Figure S3 a & b).
To directly address the role of PP2A regulation by PI3Kγ in a physiological setting, myocyte contractility was assessed in adult cardiomyocytes isolated from WT and PI3Kγinact mice administered with VEH or ISO for 7 days. Significant contractility was observed with ISO in myocytes isolated from vehicle administered WT mice which was blocked with OA pretreatment (Fig. 3c). In contrast, the contractility was reduced with acute ISO in myocytes isolated from 7 days (chronic) ISO treated WT mice (Fig. 3c). Despite chronic ISO, myocytes from the PI3Kγinact mice retained contractile capability (Fig. 3 b & c) that was abolished by pre-treatment with okadaic acid (OA) (Fig. 3 b & c). These data show that PI3Kγ negatively regulates PP2A function as inhibition of PP2A by OA results in impaired contraction in myocytes from PI3Kγinact mice.
To test whether PI3Kγ regulates β1AR-associated phosphatase activity, cells were pre-treated with Wort and β1ARs immunoprecipitated from plasma membranes. Dob simulation showed significant elevation in β1AR-associated phosphatase activity with Wort that was markedly reduced in vehicle (Fig. 3d). To further test in vivo whether β1AR-associated phosphatase activity is regulated by PI3Kγ, we used FLAG-β1AR/PI3Kγinact double transgenic mice. FLAG-β1AR was immunoprecipitated from cardiac membranes and associated phosphatase activity measured following Dob. Phosphatase activity was significantly elevated in the double Tg (Fig. 3e) compared to β1AR single Tg which displayed marked inhibition (Fig. 3e). Metabolic labeling with simultaneous inhibition of PI3Kγ by PI3Kγinact and PP2A by OA restored FLAG-β1 AR phosphorylation compared to PI3Kγ inhibition alone following agonist challenge (Fig. S3b).
PI3K has documented lipid and protein kinase activities(Carpenter et al., 1993; Dhand et al., 1994) and protein kinase activity of PI3Kγ regulates βAR internalization(Naga Prasad et al., 2005). We have used the previously characterized PI3Kγ mutants (Naga Prasad et al., 2005) containing protein (PIK-P), lipid (PIK-L), or both kinase activities (PIK-LP) to address their roles in resensitization. Cells expressing these mutants were subjected to the protocol of Dob challenge and rechallenge. Dob rechallenge resulted in β1AR desensitization with PIK-P or PIK-LP (Fig. 3f) suggesting inhibition of receptor resensitization. In contrast, significant cAMP generation was observed with PIK-L showing inability of lipid kinase component to inhibit β1AR resensitization (Fig. 3f). This suggests that protein kinase component of PI3Kγ is critical for inhibition of βAR resensitization.
PI3Kγ inhibits PP2A function at the receptor complex by phosphorylating I2PP2A
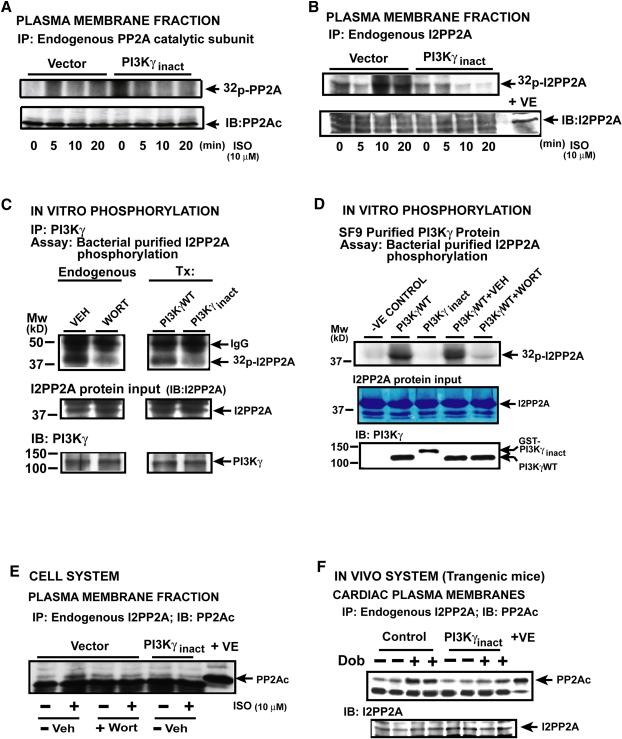
To determine the mechanism of PP2A inhibition by protein kinase component of PI3Kγ, metabolic labeling was performed in presence and absence of PI3Kγ. No appreciable phosphorylation of PP2A catalytic (Fig. 4a) or regulatory subunit (data not shown) was observed following agonist stimulation suggesting alternative mechanisms of PP2A regulation. Since PP2A activity can be inhibited by inhibitory proteins I1- or I2-PP2A(Li and Damuni, 1998), we tested whether PI3K phosphorylates I2PP2A as it is the major isoform in cardiac plasma membranes (data not shown). The mechanism by which I2PP2A inhibits PP2A is unknown and is speculated that phosphorylation of I2PP2A may lead to enhanced binding to PP2A (Pandey et al., 2003). Agonist stimulation resulted in robust plasma membrane I2PP2A phosphorylation in cells which was markedly reduced with PI3Kγinact (Fig. 4b) or Wort pre-treatment (Fig. S4a). Immunoblotting with a pan anti-phospho-serine antibody validated that PI3Kγ phosphorylates I2PP2A (Fig. S4b).
Figure 4.
