Abstract
Blood vessels deliver oxygen and nutrients to tissues and vascular networks are spatially organized to meet metabolic needs for maintaining homeostasis. In contrast, the vasculature of tumors is immature and leaky, resulting in insufficient delivery of nutrients and oxygen. Vasculogenic processes occur normally in adult tissues to repair “injured” blood vessels, leading us to hypothesize that bone marrow mononuclear cells (BMMNC) may be able to restore appropriate vessel function in tumor vasculature. Culturing BMMNC with endothelial growth medium resulted in the early outgrowth of spindle-shaped attached cells expressing CD11b/Flt1/Tie2/c-Kit/CXCR4 with pro-angiogenic activity. Intravenous administration of these cultured vascular proangiogenic cells (VPC) into nude mice bearing pancreatic cancer xenografts and Pdx1-Cre;LSL-KrasG12D;p53lox/+ genetically engineered mice that develop pancreatic ductal adenocarcinoma significantly reduced areas of hypoxia without enhancing tumor growth. The resulting vasculature structurally mimicked normal vessels with intensive pericyte coverage. Increases in the vascularized area within VPC-injected xenografts were visualized with the ultrasound diagnostic system during injection of a microbubble-based contrast agent (Sonazoid), indicating a functional “normalization” of the tumor vasculature. In addition, gene expression profiles on the VPC-transplanted xenografts revealed a marked reduction in major factors involved in drug resistance and “stemness” of cancer cells. Together, our findings identify a novel alternate approach to regulate abnormal tumor vessels, offering the potential to improve delivery and efficacy of anti-cancer drugs to hypoxic tumors.
Introduction
Blood vessels deliver oxygen and nutrients to all tissues, and the vascular network is spatially organized to meet the metabolic needs to maintain homeostasis (1). Regions of severe oxygen deprivation (hypoxia) arise in tumors due to rapid cell division and aberrant blood vessel formation (2). The vascular networks are usually disordered, chaotic, and highly leaky, resulting in an insufficient blood supply and, in general, they contribute to tumor hypoxia (3). These structural and functional abnormalities of tumor vessels are a hallmark of solid tumors, one that contributes directly to the malignant properties of cancer (2, 4). Hypoxic tumors are usually resistant to conventional chemotherapy and radiotherapies which typically target actively dividing cells (5), and accumulating evidence indicates that hypoxia has the potential to inhibit tumor cell differentiation and induce quiescence which allows cancer cells to acquire a phenotype of “stemness” (2, 4). In order to “normalize” this aberrant tumor vasculature, therapies targeting VEGF or its cognate receptor have demonstrated clinical success in various human cancers (6-8). However, anti-angiogenic therapy is not always effective and intrinsic resistance to this novel therapy has been demonstrated in some desmoplastic and hypovascular tumors including pancreatic ductal adenocarcinoma (PDAC) (9). In addition, anti-angiogenic therapy may alter the natural history of tumors by inducing an invasive and metastatic phenotype (10).
In view of the vasculogenic process that normally occurs in adult tissues under certain conditions to repair “injured” or newly formed blood vessels, bone marrow (BM) derived cells have therapeutic potential to restore appropriate vessel function. Angiogenesis has been shown to play a central role in the recovery of the injured tissues including myocardial infarction. We and others have identified BM-derived pro-angiogenic cells that accumulate in active angiogenic foci and participate in neovascularization after ischemic insults, a concept consistent with postnatal vasculogenesis (11-13). These immature BM-derived cells, which include stem/progenitor cells, can enhance angiogenesis in ischemic heart in mice and protect injured tissues from fibrosis, an unfavorable form of tissue remodeling (11). Therefore, we were curious to determine whether BMMNCs can also ‘repair’ chaotic tumor vessels and tested this hypothesis using PDAC as a model for a hypoxic tumor. We also speculated that oxygen tension may be restored if the disordered vasculature in solid tumors could be manipulated, which potentially represents a compelling therapeutic intervention against hypoxic tumors.
In the current study, we propose an alternative approach to reorganize the abnormal tumor vasculature, which can potentially improve delivery and efficacy of anti-cancer drugs against hypoxic tumors.
Materials and Methods
Cell culture
Three human pancreatic adenocarcinoma cell lines, KP-1N (from Health Science Research Resources Bank, Osaka, Japan), Panc-1, BxPC-3 (both from ATCC), and 4 extrapancreatic cancer cell lines MKN-28 (from Health Science Research Resources Bank), SW480, HepG2, and PC-9 (from ATCC) were used in this study.
The hypoxic workstation INVIVO2 400 (Ruskinn) was utilized to mimic hypoxic conditions in the tumor microenvironment. Cells were cultured at 5% O2, 5% CO2 for 1 month to adapt to hypoxic conditions and cell viability was assessed by WST-8 assay in normoxic (20 % O2) and hypoxic (5 % O2) conditions (Quick Cell Proliferation Assay Kit, Biovision) (14). Briefly, cancer cells were plated in 96-well plates (1-5 ×103 cells/well) and grown up to 72 hours, and the number of cells was quantified using a microtiter plate reader at 450 nm according to the manufacturer's instructions.
