Abstract
The cysteine precursor L-2-oxothiazolidine-4-carboxylate (OTZ, procysteine) can raise cysteine concentration, and thus glutathione levels, in some tissues. OTZ has therefore been proposed as a prodrug for combating oxidative stress. We have synthesized stable isotope labeled OTZ (i.e. L-2-oxo-[5-13C]-thiazolidine-4-carboxylate, 13C-OTZ) and tracked its uptake and metabolism in vivo in rat brain by 13C magnetic resonance spectroscopy. Although uptake and clearance of 13C-OTZ was detectable in rat brain following a bolus dose by in vivo spectroscopy, no incorporation of isotope label into brain glutathione was detectable. Continuous infusion of 13C-OTZ over 20 h, however, resulted in 13C-label incorporation into glutathione, taurine, hypotaurine and lactate at levels sufficient for detection by in vivo magnetic resonance spectroscopy. Examination of brain tissue extracts by mass spectrometry confirmed only low levels of isotope incorporation into glutathione in rats treated with a bolus dose and much higher levels after 20 h of continuous infusion. In contrast to some previous studies, bolus administration of OTZ did not alter brain glutathione levels. Even a continuous infusion of OTZ over 20 h failed to raise brain glutathione levels. These studies demonstrate the utility of in vivo magnetic resonance for non-invasive monitoring of antioxidant uptake and metabolism in intact brain. These types of experiments can be used to evaluate the efficacy of various interventions for maintenance of brain glutathione.
Keywords: Magnetic resonance, Metabolism, Cysteine, Glutathione, Taurine
Introduction
Glutathione is synthesized in two steps from its three amino acid components; glutamate, cysteine and glycine. This tripeptide is present in high concentration in most tissues and is a key component in cellular defenses against oxidative stress. Oxidative stress plays a central role in the progression of neurodegenerative diseases, and a number of approaches have been proposed to slow disease progression through the enhancement of brain glutathione content [1]. One strategy is to assure the brain is supplied with sufficient building blocks for glutathione synthesis. Of the three amino acids comprising glutathione, glutamate and glycine are abundant (millimolar concentration) in most tissues, whereas the cysteine concentration in tissue is one of the lowest of the protein amino acids (50–250 μM; [2]). For this reason, cysteine is typically thought to be the limiting amino acid for glutathione synthesis [3]. However, high concentrations of cysteine can be toxic, thereby complicating efforts to raise tissue glutathione content by administering cysteine in an unmodified form [3]. Strategies to overcome this have employed cysteine precursors such as N-acetylcysteine (NAC), a relatively nontoxic form of cysteine clinically used to treat acetaminophen overdose [4] and L-2-oxothiazolidine-4-carboxylate (OTZ), a prodrug that is metabolized to cysteine by the action of the enzyme 5-oxoprolinase [5]. Although there is ample evidence for the benefits of NAC administration in neurodegenerative diseases [6], it is not clear that NAC itself can pass through the blood brain barrier [7]. OTZ is stable, resists oxidation and has low toxicity, and thus can safely be administered at high concentrations [8]. OTZ appears to be able to cross the blood brain barrier as it has been shown to increase brain cysteine [9] and, in some cases, glutathione levels [10]. OTZ has been proposed as a therapeutic agent to combat oxidative stress in neurodegeneration [1, 11]. We have previously demonstrated in vivo monitoring of glutathione metabolism by non-invasive magnetic resonance spectroscopy methods [12]. These studies used [2-13C]-glycine to observe incorporation of a 13C isotopic label into glutathione and to report on the biosynthesis and tissue distribution of this important endogenous antioxidant. In the study reported here, we use noninvasive magnetic resonance techniques and a 13C-labeled cysteine prodrug to observe the introduction of a 13C-label into glutathione and to provide insights into the efficacy of therapeutic approaches aimed at boosting cellular oxidative stress defenses.
We have prepared L-2-oxo-[5-13C]-thiazolidine-4-carboxylate (13C-OTZ) and monitored its metabolism in rat brain by in vivo magnetic resonance spectroscopy, and in rat brain and liver by analysis of tissue extracts. This stable isotope labeling approach allows the tissue concentration of OTZ to be assessed non-invasively and also provides an insight into the chemical identity of the compounds resulting from OTZ metabolism. In vivo magnetic resonance spectroscopy provides a non-invasive, repeatable measurement well suited to time course experiments, whereas high-resolution magnetic resonance spectroscopy of ex vivo tissue extracts provides higher sensitivity for the detection of 13C-labeled metabolites. In the study reported here, we have used a combination of these methodologies to monitor the in vivo response to OTZ administration, and to unequivocally identify the principal metabolites of this compound.
Restoration of brain glutathione levels through the administration of small molecule antioxidants may play an increasing role in the treatment of neurodegenerative diseases and psychiatric disorders [13, 14]. Therefore, methods that can monitor the uptake and metabolic fate of these antioxidants will be invaluable to identifying deficient pathways and for designing strategies to best deal with these deficiencies. We demonstrate that strategic labeling of proposed therapeutic agents with isotope labels combined with magnetic resonance spectroscopy can be used to map out critical pathways in antioxidant metabolism noninvasively in intact brain.
Methods
L-2-Oxothiazolidine-4-carboxylate (OTZ), taurine and hypotaurine were obtained from Sigma Chemical Co (St. Louis, MO). [3-13C]-L-Cysteine was obtained from Cambridge Isotope Laboratories (Andover, MA). The L-2-oxo-[5-13C]-thiazolidine-4-carboxylate (13C-OTZ) was prepared from [3-13C]-L-cysteine [15].