PI3Kγ inhibits PP2A by phosphorylating I2PP2A, (a & b) Phosphorylation of plasma membrane PP2A (a) or I2PP2A (b) in [32]Pi metabolically labeled, ISO-stimulated β1AR cells co-expressing vector or PI3Kγinact (n=4). Lower panels; PP2Ac and I2PP2A immunoblotting. (c) In vitro phosphorylation assay using immunoprecipitated PI3Kγ from WT PI3Kγ and PI3Kγinact expressing cells (Tx, Transfected) as well as Wort (100 nM for 30 min) treated cells using purified I2PP2A as substrate. Input I2PP2A stained by Coomassie, PI3Kγ immunoblotted. (d) In vitro phosphorylation assay performed using purified PI3Kγ (8 pmols) or PI3Kγinact (8 pmols) and purified I2PP2A (300 pmols) as substrate for 20 min. Input I2PP2A stained by Coomassie and PI3Kγ immunoblotted. (e) Plasma membrane I2PP2A immunoprecipitates blotted for co-immunoprecipitating PP2Ac following ISO from VEH, Wort treated or PI3Kγinact expressing β1AR cells. (f) Plasma membrane I2PP2A immunoprecipitates blotted for co-immunoprecipitating PP2Ac following VEH (−) or Dob (+) (1mg/kg) treatment in PI3Kγinact Tg or littermate controls. Lower panel; I2PP2A immunoblotting. (See also Supplementary Figure S4 a-e).
To directly demonstrate that I2PP2A is a substrate for protein kinase activity of PI3Kγ, in vitro phosphorylation assays were performed on bacterially purified I2PP2A. Robust I2PP2A phosphorylation was observed with immunoprecipitated endogenous (Fig. 4c) or Baculovirus purified Wt PI3Kγ protein (Fig. 4d) which was completely abolished with PI3Kγinact (Fig. 4c & 4d). Treatment of Wt PI3Kγ protein with Wort (Fig. 4d) or immunoprecipitation of endogenous PI3Kγ from Wort pre-treated cells (Fig. 4c) resulted in marked inhibition of I2PP2A phosphorylation demonstrating that I2PP2A is a substrate for PI3Kγ. Kinetic and stoichiometric analyzes (Fig. S4 c, d & e) showed that 1.8 mol phosphate/mol of I2PP2A was transferred by PI3Kγ catalytic activity suggesting I2PP2A is phosphorylated at more than one site.
Given that PI3Kγ phosphorylates I2PP2A, we investigated whether PI3K-mediated phosphorylation is a key step in I2PP2A binding to PP2A. Endogenous I2PP2A was immunoprecipitated from plasma membranes and blotted for co-immunoprecipitating PP2A catalytic subunit. βAR stimulation resulted in significant association of PP2A with I2PP2A in VEH (Fig. 4e) that was abolished with PI3K inhibition by using pharmacologic or genetic approaches (Fig. 4e). Studies in the myocardium of PI3Kγinact Tg and controls recapitulated that PI3Kγ regulates I2PP2A binding to PP2A (Fig. 4f). Taken together our studies show that I2PP2A is a direct target for protein kinase activity of PI3Kγ unraveling the molecular basis of PP2A inhibition by I2PP2A.
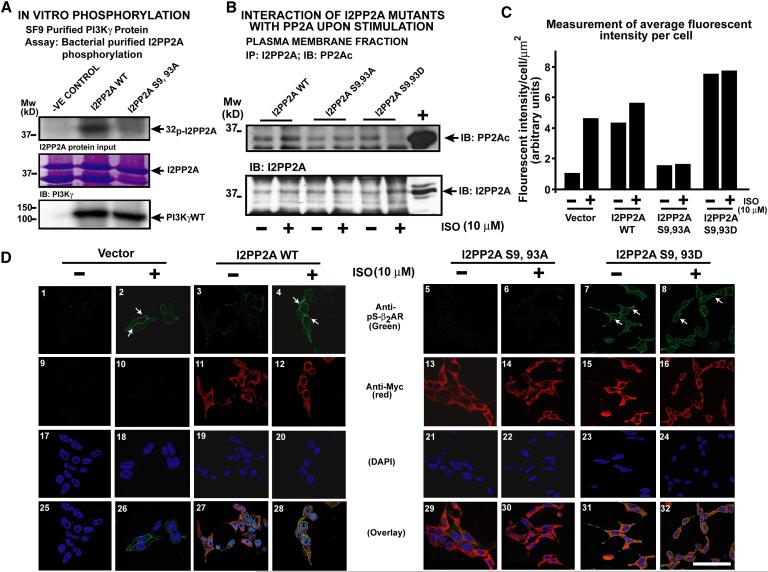
Serine 9 and 93 phosphorylation on I2PP2A by PI3Kγ regulates agonist mediated I2PP2A-PP2A interaction
A combination of mass spectrometry, bioinformatics, mutagenesis and co-immunoprecipitation (supplementary methods & Fig. S5 a-c) studies led to identification of Serine 9 and 93 as phosphorylation sites on I2PP2A by PI3Kγ. In vitro kinase assays using I2PP2A mutant with serine 9 and 93 replaced to alanines (S9, 93A) showed significant loss of phosphorylation demonstrating that PI3Kγ phosphorylates I2PP2A on these sites (Fig. 5a). To prove that phosphorylation of these two serines by PI3Kγ is critical for its interaction with PP2A, mutants of I2PP2A S9, 93A (constitutively dephosphorylated) or S9, 93D (constitutively phosphorylated, serine to aspartic acid) were expressed in cells and agonist mediated I2PP2A-PP2A interaction assessed. Significant loss in agonist mediated I2PP2A-PP2A interaction was observed with S9, 93A (Fig. 5b). Interestingly, S9, 93D mutant showed higher interaction with PP2A even at baseline which disappears from plasma membrane upon stimulation (Fig.5b) suggesting internalization of βAR complex containing I2PP2A-PP2A. To test whether differential interaction of I2PP2A mutants with PP2A alters β2AR phosphorylation confocal microscopy was performed. Upon ISO stimulation significant phosphorylation of the β2AR (green) was observed in vector and WT transfected cells (Fig. 5d panels 2 & 4, arrows). Interestingly, ISO stimulated phosphorylation of β2ARs was abolished in cells expressing S9, 93A I2PP2A (Fig. 5d, panel 6). In contrast, S9, 93D I2PP2A expressing cells showed significant β2 AR phosphorylation in unstimulated and stimulated cells (Fig.5d, panels 7 & 8, arrows). Fluorescence quantification for vector, WT and I2PP2A mutants are shown in Fig. 5c. WT and I2PP2A mutants showed similar levels of expression (Fig. S5 d & e). Consistently, cells stably expressing S9, 93A I2PP2A showed significant cAMP generation compared to cells with S9, 93D (Fig. S7a). These studies show that I2PP2A is phosphorylated by PI3Kγ on serine residues 9 and 93 resulting in PP2A regulation of βAR function.