Animals, cell transplantation and immunohistochemistry
Protocols for animal experiments were approved by the Asahikawa Medical College Institutional Animal Care and Use Committee. Cancer cells were injected subcutaneously into female CD-1 nude mice and the xenograft volume was calculated as (length × width2) × 0.5. Tumors were grown to a minimum volume of 125 mm3 before VPC transplantation. Therapeutic studies were also performed utilizing genetically engineered mice that spontaneously develop PDAC with abundant desmoplasia, Pdx1-Cre;LSL-KrasG12D;p53lox/+ mice (15), at 12-weeks-old when PDACs lesions were identified by ultrasound diagnostic system (Aplio XG, Toshiba Medical Systems).
Murine BMMNCs were isolated by density gradient centrifugation using Histopaque-1083 (Sigma) and cultured in EBM2 with supplements (EGM2-MV BulletKit, Lonza) and 10% FBS but without hydrocortisone on rat vitronectin (Sigma)-coated dishes for preparation of vascular proangiogenic cells (VPCs) (13, 16). Attached cells were suspended at 4 days and reseeded to a new culture dish for VPC transplantation into tumor bearing mice at day 7. More than 95 % of attached cells were positive for acLDL (Biomedical Technologies) uptake and BS1 lectin (Vector laboratories) binding, confirming endothelial and/or monocytic linage (13, 17). The majority of the cells express CD11b as demonstrated by flow cytometry, indicating that these cells were composed of heterogeneous populations including vascular leukocytes (18, 19). In addition to the expression of Tie2, a significant fraction of the attached BMMNCs expressed Flt-1, CXCR4 and PDGFR-β. Weak expression of the progenitor markers such as c-Kit and CD34 was also identified (antibody was purchased from Beckman Coulter and BD) (Figure S1). Although genes expressed in endothelial cells such as Flk-1 and VE-cadherin were upregulated in the BMMNC during 7 day cultures as compared to freshly isolated CD11b+ BMMNC, there was a substantial induction of PDGFR-β mRNA.
The tumor bearing mice were divided randomly into saline infused or VPC treated groups when PDAC tumors were established. The pro-angiogenic cells were prepared from BMMNCs isolated from syngenic mice and the transplantation was performed via retro-orbital cavity. Initially, 104, 105, 106 VPC were injected into nude mice bearing KP-1N xenograft (n=10 for each group) and growth of the tumors was observed for up to 6 weeks. Since we observed effects of VPC transplantation histologically when 105 – 106 cells were injected, additional studies were then performed by transplanting larger numbers of VPCs. Tumor bearing mice also received VPC or a saline injection weekly for 2-3 weeks in a 4-7 day interval before sacrifice. To clarify whether transplanted VPCs were indeed localized to tumors, we injected GFP-labeled VPCs (5 ×105 cells/mice) into mice with PDAC xenografts in some of the experiments (13).
To assess hypoxic regions, pimonidazole hydrochloride (60 mg/kg; Hypoxyprobe-1, Millipore) was injected intraperitoneally 1.5 h before killing. We harvested xenograft tumors before the lesions reached to 10-mm in diameter (the average volume was 200-300 mm3) for histological analysis. Tumor tissues were then fixed with zinc-fixative solution (IHC ZINC fixative, BD Pharmingen) for 24 hours at room temperature and embedded in paraffin for immunohistochemistry. For immunofluorescence staining in xenograft tissue, 4-μm sections were incubated with a CD31-specific antibody, MEC13.3 (1:50; BD Pharmingen), overnight at 4°C. Blood vessels were counted in 5-10 random viable fields (20x objective), and the vessel area/density was quantified using ImageJ software (ver 1.38). For other immunohistochemical studies the following antibodies were utilized; MIB-1 antibody (DAKO; 1:100), anti-NG2 (Chemicon; 1:200), anti-cleaved caspase-3 (5A1E, Cell Signal; 1:200) and anti-CA9 (Novus; 1:100). Nuclei were counterstained with 50 ng/mL DAPI (Sigma), and images were examined with a fluorescent microscope (BX-51/DP-71, Olympus).
To visualize the extracellular matrix in xenograft tumors, tissue staining was performed utilizing Masson's trichrome kit according to the manufacturer's protocol (Sigma-Aldrich, HT15-1KT).
In vivo vascular imaging by contrast enhanced ultrasound system
To visualize perfusion within tumors, ultrasound contrast agent, Sonazoid (Daiichi-Sankyo Co Ltd, Tokyo, Japan), were administered to tumor bearing mice (0.25 μL/kg). The vascularized area within tumors was imaged by an ultrasound diagnostic system (Aplio™XG, Toshiba Medical Systems Corp., Otawara, Japan) with 12MHz linear probe (PLT-1204AT). The phase modulation harmonic imaging mode (transmitted/received at 5.0/10.0 MHz) was used for the nonlinear signal extraction, and the mechanical index (MI) was set to around 0.20. The images for 30 sec after injection were recorded into the US system and transferred to consumer PC after experiments.
The arrival time parametric images were re-constructed by using the dedicated prototype software programmed by C++ (20). The software reads the Audio Video Interleaving (AVI) image obtained by the US system, calculates the enhanced intensity on the image frame by frame, and paints the colors, which correspond to the time to arrival of the enhancement. Since each color has the numeric value of the arrival, the arrival time of each part will be indicated by the still image of the resulting parametric imaging.
Quantitative real-time PCR assay
Total RNA was extracted using the RNeasy Protect Mini kit (QIAGEN) according to the manufacturer's instructions. TaqMan Gene Expression Assay primer and probe sets (Applied Biosystems) were utilized for real-time, quantitative PCR analysis. Primer sequences are summarized in Table S1. Transcript levels were normalized to the 18S rRNA. Results are expressed as normalized expression values (=2−ΔCT) or normalized expression relative to control cells or tissues without cell transplantation (=2−ΔΔCT), unless otherwise stated.