In vivo Experiments
All animal procedures were approved by the University of Florida Animal Care and Use Committee. OTZ was administered to rats as either a bolus dose by intraperitoneal injection, or as a 24 h intravenous infusion. For bolus administration a sterile solution of OTZ or 13C-OTZ (0.85 M) in 0.9% saline was prepared, adjusted to pH 7.4, and injected intraperitoneally into 10-week old female Fischer 344 rats at a dose of 1,100 mg/kg. For long-term infusion, a catheter was surgically implanted under isoflurane anaesthesia into the exterior jugular vein of the rats. The cannula was exteriorized between the scapulae. Animals recovered from anesthesia and buprenorphine (0.05–0.1 mg/kg) was administered as a component of post-surgical care. A fitted infusion harness and lines (Instech Solomon, Plymouth Meeting, PA) were employed to allow free movement of the rats after surgery and throughout the 13C-OTZ infusion period, which commenced 24 h after surgery. Intravenous 13C-OTZ was administered at a rate of 0.5 mL/h as a 84 mM solution dissolved in 0.9% saline, pH 7.4, providing a dose of 65 mg/kg/h.
Animals receiving bolus doses of 13C-OTZ were assessed by in vivo magnetic resonance spectroscopy before and during administration or at specific time points between 3 and 8 h after injection. Magnetic resonance spectroscopy was performed on animals receiving intravenous 13C-OTZ after a 20 h infusion period.
Animals were anaesthetized during magnetic resonance spectroscopy. Anesthesia was induced with 5% isoflurane in oxygen, and maintained with 1–2% isoflurane in oxygen for the duration of the magnetic resonance scanning. Respiration rates were monitored with a pressure transducer sensor placed beneath the rat and body temperature monitored by rectal thermometer. Body temperature was maintained during magnetic resonance scanning by a thermostatically controlled warm air delivery system. Animals were sacrificed immediately at the conclusion of the magnetic resonance experiments, brain and liver tissue were removed and immediately frozen in liquid nitrogen and then stored at −80°C.
Ten rats were used in a study to determine the effect of a bolus dose of unlabeled OTZ at 4 and 6 h after administration. Six rats received unlabeled OTZ at 1,100 mg/kg in 0.9% saline and four rats the saline vehicle alone. Animals were sacrificed 4 and 6 h after this dose and brain and liver tissue were harvested and immediately frozen in liquid nitrogen for biochemical analyses.
In vivo 13C Magnetic Resonance Spectroscopy
In vivo magnetic resonance data were acquired using an 11.0 T, 40 cm bore horizontal magnet (Magnex Scientific, Oxfordshire, UK) interfaced to a Bruker (Billerica, MA, USA) spectrometer and console. A 15 mm diameter 13C (118 MHz) surface coil was placed on the head of rat, and the rat was placed in a 40 mm diameter bird-cage coil tuned to the 1H frequency (470 MHz) for 1H imaging and decoupling.
Nonlocalized 13C-magnetic resonance spectra were collected using a pulse-acquire pulse sequence with 1H WALTZ decoupling. Excitation employed a nominal 90° rectangular RF pulse with a repetition time of 1.5 s over 400 averages. Data were collected into 1,024 data points with a resultant spectral width of 10 kHz. The scan duration was 10 min. Previous experiments in our tumor studies using a fiber optic thermometer determined that the 1H decoupling scheme used did not cause heating of the tissue.
Ex vivo Tissue Analysis
Tissue glutathione and cysteine content was assayed as their bimane conjugates by a method similar to that outlined previously [16]. Briefly, frozen tissue fragments (typically 4 samples from each tissue each weighing between 10 and 50 mg) were added to a solution of 0.050 M potassium phosphate, 50 μM EDTA, 100 μM acivicin, the mixture rapidly weighed, then monobromobimane in acetonitrile was added to a concentration of 5 mM. This mixture was immediately homogenized and incubated for 15 min at room temperature. Perchloric acid was then added to a concentration of 0.6 M to precipitate proteins. The extract was pH-neutralized then analyzed by liquid chromatography on a Waters Acquity Ultraperformance Liquid Chromatography (UPLC) system (Milford, MA) with UV-detection at 390 nm. Taurine and hypotaurine were assayed as their aminoquinolyl-derivatives by a modified version of the method reported by [17]. Weighed frozen tissue fragments (10–50 mg) were homogenized in 0.5 M perchloric acid. The mixture was pH-neutralized, treated with 6-aminoquinolyl-N-hydroxysuccinidyl carbamate [18] and then analyzed by UPLC. Metabolites were assayed by these methods in brain tissue from ten rats administered unlabeled OTZ or vehicle and five rats infused for 20 h with 13C-OTZ after in vivo MR studies were complete. These data are summarized in Table 1.
Table 1.
Metabolite levels in brain tissue extracts
| Glutathione (μmol/g-tissue) | Cysteine (μmol/g-tissue) | Taurine (μmol/g-tissue) | Hypotaurine (μmol/g-tissue) | |
|---|---|---|---|---|
| Control (n = 4)a | 1.22 ± 0.26 | 0.176 ± 0.050 | 3.76 ± 1.80 | 0.249 ± 0.053 |
| OTZ 4 h (n = 3)a | 1.04 ± 0.17 | 0.392 ± 0.080* | 4.35 ± 2.78 | 0.275 ± 0.103 |
| OTZ 6 h (n = 3)a | 1.05 ± 0.05 | 0.246 ± 0.061 | 4.16 ± 1.40 | 0.179 ± 0.047 |
| 13C-OTZ 20 h (n = 5)b | 1.51 ± 0.25 | 0.113 ± 0.075 | 3.93 ± 0.55 | 0.404 ± 0.147** |
P<0.005;
P<0.05 compared to controls
Bolus i.p
Continuous infusion i.v
High resolution NMR spectroscopy of tissue extracts was performed to identify the 13C-labeled brain metabolites of 13C-OTZ. Frozen tissue fragments (150–350 mg) were homogenized in 0.5 M perchloric acid containing 50 mM EDTA and 1 mM dithiothreitol. Precipitated proteins were removed and the extracts were neutralized with KOH. Extracts were freeze-dried and dissolved in 90% H2O, 10% D2O, 10 μM EDTA containing 0.025 M dioxane as a chemical shift standard (67.4 ppm). Magnetic resonance spectroscopy of tissue extracts was performed on a 11.7 T Bruker Avance DRX spectrometer. The 13C data at 125.8 MHz was collected with a pulse-acquire sequence with WALTZ16 1H-decoupling. Spectra were acquired with a sweep width of 27,777 Hz into 32 K data points with an acquisition time of 1.18 s and a relaxation delay of 1.0 s.
