Abstract
AIM: To evaluate the effect of chronic mild stress (CMS) on the emergence of gastric ulcers and possible modulation by octreotide, a synthetic somatostatin analogue.
METHODS: Adult male Wistar rats were subjected to nine different unpredictable random stress procedures for 21 d, a multifactorial interactional animal model for CMS. Octreotide was administered daily for 21 d at two dose levels (50 and 90 μg/kg) before exposure to stress procedure. Macro- and microscopical assessments were made, in addition to quantification of plasma corticosterone and gastric mucosal inflammatory, oxidative stress, and apoptotic biomarkers.
RESULTS: Exposure to CMS elevated plasma corticosterone (28.3 ± 0.6 μg/dL, P = 0.002), an event that was accompanied by gastric lesions (6.4 ± 0.16 mm, P = 0.01) and confirmed histopathologically. Moreover, the insult elevated gastric mucosal lipid peroxides (13 ± 0.5 nmol/g tissue, P = 0.001), tumor necrosis factor-α (3008.6 ± 78.18 pg/g tissue, P < 0.001), prostaglandin E2 (117.1 ± 4.31 pg/g tissue, P = 0.002), and caspase-3 activity (2.4 ± 0.14 OD/mg protein, P = 0.002). Conversely, CMS mitigated interleukin-10 (627.9 ± 12.82 pg/g tissue, P = 0.001). Furthermore, in animals exposed to CMS, octreotide restored plasma corticosterone (61% and 71% from CMS, P = 0.002) at both dose levels. These beneficial effects were associated with a remarkable suppression of gastric lesions (38% and 9% from CMS, P = 0.01) and reversal of derangements in gastric mucosa.
CONCLUSION: The current investigation provides evidence that exposure to CMS induces gastric ulceration, which was alleviated by administration of octreotide possibly possessing antioxidant, anti-inflammatory, and anti-apoptotic actions.
Keywords: Gastric ulcer, Chronic mild stress, Octreotide, Inflammation, Oxidative stress, Apoptosis, Histopathology
INTRODUCTION
Severe life stressors frequently antedate the onset of functional gastrointestinal disorders. The stomach, in particular, is extremely sensitive to various stress stimuli and peptic ulcer has often been described as a stress disease[1]. Stress per se, alters the mechanisms of neurohormonal regulation, which results in lesions earliest found in the stomach, as a consequence of a general adaptation syndrome[2]. Chronic mild stress (CMS) is a paradigm where animals are exposed to a combination of mild unpredictable stressors[3]. Moreover, in the CMS model, several reports implicate important roles of reactive oxygen species (ROS)[4-6], and enhanced production of inflammatory mediators, which are also prime events in acute stress models[7,8]. Markedly, stress induces gastric ulceration via different factors, viz. stimulation of brain gut axis, reduction of mucosal blood flow, and leukocyte infiltration[7,9,10]. The latter contributes to free radical and pro-inflammatory cytokines formation, which further recruit more inflammatory cells, thus augmenting ROS production and maximizing mucosal damage[7,8,11-13]. Imbalances in the production of interleukin (IL)-10 and tumor necrosis factor (TNF)-α play crucial roles in gastric ulceration[7,14]. Furthermore, TNF-α activates extrinsic apoptotic pathway via caspase-3 induction, ultimately resulting in gastric injury[15]. On the contrary, inhibition of TNF-α via the cytoprotective prostraglandin (PG) highlights its anti-inflammatory properties in the gastric mucosa[13] .
Somatostatin is secreted from D-cells in the stomach where it suppresses acid secretion directly from parietal cells and indirectly by inhibiting the release of histamine and gastrin[16]. Furthermore, somatostatin inhibits pepsin secretion and reduces gastroduodenal mucosal blood flow, which are important entities in the pathophysiology of peptic ulcer bleeding[17]. Conversely, gastric ulcers are linked to decreased levels of this hormone[18,19]. The effectiveness of synthetic somatostatin analogues as gastroprotective agents is advocated by inhibition of leukocyte adhesion[20] and antioxidant[21] properties, beside their antisecretory potential[22].
The possibility that animals exposed to chronic stressors may develop gastric lesions has not been extensively investigated. Hence, the present study aimed to assess the potential modulatory effect of CMS on the stomach integrity assessed macro- and microscopically. In addition, the gastric mucosal redox status, as well as the inflammatory process that might accompany exposure to CMS were determined herein. Moreover, the study evaluated the effect of octreotide, a synthetic cyclic octapeptide somatostatin analogue, on CMS-induced gastric mucosal alterations.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (175 ± 15 g; National Research Center Laboratory, Cairo, Egypt) were kept in a controlled environment, at a constant temperature (23 ± 2°C), humidity (60% ± 10%), and light/dark (12/12 h) cycle, lights on at 5:00 am. Rats were singly housed and acclimatized for 1 wk before any experimental procedures and were allowed standard rat chow and tap water ad libitum. Experimental protocols were approved by the Research Ethical Committee of Faculty of Pharmacy Cairo University (Cairo, Egypt).
