Abstract
AIM: To determine the role of CD133 in cholangiocarcinoma progression.
METHODS: CD133 protein expression was evaluated by immunohistochemistry in 34 cholangiocarcinoma specimens. In addition, proliferation, chemoresistance and invasive properties of CD133-enriched (CD133+) and CD133-depleted (CD133-) RMCCA1 cholangiocarcinoma cells were studied and compared.
RESULTS: Strong CD133 expression was observed in 67.6% (23/34) of the cholangiocarcinoma specimens. Strong expression of CD133 was significantly associated with nodal metastasis (P = 0.009) and positive surgical margin status (P = 0.011). In the in vitro study, both the CD133+ and CD133- cells had similar proliferation abilities and resistance to chemotherapeutic drugs. However, the CD133+ cells had a higher invasive ability compared with CD133- cells.
CONCLUSION: CD133+ cells play an important role in the invasiveness of cholangiocarcinoma. Targeting of the CD133+ cells may be a useful approach to improve treatment against cholangiocarcinoma.
Keywords: CD133, Cholangiocarcinoma, Immunohistochemistry, Invasion, Metastasis
INTRODUCTION
Cholangiocarcinoma is known as one of the most aggressive malignant tumors associated with local invasiveness and a high rate of metastasis. It is also known to be one of the most common causes of cancer death in Thailand[1]. Three-year survival rates of 35% to 50% are achieved only in a subset of patients who have negative histological margins at the time of surgery[2-4]. Palliative therapeutic approaches, consisting of percutaneous and endoscopic biliary drainage, have usually been used for these patients because there is no effective chemotherapeutic treatment for this type of cancer. Therefore, identification of the molecules involved in cholangiocarcinoma cell progression is crucial for the development of novel drug treatments for this disease.
CD133, a human homologue of a mouse Prominin-1, is a 5-transmembrane cell-surface glycoprotein[5]. It has been detected in an enrichment of hematopoietic stem cells derived from fetal liver or bone marrow neuroepithelial cells, embryonic epithelial cells and adult immature epithelial cells[5]. Furthermore, CD133 has been successfully used for the identification of cancer stem cell (CSC) niches in glioblastoma[6], colon cancer[7] and other solid carcinomas[8]. Recently, CD133 was identified in many kinds of cancer specimens, including hepatocellular carcinoma[9] and colon cancer[10], and was associated with poor prognoses. To understand the roles of CD133 in cholangiocarcinoma cells, the characteristics of CD133+ and CD133- cholangiocarcinoma cells must be investigated; such studies have not been carried out with CD133+ cholangiocarcinoma cells. In the present study, we investigated the clinicopathological significance of CD133 expression in human cholangiocarcinoma specimens. In addition, we studied the cell proliferation, chemoresistance and invasiveness of CD133+ and CD133- cholangiocarcinoma cells from the RMCCA1 cholangiocarcinoma cell line.
MATERIALS AND METHODS
Human cholangiocarcinoma tissue samples
The cholangiocarcinoma tissue samples analyzed in this study were obtained from cholangiocarcinoma patients who underwent a surgical resection at Rajavithi Hospital in Bangkok, Thailand from 2008 to 2010. The study was approved by the ethics committee of Rajavithi Hospital.
Immunohistochemical staining
Paraffin wax sections of cholangiocarcinoma specimens were dewaxed in xylene, and transferred to alcohol. Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol, and the sections were boiled in 10 mmol/L citrate buffer (pH 6.0) in a microwave oven (750 W) for antigen retrieval. Nonspecific binding was blocked by incubating with 3% normal horse serum for 20 min. Sections were incubated overnight at 4°C with a 1/1000 dilution of mouse monoclonal antibody for CD133. Biotinylated rabbit anti-mouse IgM (Dako, Glostrup, Denmark) was applied to the sections, followed by an avidin-biotin-peroxidase conjugate (ABC Elite; Vector Laboratories, Burlingame, California, USA) for 30 min at room temperature. The immunohistochemical reaction was developed with freshly prepared reagents from a Histofine streptavidin-biotin complex peroxidase (SAB-PO) kit (Nichirei Inc., Tokyo, Japan). The immunohistochemical reactions were then visualized under high power magnification (× 400) using an Olympus BH2 microscope (field width, 0.5 mm) and scored into 3 categories based on the percentage of positively stained cells, as follows: (1) negative, < 5%; (2) weak, 5%-50%; and (3) strong > 50%.