Isotopic fractional enrichment data was determined by mass spectrometry by trapping reduced glutathione using N-ethylmaleimide. Briefly, frozen tissue fragments were suspended in 10 mM N-ethylmaleimide in distilled water and tissue dispersed with a tissue homogenizer. After sitting at room temperature for 15 min, suspended solids were removed by centrifugation and the supernatant analyzed by liquid chromatography/mass spectrometry (LC/MS) in the Genome Sciences Laboratory of NC State University. LC/MS analyses were performed with a Thermo Surveyor liquid chromatograph coupled to a Thermo LTQ linear ion trap mass spectrometer. Chromatographic separations were achieved with a Thermo Hypersil Gold (150 mm × 2.1 mm I.D., 5 um particle size, 175 Å pore size) reverse-phase column. The mass spectrometer was operated in positive mode with electrospray ionization. The integrated intensities of the peaks at mass-to-charge (m/z) 433 (M + H), m/z 434 and m/z 435 due to S-(N-ethylmaleimidyl)-glutathione were measured and compared to the theoretical intensities for S-(N-ethylmaleimidyl)-glutathione (C16N4O8H24S) calculated using a mass spectrometry webtool [19]. This was converted to fractional enrichment. Similarly, the isotopic distribution in cysteine in the tissue samples was calculated by analyzing the isotope distribution pattern for the S-(N-ethylmaleimidyl)-cysteine at m/z 247, 248, 249.
Results
Table 1 shows the effects of bolus intraperitoneal injection of unlabeled OTZ and intravenously infused 13C-OTZ on the concentrations of glutathione, cysteine, taurine and hypotaurine determined from chromatographic analyses of tissue extracts. No statistically significant change in brain glutathione concentration was observed at 4 or 6 h after bolus OTZ administration. Cysteine content was elevated at 4 h post-bolus, but this had normalized to control levels by 6 h. Taurine and hypotaurine levels showed no statistically significant differences between treated and control animals following bolus administration. Intravenous infusion of 13C-OTZ over a 20 h period had no effect on the concentration of brain glutathione, cysteine or taurine, however a statistically significant increase in hypotaurine content was observed.
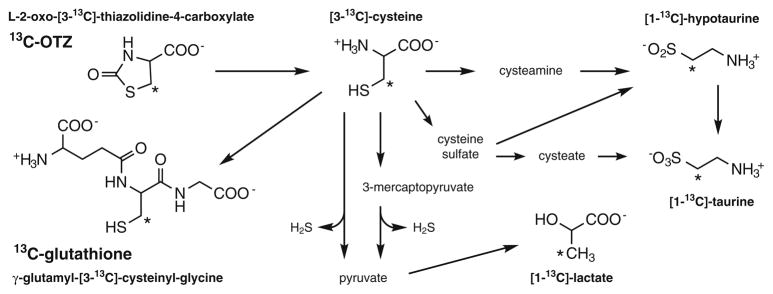
Although the steady state concentrations of these compounds were mainly unperturbed by OTZ administration all of those metabolites are continually being synthesized and degraded and the rate at which these processes occur can reflect brain health. The expected metabolic pathways for 13C-OTZ conversion into cysteine, glutathione and other metabolites are shown in Fig. 1.
Fig. 1.