Induction of CMS, treatment, and experimental groups
Rats were randomly assigned to 6 groups (n = 10-12; each). Animals in group I received an ip injection of saline and served as control group, while groups II and III rats received octreotide (Novartis Pharmaceuticals, Basle, Switzerland) at two dose levels (50 and 90 μg/kg, ip)[23]. Group IV rats were exposed to CMS, as detailed below, while animals of groups V and VI were subjected to CMS during daily treatment with octreotide at the indicated doses. The somatostatin analogue was dissolved in saline and its administration started from the first day 2 h before exposure to stressors between 9:00 am and 12:00 pm. CMS was induced by exposure of animals to unpredictable repetitive random stress procedures for 21 d following the protocol described by Bekris et al[24]. Briefly, the stressors comprised high-speed agitation for 10 min; deprivation of either food and/or water for 24 h; either 45°C heat stimulus for 5 min or 4°C cold exposure for 1 h;immobilization for 2 h; interrupted noise for 3 h; continuous illumination for 24 h; and tilted cage for 12 h. On the other hand, unstressed control animals were housed undisturbed under constant conditions, without contact with the stressed animals.
Plasma corticosterone level measurement
At the beginning of the following dark cycle, blood was collected in chilled EDTA-tubes. Plasma corticosterone levels were determined using a commercially available radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA).
Measurement of ulcer index
Rats were euthanized and their stomachs were rapidly removed and opened along the greater curvature to assess the extent of gastric damage in a double blind fashion. The length of each lesion along its greatest diameter was measured and the sum of lengths was expressed as ulcer index (mm)[8].
Biochemical determinations
Gastric mucosa was scraped and homogenized either in ice-cold saline for assessment of lipid peroxides, total antioxidant capacity (TAC), TNF-α, IL-10, and capase-3 or in 0.1 mol/L phosphate (pH 7.4) buffer, containing 1 mmol/L EDTA and 0.1 μmol/L indomethacin for PGE2 measurement and frozen at -70°C until assayed.
Lipid peroxides and TAC
The thiobarbituric acid reaction of Mihara and Uchiyama[25] was adopted for estimation of lipid peroxides level, using malondialdehyde (MDA) as a standard. The method for the assessment of TAC of gastric mucosa was based on that of Koracevic et al[26].
TNF-α, IL-10, PGE2, and caspase-3 activity estimations
Gastric mucosal TNF-α, IL-10, and PGE2 were measured by ELISA kits purchased from Invitrogen (California, USA), Bender MedSystems (Vienna, Austria), and Cayman Chemical (MI, USA), respectively. In addition, the activity of gastric mucosal caspase-3 was measured by using a colorimetric assay kit (Biosource International, California, USA). Briefly, the levels of the chromophore p-nitroanilide (pNA) released by caspase-3 activity in the tissue lysates were quantified spectrophotometrically at 405 nm. All the procedures of the used kits were performed following the manufacturer’s instruction manual.
Stomach histopathological examination
The stomach was removed from representative animals (n = 4) in each group and immediately fixed in 10% phosphate buffered formaldehyde. Subsequently sections were embedded in paraffin, and 5 μm sections were prepared. The sections were stained with haematoxylin and eosin (HE) and examined microscopically.
Statistical analysis
Data were expressed as mean of 6-8 experiments ± SE. The comparisons of data were carried out using one-way analysis of variance (ANOVA) followed by Tukey-Kramer Multiple Comparisons Test. All analysis utilized SPSS 16.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA). A probability level of less than 0.05 was accepted as statistically significant.
RESULTS
Exposure of animals to CMS elevated plasma corticosterone (28 ± 0.6 μg/dL, P = 0.002, Table 1), an effect that was associated with erosion formation in the glandular region of the stomach, manifested by gross inspection (6.4 ± 0.16 mm, P = 0.01), as well as microsopically (Table 1 and Figure 1). Treatment with 50 and 90 μg/kg octreotide, on the contrary, restored plasma corticosterone level (61% and 71%, P = 0.002, respectively) and ameliorated gastric lesion formation (38%, P = 0.01, 50 μg/kg), which was more evident at the higher dose (9%, P = 0.01) in rats subjected to CMS.
Table 1.
Effect of octreotide on ulcer index and plasma corticosterone level in chronic mild stressed rats
| Groups |
Parameters |
|
| Ulcer index (mm) | Plasma corticosterone (μg/dL) | |
| Control | 0 ± 0 | 15.4 ± 1.0 |
| OCT (50 μg/kg) | 0 ± 0 | 17.3 ± 0.7 |
| OCT (90 μg/kg) | 0 ± 0 | 16.0 ± 1.2 |
| CMS | 6.4 ± 0.16a | 28.4 ± 0.6a |
| CMS + OCT (50 μg/kg) | 2.4 ± 0.06a,b | 17.2 ± 0.7b |
| CMS + OCT (90 μg/kg) | 0.6 ± 0.01a,b | 20.4 ± 1.1b |
Rats were subjected to mild stressors for 21 d; octreotide was given 2 h before the insults or to normal animals. Data are mean of 6-8 rats ± SE.
P < 0.05 compared to control and chronic mild stress (CMS) groups respectively. For comparisons among treatment groups, one-way ANOVA followed by Tukey-Kramer Multiple Comparisons Test was used. OCT: Octreotide.
Figure 1.