Cell culture and materials
The human cholangiocarcinoma RMCCA1 cells[11] were routinely grown in Ham’s F12 medium supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) at 37°C in a 5% CO2 humidified atmosphere. For experiments, cells were grown in Ham’s F12 medium supplemented with 1% FBS.
Enrichment of CD133+ cholangiocarcinoma cells
Cells were harvested by treatment with 0.25% trypsin (Gibco, LA, USA) in the logarithmic phase of growth followed by centrifugation at 300 g for 5min. The cells were resuspended in 100 μL buffer (phosphate-buffered saline (PBS), pH7.2, 0.5% bovine serum albumin, 2 mmol/L ethylenediaminetetraacetic acid). Single cells were magnetically labeled with anti-CD133 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) in the dark at 4°C for 30 min and applied to a prepared MS Column (Miltenyi Biotec, Bergisch Gladbach, Germany). CD133- cells were collected in the flow-through of the column; CD133+ cells bound to the beads were flushed out by applying the plunger supplied with the column. The percentage of CD133-expressing cells in the original cell populations, the flow-through and the flushed-out fractions was analyzed by fluorescence-activated cell sorting (FACS) with fluorescein isothiocyanate (FITC)-conjugated anti-CD133 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were stained at a concentration of 1 × 106 cells per 90 μL buffer and 10 μL antibody at 4°C for 25 min before FACS analysis.
Cell proliferation assay
For proliferation assays, cells were seeded into 96-well culture plates at a density of 10 000 cells per well. For cancer chemoresistance studies, cells were treated with vehicle (PBS) or 5-50 μmol/L cisplatin. Cells were then incubated for 48 hours before applying the water soluble tetrazolium salts (WST)-1 cell proliferation assay reagent (Roche Diagnostics, Laval, Quebec, Canada) according to the manufacturer’s recommendations. The degree of cell proliferation was assessed by determining the A450 nm of the cell culture medium after addition of WST-1 for 2 h. Results are reported as the percentage of inhibition of cell proliferation, where the optical density measured for vehicle-treated cells was considered to represent 100% proliferation.
Cell invasion assay
The invasiveness of cholangiocarcinoma cells was assayed in a 24-well Biocoat Matrigel invasion chamber (8 μm; Becton Dickinson, Bioscience, Bedford, MA, USA). There were 50 000 cells seeded in the upper chamber. The bottom chamber contained 10% FBS. After 24 h of incubation, the invading cells at the lower surface of the Matrigel-coated membrane were fixed with 70% ethanol, stained with crystal violet and counted in 5 random 200 × power fields under a light microscope.
Statistical analysis
Statistical analysis of the association between clinicopathological findings and the expression of CD133 was performed by means of the χ2 test or Fisher’s exact test. The Kaplan-Meier method was used to estimate survival as a function of time, and the survival differences were analyzed by log-rank test. The Cox regression model was used for multivariate analysis of prognostic factors. The experiments in the cell proliferation and invasiveness assays were all performed in triplicate, and each result is reported as the mean with standard deviation. Data were compared using the Student t-test. A P-value < 0.05 was considered statistically significant.
RESULTS
Expression of CD133 in paraffin-embedded cholangiocarcinoma samples
Among the 34 cholangiocarcinoma specimens, 17 specimens were derived from the patients who received extended right hepatectomy, 7 specimens were derived from the patients who received left hepatectomy and 10 specimens were derived from the tissue biopsy of unresectable cholangiocarcinoma patients. Expression of CD133 was detected by immunohistochemistry in these 34 paraffin-embedded cholangiocarcinoma specimens. In non-cancerous bile duct tissues, the immunohistochemical signal for CD133 was negative. In cancerous tissues, specific CD133 signals were localized mainly in the cell membrane and cytoplasm of cholangiocarcinoma cells (Figure 1). We found that all cholangiocarcinoma specimens (34/34) exhibited signals indicating CD133 expression (> 5% of cell staining). Applying the criteria for intensity of immunohistochemical staining for CD133, strong expression (> 50% of cell staining) of CD133 was noted in 67.6% (23/34) of cholangiocarcinoma specimens. In addition, we demonstrated that 75% (9/12) of perihilar and 63.6% (14/22) of intrahepatic cholangiocarcinoma samples strongly expressed CD133.