Statistical analysis
All results are expressed as mean ± standard deviation unless otherwise noted. Statistical significance of differences was determined using a two-tailed Student's t-test.
Results and Discussion
Transplantation of cultured vascular proangiogenic cells (VPCs) into tumor bearing mice induces vascular remodeling and reduces tumor hypoxia
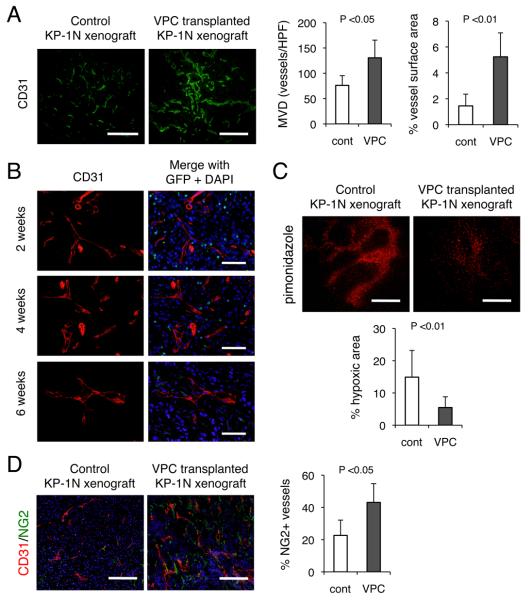
In order to determine whether cultured CD11b+ VPCs possess a capacity to ‘repair’ tumor vessels, we performed a series of investigations utilizing spindle-shaped attached BM mononuclear cells cultured in EGM2 medium as a crude pro-angiogenic cell (Figure S1). Pancreatic ductal adenocarcinoma (PDAC) was selected as a model for a hypoxic tumor to test our hypothesis that certain malignant phenotypes associated with hypoxia may be abolished if abnormalities of the tumor vasculature could be appropriately manipulated. Intravenous administration of 5 ×105 VPCs into nude mice bearing KP-1N human pancreatic cancer xenografts significantly increased tumor microvessel density (Figure 1A). The transplanted cells localized to the peri-vascular area, closely associating with the tumor vasculature rather than directly differentiating into vascular endothelial cells (Figure 1B). In addition, the number of GFP+ transplanted cells was significantly attenuated at 6 weeks after transplantation, suggesting that the ex vivo cultured VPC may not constitute blood vessels for long term. In contrast to narrowed and fragmented vasculature in control xenografts, the vessel surface area in VPC-transplanted tumors was dramatically increased (1.5 ± 0.9 % vs. 5.3 ± 1.8 %; p <0.01). The enlarged tumor vasculature appeared to be functional because it was accompanied by reduced areas of tumor hypoxia as represented by pimonidazole-positive areas within the tumor (Figure 1C). In addition, an increase in the vascularized (perfused) area within VPC-injected xenografts was observed by arrival time parametric imaging reconstructed from images by ultrasound diagnostic system with 12MHz linear probe during injection of contrast agents (Sonazoid) when serial intravenous injections of 105 VPCs three time at 4 day intervals were performed (Figure S2, Supplementary Material 1, 2), indicating a functional “reorganization/remodeling” of the abnormal tumor vasculature.
Figure 1. Intravenous transplantation of ex vivo cultured CD11b+ vascular proangiogenic cells (VPCs) induces vascular remodeling and reduces tumor hypoxia in KP-1N xenograft.
(A) VPC transplantation (5 ×105 cells) was performed in CD-1 nude mice bearing KP-1N xenografts, and mice were sacrificed 2 weeks after transplantation. The tumor sections were stained with CD31. The data are shown as mean ± SEM microvessel density (MVD) and vessel surface area. (B) In order to trace transplanted cells, VPCs prepared using GFP-labeled BMMNC were intravenously injected to mice with KP-1N xenografts. Mice were sacrificed 2-6 week after and the tumor sections were stained with anti-CD31 and anti-GFP. Scale bars; 100 μm. (C) To assess hypoxic regions, frozen sections were stained with anti-Hypoxyprobe-1 antibody. Positive staining area was shown as hypoxic area in xenografts. (D) Double immunofluorescent staining with NG2 with CD31. The number of CD31+ microvessels covered with NG2 positive pericytes is shown. Scale bars; 500 μm.
We next confirmed the tumor vessels in the VPC-transplanted tumor were structurally mature. Since pericytes play an essential role in the integrity of structural vessels, immunohistochemical staining for NG2 and CD31 was then performed (Figure 1D). Increased numbers of CD31+ microvessels were covered with NG2+ pericytes, indicating that the resulting tumor vasculature structurally mimicked normal vessels with a high pericyte coverage ratio.