Figure. 5.
I2PP2A phosphorylation on Serine 9 and 93 by PI3Kγ is critical for its interaction with PP2A. (a) In vitro phosphorylation using purified PI3Kγ and purified I2PP2A WT or mutants as substrates. Input I2PP2A stained by Coomassie and PI3Kγ immunoblotted. (b) Plasma membrane I2PP2A immunoprecipitates blotted for co-immunoprecipitating PP2Ac (Upper panel) following ISO from cells transfected with either I2PP2A WT or I2PP2A mutants (S9, 93A or S9, 93D). Immunoprecipitations were confirmed blotting for I2PP2A. (c) Fluorescence quantitation of the confocal images depicting fluorescent intensity/cell/ μm2, (n=70-100 cells/ experiment). d) Confocal microscopy showing phospho-β2AR (green) (panels 1-8), anti-Myc I2PP2A WT (red) (panels 11 & 12) or I2PP2A mutants (red) (panels 13-16) and nuclei (DAPI) (17-24) staining in presence and absence of ISO. Overlay is shown in panels 25-32. (See also Supplementary Figure S5 a-5e and S7a). Scale, 10 μm.
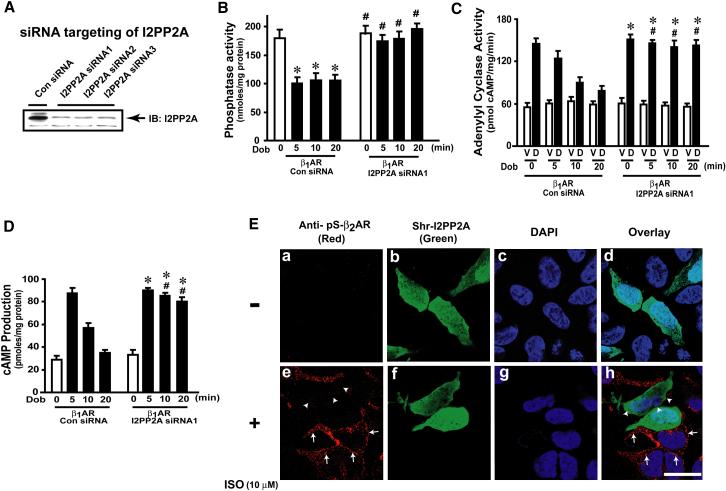
I2PP2A knock down results in sustained βAR signaling
Since PI3Kγ inhibits PP2A activity via I2PP2A driving βAR desensitization, we postulated that siRNA mediated depletion of I2PP2A should result in receptor resensitization. Three different siRNAs targeting I2PP2A effectively depleted I2PP2A expression (Fig. 6a) leading to concomitant increase in plasma membrane phosphatase activity (Fig. 6b & S6a). Consistently, knock down of I2PP2A resulted in significant increase in adenylyl cyclase activity (Fig. 6c) and cAMP generation despite agonist (Fig. 6d & S6b).
Figure 6.
Depletion I2PP2A results in βAR resensitization. (a) Knock-down of I2PP2A by 3 different siRNAs. (b) Plasma membrane Serine-Threonine phosphatase activity in β1AR cells co-expressing control (Con) or I2PP2A siRNA1 following Dob (black bars) (n=3). *p< 0.001, versus 0 min, #p<0.001, versus Con siRNA. (c) Basal (White) and in vitro Dob-stimulated (black, 5 min) adenylyl cyclase activity in Con or I2PP2A siRNA1 expressing β1AR cells following pre-treatment with Dob (0-20 min, n=4). *p< 0.001 versus VEH, #p<0.001 versus 10 and 20 min Dob-challenged control siRNA. (d) cAMP generation in β1AR cells co-expressing control or I2PP2A siRNA1 following Dob (n=3). *p< 0.001, versus VEH in I2PP2A siRNA1cells, #p<0.001, versus Dob treated controls (10 and 20 min). (e) Confocal microscopy showing phospho-β2AR (red), short hairpin RNA (Shr-I2PP2A) (green) and nuclei (DAPI) upon ISO treatment. (See also Supplementary Figure S6 a-b & S7b). Scale, 10 μm
To comprehensively study the of effect I2PP2A knock down, we generated short-hairpin RNA (Shr-I2PP2A) from siRNA1. β2AR expressing cells were transfected with Shr-I2PP2A and confocal microscopy was carried out to assess β2AR dephosphorylation. In the absence of ISO, no β2AR phosphorylation was observed (Fig. 6e, panels a, b & d). ISO treatment resulted in significant phosphorylation of β2ARs (red) (Fig. 6e, panels e & h, arrows) in cells without Shr-I2PP2A. In contrast, β2AR phosphorylation was completely abolished (Fig. 6e, panel e & h, arrowheads) in neighboring cells expressing Shr-I2PP2A (green) (Fig. 6e, panel f & h). To test whether I2PP2A regulates βAR function in myocytes, HL-1 cardiomyocyte cell line was transfected with Shr-I2PP2A. Significant cAMP generation was observed following agonist stimulation in I2PP2A knock down cells (Fig. S7b). These studies demonstrate that I2PP2A is a critical link in PI3Kγ-mediated inhibition of PP2A resulting in plasma membrane receptor desensitization.