Figure 1.

Representative immunohistochemical staining for CD133 in cholangiocarcinoma specimens. A: Normal bile duct cells (red arrow) demonstrated negative staining, whereas cholangiocarcinoma cells had strong cytoplasmic staining (black arrow); B: Moderately differentiated cholangiocarcinoma with overexpression of CD133. Positive staining was observed in the cytoplasm of cholangiocarcinoma cells (200 × magnification).
When comparing clinicopathological variables, nodal metastasis (P = 0.009) and positive surgical margin status (P = 0.011) were more frequent in the CD133 strong expression group compared with the CD133 weak expression group (Table 1).
Table 1.
Relation between clinicopathological features and immunohistochemical staining of CD133 in cholangiocarcinoma specimens
| Variable | Total(n = 34) |
CD133 expression |
P-value | |
| Weak | Strong | |||
| Age | ||||
| < 60 | 18 | 7 | 11 | 0.477 |
| > 60 | 16 | 4 | 12 | |
| Sex | ||||
| Male | 22 | 8 | 14 | 0.705 |
| Female | 12 | 3 | 9 | |
| Tumor differentiation | ||||
| Well | 17 | 7 | 10 | 0.465 |
| Moderate/poor | 17 | 4 | 13 | |
| Node | ||||
| Negative | 16 | 9 | 7 | 0.0091 |
| Positive | 18 | 2 | 16 | |
| Distant metastasis | ||||
| Negative | 24 | 10 | 14 | 0.113 |
| Positive | 10 | 1 | 9 | |
| Surgical resection margin2 | ||||
| R0 | 20 | 10 | 10 | 0.0111 |
| R1, R2 | 14 | 1 | 13 | |
| Location of tumor | ||||
| Perihilar | 12 | 3 | 9 | 0.705 |
| Intrahepatic | 22 | 8 | 14 | |
| Neurovascular invasion3 | ||||
| Positive | 15 | 8 | 7 | 0.210 |
| Negative | 9 | 2 | 7 | |
Statistically significant;
Surgical resection margin: R0 = negative resection margin, R1 = microscopic positive resection margin and R2 = macroscopic positive resection margin;
To study the presence of neurovascular invasion, we excluded 10 specimens derived from tissue biopsy.
CD133 expression and patient survival
Univariate analysis revealed that the median survival time was 11 mo in patients with strong CD133 expression and 14 mo in patients with weak expression (P = 0.23). We also found that the median survival time in patients with a negative surgical margin was significantly better than in patients with a positive surgical margin (15 mo vs 8 mo, P = 0.009) (Figure 2). In a multivariate Cox regression analysis, only surgical margin status was indicated as an independent risk factor for survival (P = 0.04, Table 2).
Figure 2.

Kaplan-Meier cumulative overall survival curves. A: Survival curves of patients with strong and weak CD133 expression (P = 0.23); B: Survival curves of patients with positive and negative surgical margin status (P = 0.009).
Table 2.
Survival analysis on clinicopathological parameters by Cox’s multivariate model
| Variables | P-value | Hazard ratio |
95% CI |
|
| Lower limit | Upper limit | |||
| Tumor differentiation | 0.57 | 0.77 | 0.32 | 1.86 |
| Lymph node metastasis | 0.81 | 1.15 | 0.36 | 3.66 |
| Distant metastasis | 0.97 | 1.03 | 0.22 | 4.53 |
| Neurovascular invasion | 0.33 | 1.48 | 0.67 | 3.26 |
| CD133 expression | 0.35 | 0.53 | 0.14 | 2.02 |
| Surgical resection status | 0.041 | 4.09 | 1.06 | 15.72 |
Statistically significant; CI: Confidence interval.
Enrichment of CD133+ cholangiocarcinoma cells
To study the characteristics of CD133+ cholangiocarcinoma cells, we enriched the CD133+ and CD133- cells from RMCCA1 cholangiocarcinoma cell lines using magnetic cell sorting technology. The percentage of CD133+ cells in CD133-enriched cells was 91.9%, and the percentage of CD133+ cells in the CD133-depleted cells was 11.2% (Figure 3A). As revealed by morphological studies, no cell morphology differences were observed between CD133+ and CD133- RMCCA1 cells (Figure 3B).