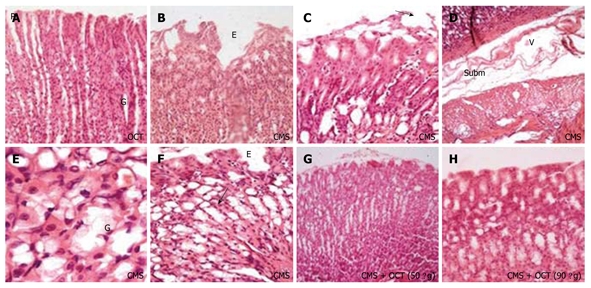
Representative photomicrographs of stomach fundus mucosa obtained at the beginning of the following dark cycle after last exposure to mild stressors with or in absence of octreotide (50 and 90 μg/kg, ip) treatment. A: Octreotide (OCT) administered to normal rats showing normal gastric architecture with gastric pits (P) and gastric glands (G); B: Chronic mild stress (CMS) sections showing mucosal erosion designated by letter E; C: CMS showing hydropic degeneration with erosion and epithelial desquamation (arrow); D: CMS with submucosal edema (V) as represented by wide separation of connective tissue between mucosa and muscularis mucosa infiltrated macrophages and eosinophils (E); E: CMS showing goblet cell (G) metaplasia indicative of mucous secretion and increase in activity of mucous secretion due to fasting, irritation and stress; F: CMS showing surface erosions (E) and marked goblet cell metaplasia with inflammatory cell infiltration (arrow) in the deep mucosa; G and H: Improvement of epithelial lining as indication for surface regeneration with OCT at both dose levels in CMS rats (HE stain, × 40 and 100).
Daily administration of octreotide at the indicated dose levels (50 and 90 μg/kg) for 3 wk to rats did not show any statistical difference from non-treated control animals except for a decrease in PGE2 (64.9 ± 2.2 and 62.95 ± 3.57 pg/g tissue, P = 0.002, respectively) and increased IL-10 (1128.5 ± 94.1 and 1250.53 ± 95.21 pg/g tissue, P = 0.001, respectively) levels (Figures 2, 3, 4, 5).
Figure 2.
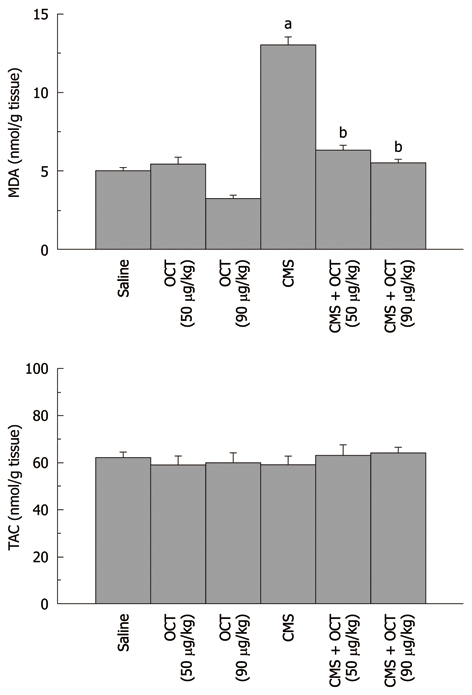
Effect of octreotide (50 and 90 μg/kg, ip) given 2 h prior to chronic mild stress exposure on mucosal malondialdehyde content (upper panel) and total antioxidant capacity (lower panel) in normal rats and those subjected to chronic mild stress. Values are mean ± SE, n = 6-8 each. a,bP < 0.05 vs control and chronic mild stress (CMS), respectively, using one-way ANOVA followed by Tukey-Kramer Multiple Comparisons Test. OCT: Octreotide; MDA: Malondialdehyde content; TAC: Total antioxidant capacity.
Figure 3.
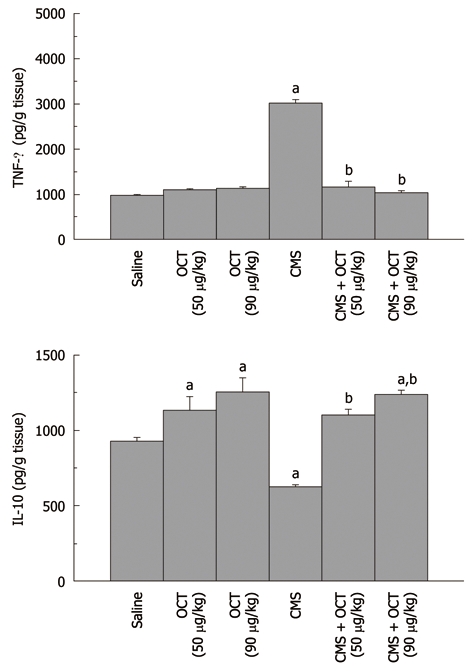
Effect of octreotide (50 and 90 μg/kg, ip) on gastric mucosal tumor necrosis factor-α (upper panel) and interleukin-10 (lower panel) content in normal rats and those subjected to chronic mild stress, where octreotide was administered 2 h before chronic mild stress exposure. Values are mean ± SE, n = 6-8 each. a,bP < 0.05 vs control and chronic mild stress (CMS), respectively, using one-way ANOVA followed by Tukey-Kramer Multiple Comparisons Test. OCT: Octreotide; TNF: Tumor necrosis factor; IL: Interleukin.
Figure 4.