Pathways of 13C-OTZ metabolism, with the structure of 13C-labeled compounds observed in magnetic resonance spectra. Asterisks denote the position of the 13C-labels. Adapted from [2]
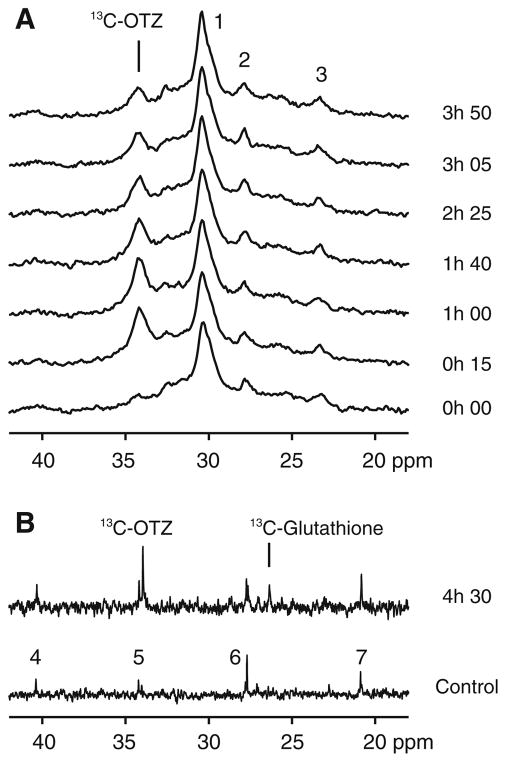
A portion of a typical in vivo brain 13C magnetic resonance spectrum is shown in Fig. 2a (bottom spectrum, timepoint 0 h 00). The surface coil placed on the head of these rats detects the natural abundance 13C signal principally from brain, but also from the adjacent skin, subcutaneous fat and muscle. The spectrum shown at the zero timepoint in Fig. 2a is dominated by a broad envelope of signals originating primarily from subcutaneous lipid centered at ~30 ppm. Other much smaller but broad envelopes of resonances due to other lipid and macromolecules within the rat head are centered near 24 and 28 ppm. After acquisition of the background spectrum in Fig. 2a, the rat was injected with a bolus of 13C-OTZ and placed back in the magnet and a series of spectra were obtained sequentially, starting at the time after injection shown to the right of each spectrum. This time course shows the buildup of the 13C-OTZ signal at 34.2 ppm over the first two spectra (t = 0 h 15 and 1 h 00 post injection) and then a decrease in signal intensity as the 13C-OTZ is cleared from the tissue. No other metabolites are immediately apparent. At the end of this experiment, 4.5 h after injection, the brain tissue was frozen and extracted. The high-resolution magnetic resonance spectrum of the tissue extract is shown in Fig. 2b (time 4 h 30). This spectrum shows the presence of the 13C-OTZ at 34.2 ppm and a small resonance at 26.5 ppm, corresponding to the 3-carbon of the cysteinyl-residue of glutathione (i.e. γ-glutamyl-[3-13C]-cysteinyl-glycine; hereafter referred to as 13C-glutathione). These peak positions and identities were established from high-resolution studies of standard compounds in water pH 7.4 referenced to dioxane (67.4 ppm). All other resonances in the extract spectrum are from the natural abundance 13C metabolites that are present in brain tissue from control rats not infused with 13C-OTZ. An example of the background natural abundance resonances visible in the extract is shown in the spectrum in Fig. 2b (bottom) from a control rat. The major differences between the spectra in Fig. 2b are due to the presence of 13C-OTZ and 13C-glutathione. The level of 13C-glutathione detected in the extract spectrum in Fig. 2b (top) was not sufficiently high enough for unambiguous detection in the in vivo spectra shown in Fig. 2a. Mass spectrometry analysis of this extract indicates that the fractional enrichment of glutathione in the brain 4 h 30 after bolus injection of 1,100 mg/kg 13C-OTZ was 0.0833.
Fig. 2.
a A portion of the in vivo rat brain 13C magnetic resonance spectra acquired before and (bottom, t = 0 h 00) at six time points (15 min–3 h 50) after bolus injection of 1,100 mg/kg 13C-OTZ. b A portion of the high-resolution 13C magnetic resonance spectrum of the acid extract of brain tissue from the same rat (upper), and from a control rat (lower). Signals are detected from 13C-OTZ (34.2 ppm), 13C-glutathione (26.5 ppm), and natural abundance metabolites (including CH2 carbons of long chain fatty acids, principally (–CH2–)n (peak 1), –CH2–CH=CH–(peak 2) and CH2-CH3 (peak 3) carbons). Natural abundance resonances in the control brain tissue extract spectrum are assigned to N-acetyl aspartate (peaks 4 and 7), and glutamate/glutamine (peaks 5 and 6)
There is the possibility that label incorporation into glutathione may take longer than the 4.5 h used in these studies. A series of experiments were performed where rats were injected with 1,100 mg/kg 13C-OTZ and placed in themagnet at 3 and 7 h post injection. Results were similar to those shown in Fig. 2a up to 5 h, i.e. showing only the 13C-OTZ peak in the in vivo spectra (data not shown). After the 7 h time points, brain tissue was frozen and high-resolution extract data was obtained and no 13C-label above natural abundance was detected for 13C-glutathione (data not shown).
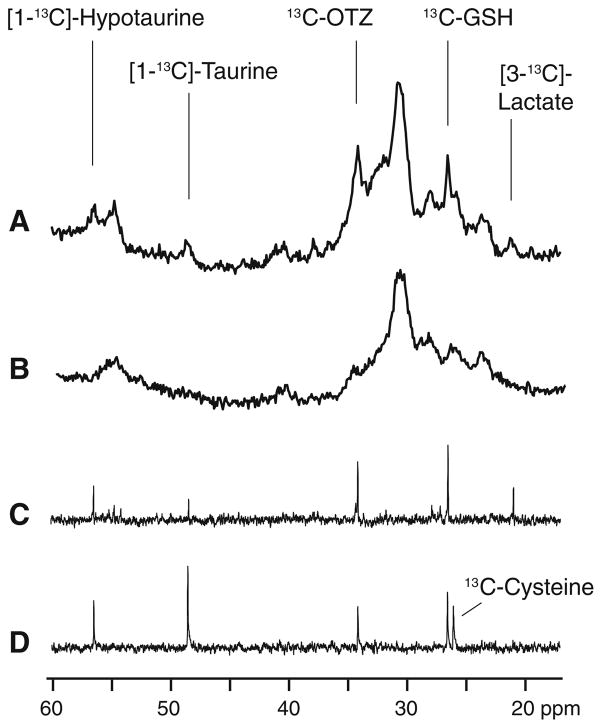
These bolus studies demonstrated that 13C-OTZ is cleared before 13C-labeled metabolites formed from 13C-OTZ are present at high enough concentration to be detected in the in vivo spectra. In order to increase the time available for 13C-OTZ to be converted to 13C-labeled metabolites, we switched to a chronic infusion model where 13C-OTZ is administered over 20 h. Figure 3b shows an in vivo rat brain spectrum acquired from an untreated control animal, and is similar in appearance to the pre-treatment spectra shown in Fig. 2a (t = 0 h 00). Figure 3a shows an in vivo spectrum from rat brain after chronic infusion of 13C-OTZ for 20 h. In this spectrum, 13C-OTZ is visible as observed in the bolus experiments (34.2 ppm) and additional signals are detected as well at 21.0, 26.5, 48.5 and 56.5 ppm. These peak positions are assigned to 13C-lactate, 13C-glutathione, 13C-taurine and 13C-hypotaurine respectively (Fig. 3a). These assignments were determined after comparison with spectra from tissue extracts. Figure 3c shows a spectrum from an extract of the brain of this same rat, confirming the peak positions and the identity of the 13C-labeled metabolites visible in the in vivo spectrum. The assignments of the metabolites in the extracts were determined by comparisons with spectra of authentic compounds. An extract from the liver of this rat is shown in Fig. 3d. Except for lactate, the same peaks are present as in the brain. An extra peak shown in Fig. 3d is from the addition of a small amount of a standard solution of 13C-cysteine to the extract. This peak appears at 26.1 ppm and is clearly distinct from that of glutathione at 26.5 ppm. In the brain extract and liver extract prior to addition of the standard, no labeled 13C-cysteine is detectable.