Figure 3.
Isolation of CD133+ and CD133- cholangiocarcinoma cells. A: The percentage of CD133-expressing cells in the original RMCCA1 cell populations, the flushed-out fractions (CD133+ RMCCA1 cells) and the flow-through (CD133- RMCCA1 cells) were analyzed by fluorescence-activated cell sorting; B: The morphology of original, CD133+ and CD133- RMCCA1 cells is demonstrated under a phase contrast microscope at 200 × magnification. FITC: Fluorescein isothiocyanate.
Proliferation of CD133+ and CD133- cholangiocarcinoma cells
Because previous reports demonstrated that CD133+ cells have higher proliferative potential than CD133- cells[12], we investigated the rate of cell proliferation in CD133+ and CD133- RMCCA1 cells cultured for 3 days. The results showed that there was no statistically significant difference in cell proliferation between CD133+ and CD133- RMCCA1 cells (Figure 4A).
Figure 4.
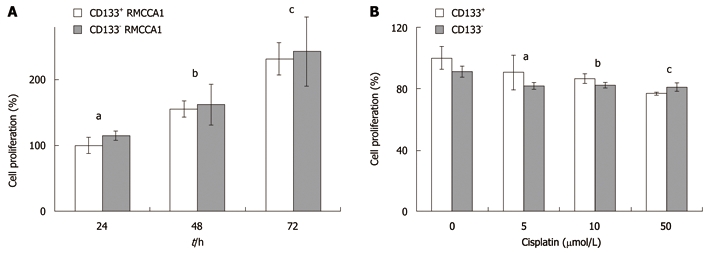
Cell proliferation assays of CD133+ and CD133- RMCCA1 cells. A: The proliferation rates of CD133+ and CD133- cholangiocarcinoma cells. CD133+ and CD133- RMCCA1 cells were cultured for 24-72 h. A cell proliferation assay was performed using WST-1. Results are reported as a percentage of cell proliferation, where the optical density values from CD133+ cells cultured for 24 h are set as 100% of proliferation. Data is represented as the mean ± SD of 3 independent experiments. (aP = 0.095, bP = 0.578, cP = 0.544 vs the same time of culturing); B: Effect of cisplatin on cholangiocarcinoma cells. CD133+ and CD133- RMCCA1 cells were treated with cisplatin at various concentrations (0, 5, 10 and 50 μmol/L) for 2 d. Effects on cell proliferation were measured by WST-1. Results are reported as percentage of cell proliferation, where the optical density values from vehicle-treated cells (0 μmol/L cisplatin) are set as 100% proliferation. Data is presented as the mean ± SD of 3 independent experiments. (aP = 0.060, bP = 0.056, cP = 0.053 vs the same concentration of cisplatin). WST: Water soluble tetrazolium salts.
Chemoresistance of CD133+ cholangiocarcinoma cells
Previous studies have proposed that CD133+ enrichment of cancer cells is a source of chemotherapy resistance[13]. Therefore, we investigated the role of CD133 in cholangiocarcinoma cell resistance to chemotherapeutic drugs. CD133+ and CD133- RMCCA1 cells were treated with 0-50 μmol/L cisplatin for 2 d before cell proliferation assays were performed. The percentage of cell proliferation was set to 100% when cells were treated with vehicle (PBS). Both CD133+ and CD133- cells displayed similar degrees of resistance to chemotherapeutic drugs. There was no statistically significant difference in cell proliferation between CD133+ and CD133- RMCCA1 cells (Figure 4B).
Cell invasiveness of CD133+ cholangiocarcinoma cells
Because the expression of CD133 in cholangiocarcinoma specimens is significantly correlated with the lymph node metastatic status, we performed an in vitro invasion assay. When the percentage of CD133+ RMCCA1 cell invasion was set at 100%, the percentage of CD133- RMCCA1 cell invasion was 58.3% ± 19.91%. CD133+ RMCCA1 cells showed significantly higher invasive ability than CD133- RMCCA1 cells (P < 0.001, Figure 5).
Figure 5.