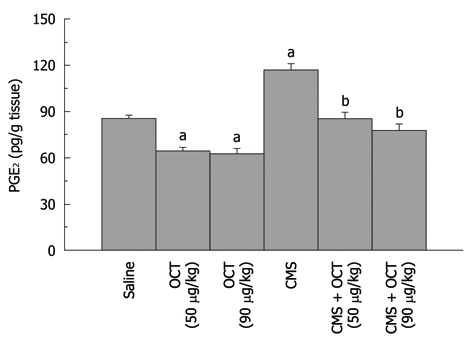
Effect of octreotide (50 and 90 μg/kg, ip) on gastric mucosal PGE2 content in normal rats and those subjected to chronic mild stress (mean of 6-8 animals ± SE). Octreotide was administered 2 h before chronic mild stress (CMS) exposure. a,bP < 0.05 vs control and CMS, respectively, using one-way ANOVA followed by Tukey-Kramer Multiple Comparisons Test. OCT: Octreotide.
Figure 5.
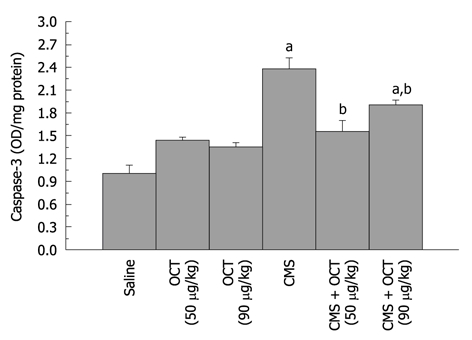
Effect of octreotide (50 and 90 μg/kg, ip) on gastric mucosal caspase-3 activity in normal rats and those subjected to chronic mild stress (mean of 6-8 animals ± SE). Octreotide was administered 2 h before chronic mild stress (CMS) exposure. a,bP < 0.05 vs control and CMS, respectively, using one-way ANOVA followed by Tukey-Kramer Multiple Comparisons Test. OCT: Octreotide.
CMS elevated gastric mucosal MDA (13 ± 0.5 nmol/g tissue, P = 0.001, Figure 2), TNF-α (3008.6 ± 78.18 pg/g tissue, P < 0.001, Figure 3), PGE2 (117.1 ± 4.31 pg/g tissue, P = 0.002, Figure 4), and caspase-3 (2.4 ± 0.14 OD/mg protein, P = 0.002, Figure 5) as compared to control values. Furthermore, reduction in the level of IL-10 (627.9 ± 12.82 pg/g tissue, P = 0.001, Figure 3) in the gastric mucosa was detected, while TAC (P = 0.099) was not altered significantly (Figure 2). Octreotide at both dose levels (50 and 90 μg/kg) suppressed gastric mucosal MDA (58% and 50%, P = 0.001, respectively, Figure 2), TNF-α (39% and 34%, P < 0.001, respectively, Figure 3), PGE2 (73% and 67%, P = 0.002, respectively, Figure 4) and caspase-3 activity (67% and 79%, P = 0.002, respectively, Figure 5), as compared to animals subjected to CMS. Furthermore, octreotide evoked an increment in gastric mucosal IL-10 (175% and 197%, P = 0.001, respectively, Figure 3), as compared to stressed rats; however TAC (P = 0.099, Figure 2) was unaltered.
DISCUSSION
Previous studies have implicated the influence of acute stress exposure in gastric induced ulcerations[7,8,10]; however the effect of chronic exposure to mild environmental stressors on gastric mucosal integrity have not been fully delineated. The current investigation extends previous findings, using other stress procedures[6,27,28], that CMS exposure induces gastric lesions as evidenced by macro- and microscopical besides mechanistic pathways. The most important findings of the current study demonstrate that CMS for 21 d (1) increased plasma corticosterone; (2) induces gastric mucosal erosions; (3) deranges mucosal oxidant status, as well as pro- and anti-inflammatory cytokines; (4) activates caspase-3 mediated apoptosis; and (5) surprisingly enhances PGE2 production in the gastric mucosa. On the other hand, concomitant administration of octreotide reduced ulcer formation and efficiently reinstated most of the changes associated with CMS.
Stress, on one hand, causes the activation of the brain gut axis and stimulates the stomach both sympathetically and parasympathetically[29]. The former produces arteriolar vasoconstriction, thus reducing blood flow to the stomach, while the latter enhances gastric motility and muscular contraction leading to vascular compression with consequent mucosal ischemia[30]. Interestingly, in the present study we demonstrate hydropic degeneration with erosion and epithelial desquamation suggestive of ischemic outcome. Consequently, following the ischemic event, superoxide anion (O2-) leakage from mitochondrial electron transport chain is triggered, which further augments hydroxyl radical (OH.) production, with subsequent oxidative damage of macromolecules[31].