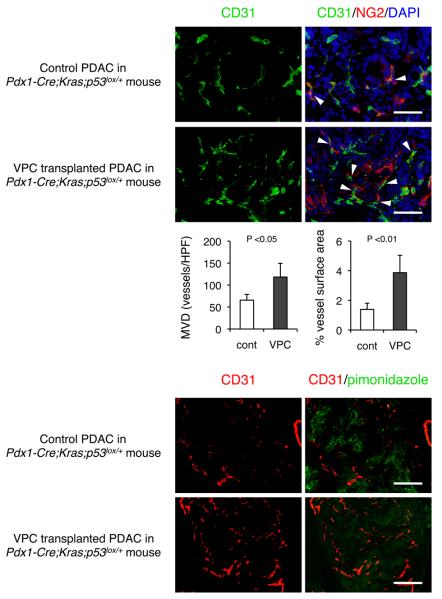
Similar observations were demonstrated in genetically engineered mice Pdx1-Cre;LSL-KrasG12D;p53lox/+ which spontaneously develop desmoplastic PDAC (15), and undifferentiated tissues with abundant desmoplasia were selected for histological analysis. Poor tissue perfusion was also successfully corrected by VPC transplantation in this mouse model (Figure 2). Therefore, the vascular regeneration/remodeling through the cell-mediated approach is not limited to artificial xenograft tumors but is also capable of manipulating abnormal blood perfusion in spontaneously developing desmoplastic PDAC tumors in mice.
Figure 2. Transplantation of vascular proangiogenic cells (VPCs) promote maturation of the tumor vasculature resulted unreduced hypoxic area in spontaneously developed PDAC.
Sequential VPC transplantation (5 ×105; twice) was performed in Pdx1-Cre;LSL-KrasG12D;p53lox/+ mice (12 weeks old), and mice were sacrificed 2 weeks after transplantation. (A) Double immunofluorescent staining with NG2 with CD31 in Zn-fixed sections. Arrowheads indicate NG2+ pericyte-covered microvessels. (B) Double immunofluorescent staining with anti-Hypoxyprobe-1 with anti-CD31 in frozen sections. Scale bars; 100 μm.
Transplantation of cultured CD11b+ VPC does not enhance tumor growth but instead temporarily delays the outgrowth
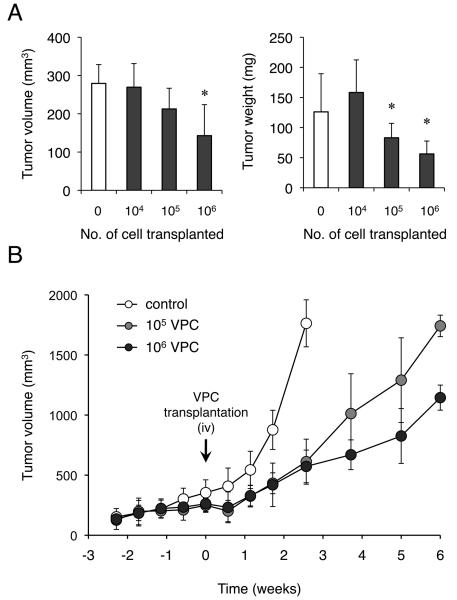
Since tumor outgrowth is generally dependent upon angiogenesis, we initially speculated that enhanced blood perfusion may promote tumor growth and metastasis. However, to our surprise, growth of PDAC xenografts was significantly inhibited when more than 105 VPCs were transplanted (Figure 3A). When tumor growth was observed for 6 weeks post-transplantation, growth of xenograft tumors was temporarily slowed by VPC transplantation although they started to re-grow within 3 weeks (Figure 3B). In order to determine whether this reduction of tumor growth can also be observed in other cell types, additional xenograft experiments were then performed using various human cancer cell lines (Table S2). Serial intravenous injections of 5 ×105 VPCs were performed subsequent to xenograft establishment in 7 day intervals. A significant reduction in tumor growth was observed in other human pancreatic cancer cells, Panc-1 and BxPC-3. Additionally, growth inhibition of xenograft tumors were also demonstrated in MKN-24 (human gastric cancer cell) and PC-9 (human lung cancer cell). Of note, enhancement of tumor growth was not induced by VPC transplantation in cells tested and metastatic outgrowth was not demonstrated even in SW480 and hypervascular HepG2 xenografts.
Figure 3. VPC transplantation dose not stimulate tumor outgrow in KP-1N xenografts.
104, 105, 106 VPC were injected into nude mice bearing KP-1N xenograft (n=10 for each group). (A) Tumor volume (left panel) and weight (right panel) of KP-1N xenografts with or without VPC transplantation (* P < 0.05, versus control mice; PBS injected). (B) Growth of KP-1N xenografts with or without VPC transplantation was observed for up to 6 weeks (PBS, 105 or 106 VPC injected; n=6 for each). Mice were humanely killed following development of a tumor larger than 2,000 mm3 or a tumor harboring an ulcer.
Oxygen and nutrients are required for any tissue including tumors, but cancer cells can survive even in severe hypoxia (21). It is well known that most cancer cells rely on aerobic glycolysis rather than mitochondrial oxidative phosphorylation to generate energy needed for cellular processes (22). However, it should be noted that clinical trials have shown that reducing tissue hypoxia either by blood transfusion or erythropoietin could be associated with an improved response to radiotherapy and may improve the survival of cancer patients (23). In addition, a recent study demonstrated that Ang-1-mediated maturation of blood vessel can inhibit tumor growth through a suppression of permeability in tumors with pericyte-rich blood vessels (24). We therefore sought to address the question as to whether the tumor microenvironment with abundant oxygen delivered by reorganized blood vessels is favorable for tumor cells or not.