Invasion assays of CD133+ and CD133- RMCCA1 cells. A: Invasion assays of CD133+ and CD133- RMCCA1 cells were performed using the Biocoat Matrigel invasion chamber. Results are reported as the percentage of cell invasion, where the number of cell invasion from CD133+ RMCCA1 cells is set as 100% cell invasion. Data is represented as the mean ± SD of three independent experiments (bP < 0.001); B: The morphology of CD133+ and CD133- RMCCA1 cell invasion is demonstrated under a phase contrast microscope at 200 × magnification.
DISCUSSION
In this study, we performed immunohistochemical staining to detect the expression of CD133 in cholangiocarcinoma specimens. We demonstrated that all of the cholangiocarcinoma specimens expressed CD133, and 67.6% of the cholangiocarcinoma specimens demonstrated strong expression of CD133. In addition, strong expression of CD133 was significantly associated with lymph node metastasis and surgical margin status. It is well known that lymph node metastasis and surgical margin status are the major prognostic factors for cholangiocarcinoma[3,4]. These findings are consistent with a previous study, which demonstrated that cholangiocarcinoma that highly expressed CD133 tended to be related to higher incidences of metastasis and to worse prognoses[14]. In this study, the multivariate Cox regression analysis demonstrated that surgical margin status is an independent prognostic predictor in our patients. Although we found that patients with high expression of CD133 had a lower median survival time than patients with low expression of CD133 in cholangiocarcinoma specimens, this did not reach the statistical difference. We suggest that this could be attributable to the small sample size of our study. A further study which includes a larger cohort of cholangiocarcinoma patients should be performed before using CD133 as a prognostic marker for cholangiocarcinoma.
Previous studies have demonstrated that CD133+ cells exhibit more aggressive behavior, including increased cell proliferation[12] and invasive abilities[15,16]. Here, we studied the characteristics of CD133 in cholangiocarcinoma cell lines using cell proliferation and invasion assays. Our results suggest that CD133+ RMCCA1 cells have higher invasive activity than CD133- RMCCA1 cells. A previous study indicated that C-X-C chemokine receptor type 4 (CXCR4) is markedly expressed in CD133+ pancreatic cancer cells and may be responsible for the increased invasive ability of cells cocultured with pancreatic stromal cells, which express stromal derived factor-1 (CXCL12), the ligand for CXCR4[15]. Further studies focusing on the molecular mechanisms that enhance CD133+ cholangiocarcinoma cell invasiveness should be performed in the future.
A previous study indicated that enriched CD133+ lung cancer cells were resistant to cisplatin treatment[13]. In contrast, Meng et al[17] demonstrated that both CD133+ and CD133- A549 lung cancer cells exhibited similar degrees of resistance to chemotherapeutic drugs. Our data are consistent with those of Meng et al[17]: CD133+ and CD133- cells exhibited similar cell proliferation abilities and resistance to chemotherapeutic drugs[18]. We previously demonstrated that activation of the phosphatidylinositol 3-kinase (PI3K) pathway in cholangiocarcinoma cells protects the cells from cytotoxicity induced by platinum-based chemotherapy. In addition, we also found that there was no difference between Akt and mTOR (mammalian target of rapamycin) activation in CD133+ and CD133- cholangiocarcinoma cells (data not shown). These results indicate that the proliferative ability and resistance to chemotherapeutic drugs in cholangiocarcinoma cells are not unique to CD133+ cells.
In conclusion, our findings indicate that CD133+ cells have higher invasive abilities than CD133- cells. CD133 expression can be found in cholangiocarcinoma, and it significantly correlates with lymph node metastasis and surgical margin status. CD133 may be a potential prognostic factor of cholangiocarcinoma.
COMMENTS
Background
Cholangiocarcinoma is a malignancy arising from the biliary tract. Cholangiocarcinoma is one of the most common causes of cancer death in Thailand. A recent study suggested that expression of CD133 in cholangiocarcinoma specimens is associated with the severity of this disease.
Research frontiers
CD133+ cholangiocarcinoma cells have a high ability to invade through Matrigel. In addition, expression of CD133 in cholangiocarcinoma specimens is associated with cholangiocarcinoma progression.
Innovations and breakthroughs
This is believed to be the first report to show that, in cholangiocarcinoma cell lines with high CD133 expression, the neoplastic behavior is more aggressive than in CD133- cells.
Applications
Strong CD133 expression was observed in 67.6% of the cholangiocarcinoma specimens and was significantly associated with nodal metastasis and positive surgical margin status. Therefore, the authors suggest that CD133+ cells play an important role in the invasiveness and progression of cholangiocarcinoma. Targeting of the CD133+ cholangiocarcinoma cells may be a useful approach to improve therapies directed against cholangiocarcinoma.