Fig. 3.
a A portion of the in vivo 13C magnetic resonance spectrum showing signals originating from subcutaneous fat and brain from a rat after 20 h of 13C-OTZ infusion at a total dose of 1,300 mg/kg. b The natural abundance in vivo 13C magnetic resonance spectrum showing signal originating from subcutaneous fat and brain from a control rat. c A portion of the high-resolution 13C magnetic resonance spectrum of the acid extract of brain tissue from the same rat used in a. d A portion of the high-resolution 13C magnetic resonance spectrum of the acid extract of liver tissue from the same rat used in a and spiked with a standard solution of [3-13C]-cysteine. In addition to resonances identified in Fig. 2, [3-13C]-lactate is observed at 21.0 ppm, [1-13C]-taurine at 48.5 ppm, [1-13C]-hypotaurine at 56.5 ppm. The liver sample shows an additional resonance from the added [3-13C]-cysteine at 26.1 ppm
UPLC analysis of brain tissue extracts from these intravenously infused animals demonstrated glutathione levels that did not differ significantly from control data. In addition, brain cysteine concentration showed a degree of variability reflected in the larger standard deviation compared to other metabolite concentration measurements, and hypotaurine levels were significantly elevated compared to controls (Table 1).
In brain tissue, the fractional enrichment of glutathione after 20 h of infusion of 13C-OTZ was 0.348 ± 0.071 (n = 5), and the enrichment of cysteine in the brain tissue was 0.434 ± 0.113 (n = 5).
Discussion
The data in Table 1 show that glutathione levels were unchanged 4 or 6 h after a 1,100 mg/kg bolus dose of OTZ. This is unlike the 40% increase in brain glutathione that peaked 4 h after a 1,170 mg/kg dose in the study of Mesina et al. [10] or 6 h after a dose of 735 mg/kg as observed by Gerard-Monnier et al. [20]. Another study reported that a 1,170 mk/kg dose of OTZ resulted in a large regional variation in glutathione across the brain [21]. Interestingly, in that study, all regions of the brain showed a decrease in glutathione immediately after intraperitoneal injection but then recovered or exceeded control levels. A maximal level of 34% above controls was found in the hippocampus 6 h after injection but most other regions saw smaller increases (3–15%) 4–8 h after injection [21]. The glutathione concentration data presented in Table 1 were determined from at least 4 tissue samples from randomly selected regions of the brain and therefore would likely reflect the average brain glutathione/metabolite content. Although our results are not consistent with those three studies, our observed increases in brain cysteine concentration after 4 h is in line with two other studies showing elevated cysteine levels that peak approximately 4 h after OTZ administration without an increase in glutathione [9, 22]. Even after continuous infusion of 13C-OTZ for 20 h, we found that the glutathione levels determined in brain tissue extracts were not statistically significantly different from the control levels. These results obtained from tissue extracts show that in vivo detection of glutathione in the brain by magnetic resonance spectroscopy will not be facilitated by increasing the level of this metabolite but will rely upon metabolic turnover of the endogenous pool to incorporate sufficient isotope label.
The results shown in Table 1, and those of others [9, 22], suggest that although bolus OTZ administration significantly increases cysteine concentration in brain tissue, a subsequent rapid elevation of glutathione levels is not observed. This is consistent with modeling studies in liver showing that feedback inhibition of glutamate cysteine ligase (the rate-limiting enzyme in glutathione biosynthesis) results in slower than expected increases in glutathione concentration when the cysteine concentration is raised [23]. After this initial increase in tissue cysteine seen 4 h after bolus dosing, cysteine levels are no longer significantly different from control levels at 6 or 20 h (Table 1). This suggests the brain acts to maintain low cysteine by shunting this amino acid down other pathways such as that leading to increased tissue hypotaurine.
Our data shows that the cysteine pool doubled to ~0.4 μmol/g-tissue 4 h following a bolus dose of unlabeled OTZ (Table 1). This increase in cysteine should also occur 4 h after a bolus dose of 13C-OTZ. However, using in vivo magnetic resonance spectroscopy, we could not detect any 13C-labeled cysteine approximately 4 h after dosing even though we can detect uptake of the 13C-OTZ (Fig. 2a). If we assume that any additional cysteine present in vivo originates entirely from the 13C-labeled OTZ, then the bolus dose should produce ~0.2 μmol/g-tissue of 13C-cysteine. Since a peak due to 13C-cysteine was not observed in our in vivo 13C magnetic resonance spectra (Fig. 2a), this concentration is likely to be below the minimum level for detection under our acquisition parameters. In fact, 13C-cysteine was not observed in the in vivo spectra presented in both Figs. 2 and 3. In addition, 13C-cysteine was not observed in the tissue extracts studied by high-resolution magnetic resonance spectroscopy where both sensitivity and resolution are improved over the in vivo methods due to the longer acquisition times possible.