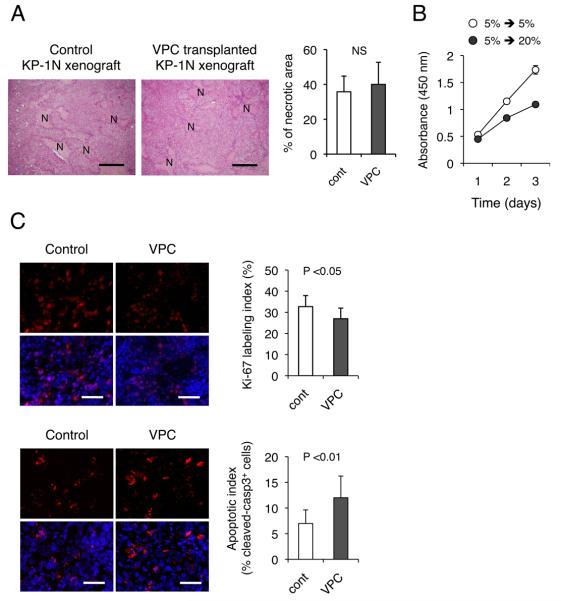
Histopathological analysis revealed that transplantation of the cultured CD11b+ VPCs did not significantly increase areas of necrosis (Figure 4A). Masson's trichrome staining demonstrated that xenograft tumors in the VPC-transplanted mice were depleted of desmoplastic stroma, resulting in densely packed ductal adenocarcinoma cells (Figure S3), and the number of cells per area was increased by VPC transplantation, suggesting that VPCs increase the density of cells. This could thereby facilitate the blood perfusion in PDAC indirectly. Similar observations have been demonstrated by VPC transplantation into mouse models of acute coronary ischemia (25, 26); i.e., tissue remodeling composed of extensive fibrosis in infarct heart and cirrhotic liver could be attenuated by transplantation of BMMNCs manipulated by a similar ex vivo differentiation protocol (27).
Figure 4. Transplantation of ex vivo cultured VPC impairs growth of KP-1N xenografts.
(A) KP-1N xenografts were treated with or without intravenous administration of cultured VPCs (5 ×105 cells; twice) and mice were sacrificed 2 weeks after the procedure. H-E stainings for xenograft sections are shown and % necrotic area (N) was measured. Scale bars; 200 μm. (B) KP-1N cells adapted to chronic hypoxia (5 % O2 in hypoxia workstation INVIVO400) were culture wither in normoxic (20 % O2) or hypoxic conditions (5 % O2) for 3 days. Cell growth was quantified by WST-8 assay. (C) Xenograft tissues were stained with anti-Ki-67 (upper panel) and anti-cleaved caspase-3 (lower panel). Quantification of proliferating/apoptotic is shown as percent positive cells in 5 viable fields from 10 sections. Scale bars; 100 μm. Proliferation/Apoptotic index are shown (mean ± SEM).
To directly address the impact of oxygen during reperfusion (reoxygenation) in hypoxic tumors, we performed an in vitro assay utilizing PDAC cells adapted to long term hypoxic conditions under low oxygen tension (5 % O2) by utilizing a hypoxia workstation. Although hypoxia generally downregulates cell proliferation (28), the KP-1N human PDAC line adapted to 5 % O2 (chronic hypoxia) can grow equally with a comparable doubling time as cells cultured in normoxic conditions (20 % O2) (doubling time in chronic hypoxia and normoxic conditions were 21.0 ± 2.8 h and 22.8 ± 3.1 h, respectively). However, proliferation of the hypoxia-conditioned KP-1N cells was significantly attenuated when cells were placed again into normoxic conditions (doubling time; 31.7 ± 4.8 h, P < 0.01) (Figure 4B). A similar observation was also demonstrated in primary mouse PDAC cells from Pdx1-Cre;LSL-KrasG12D;p53lox/+ mice (doubling in chronic hypoxia was 13.6 ± 2.5 h and whereas 16.7 ± 2.9 h in reoxygenated cells; P< 0.05). These results may account for the attenuated tumor growth when the tumor vasculature was reorganized/remodeled by the ex vivo cultured CD11b+ VPC transplantation that liberated PDAC cells from hypoxic conditions.
We therefore examined impact of serial VPC transplantation (5 ×105 cells; 7 day intervals) on proliferation kinetics of xenograft tumors in vivo. There was a modest but statistically significant difference in the Ki-67 labeling index (Figure 4C), consistent with the growth retardation by VPC transplantation. Since staining the xenograft tissues for Ki-67 demonstrated a reduced proliferation of cancer cells by only 17.6 %, additional immunostaining using anti-cleaved caspase-3 was then performed. The apoptotic fraction was markedly increased by 1.72-fold in xenograft tumors when cultured VPCs were serially transplanted (Figure 4C). These results indicate that enhanced blood perfusion may impair the tumor cells' ability to rapidly grow. The remodeling of abnormal tumor vasculature induces reperfusion of hypoxic tissue and reduced areas of significant hypoxia (Figure 1, 2). This potentially leads to an increase in free-radical concentrations, resulting in growth suppression through an induction of stress-response genes (21, 29).