Stress ulcers, on the other hand, are linked to leukocyte infiltration, which further exacerbates free radicals production and TNF-α generation[7,8,10]. This inflammatory cytokine further recruits more neutrophils resulting in a feed forward damaging cycle[7]. Both effects were evidenced in the current study by an increase in lipid peroxides, as well as TNF-α. The increment in lipid peroxides corroborates similar findings in other organs when exposed to CMS[4,5] and several reports utilizing acute stress models[8,31], as well as another chronic restraint stress ulcer model[6]. Notably, lipid peroxides formation is an indicative marker of a vicious ROS cycle; however, there was no change in TAC levels from baseline. A plausible explanation for the latter is the adaptation of the gastric mucosa to CMS-induced ROS, where the presence of either decreased[6,8,31] or increased[31,32] levels of endogenous antioxidants, with a net unchanged concentration, was previously reported in ulcer models including acute stress. Meanwhile, we observed a decline in the level of the anti-inflammatory cytokine IL-10, which accounts for the overwhelming antagonistic effect of TNF-α as reported by Brossart et al[33], which may additionally aggravate gastric lesion formation. Recently, genetic IL-10 polymorphism has been found to predispose individuals to peptic ulcer[34], thus lending further support to our findings.
Evidence exists that the synthetic somatostatin analogue, octreotide, possesses antiulcerogenic activity in other gastric lesions models[20,21]. Notably, this protective effect may be attributed to maintaining of mucosal blood flow, an essential gastroprotective factor thus preserving tissue integrity. The gastroprotective effect was associated with reduction in MDA levels, which is in line with the reported findings utilizing octreotide in another model of gastric injury by Sener et al[21]. In addition, the study of Scheiman et al[20] provided evidence that octreotide affords gastroprotection by inhibition of neutrophil infiltration, which lends further support to the reduction in inflammatory cells recruitment in the gastric mucosa, more evident with the higher dose of octreotide in this study.
Octreotide also reinstated cytokine levels in the gastric mucosa of animals subjected to a combination of various stressors. Somatostatin analogues were shown to have significant anti-inflammatory effects in vivo associated with suppression of inflammatory cytokines as TNF-α[35]. Meanwhile, a recent in vitro report of ter Veld et al[36] displayed that octreotide increased IL-10 dose-dependently, an effect that supports the current finding in animals treated with the somatostatin analogue.
Apoptosis is largely implicated in the pathogenesis of gastric ulcers[37]. Increased TNF-α, as well as free radicals activate caspase-3, one of the effector caspases involved in apoptotic cell death[38]. Caspases, in turn, elicit neutrophil activation through increased expression of chemoattractants[10] thus a vicious cycle exists, which further aggravates gastric damage. Therefore, the enhanced caspase-3 activity by CMS is consistent with the noted increase in TNF-α content of the gastric mucosa and the disturbance in oxidants/antioxidants mucosal homeostasis. Moreover, Esplugues et al[39] depicted that stress itself inhibits gastric acid secretion through a central nervous reflex mechanism; however, the present study documents that atrophy and degeneration of gastric glands as shown in photomicrographs of gastric mucosa of rats subjected to CMC may also be a cause. Additionally, this event, as well as sloughing of the gastric epithelial layer, may thus pin down the contribution of cell death in this study. Although somatostatin has been been reported as an inducer of apoptosis [40], evidence supports its ability to upregulate Bcl-2, a major inhibitor of apoptosis[41].
Despite the fact that some studies supported an ulcerogenic action of the endogenous glucocorticoids[42], other reports[42,43] showed that these steroids are released as an adaptive response to stress rather than being a significant ulcerogenic component of the brain-gut axis. The present study supports this notion, where CMS was shown to increase corticosterone level. In agreement with the adaptive response to stress, the current investigation revealed that rats subjected to CMS surprisingly elevated PGE2, an action that is similar to cold-restraint stress[44]. Exposure to mild stressors is known to cause preconditioning, which contributes to gastroprotective effects against more severe stressors in several gastric ulcer models including exposure to stressful conditions[45]. Under stress conditions, the enhanced resistance to subsequent challenges by other irritants is attributed, in part, to increased endothelial growth factor (EGF) expression and release, as well as gastric mucosal cell proliferation[46]. Moreover, phospholipase A2 is activated by mild stress, which releases arachidonic acid to be metabolized to PGs by both COX-1 and -2 activation[28]. Hence, PGs formed by preconditioning stress may attenuate stress induced gastric injury[47]. Such an action could thus account for the erosions seen in the histopathological study rather than deep ulcerations that invade the muscularis layer. PGE2 is among the factors that regulate gastric blood flow[47] and enhance mucus, as well as bicarbonate synthesis[48], which are important gastric defensive factors. The current study shows dilated gastric pits, which reflect increased mucus production in the gastric mucosa of rats exposed to CMS. Such an effect may be a consequence of elevated PGE2 levels, hence confirming the adaptation theory. Since an imbalance between protective and aggressive factors in the stomach accounts for peptic ulcer formation, the present diverse conditions may favor ulcer formation rather than gastroprotection. On the other hand, treatment with octreotide restored PGE2 in the gastric mucosa, an effect that highlights its efficacy in intercepting preconditioning of the gastric mucosa to mild stressful procedures.
This study reveals that exposure to chronic mild stressors, such as those present in the environment, may increase susceptibility of the gastric mucosa to aggressive factors, resulting ultimately in gastric lesions. Hence, rats subjected to CMS could serve as a chronic model for stress-induced peptic ulceration that can be used for the evaluation of compounds possessing antiulcer activity. Gastroprotective mechanisms of octreotide are probably due to its antioxidant capacity with concomitant anti-inflammatory and anti-apoptotic effects.