Terminology
CD133, a human homologue of a mouse prominin-1, is a 5-transmembrane cell-surface glycoprotein. It has been detected in an enrichment of hematopoietic stem cells derived from fetal liver or bone marrow neuroepithelial cells, embryonic epithelial cells and adult immature epithelial cells.
Peer review
This study provides evidence that high CD133 expression in cholangiocarcinoma cells may be used as a marker for targeted cancer therapy. This was a well performed and clearly presented study.
Footnotes
Supported by Rajavithi Hospital Project Grant and Thailand Research Fund (RSA52)
Peer reviewers: Yoshiaki Murakami, MD, Department of Surgery, Division of Clinical Medical Science, Graduate School of Biomedical Science, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan; Ching Chung Lin, MD, MMS, Division of Gastroenterology, Department of Internal Medicine, Mackay Memorial Hospital, Taipei 111, Taiwan, China
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
References
- 1.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akoad M, Jenkins R. Proximal biliary malignancy. Surg Clin North Am. 2008;88:1409–1428, x-xi. doi: 10.1016/j.suc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Sano T, Shimada K, Sakamoto Y, Ojima H, Esaki M, Kosuge T. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol. 2008;15:590–599. doi: 10.1245/s10434-007-9687-y. [DOI] [PubMed] [Google Scholar]
- 4.Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol. 2007;96:160–165. doi: 10.1002/jso.20792. [DOI] [PubMed] [Google Scholar]
- 5.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 6.Denysenko T, Gennero L, Roos MA, Melcarne A, Juenemann C, Faccani G, Morra I, Cavallo G, Reguzzi S, Pescarmona G, et al. Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct. 2010;28:343–351. doi: 10.1002/cbf.1666. [DOI] [PubMed] [Google Scholar]
- 7.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Wu PY. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009;18:1127–1134. doi: 10.1089/scd.2008.0338. [DOI] [PubMed] [Google Scholar]
- 9.Song W, Li H, Tao K, Li R, SongZ , ZhaoQ , ZhangF , DouK Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–1218. doi: 10.1111/j.1742-1241.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 10.Li CY, Li BX, Liang Y, Peng RQ, Ding Y, Xu DZ, Zhang X, Pan ZZ, Wan DS, Zeng YX, et al. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. J Transl Med. 2009;7:56. doi: 10.1186/1479-5876-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rattanasinganchan P, Leelawat K, Treepongkaruna SA, Tocharoentanaphol C, Subwongcharoen S, Suthiphongchai T, Tohtong R. Establishment and characterization of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient. World J Gastroenterol. 2006;12:6500–6506. doi: 10.3748/wjg.v12.i40.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin K, Jiang X, Zou Y, Wang J, Qin L, Zeng Y. Study on the proliferation and drug-resistance of human brain tumor stem-like cells. Cell Mol Neurobiol. 2010;30:955–960. doi: 10.1007/s10571-010-9525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimada M, Sugimoto K, Iwahashi S, Utsunomiya T, MorineY , ImuraS , IkemotoT CD133 expression is a potential prognostic indicator in intrahepatic cholangiocarcinoma. J Gastroenterol. 2010;45:896–902. doi: 10.1007/s00535-010-0235-3. [DOI] [PubMed] [Google Scholar]
- 15.Moriyama T, Ohuchida K, Mizumoto K, CuiL , IkenagaN , Sato N, TanakaM Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010;116:3357–3368. doi: 10.1002/cncr.25121. [DOI] [PubMed] [Google Scholar]
- 16.Ding W, You H, Dang H, LeBlanc F, GaliciaV , LuSC , StilesB , RountreeCB Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology. 2010;52:945–953. doi: 10.1002/hep.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng X, Li M, Wang X, Wang Y, Ma D. Both CD133+ and CD133- subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer Sci. 2009;100:1040–1046. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leelawat K, Narong S, Udomchaiprasertkul W, Leelawat S, Tungpradubkul S. Inhibition of PI3K increases oxaliplatin sensitivity in cholangiocarcinoma cells. Cancer Cell Int. 2009;9:3. doi: 10.1186/1475-2867-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]