Transplantation of cultured CD11b+ VPCs attenuates angiogenic cytokine production from cancer cells during reperfusion in PDAC xenografts
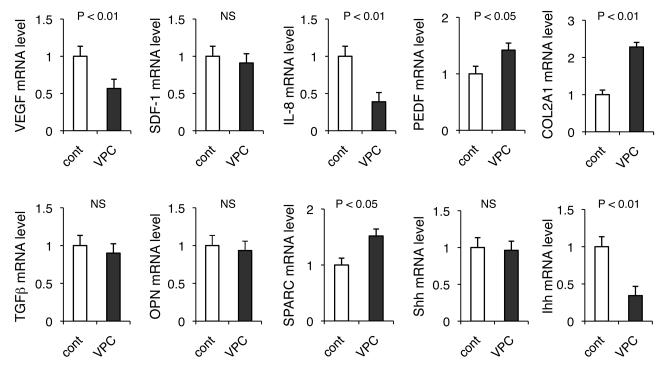
The VPCs transplantation induced “repair” of the abnormal tumor vasculature and reduced tumor desmoplasia, which could further facilitate blood perfusion. This histological remodeling may also attenuate oxygen consumption by the microenvironment. We therefore speculated that transplantation of the cultured CD11b+ VPCs may alter the imbalance between pro- and anti-angiogenic cytokines released from tumor cells. In general, cancer cells express excess amounts of various pro-angiogenic factors, which primarily regulate the abnormal tumor vasculature. In order to test this possibility, we quantified mRNA levels of pro-angiogenic factors from cancer cells in xenografts by utilizing human-specific probes. Consistent with the marked reduction in tumor hypoxia, the VEGF mRNA levels were significantly attenuated (Figure 5). IL-8, another pro-angiogenic cytokine that can be induced by hypoxia in cells with oncogenic Kras (30), was also strongly downregulated. Pigment epithelium-derived factor (PEDF) is a potent angiogenic inhibitor in the pancreas, expressed by both epithelial and stromal compartments, and regulated by hypoxia. The expression has been shown to be downregulated during pancreatic tumorigenesis, at least in part playing a role in neovascularization and metastatic outgrow in PDAC (31, 32). Curiously, VPC transplantation restored the expression in cancer cells, which may account for not only the reduction in aberrant tumor angiogenesis but also the inhibition of proliferation of tumor cells.
Figure 5. Transplantation of VPCs terminates aberrant neovasularization by attenuating angiogenic cytokine production from cancer cells.
RNA was extracted from xenograft tissue treated with or without VPC transplantaion and mRNA levels of pro- and anti-angiogenic factors in cancer cells were analyzed by TaqMan qPCR. Alterations in gene expression associated with desmoplasia are also demonstrated.
Since Masson's trichrome staining demonstrated that the transplantation of cultured VPCs significantly reduced the amount of desmoplastic stroma, we speculated that genes involved in fibrosis may also be altered. There was no significant difference in mRNA levels of cancer cell derived TGF-β and osteopontin (OPN) (27, 33), known fibrosis mediators, and the precise mechanisms by which VPC transplantation reduced PDAC desmoplasia needs to be further elucidated. However, Ihh hedgehog morphogen, but not Shh, which plays a role in pancreatic fibrosis through an activation of pancreatic stellate cells (34), was downregulated by regeneration of the tumor vasculature in PDAC xenografts. There was upregulation of desmoplasia related α2-(I)-pro-collagen (COL2A1) and secreted protein acidic and rich in cysteine (SPARC) mRNA genes in tumor cells when VPCs were transplanted; however, the levels were modest when compared to their stromal expression, where VPC transplantation had no influence Taken together, transplanted VPCs localized to hypoxic areas either in tumors or in infarcted (ischemic) heart may have the capacity to terminate abnormal tissue remodeling including aberrant angiogenesis.
Cancer cells released from hypoxia represent a phenotype with less resistance to chemotherapy and reduced ‘stemness’ phenotype
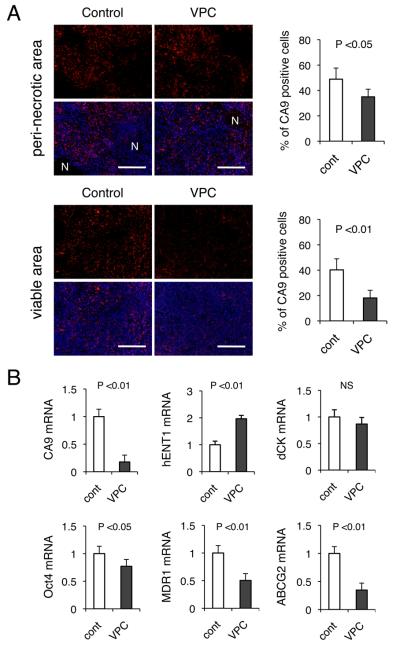
Expression of carbonic anhydrase 9 (CA9), one of the hypoxia inducible factor (HIF)-target genes, has been proposed as a marker of prolonged hypoxia (35). CA9 plays a role in maintaining an alkaline intracellular pH and an acidic extracellular pH(36, 37) and in anchorage-independent tumor cell growth, facilitating invasion of cancer cells into the extracellular matrix by modulating the functions of E-cadherin (38). We therefore examined CA9 expression by immunohistochemistry in VPC-transplanted tumors (Figure 6A). In line with a significant reduction in pimonidazole-positive areas in xenografts receiving VPC injections, the resulting vascular remodeling also attenuated the number of CA9-positive cells dramatically. The reduction was more prominent in viable areas as compared to peri-nectotic areas.
Figure 6. Reduction in tumor hypoxia reduces CA9 expression and gene expression related drug resistance and ‘stemness’.