COMMENTS
Background
Stress has been linked to the etiopathogenesis of various diseases, ranging from psychiatric disorders to several ailments of the gastrointestinal tract. Evidence exists that exposure to acute, as well as chronic, stressful conditions is linked to gastric injury. However, animal models for chronic induction of gastropathy are limited. Izgüt-Uysal et al showed that a chronic restrained model for 21 d induced gastric lesions. Moreover, Bhattacharya et al introduced another chronic mild stress (CMS) procedure using a rat footshock model as a modification of that adopted by Conti et al by adding the element of unpredictability. This model, as well as other stress procedures have been correlated to gastric injury. The current study aimed to evaluate the effect of random exposure to nine different unpredictable stress procedures for 21 d, a multifactorial interactional animal model for CMS, on the emergence of hemorrhagic gastric ulcers and the possible modulatory effects of octreotide, a synthetic somatostatin analogue.
Research frontiers
Gastric ulcer formation is attributed to an imbalance between aggressive and protective factors overweighing the effect of the former. Several hormones regulate gastric mucosal functions among which is somatostatin, a hormone secreted from D-cells in the stomach. Somatostatin suppresses acid secretion directly from parietal cells and indirectly by inhibiting the release of histamine and gastrin. It is used clinically in peptic ulcer bleeding due to its inhibitory effects on pepsin secretion and gastroduodenal mucosal blood flow. Conversely, gastric ulcers are linked to decreased levels of this hormone. However, somatostatin has a short half life, thus synthetic analogues are used efficiently. The effectiveness of synthetic somatostatin analogues as gastroprotective agents is advocated by inhibition of leukocyte adhesion and antioxidant properties, beside their antisecretory potential. Thus the current study utilized octreotide, a synthetic cyclic octapeptide somatostatin analogue, on a chronic model of mild stress exposures for its well documented gastroprotective effect.
Innovations and breakthroughs
A vast majority of acute models for stress-induced gastric ulcerations exists; however, workable models for chronicity and excessive exposure to stressors are limited. To further understand mechanistic pathways and identify new targets for ulcer treatments, the current study aimed to evaluate the effect of random exposure to nine different unpredictable stress procedures for 21 d, a multifactorial interactional animal model for CMS, on the emergence of hemorrhagic gastric ulcers and the possible modulatory effects of a somatostatin-analogue, octreotide, utilizing two dose levels.
Applications
The introduction of a CMS gastric lesion model can give better insights for understanding the mechanisms involved in exposure to stressful stimuli in the environment on daily basis that produces gastric injury, as well to facilitate management of such hassle events.
Peer review
The authors presented data of some pathophysiological and morphologic alterations of gastric mucosa associated with experimental chronic stress in rats. They also tried to use a somatostatin analogue, octreotide, to protect the animals from stress-caused ulceration in gastric mucosa. The results appear to be very interesting. They may be helpful for fully understanding chronic stress and gastric lesions. It may indicate a possible therapeutic usage of similar chemicals in prevention of stress-associated gastric ulcer in high-risk individuals.
Acknowledgments
The authors would like to thank Dr. Rawhia E Doghaim, Professor, Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt, for her valuable assistance with the histopathological testing and photomicrography interpretation.
Footnotes
Peer reviewer: Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Filaretova L. The hypothalamic-pituitary-adrenocortical system: Hormonal brain-gut interaction and gastroprotection. Auton Neurosci. 2006;125:86–93. doi: 10.1016/j.autneu.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 3.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 4.Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PM, Papadopoulou-Daifoti Z. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98:215–222. doi: 10.1016/j.physbeh.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F, Gavioli EC, Dal-Pizzol F, Quevedo J. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J Psychiatr Res. 2009;43:864–869. doi: 10.1016/j.jpsychires.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Izgüt-Uysal VN, Bülbül M, Tan R, Derin N, Ustünel I, Ağar A, Yargiçoğlu P. Effect of chronic stress and L-carnitine on rat stomach. J Physiol Sci. 2007;57:187–192. doi: 10.2170/physiolsci.RP004707. [DOI] [PubMed] [Google Scholar]
- 7.Jia YT, Ma B, Wei W, Xu Y, Wang Y, Tang HT, Xia ZF. Sustained activation of nuclear factor-kappaB by reactive oxygen species is involved in the pathogenesis of stress-induced gastric damage in rats. Crit Care Med. 2007;35:1582–1591. doi: 10.1097/01.CCM.0000266824.82280.17. [DOI] [PubMed] [Google Scholar]