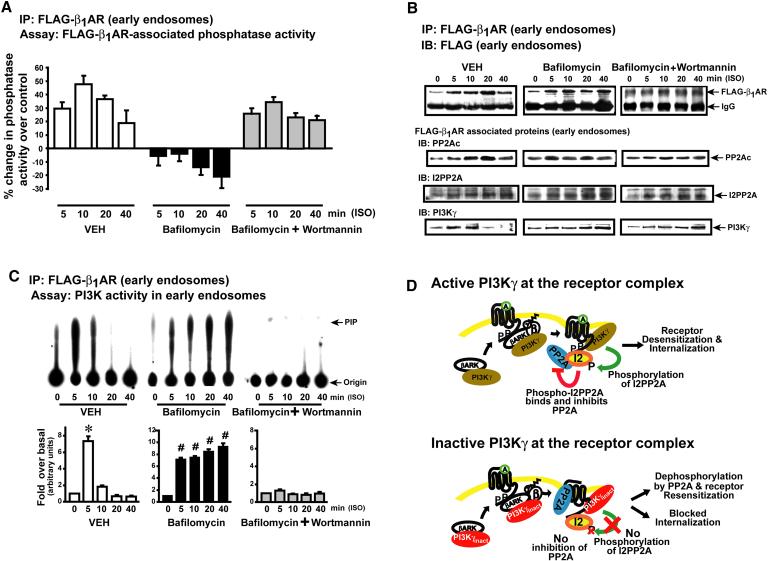
Endosomal acidification leads to loss of PI3K enhancing PP2A activity
As internalized receptors undergo dephosphorylation in endosomes(Krueger et al., 1997), we postulated that acidification of the endosomes may be a prerequisite step in loss of PI3K. The loss of PI3K with acidification could release the inhibitory effect on PP2A resulting in dephosphorylation and resensitization of βARs. To test this hypothesis, β1AR cells were assessed for receptor-associated phosphatase activity, PI3K activity, and co-immunoprecipitating PP2A, I2PP2A and PI3Kγ in endosomes following stimulation. Agonist stimulation of VEH treated cells showed marked increase in β1AR-associated phosphatase activity (Fig. 7a) with significant reduction in PI3K activity (Fig. 7c). The loss of β1AR-associated PI3K activity at 10 and 20 min is due to dissociation of PI3K from receptor complex with endosomal acidification (Fig. 7b, lower panel). Importantly, blocking endosomal acidification by bafilomycin rescued PI3K activity (Fig. 7c), its interaction with β1AR (Fig. 7b, lower panel) and reduced β1AR-associated phosphatase activity (Fig. 7a). In contrast, PI3K inhibition by Wort (Fig. 7c) along with bafilomycin resulted in rescue of β1AR-associated phosphatase activity (Fig. 7a) despite continued interaction of PI3Kγ and the receptor (Fig. 7b). Receptor association of PP2A and I2PP2A is not markedly altered over time (Fig. 7b) suggesting that acidification drives disengagement of PI3Kγ from the complex unlocking the inhibition on PP2A.
Figure. 7.
Regulation of phosphatase activity by PI3K in early endosomes. (a) β1AR-associated Serine-Threonine phosphatase activity (% change over un-stimulated), (n=6-8). (b) co-immunoprecipitated PP2Ac, I2PP2A and PI3Kγ following immunoprecipitation of FLAG-β1AR. (c)β1AR-associated PI3K activity from early endosomes after ISO in control, Bafilomycin or Bafilomycin and Wort pre-treated β1AR cells., (n=6-8), *p< 0.00001 versus other time points, #p<0.00001 versus 0 time point. (d) Illustration depicting PI3Kγ regulation of βAR-associated PP2A by I2PP2A leading to receptor desensitization/resensitization.
Discussion
We show that PI3Kγ negatively regulates βAR resensitization through a tightly coupled process. Using a dominant negative strategy (PI3Kγinact), we show a significant amelioration of cardiac dysfunction due to preservation of βAR function (resensitization). Inhibition of PI3Kγ showed reduced βAR phosphorylation that was associated with significant increase in PP2A activity. Simultaneous inhibition of PP2A (by okadaic acid) and PI3Kγ (by PI3Kγinact) restored β1AR phosphorylation in cells and reduced myocyte contractility. PI3Kγ inhibits PP2A activity by phosphorylating I2PP2A, an endogenous inhibitor of PP2A. I2PP2A phosphorylation results in enhanced binding to PP2A catalytic subunit inhibiting phosphatase activity driving βAR desensitization.