KP-1N xenografts were treated with or without intravenous administration of cultured VPC (105 cells for 3 times) and mice were sacrificed 2 weeks after the procedure. (A) Xenograft tissues were stained with anti-CA9 either in peri-necrotic (upper panel) or in viable area (lower panel). Quantification of CA9 positive cells is shown in 5 viable fields from 6 sections. Scale bars; 500 μm. “N” indicates necrotic area. (B) RNA was extracted from xenograft tissue and mRNA levels of CA9, Oct-4, hENT1, dCK, MDR-1 (ABCB1) and ABCG2 in cancer cells in xenografts were analyzed by TaqMan qPCR.
Hypoxia and HIFs have been shown to activate signaling pathways that control stem cell self-renewal and multipotency (39). In addition to hematological malignancies, solid tumors can also develop from a small number of self-renewing transformed cells, the so-called tumor initiating cells (40). Accumulating evidence has indicated that these rare types of cancer cells with “stemness” properties can localize in a ‘hypoxic niche’, giving rise to a resistance to chemotherapy and radiotherapy (4). This implies that regional hypoxia plays a fundamental role in both regulating “stemness” properties of cancer cells and diminishing therapeutic response, and both of the hypoxia-induced phenotypes could be reversible. We therefore sought to determine whether vascular remodeling induced by VPC transplantation may alter such “stemness” and resistant phenotypes of hypoxic PDAC cells. Xenograft tumors were utilized to specifically analyze gene expression in cancer cells rather than stromal cells, utilizing human-specific probes for TaqMan qPCR assays (Figure 6B). There was a significant reduction in CA9 mRNA levels in VPC-transplantated xenografts, consistent with a massive reduction in CA9 immunostaining (Figure 6A). Octamer-binding transcription factor 4 (Oct-4), one of the “stemness” genes that can induce puripotency in differentiated cells (41), was downregulated by 22.9 %. Since HIF-2α can directly regulate Oct-4, it is therefore possible that hypoxia mediates its effects on stem cell function by altering the stemness genes (42). Considering that Oct-4 is involved in tumor progression and motility (43), the downregulation of Oct-4 would be beneficial to control the malignant phenotype caused by hypoxia. Additionally, the expression levels of MDR1 (ABCB1) and ABCG2, genes that play a major role in ‘resistance’ to chemotherapy, were also dramatically downregulated by VPC transplantation (Figure 6B).
Gemcitabine is the standard chemotherapeutic reagent for locally advanced or metastatic PDAC (44), and a recent study performed on cultured PDAC cells indicated that human equilibrative nucleoside transporter-1 (hENT1) is the major transporter for gemcitabine and increased hENT1 abundance facilitates efficient cellular entry of the drug and confers its increased cytotoxicity (14, 45). We found that there was 2-fold induction of hNET1 mRNA in tumor cells when xenograft bearing mice were treated with the cultured CD11b+ VPCs, consistent with the observation that hENT could be downregulated by hypoxia (46). Therefore, although we did not observe any differences in dCK mRNA, another important intracellular modulator of gemcitabine, PDACs with normalized blood vessels by VPC transplantation may be more sensitive to gemcitabine as compared to hypoxic tumors. Taken together, remodeling of an unstable tumor vasculature leads to a significant reduction in expression of genes associated with the “stemness” of cancer cells as well as an increase in sensitivity to conventional chemotherapy. We are currently testing this hypothesis by studying a combination of chemotherapeutic agents such as gemcitabine and VPC transplantation.
Conclusion
Bone marrow (BM) cells are thought to play a role in tumor development (47), and various types of BM-derived hematopoietic cells have been observed to closely associate with the tumor neovasculature (18, 48). Indeed, a small number of BM-derived progenitor cells were demonstrated to incorporate into the lumen of a growing vasculature where they differentiate into endothelial cells in a mouse metastasis model (49). The BM-derived cells generally have been thought to augment the malignant phenotype of tumor; however, our data support the notion that certain immature myeloid cells from the BM may have the capacity to repair an abnormal microenvironment if they are appropriately differentiated ex vivo. In support of our results, others have also demonstrated increases in blood flow within tumor xenografts when ES cell derived VPCs were transplanted, and no enhancement of tumor growth was observed (50). In the current study, we observed a considerable number of transplanted CD11b+VPCs localize to the peri-vascular area, and therefore they did not appear to induce angiogenesis (vasculogenesis) by directly differentiating into vascular ECs. Those transplanted cells have been demonstrated to promote neovascularization indirectly through paracrine stimulation/stabilization of neovessels (11). We found that, in addition to VEGF and Ang-1, the cultured VPCs also express significant levels of anti-angiogenic factors (manuscript in preparation), suggesting their potential role in terminating aberrant neovascularization. Further studies are required to address the precise mechanisms by which these ex vivo cultured BMMNCs influence the chaotic blood vessels and tumor microenvironment. Our study also implies that the potential risk of enhancing tumor growth may not be an issue during cell therapy utilizing progenitors, at least if cultured (manipulated) cells are used.
Collectively, we have identified an alternative approach to regulate the abnormal tumor vasculature. Tumor vessels remodeled by ex vivo cultured CD11b+ VPCs exhibited maturation of the ‘abnormal’ vasculature, resulting in a significant reduction in tumor hypoxia. The cancer cells appeared to have a distinct phenotype in the reperfused/reoxygenated microenvironment with decreased “stemness” related gene expressions. This approach may not only attenuate innate resistance to chemotherapy/radiotherapy but also potentially improve delivery of anti-cancer drugs to hypoxic tumors.