- 8.Nishida K, Ohta Y, Ishiguro I. Relation of inducible nitric oxide synthase activity to lipid peroxidation and nonprotein sulfhydryl oxidation in the development of stress-induced gastric mucosal lesions in rats. Nitric Oxide. 1998;2:215–223. doi: 10.1006/niox.1998.0178. [DOI] [PubMed] [Google Scholar]
- 9.Glavin GB, Gerrard JM. Characterization of the gastroprotective effects of N,N-diethyl-2-[4-(phenylmethyl)phenoxy]-ethanamine hydrochloride, a non-H1/non-H2 histamine antagonist. Digestion. 1990;47:143–148. doi: 10.1159/000200489. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Harada N, Sobue K, Katsuya H, Okajima K. Insulin-like growth factor-I reduces stress-induced gastric mucosal injury by inhibiting neutrophil activation in mice. Growth Horm IGF Res. 2009;19:136–145. doi: 10.1016/j.ghir.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39–50. [PubMed] [Google Scholar]
- 12.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Horikawa Y, Matsuhashi T, Hatakeyama N, Oyake J, Ohba R, et al. Attenuation of gastric mucosal inflammation induced by aspirin through activation of A2A adenosine receptor in rats. World J Gastroenterol. 2006;12:568–573. doi: 10.3748/wjg.v12.i4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konturek PC, Brzozowski T, Duda A, Kwiecien S, Löber S, Dembinski A, Hahn EG, Konturek SJ. Epidermal growth factor and prostaglandin E(2) accelerate mucosal recovery from stress-induced gastric lesions via inhibition of apoptosis. J Physiol Paris. 2001;95:361–367. doi: 10.1016/s0928-4257(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 14.Abuzarova ER, Gorshkov OV, Chernova OA, Chernov VM, Akberova NI, Abdulkhakov RA. [Peculiarities of genotype distribution of interleukins (IL-1 and IL-10) in patients with peptic ulcer disease and their associations with persistence of Mycoplasma hyorhinis and Helicobacter pylori genotypes] Eksp Klin Gastroenterol. 2008:27–31. [PubMed] [Google Scholar]
- 15.Slomiany BL, Piotrowski J, Slomiany A. Downregulation of endothelin-1 by interleukin-4 during gastric ulcer healing. Biochem Biophys Res Commun. 1999;263:591–595. doi: 10.1006/bbrc.1999.1406. [DOI] [PubMed] [Google Scholar]
- 16.Van Op den bosch J, Van Nassauw L, Van Marck E, Timmermans JP. Somatostatin modulates mast cell-induced responses in murine spinal neurons and satellite cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G406–G417. doi: 10.1152/ajpgi.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sgouros SN, Bergele C, Viazis N, Avgerinos A. Somatostatin and its analogues in peptic ulcer bleeding: facts and pathophysiological aspects. Dig Liver Dis. 2006;38:143–148. doi: 10.1016/j.dld.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Paula AC, Gracioso JS, Toma W, Bezerra R, Saad MA, De Lucca IM, Carneiro EM, Souza Brito AR. Is gastric ulceration different in normal and malnourished rats? Br J Nutr. 2005;93:47–52. doi: 10.1079/bjn20041291. [DOI] [PubMed] [Google Scholar]
- 19.Sun FP, Song YG, Cheng W, Zhao T, Yao YL. Gastrin, somatostatin, G and D cells of gastric ulcer in rats. World J Gastroenterol. 2002;8:375–378. doi: 10.3748/wjg.v8.i2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheiman JM, Tillner A, Pohl T, Oldenburg A, Angermüller S, Görlach E, Engel G, Usadel KH, Kusterer K. Reduction of non-steroidal anti-inflammatory drug induced gastric injury and leucocyte endothelial adhesion by octreotide. Gut. 1997;40:720–725. doi: 10.1136/gut.40.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sener G, Paskaloglu K, Kapucu C, Cetinel S, Contuk G, Ayanoğlu-Dülger G. Octreotide ameliorates alendronate-induced gastric injury. Peptides. 2004;25:115–121. doi: 10.1016/j.peptides.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Nie Y, Li Y, Sha W, Dai S, She Q, Wu H. [Effect of octreotide on intragastric pH in patients with duodenal ulcer bleeding] Zhonghua Yixue Zazhi. 2001;81:520–522. [PubMed] [Google Scholar]
- 23.Lai HS, Chen Y. Effect of octreotide on postoperative intraperitoneal adhesions in rats. Scand J Gastroenterol. 1996;31:678–681. doi: 10.3109/00365529609009149. [DOI] [PubMed] [Google Scholar]
- 24.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 26.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overmier JB, Murison R. Anxiety and helplessness in the face of stress predisposes, precipitates, and sustains gastric ulceration. Behav Brain Res. 2000;110:161–174. doi: 10.1016/s0166-4328(99)00193-x. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka A, Hatazawa R, Takahira Y, Izumi N, Filaretova L, Takeuchi K. Preconditioning stress prevents cold restraint stress-induced gastric lesions in rats: roles of COX-1, COX-2, and PLA2. Dig Dis Sci. 2007;52:478–487. doi: 10.1007/s10620-006-9394-8. [DOI] [PubMed] [Google Scholar]
- 29.Stoíko IuM, Kurygin AA, Musinov IM. [Vagotomy in the treatment of acute ulcerations of the stomach complicated by severe hemorrhage] Vestn Khir Im I I Grek. 2001;160:25–29. [PubMed] [Google Scholar]