GPCR function is regulated by desensitization (phosphorylation) and resensitization (dephosphorylation). Desensitized βARs are internalized and resensitized by PP2A in acidic environment of early endosomes (Krueger et al., 1997; Rockman et al., 2002). Our studies suggest that receptors can be resensitized at the plasma membrane without undergoing internalization; a process which we believe is dynamically regulated by PI3Kγ. Such a view is supported by our studies with PI3K inhibition, PP2A inhibition and loss of I2PP2A function. Importantly, reversible β2AR phosphorylation observed with I2PP2A mutants (Fig. 5d) further strengthens this paradigm. Therefore, the role of receptor internalization for resensitization is an enigma that raises the question whether desensitization and internalization are independent or interdependent. Critically, data from our studies ascertain that desensitization and internalization are two independent processes. In a recent study, dephosphorylation of TRH receptors was observed despite inhibition of receptor internalization (Jones and Hinkle, 2005) consistent with our view that receptors can be dephosphorylated at the plasma membrane. This phenomenon may allow for more rapid resensitization at plasma membrane following agonist-induced desensitization than for an internalized receptor. Our studies show that by specifically targeting PI3Kγ or I2PP2A we can uncouple the overlapping processes of internalization and resensitization.
The recognition that internalization and resensitization could be independent processes then begs the question as to why in all the previous studies, inhibition of internalization also blocked receptor resensitization? We believe the connecting link between these two processes is PI3Kγ which is recruited to βAR complex and facilitates receptor internalization(Naga Prasad et al., 2002). Our current study shows that agonist mediated recruitment of PI3Kγ to the βAR complex inhibits PP2A-mediated receptor dephosphorylation leading to desensitization. Pharmacologic or genetic inhibition of PI3Kγ results in βAR resensitization and is potentially the underlying cause for amelioration of cardiac dysfunction observed in FLAG-β1AR/PI3Kγinact double Tg mice and PI3Kγinact Tg mice(Nienaber et al., 2003) with cardiac stress. Consistent with this conjecture, inhibition of PP2A activity by fostriecin results in the loss of resensitization despite the presence of PI3Kγinact. Accordingly, loss in β1AR phosphorylation observed with expression of PI3Kγinact was rescued with inhibition of PP2A by okadaic acid showing that PP2A is downstream of PI3Kγin regulating receptor function. In this context, studies on myocytes isolated from PI3Kγinact Tg mice showed reduction in ISO mediated contractility following PP2A inhibition by okadaic acid. This is in contrast to the preserved myocyte contractile response observed upon ISO in myocytes from PI3Kγinact Tg mice compared to littermate controls. Indeed, inhibition of PI3K leads to significant increase in βAR-associated phosphatase activity showing that PI3K inhibits PP2A activity with significant implications in cellular signaling(Sontag, 2001; Virshup and Shenolikar, 2009).
Inhibition of PP2A activity by PI3K is a powerful regulatory mechanism that may have profound effects on signaling due to stoichiometric differences between kinases and phosphatases. While there are more than 400 serine/threonine kinases, there are only 13 phospho-protein phosphatase coding genes in the human genome (Virshup and Shenolikar, 2009). Therefore, it becomes critical to understand the molecular mechanism of PP2A inhibition by PI3K as it may have significant implications in various cellular events. Recent studies have shown that PKA phosphorylation of PP2A regulatory subunit results in its activation(Ahn et al., 2007), while Src mediated phosphorylation of the catalytic subunit results in inhibition of PP2A activity(Chen et al., 1992). Protein kinase mutants of PI3Kγ recapitulates desensitization observed with endogenous PI3Kγ thereby, indicating that protein kinase component of PI3Kγ regulates PP2A function. Importantly, our metabolic labeling studies showed no differential phosphorylation of PP2A catalytic/regulatory subunits in the presence or absence of active PI3K suggesting alternative mechanisms of PP2A regulation.
A well known alternative mechanism of PP2A regulation is through endogenous inhibitors of PP2A, I1 and I2PP2A(Li and Damuni, 1998). It is known that inhibition of PP2A activity occurs by binding of I1- or I2PP2A(Li and Damuni, 1998). I2PP2A is the dominant isoform expressed in the cardiac membranes (data not shown). Although a recent study shows the recruitment of I2PP2A to muscarinic acetylcholine receptor (Simon et al., 2006), its function at the receptor complex is not well established. We show in our studies that I2PP2A at the βAR complex regulates receptor resensitization by inhibiting PP2A activity (Fig. 6). Importantly, very little it known about I2PP2A regulation and it is thought that phosphorylation of I2PP2A enhances its ability to bind and inhibit PP2A activity (Adachi et al., 1994). Furthermore, the kinase that catalyzes phosphorylation of I2PP2A is currently unknown. We show that PI3Kγ phosphorylates I2PP2A on serine 9 and 93 residues resulting in enhanced interaction of I2PP2A with PP2A. These phosphorylation sites are critical as S9, 93A I2PP2A mutant is unable to bind efficiently to PP2A following agonist stimulation. Furthermore, stoichiometric analysis shows that PI3K phosphorylates I2PP2A on more than one site. In addition to previously known phosphorylation on serine 9 of I2PP2A (Adachi et al., 1994; ten Klooster et al., 2007), we have also identified that phosphorylation on serine 93 is critical for its interaction with PP2A. These two serine phosphorylation sites on I2PP2A together have significant consequences on βAR phosphorylation and function. Consistently, I2PP2A depletion by siRNA led to sustained receptor signaling. Our study identifies I2PP2A as a protein kinase substrate for PI3Kγ adding to the limited list of known substrates (Dhand et al., 1994) (Pirola et al., 2001) (Naga Prasad et al., 2005).