The ability to detect 13C-labeling of metabolites such as cysteine and glutathione in a bolus dose protocol is dependent on the rate of metabolite production from 13C-OTZ exceeding the rate of clearance of both this precursor and metabolites. One study has determined that the turnover rate of brain glutathione is 0.06 μmol/g h [24]. Assuming our minimal detectable concentration of 13C-labeled metabolite to be ~0.2 μmol/g-tissue, it would take just over 3 h to generate sufficient labeled glutathione in the brain to allow in vivo detection by our methods. The data shown in Fig. 2a show that the 13C-OTZ is cleared from the brain relatively rapidly, and so may not be maintained at sufficiently high levels to allow appreciable amounts of label to be incorporated into glutathione. Only upon examination of the tissue extract was 13C label detected in glutathione (Fig. 2b). The mass spectrometry analysis of this extract indicates just over 8% of the glutathione is enriched with 13C label. Assuming glutathione is present at 1.0–1.2 μmol/g-tissue (Table 1), at this enrichment level, labeled 13C-glutathione would be present at 0.08–0.10 μmol/g-tissue. This level of 13C-glutathione would not be detectable by in vivo magnetic resonance spectroscopy under our experimental conditions. For this reason we altered our administration protocol to deliver 13C-OTZ over a 20 h period, to allow more time for the 13C-label to be incorporated into cysteine and glutathione.
There is little precedent on the appropriate dose for long-term infusion of OTZ, so the infusion protocol used was based on our previous studies in tumors that employed a chronic infusion of 13C-glycine for label incorporation into glutathione that was visible by in vivo magnetic resonance detection [12]. The in vivo data shown in Fig. 3 employed a total 13C-OTZ dose of 1,300 mg/kg delivered over 20 h, and show that infusion of 13C-OTZ at this level provides a sufficient 13C-cysteine concentration over that period for label uptake into glutathione and other metabolites. Surprisingly, this infusion did not significantly elevate glutathione or cysteine concentration above control levels, as shown in Table 1. Ex vivo analysis of brain (Fig. 3c) and liver (Fig. 3d) tissue extracts detected little or no [3-13C]-cysteine, but substantial labeling of downstream metabolites such as glutathione, lactate, taurine and hypotaurine. This suggests that the cysteine provided by 13C-OTZ does not accumulate. This is likely due to the presence of homeostatic mechanisms that can quickly shuttle cysteine through a number of metabolic pathways before toxic levels are reached. Cysteine dioxygenase has been proposed as a regulator of cysteine levels and, subsequently, glutathione content [25]. Activity of cysteine dioxygenase can lead to production of both taurine and hypotaurine via a cysteine sulfinate intermediate (Fig. 1) [26]. The significantly increased hypotaurine levels observed in our study after OTZ infusion suggests that flux through the cysteine dioxygenase pathway may have increased. Although flux through cysteine dioxygenase is well known in the liver, the enzymes are also present in the brain and may be available for regulating cysteine levels [27, 28]. Alternatively, brain hypotaurine and taurine may be produced from cysteine via cysteamine (Fig. 1), but activity through this pathway is thought to be lower than through cysteine dioxygenase [29]. There is also the possibility that the labeled hypotaurine and taurine can be synthesized in the liver and transported to the brain. Taurine biosynthesis does occur in liver at a faster rate than in the brain and circulating taurine is taken up by brain tissue. On the other hand, because synthesis of taurine does occur in the brain, local metabolism may be responsible for the majority of this labeled metabolite found in the brain [30].
Label from 13C-OTZ also was detected in [3-13C]-lactate in both extract and in vivo spectra. There are several possible pathways for metabolism of 13C-OTZ to [3-13C]-lactate through [3-13C]-cysteine, with [3-13C]-pyruvate as an intermediate (Fig. 1) [2]. Pyruvate production can occur via cysteine sulfinic acid or by other pathways that also release cysteine sulfur as hydrogen sulfide, a multifunctional signaling molecule [31]. Much of the hydrogen sulfide and pyruvate production in the brain was thought to occur through the action of cystathionine β-lyase, but recently it has been shown that reactions catalyzed by cysteine amino transferase and mercaptopyruvate sulfur-transferase, with mercaptopyruvate as an intermediate, may be a major route of cysteine metabolism [32]. The in vivo detection of lactate labeling therefore offers a monitor of flux through another cysteine catabolic pathway.
We must emphasize that the in vivo magnetic resonance data presented in Figs. 2 and 3 were obtained from 15 mm diameter rat head coil without any further localization. Therefore the data samples metabolites principally from brain, but also from skin, subcutaneous fat and muscle. The fat tissue contains little glutathione and rat skin and muscle levels are<0.8 μmol/g-tissue [33]. From 1H images of the rat head in the region of the surface coil, we conservatively estimate that >80% of the soft tissue sampled by this coil is from the brain, and good agreement is observed between the in vivo spectrum and the brain extract spectrum shown in Fig. 3a and c. Localization to the brain is possible using methods such as 13C chemical shift imaging (CSI) as we have demonstrated in tumor studies [12]. However, methods such as CSI result in lower signal levels as less tissue is sampled. Using the current hardware and infusion protocols, we were unable to obtain good CSI data from the brain. Although it is a goal to investigate heterogeneity in glutathione metabolism across the brain, currently we are limited to global assessment of the uptake of agents such as 13C-OTZ and monitoring their downstream metabolism.