- 30.Ito M, Shichijo K, Sekine I. Gastric motility and ischemic changes in occurrence of linear ulcer formation induced by restraint-water immersion stress in rat. Gastroenterol Jpn. 1993;28:367–73. doi: 10.1007/BF02776980. [DOI] [PubMed] [Google Scholar]
- 31.Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. Hydroxyl radical is the major causative factor in stress-induced gastric ulceration. Free Radic Biol Med. 1997;23:8–18. doi: 10.1016/s0891-5849(96)00547-3. [DOI] [PubMed] [Google Scholar]
- 32.Amanvermez R, Tunçel OK, Demir S, Kefeli M, Bek Y, Celik C. Protective effects of cysteine, methionine and vitamin C on the stomach in chronically alcohol treated rats. J Appl Toxicol. 2008;28:591–598. doi: 10.1002/jat.1308. [DOI] [PubMed] [Google Scholar]
- 33.Brossart P, Zobywalski A, Grünebach F, Behnke L, Stuhler G, Reichardt VL, Kanz L, Brugger W. Tumor necrosis factor alpha and CD40 ligand antagonize the inhibitory effects of interleukin 10 on T-cell stimulatory capacity of dendritic cells. Cancer Res. 2000;60:4485–4492. [PubMed] [Google Scholar]
- 34.Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Sugimura H, Hishida A. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol. 2007;22:1443–1449. doi: 10.1111/j.1440-1746.2006.04613.x. [DOI] [PubMed] [Google Scholar]
- 35.Karalis K, Mastorakos G, Chrousos GP, Tolis G. Somatostatin analogues suppress the inflammatory reaction in vivo. J Clin Invest. 1994;93:2000–2006. doi: 10.1172/JCI117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ter Veld F, Rose B, Mussmann R, Martin S, Herder C, Kempf K. Effects of somatostatin and octreotide on cytokine and chemokine production by lipopolysaccharide-activated peripheral blood mononuclear cells. J Endocrinol Invest. 2009;32:123–129. doi: 10.1007/BF03345700. [DOI] [PubMed] [Google Scholar]
- 37.Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111–1115. [PubMed] [Google Scholar]
- 38.Szabó I, Tarnawski AS. Apoptosis in the gastric mucosa: molecular mechanisms, basic and clinical implications. J Physiol Pharmacol. 2000;51:3–15. [PubMed] [Google Scholar]
- 39.Esplugues JV, Barrachina MD, Beltrán B, Calatayud S, Whittle BJ, Moncada S. Inhibition of gastric acid secretion by stress: a protective reflex mediated by cerebral nitric oxide. Proc Natl Acad Sci USA. 1996;93:14839–14844. doi: 10.1073/pnas.93.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasquali D, Rossi V, Conzo G, Pannone G, Bufo P, De Bellis A, Renzullo A, Bellastella G, Colao A, Vallone G, et al. Effects of somatostatin analog SOM230 on cell proliferation, apoptosis, and catecholamine levels in cultured pheochromocytoma cells. J Mol Endocrinol. 2008;40:263–271. doi: 10.1677/JME-08-0012. [DOI] [PubMed] [Google Scholar]
- 41.Kang BN, Jeong KS, Park SJ, Kim SJ, Kim TH, Kim HJ, Ryu SY. Regulation of apoptosis by somatostatin and substance P in peritoneal macrophages. Regul Pept. 2001;101:43–49. doi: 10.1016/s0167-0115(01)00264-6. [DOI] [PubMed] [Google Scholar]
- 42.Filaretova L, Podvigina T, Bagaeva T, Morozova O. Dual action of glucocorticoid hormones on the gastric mucosa: how the gastroprotective action can be transformed to the ulcerogenic one. Inflammopharmacology. 2009;17:15–22. doi: 10.1007/s10787-008-8046-3. [DOI] [PubMed] [Google Scholar]
- 43.Filaretova LP, Bagaeva TR, Amagase K, Takeuchi K. Contribution of glucocorticoids to protective influence of preconditioning mild stress against stress-induced gastric erosions. Ann N Y Acad Sci. 2008;1148:209–212. doi: 10.1196/annals.1410.005. [DOI] [PubMed] [Google Scholar]
- 44.Pizzuto G, Surgo D, Clementi M, Marsico R, Genco A, Materia A, Basso N. Differential effect of stress on gastric somatostatin, prostaglandin E and gastrin release in the rat. Ital J Gastroenterol Hepatol. 1997;29:143–147. [PubMed] [Google Scholar]
- 45.Konturek PC, Duda A, Brzozowski T, Konturek SJ, Kwiecien S, Drozdowicz D, Pajdo R, Meixner H, Hahn EG. Activation of genes for superoxide dismutase, interleukin-1beta, tumor necrosis factor-alpha, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand J Gastroenterol. 2000;35:452–463. doi: 10.1080/003655200750023697. [DOI] [PubMed] [Google Scholar]
- 46.Brzozowski T, Konturek SJ, Pytko-Polonczyk J, Warzecha Z. Gastric adaptation to stress: role of sensory nerves, salivary glands, and adrenal glands. Scand J Gastroenterol. 1995;30:6–16. doi: 10.3109/00365529509093229. [DOI] [PubMed] [Google Scholar]
- 47.Brzozowski T, Konturek PC, Konturek SJ, Drozdowicz D, Pajdo R, Pawlik M, Brzozowska I, Hahn EG. Expression of cyclooxygenase (COX)-1 and COX-2 in adaptive cytoprotection induced by mild stress. J Physiol Paris. 2000;94:83–91. doi: 10.1016/s0928-4257(00)00145-5. [DOI] [PubMed] [Google Scholar]
- 48.Flemström G, Knutson L, Kivilaakso E. Gastroduodenal mucosal secretion of bicarbonate and mucus: physiological control and role in protection. Klin Wochenschr. 1986;64 Suppl 7:107–111. [PubMed] [Google Scholar]