Agonist activation of receptor results in PI3Kγ-mediated PP2A inhibition shifting the equilibrium towards desensitization and internalization. Our data shows that acidification of the endosome results in loss of PI3K activity releasing the inhibitory effect on PP2A accounting for resensitization of receptors. This potentially may be the mechanism utilized by parathyroid hormone (PTH) receptors in sustaining adenylyl cyclase activity in early endosomes in response to PTH(Ferrandon et al., 2009). Our studies demonstrate a critical role for PI3Kγ in receptor resensitization that is mediated by regulation of PP2A activity. PI3Kγ regulates resensitization of βARs by modulating I2PP2A/PP2A interaction at the resensitizome (resensitization machinery). We believe this mechanism to be universal as the key players in this process are recruited to various G-protein coupled receptors(Pullar et al., 2003; Rockman et al., 2002; Simon et al., 2006). Our study shows that βAR resensitization is not a cursory response but a well orchestrated dynamic process tightly regulated by interplay of kinases and phosphatases. Since studies suggest that receptor internalization is pathological(Lefkowitz and Shenoy, 2005), our findings open up exciting possibilities for pharmacologic or genetic interventions to improve receptor resensitization by targeting PI3Kγ or I2PP2A . Finally, our study has broad implications on cellular signaling which warrants further investigation as activation of PI3Kγ may result in PP2A inhibition prolonging downstream signaling.
Supplementary Material
Acknowledgements
We would like to thank Dr. David Pallas (Emory University) for I2PP2A antibody. We thank Dr. Sadashiva Karnik for critical review of the manuscript. Drs. Dianne Perez (Cleveland Clinic) and Edward Plow(Cleveland Clinic) for helpful discussions. Dr. Howard A. Rockman (Duke University) for insightful thoughts and discussion on the data. Drs. Belinda Willard and Mike Kinter for mass spectrometry analysis. This work is supported by NIH grant HL089473, HL089473-02S1 (S.V.NP) and AHA post-doctoral fellowship (N.T.V). N.T.V., M.L.M., M.K.G., A.F.H. and S.V. NP designed and carried out experiments. N.T.V., M.L.M, M.K.G and S.V. NP analyzed experimental data. N.T.V and S.V. NP wrote the manuscript and was critically reviewed by M.L.M and M.K.G.
Appendix
MATERIALS AND METHODS
Experimental Animals
C57/BL6 WT and Tg mice of either sex 3-6 months of age were used. Flag-β1AR(Noma et al., 2007) and PI3Kγinact(Nienaber et al., 2003) (Gift from Dr. Howard A. Rockman, Duke University) transgenic mice were bred to generate double Tg mice expressing Flag-β1AR and PI3Kγinact in the heart. ISO (30 mg/kg/day) was infused for 7 days (Casey et al., 2010) for assessment of cardiac and βAR function.
Echocardiography
Echocardiography was performed on anesthetized vehicle/ISO treated mice using a Vevo770 (VisualSonics) (Noma et al., 2007) pre- and post-treatment. M-mode views were recorded including left ventricular systolic and diastolic dimensions.
Cell culture, immunoprecipitation, western immunoblotting and plasmid constructs
Standard procedures for cell culture, western immunoblotting and immunoprecipitations were followed. In experiments using β1AR agonist dobutamine (Dob), the cells were pre-treated with β2AR antagonist ICI 118,551 to block endogenousβ2ARs. Detailed description can be found in supplemental methods. FLAG-β1AR, FLAG-β2AR, FLAG-PIK-P, FLAG-PIK-L and FLAG-PIK-LP, HA-PI3Kγ WT and HA-PI3Kγinact mutants constructs were described previously(Naga Prasad et al., 2005). Human I2PP2A in pBud Vector (Invitrogen) was a gift from Dr. Karnik, CCF which was subcloned into various expression vectors (details in supplemental methods). Quick change lightning site directed mutagenesis kit (Agilent technologies) was used for generating I2PP2A mutants (details in supplemental methods).
Isolation of Plasma Membranes and Early Endosomes
Plasma membranes and early endosomes were isolated as previously described(Perrino et al., 2005). Plasma membranes were prepared by homogenization of samples in lysis buffer (5mmol/L Tris-HCl pH 7.5, 5 mM EDTA, 1 mM PMSF, and 2 μg/mL Leupeptin and Aprotinin). Cell debris/nuclei were removed by centrifugation at 1000 g for 5 minutes and the supernatant was centrifuged at 37, 000 g for 20 minutes. Pellet representing membrane fraction was resuspended in 75 mM Tris-HCl pH 7.5, 2 mM EDTA, and 12.5 mM MgCl2 while supernatant was centrifuged for 1 hour at 300, 000 g to obtain early endosomes.
βAR density, adenylyl cyclase activity and cAMP assays
βAR density was determined by incubating 25 μg of the membranes with saturating concentrations of 125I Cyanopindolol or 40 μM Alprenolol (non-specific binding) as previously described(Naga Prasad et al., 2001). Adenylyl cyclase assays were carried out by incubating 20 μg of membranes at 37°C for 15 min with isoproterenol or NaF (G-protein activator) assay mixture containing 20 mM Tris-HCl, 0.8 mM MgCl2, 2 mM EDTA, 0.12mM ATP, 0.05 mM GTP, 0.1 mM cAMP, 2.7 mM phosphoenolpyruvate, 0.05 IU/ml myokinase, 0.01 IU/ml pyruvate kinase and 32Pγ ATP and generated cAMP quantified(Choi et al., 1997). The cAMP content in the lysates was determined either by Bio track [3H] cAMP (GE Healthcare) kit (Rapacciuolo et al., 2003) or catch point cAMP immunoassay kit (Molecular Devices).
Metabolic labeling and receptor phosphorylation
β1AR stable cells transfected with vector or PI3Kγinact were starved in phosphate-free media for 2h, treated with 100 μCi/ml of [32]Pi for 1h and endocytosis inhibitors (0.45M sucrose and 2% Cyclodextrin). Following stimulation, anti-Flag, anti-PP2Ac or anti-I2PP2A antibody was used for immunoprecipitation from plasma membranes. Phosphorylation was visualized following SDS-PAGE and autoradiography. Non-radioactive labeling was performed as described above except that β2AR stable cells were treated with serum-free media containing non-radioactive phosphates and phosphorylation assessed by anti-phospho-Serine antibody immunoblotting (1:750; Chemicon, Pittsburgh, PA).