Supplementary Material
Acknowledgements
We thank Shizuo Kato for his assistance in tissue section preparation, Yasuhiko Nagasaka for technical assistance in flow cytometry analysis and Kotoe Shibusa for BMMNC collection and cell sorting. We also thank to Azusa Tsukada (Toshiba Medical Systems) for assistance in obtaining contrast-enhanced ultrasound images and Dr. Hideki Kato (Hamamatsu University School of Medicine) for genetic testing of mice and helpful discussions.
Grant Support: This work was supported by grant to Y.M. from New Energy and Industrial Technology Development Organization of Japan (07A05010a).
Abbreviations
- VPC
vascular proangiogenic cell
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 2.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–72. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–30. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 5.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–68. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–40. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 10.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ii M, Nishimura H, Iwakura A, et al. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–20. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki M, Nakamura K, Mizukami Y, et al. Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Sci. 2008;99:1131–8. doi: 10.1111/j.1349-7006.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, Sasajima J, Mizukami Y, et al. Hedgehog Promotes Neovascularization in Pancreatic Cancers by Regulating Ang-1 and IGF-1 Expression in Bone-Marrow Derived Pro-Angiogenic Cells. PLoS One. 2010;5:e8824. doi: 10.1371/journal.pone.0008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano Y, Tanno S, Koizumi K, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–63. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardeesy N, Aguirre AJ, Chu GC, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ii M, Takenaka H, Asai J, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 17.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 18.Conejo-Garcia JR, Buckanovich RJ, Benencia F, et al. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–81. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Kim JS, Papadopoulos J, et al. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–80. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimoto K, Moriyasu F, Kamiyama N, Metoki R, Iijima H. Parametric imaging of contrast ultrasound for the evaluation of neovascularization in liver tumors. Hepatol Res. 2007;37:464–72. doi: 10.1111/j.1872-034X.2007.00060.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Cairns R, Papandreou I, Koong A, Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the warburg effect. PLoS One. 2009;4:e7033. doi: 10.1371/journal.pone.0007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rades D, Tribius S, Yekebas EF, et al. Epoetin alfa improves survival after chemoradiation for stage III esophageal cancer: final results of a prospective observational study. Int J Radiat Oncol Biol Phys. 2006;65:459–65. doi: 10.1016/j.ijrobp.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Satoh N, Yamada Y, Kinugasa Y, Takakura N. Angiopoietin-1 alters tumor growth by stabilizing blood vessels or by promoting angiogenesis. Cancer Sci. 2008;99:2373–9. doi: 10.1111/j.1349-7006.2008.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 26.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–69. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T, Torimura T, Sakamoto M, et al. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology. 2007;133:91–107 e1. doi: 10.1053/j.gastro.2007.03.110. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 29.Yu G, Tseng GC, Yu YP, et al. CSR1 suppresses tumor growth and metastasis of prostate cancer. Am J Pathol. 2006;168:597–607. doi: 10.2353/ajpath.2006.050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–7. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 31.Doll JA, Stellmach VM, Bouck NP, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–80. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 32.Uehara H, Miyamoto M, Kato K, et al. Expression of pigment epithelium-derived factor decreases liver metastasis and correlates with favorable prognosis for patients with ductal pancreatic adenocarcinoma. Cancer Res. 2004;64:3533–7. doi: 10.1158/0008-5472.CAN-03-3725. [DOI] [PubMed] [Google Scholar]
- 33.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, et al. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009;119:1583–94. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinozaki S, Ohnishi H, Hama K, et al. Indian hedgehog promotes the migration of rat activated pancreatic stellate cells by increasing membrane type-1 matrix metalloproteinase on the plasma membrane. J Cell Physiol. 2008;216:38–46. doi: 10.1002/jcp.21372. [DOI] [PubMed] [Google Scholar]
- 35.Olive PL, Aquino-Parsons C, MacPhail SH, et al. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001;61:8924–9. [PubMed] [Google Scholar]
- 36.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–6. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 38.Svastova E, Zilka N, Zat'ovicova M, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res. 2003;290:332–45. doi: 10.1016/s0014-4827(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 39.Soeda A, Park M, Lee D, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 40.Saini V, Shoemaker RH. Potential for therapeutic targeting of tumor stem cells. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–9. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Covello KL, Kehler J, Yu H, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang CL, Tsai YC, He L, Wu TC, Hung CF. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer Res. 2007;67:10047–57. doi: 10.1158/0008-5472.CAN-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, Pastor-Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2′,2′-difluorodeoxycytidine- induced cytotoxicity. Clin Cancer Res. 2003;9:5000–8. [PubMed] [Google Scholar]
- 46.Lam W, Leung CH, Bussom S, Cheng YC. The impact of hypoxic treatment on the expression of phosphoglycerate kinase and the cytotoxicity of troxacitabine and gemcitabine. Mol Pharmacol. 2007;72:536–44. doi: 10.1124/mol.106.033472. [DOI] [PubMed] [Google Scholar]
- 47.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 50.Yurugi-Kobayashi T, Itoh H, Yamashita J, et al. Effective contribution of transplanted vascular progenitor cells derived from embryonic stem cells to adult neovascularization in proper differentiation stage. Blood. 2003;101:2675–8. doi: 10.1182/blood-2002-06-1877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.