The 13C-labeled metabolites downstream of 13C-OTZ detected in this in vivo study match those identified in extracts from in vitro studies of primary rat astrocytes incubated with medium supplemented with [3-13C]-cysteine [34]. However, the in vitro studies detected far more 13C-label incorporation into hypotaurine and taurine than into glutathione. This may be due to the high concentration (0.8 mM) of [3-13C]-cysteine used in those studies spurring an increase in activity of cysteine dioxygenase and increased shunting of cysteine into the hypotaurine/taurine pathways.
The lack of cysteine accumulation and the absence of glutathione concentration elevation have potential consequences for methods that aim to boost tissue glutathione content via cysteine prodrug administration. Our studies and other work in the field show either modest or no increase in brain glutathione content upon OTZ administration. However, although glutathione concentration may remain unchanged, the incorporation of a 13C label into glutathione demonstrates that metabolism of 13C-OTZ into cysteine, glutathione, lactate, taurine and hypotaurine can be followed in intact brain. Although not done in this study, quantitation of isotope labeled components in intact brain could be accomplished by the use of an external standard positioned near the surface coil and calibration with 13C-labeled compounds of known concentration in phantoms. In this study, we have assumed a majority of the observed metabolites are from the brain (see above), but quantitation can only be accomplished in combination with localization methods such as CSI where the location and the volume of sampled tissue can be measured. In these initial studies, we have assessed the ability of magnetic resonance to track uptake and transformation of these prodrugs and whether sufficient amounts are incorporated into glutathione for detection. By using isotope labeling, these types of experiments can track both the rate of metabolic transformation and the identity of metabolic pathways present in intact tissue. Although steady-state levels of antioxidants may not change in response to oxidative stress, flux rates through these pathways may change. Changes in metabolic rates may precede alterations in glutathione steady-state levels that are often observed in psychiatric disorders [13] and at early stages of Parkinson’s disease [14]. This work paves the way for investigations of the extent to which OTZ and other small molecule antioxidants can be used to probe metabolic pathways in intact tissue and how these agents can strengthen tissue defenses against oxidative stress.
Acknowledgments
This research was supported by NIH grants R21 AG029994 (M.P.G.) and R01 CA16783 (S.M.L.) and the Medical Research Council, UK (Grant ID 87867, P.E.T.).
Contributor Information
Michael P. Gamcsik, Email: mgamcsi@ncsu.edu, UNC/NCSU Joint Department of Biomedical Engineering, Campus Box 7115, Raleigh, NC 27695, USA
M. Daniel Clark, Department of Neuroscience, McKnight Brain Institute, University of Florida, Gainesville, FL 32610, USA.
Susan M. Ludeman, Departments of Arts and Sciences and Pharmaceutical Sciences, Albany College of Pharmacy and Health Sciences, Albany, NY 12208, USA
James B. Springer, Department of Medicine, Duke University Medical Center, Durham, NC 27710, USA
Michael A. D’Alessandro, Departments of Arts and Sciences and Pharmaceutical Sciences, Albany College of Pharmacy and Health Sciences, Albany, NY 12208, USA
Nicholas E. Simpson, Department of Medicine, University of Florida, Gainesville, FL 32610, USA
Roxana Pourdeyhimi, UNC/NCSU Joint Department of Biomedical Engineering, Campus Box 7115, Raleigh, NC 27695, USA.
C. Bryce Johnson, UNC/NCSU Joint Department of Biomedical Engineering, Campus Box 7115, Raleigh, NC 27695, USA.
Stephanie D. Teeter, UNC/NCSU Joint Department of Biomedical Engineering, Campus Box 7115, Raleigh, NC 27695, USA
Stephen J. Blackband, Department of Neuroscience, McKnight Brain Institute, University of Florida, Gainesville, FL 32610, USA
Peter E. Thelwall, Newcastle Magnetic Resonance Centre, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne NE4 5PL, UK
References
- 1.Schulz JB, Lindenau J, Seyfried J, et al. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 2.Osman LP, Mitchell SC, Waring RH. Cysteine, its metabolism and toxicity. Sulfur Rep. 1997;20:155–172. [Google Scholar]
- 3.Meister A. A brief history of glutathione and a survey of its metabolism and functions. In: Dolphin D, Poulson R, Avramovic O, editors. Glutathione. Chemical biochemical and medical aspects, part A. Wiley; New York: 1989. pp. 1–48. [Google Scholar]
- 4.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–289. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson JM, Meister A. Stimulation of hepatic glutathione formation by administration of L-2-oxothiazolidine-4-carboxylate, a 5-oxo-L-prolinase Substrate. Proc Natl Acad Sci USA. 1981;78:936–939. doi: 10.1073/pnas.78.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arakawa M, Ito Y. N-acetylcysteine and neurodegenerative diseases: basic and clinical pharmacology. Cerebellum. 2007;6:308–314. doi: 10.1080/14734220601142878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arfsten DP, Johnson EW, Wilfong ER, et al. Distribution of radio-labeled N-acetyl-L-cysteine in Sprague-Dawley rats and its effect on glutathione metabolism following single and repeat dosing by oral gavage. Cutan Ocul Toxicol. 2007;26:113–134. doi: 10.1080/15569520701212233. [DOI] [PubMed] [Google Scholar]