Confocal Microscopy
FLAG-β2AR cells were transfected with vector or HA-tagged PI3Kγ and plated onto coverslips treated with poly L-Lysine(Naga Prasad et al., 2005). Cells were serum starved for 2 hours along with endocytosis inhibitors, stimulated, fixed (4% para-formaldehyde) and incubated in 1% BSA in PBS. Anti-phospho 355/356 β2AR (Tran et al., 2007), 1:200 (Santacruz) or anti-HA (1: 100; Roche) were used as primary antibodies while, goat anti-rabbit AlexaFlour 488 (1:500; Molecular probes) and anti-mouse AlexaFlour 568 (1: 500; Molecular probes) were used as secondary. Samples were visualized using sequential line excitation at 488 and 568 nm for green and red respectively. 70 to 100 positive cells were analyzed in each experiment and quantitation was performed using IMAGE PRO PLUS7 (Media Cybernetics, Inc).
Phosphatase assay
Phosphatase activity was measured using the Serine-Threonine phosphatase kit (Upstate Biotechnology). 25 μg of plasma membrane or immunoprecipitated samples were resuspended in the phosphate free assay buffer and incubated in presence or absence of Serine-Threonine specific phospho-peptide substrate for 10 minutes. The reaction mix was incubated with acidic malachite green solution and absorbance was measured at 630 nm in a plate reader.
Protein kinase assays
Assays were performed as previously described(Naga Prasad et al., 2005). Cells were solubilized in Triton X-100 lysis buffer (0.8% Triton X-100, 20 mM Tris-Cl pH 7.4, 300 mM NaCl, 1 mM EDTA, 20% glycerol, 0.1 mM PMSF, 10 μg ml−1 each of Leupeptin, and Aprotinin). PI3Kγ was immunoprecipitated, beads were washed with lysis buffer and re-suspended in 45 μl reaction buffer (20 mM HEPES, (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, 5 μM ATP, 1 mM DTT, 0.2 mM EDTA containing 10 μCi of [32]P-γ -ATP with purified I2PP2A as substrate. The assay was performed at 30°C for 20 min, the reaction was stopped by gel-loading buffer and phosphorylation was assessed by autoradiography. Assessment kinetics and stoichiometry of phosphorylation is described in supplementary methods.
Isolation of adult cardiac myocytes and contractility studies
The mice were anesthetized; the excised heart was immediately cannulated with 20-guage needle and mounted to perfusion apparatus. The perfusion buffer contained 113 mM NaCl, 4.7 mM KCl, 0.6 mM KH2PO4, 0.6 mM Na2PO4, 1.2 mM MgSO4, 0.5 mM MgCl2, 10 mM HEPES, 20 mM D-glucose, 30 mM taurine and 20 μM Ca2+ at pH 7.4. Following perfusion for 4 minutes, 150 units/ml of type II collagenase was perfused for 15 minutes. All the solutions were maintained at 34 °C and continuously bubbled with 95% O2 and 5% CO2. Left ventricular tissue was separated from the atria and right ventricle, minced, and digested in perfusate for 15 min. The digested heart was filtered through 200 μm nylon mesh, placed in a conical tube, and spun at 100 rpm to allow viable myocytes to settle. Serial washes were used to remove nonviable myocytes and digestive enzymes, and the concentration of Ca2+ was gradually increased to 1.8 mM for myocyte contractility studies. Myocytes were plated on glass chamber slides and placed on the microscopic stage (Leica) connected to field stimulator specifically designed for driving isolated myocytes (MyoPace, IonOptix). Cardiomyocytes were stimulated at 0.5 Hz and imaged with variable field-rate camera (MyoCam, IonOptix) using edge-detection and sarcomere length technology. Peak contraction was measured as the % of peak cell shortening. Myocytes were treated with ISO or ISO and OA for 10 minutes for each of the experiments. Each data point represents myocytes isolated from different animals with at least 15-20 cells averaged per treatment and about > 6 contractions included for each myocyte.
siRNA & Shr-RNA knockdown of I2PP2A
siRNA duplexes were generated from human I2PP2A cDNA sequence using QIAGEN and Ambion siRNA designing program. The target 21-mer sequence for siRNAs were si(1) RNA (region 265-272 base pairs (bp) AACCATCCACAAGTGTCTGCA; si(2)RNA (region 299-306 bp) AAGATGAAGAGGCACTGCATT; si(3)RNA (region 505-512 bp) AAACGTTCGAGTCAAACGCAG respectively. All star negative control siRNA and siRNA(1,2 and 3) were custom made from QIAGEN. The siRNA duplexes were resuspended and transfected using Hiperfect (QIAGEN) into β1AR stable cells at 150 nM concentration. All assays were performed 48 hours post-transfection. The short hairpin RNA (Shr-I2PP2A) sequence corresponding to siRNA1 (GGATCCCAGTGCAGACACTTGTGGATGGTTGATATCCGCCATCCACAAGTGTCTGCACTTTTTTTCCAAAAGCTT) was cloned into pRNAT-U6.1/Hygro vector (GenScript).
GST fusion protein expression and pull-down experiment
Glutathione-S-transferase (GST)-I2PP2A fusion protein was generated with pGEX4T1 bacterial expression system in BL21 cells using 0.1 mM IPTG induction. Cells were pelleted, lysed, and GST–I2PP2A fusion protein was isolated from the supernatant using Glutathione-Sepharose beads. GST beads were washed and resuspended in PBS. Thrombin cleaved purified I2PP2A was used as a substrate for in vitro phosphorylation assays.
Statistics
Data are expressed as mean ± s.e.m. Statistical comparisons were performed using an unpaired Student’s t-test and analysis of variance (ANOVA) when appropriate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi Y, Pavlakis GN, Copeland TD. Identification of in vivo phosphorylation sites of SET, a nuclear phosphoprotein encoded by the translocation breakpoint in acute undifferentiated leukemia. FEBS Lett. 1994;340:231–235. doi: 10.1016/0014-5793(94)80144-4. [DOI] [PubMed] [Google Scholar]
- Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Auger KR, Duckworth BC, Hou WM, Schaffhausen B, Cantley LC. A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol. 1993;13:1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, et al. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107:532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Choi DJ, Koch WJ, Hunter JJ, Rockman HA. Mechanism of beta-adrenergic receptor desensitization in cardiac hypertrophy is increased beta-adrenergic receptor kinase. J Biol Chem. 1997;272:17223–17229. doi: 10.1074/jbc.272.27.17223. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, et al. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Dhand R, Hiles I, Panayotou G, Roche S, Fry MJ, Gout I, Totty NF, Truong O, Vicendo P, Yonezawa K, et al. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. Embo J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009 doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler D, Crepieux P, Poupon A, Clement F, Fages F, Reiter E. Towards a systems biology approach of G protein-coupled receptor signalling: challenges and expectations. C R Biol. 2009;332:947–957. doi: 10.1016/j.crvi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Jones BW, Hinkle PM. Beta-arrestin mediates desensitization and internalization but does not affect dephosphorylation of the thyrotropin-releasing hormone receptor. J Biol Chem. 2005;280:38346–38354. doi: 10.1074/jbc.M502918200. [DOI] [PubMed] [Google Scholar]
- Krueger KM, Daaka Y, Pitcher JA, Lefkowitz RJ. The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J Biol Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- Leblais V, Jo SH, Chakir K, Maltsev V, Zheng M, Crow MT, Wang W, Lakatta EG, Xiao RP. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes. Circ Res. 2004;95:1183–1190. doi: 10.1161/01.RES.0000150049.74539.8a. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Li M, Damuni Z. I1PP2A and I2PP2A. Two potent protein phosphatase 2A-specific inhibitor proteins. Methods Mol Biol. 1998;93:59–66. doi: 10.1385/0-89603-468-2:59. [DOI] [PubMed] [Google Scholar]
- Naga Prasad SV, Barak LS, Rapacciuolo A, Caron MG, Rockman HA. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem. 2001;276:18953–18959. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- Naga Prasad SV, Jayatilleke A, Madamanchi A, Rockman HA. Protein kinase activity of phosphoinositide 3-kinase regulates beta-adrenergic receptor endocytosis. Nat Cell Biol. 2005;7:785–796. doi: 10.1038/ncb1278. [DOI] [PubMed] [Google Scholar]
- Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J Cell Biol. 2002;158:563–575. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienaber JJ, Tachibana H, Naga Prasad SV, Esposito G, Wu D, Mao L, Rockman HA. Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AV, Mellon SH, Miller WL. Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem. 2003;278:2837–2844. doi: 10.1074/jbc.M209527200. [DOI] [PubMed] [Google Scholar]
- Penn RB. Agonizing over agonism: should asthmatics turn their beta-receptors on or off? Proc Natl Acad Sci U S A. 2009;106:2095–2096. doi: 10.1073/pnas.0812935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C, Naga Prasad SV, Schroder JN, Hata JA, Milano C, Rockman HA. Restoration of beta-adrenergic receptor signaling and contractile function in heart failure by disruption of the betaARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–2587. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- Perrino C, Schroder JN, Lima B, Villamizar N, Nienaber JJ, Milano CA, Naga Prasad SV. Dynamic regulation of phosphoinositide 3-kinase-gamma activity and beta-adrenergic receptor trafficking in end-stage human heart failure. Circulation. 2007;116:2571–2579. doi: 10.1161/CIRCULATIONAHA.107.706515. [DOI] [PubMed] [Google Scholar]
- Pirola L, Zvelebil MJ, Bulgarelli-Leva G, Van Obberghen E, Waterfield MD, Wymann MP. Activation loop sequences confer substrate specificity to phosphoinositide 3-kinase alpha (PI3Kalpha ). Functions of lipid kinase-deficient PI3Kalpha in signaling. J Biol Chem. 2001;276:21544–21554. doi: 10.1074/jbc.M011330200. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Chen J, Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278:22555–22562. doi: 10.1074/jbc.M300205200. [DOI] [PubMed] [Google Scholar]
- Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Simon V, Guidry J, Gettys TW, Tobin AB, Lanier SM. The proto-oncogene SET interacts with muscarinic receptors and attenuates receptor signaling. J Biol Chem. 2006;281:40310–40320. doi: 10.1074/jbc.M603858200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13:7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster JP, Leeuwen I, Scheres N, Anthony EC, Hordijk PL. Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET. Embo J. 2007;26:336–345. doi: 10.1038/sj.emboj.7601518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TM, Friedman J, Baameur F, Knoll BJ, Moore RH, Clark RB. Characterization of beta2-adrenergic receptor dephosphorylation: Comparison with the rate of resensitization. Mol Pharmacol. 2007;71:47–60. doi: 10.1124/mol.106.028456. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Baines CP, Liu GS, Armstrong SC, Ganote CE, Walsh AH, Honkanen RE, Cohen MV, Downey JM. Fostriecin, an inhibitor of protein phosphatase 2A, limits myocardial infarct size even when administered after onset of ischemia. Circulation. 1998;98:899–905. doi: 10.1161/01.cir.98.9.899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.