- 8.Cudkowicz ME, Sexton ME, Ellis T, et al. The pharmacokinetics and pharmaco-dynamics of Procysteine in amyotrophic lateral sclerosis. Neurology. 1999;52:1492–1494. doi: 10.1212/wnl.52.7.1492. [DOI] [PubMed] [Google Scholar]
- 9.Anderson ME, Meister A. Marked increase of cysteine levels in many regions of the brain after administration of 2-oxothiazolidine-4-carboxylate. FASEB J. 1989;3:1632–1636. doi: 10.1096/fasebj.3.5.2920877. [DOI] [PubMed] [Google Scholar]
- 10.Mesina JE, Page RH, Hetzel FW, et al. Administration of L-2-oxothiazolidine-4-carboxylate increases glutathione levels in rat brain. Brain Res. 1989;478:181–183. doi: 10.1016/0006-8993(89)91494-7. [DOI] [PubMed] [Google Scholar]
- 11.Park SW, Kim SH, Park KH, et al. Preventive effects of antioxidants in MPTP-induced mouse model of Parkinson’s disease. Neurosci Lett. 2004;363:243–246. doi: 10.1016/j.neulet.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 12.Thelwall PE, Yemin AY, Gillian TL, et al. Noninvasive in vivo detection of glutathione metabolism in tumors. Cancer Res. 2005;65:10149–10153. doi: 10.1158/0008-5472.CAN-05-1781. [DOI] [PubMed] [Google Scholar]
- 13.Berk M, Ng F, Dean O, et al. Glutathione; a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29:346–351. doi: 10.1016/j.tips.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room? Biomed Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Amoyaw PNA, Springer JB, Gamcsik MP, et al. Synthesis of 13C-labelled derivatives of cysteine for magnetic resonance imaging studies of drug uptake and conversion to glutathione in rat brain. J Labelled Compds Radiopharm. 2010 (in press) [Google Scholar]
- 16.Millis KK, Lesko SA, Gamcsik MP. Formation, intracellular distribution and efflux of glutathione-bimane conjugates in drug-sensitive and -resistant MCF-7 cells. Cancer Chemother Pharmacol. 1997;40:101–111. doi: 10.1007/s002800050633. [DOI] [PubMed] [Google Scholar]
- 17.Boogers I, Plugge W, Stokkermans YQ, et al. Ultra-performance liquid chromatographic analysis of amino acid in protein hydrolysates using an automated pre-column derivatisation method. J Chromatogr A. 2008;1189:406–409. doi: 10.1016/j.chroma.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SA, DeAntonis K, Michaud DP. Compositional protein analysis using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, a novel derivatization reagent. In: Angeletti RH, editor. Techniques in protein chemistry IV. Academic Press, Inc; San Diego: 1993. pp. 289–298. [Google Scholar]
- 19.Manura JJ, Manura DJ. Isotope distribution calculator and mass spec plotter. 2009 www.sisweb.com/mstools/isotope.htm.
- 20.Gerard-Monnier D, Fougeat S, Gourvest JF, et al. Partial prevention of glutathione depletion in rats following acute intoxication with diethylmaleate. Clin Physiol Biochem. 1993;10:36–42. [PubMed] [Google Scholar]
- 21.Shivakumar BR, Ravindranath V. Selective modulation of glutathione in mouse brain regions and its effect on acrylamide-induced neurotoxicity. Biochem Pharmacol. 1992;43:263–269. doi: 10.1016/0006-2952(92)90287-s. [DOI] [PubMed] [Google Scholar]
- 22.Pileblad E, Magnusson T. Increase in rat brain glutathione following intracerebroventricular administration of γ-glutamyl-cysteine. Biochem Pharmacol. 1992;44:895–903. doi: 10.1016/0006-2952(92)90121-x. [DOI] [PubMed] [Google Scholar]
- 23.Reed MC, Thomas RL, Pavisic J, et al. A mathematical model of glutathione metabolism. Theor Biol Med Modeling. 2008;5:8. doi: 10.1186/1742-4682-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi I-Y, Gruetter R. Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1–13C] glucose in the rat brain in vivo. J Neurochem. 2004;91:778–787. doi: 10.1111/j.1471-4159.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominy JE, Jr, Hwang J, Stipanuk MH. Overexpression of cysteine dioxygenase reduces intracellular cysteine and glutathione pools in HepG2/C3A cells. Am J Physiol Endocrinol Metab. 2007;293:E62–E69. doi: 10.1152/ajpendo.00053.2007. [DOI] [PubMed] [Google Scholar]
- 26.Stipanuk MH, Dominy JE, Jr, Lee JI, et al. Mammalian cysteine metabolism: new insights into regulation and cysteine metabolism. J Nutr. 2006;136:1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 27.Beetsch JW, Olson JE. Taurine synthesis and cysteine metabolism in cultured rat astrocytes: effects of hyperosmotic exposure. Am J Physiol (Cell Physiol) 1998;274:C866–C874. doi: 10.1152/ajpcell.1998.274.4.C866. [DOI] [PubMed] [Google Scholar]
- 28.Stipanuk MH, Londono M, Lee JI, et al. Enzymes and metabolites and cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002;132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- 29.Dominy J, Eller S, Dawson R., Jr Building biosynthetic schools: Reviewing compartmentation of CNS taurine synthesis. Neurochem Res. 2004;29:97–103. doi: 10.1023/b:nere.0000010437.81860.d5. [DOI] [PubMed] [Google Scholar]
- 30.Pasantes-Morales H, Chatagner F, Mandel P. Synthesis of taurine in rat liver and brain in vivo. Neurochem Res. 1980;5:441–451. doi: 10.1007/BF00964232. [DOI] [PubMed] [Google Scholar]
- 31.Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibuya N, Tanaka M, Yoshida M, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 33.Potter DW, Tran TB. Apparent rates of glutathione turnover in rat tissues. Toxicol Appl Pharmacol. 1993;120:186–192. doi: 10.1006/taap.1993.1102. [DOI] [PubMed] [Google Scholar]
- 34.Brand A, Leibfritz D, Hamprecht B, et al. Metabolism of cysteine in astroglial cells: synthesis of hypotaurine and taurine. J Neurochem. 1998;71:827–832. doi: 10.1046/j.1471-4159.1998.71020827.x. [DOI] [PubMed] [Google Scholar]