Abstract
AIM: To quantitatively assess the relationship between coffee consumption and incidence of pancreatic cancer in a meta-analysis of cohort studies.
METHODS: We searched MEDLINE, EMBASE, Science Citation Index Expanded and bibliographies of retrieved articles. Studies were included if they reported relative risks (RRs) and corresponding 95% CIs of pancreatic cancer with respect to frequency of coffee intake. We performed random-effects meta-analyses and meta-regressions of study-specific incremental estimates to determine the risk of pancreatic cancer associated with a 1 cup/d increment in coffee consumption.
RESULTS: Fourteen studies met the inclusion criteria, which included 671 080 individuals (1496 cancer events) with an average follow-up of 14.9 years. Compared with individuals who did not drink or seldom drank coffee per day, the pooled RR of pancreatic cancer was 0.82 (95% CI: 0.69-0.95) for regular coffee drinkers, 0.86 (0.76-0.96) for low to moderate coffee drinkers, and 0.68 (0.51-0.84) for high drinkers. In subgroup analyses, we noted that, coffee drinking was associated with a reduced risk of pancreatic cancer in men, while this association was not seen in women. These associations were also similar in studies from North America, Europe, and the Asia-Pacific region.
CONCLUSION: Findings from this meta-analysis suggest that there is an inverse relationship between coffee drinking and risk of pancreatic cancer.
Keywords: Coffee, Cohort study, Meta-analysis, Pancreatic neoplasm
INTRODUCTION
Coffee is one of the most widely consumed beverages in the world, with a yearly world average consumption of 1.1 kg per capita, which reaches 4.5 kg in industrialized countries[1]. More recently, coffee consumption has been associated with a reduction in the risk of several chronic diseases, including type 2 diabetes mellitus, Parkinson’s disease and liver disease[2-4]. Of these associations, the relationship between coffee drinking and cancer risk is of great interest.
Coffee consumption may exert an anticarcinogenic effect in some organs. For example, a reduction in cholesterol, bile acid, and neutral sterol secretion in the colon is a direct effect of coffee consumption as is increased colonic motility, which can reduce exposure of epithelium to carcinogens[5]. The components of coffee which have received attention are caffeine (a purine alkaloid), cafestol (a diterpene), kahweol (another diterpene), and chlorogenic acid (a dietary phenol). Cafestol and kahweol, which are active ingredients in the coffee oil, decrease mutagenesis and tumorigenesis in animal models. Diterpenes, found in coffee, reduce genotoxicity of carcinogens and lower DNA adduct formation. Caffeic acid and chlorogenic acid are antioxidants and have been reported to decrease DNA methylation[6]. More subtly, caffeine itself appears to be protective-affecting cell cycle, proliferation, and apoptosis[7,8].
Pancreatic cancer is one of the most aggressive and treatment-refractory malignancies in humans. Given that there is no screening test for the early detection of this cancer and no effective treatment to prolong survival time, primary prevention appears to be the most important way of reducing pancreatic cancer mortality. Over the last 4 decades, a number of epidemiologic studies have estimated the association between coffee consumption and pancreatic cancer occurrence. However, the results of these studies were inconsistent. Data from case-control studies may be subject to recall bias with respect to coffee consumption and selection bias with respect to the control group. Additional prospective cohort studies excluding those biases would be more useful for assessing coffee-cancer associations. We, therefore, systematically reviewed and performed a meta-analysis of prospective cohort studies to quantitatively assess the association between coffee intake and pancreatic cancer risk in humans. Because of the high consumption of coffee, even small effects on pancreatic cancer occurrence could have a large impact on public health.
MATERIALS AND METHODS
Literature search
We searched the electronic databases MEDLINE (1966 to August 2010), EMBASE (1985 to August 2010), and Science Citation Index Expanded (1945 to August 2010), using the Medical Subject Heading (MeSH) term coffee combined with pancreatic cancer or pancreatic neoplasm or pancreatic carcinoma. Furthermore, we reviewed reference lists of retrieved articles to search for more studies. Articles published in any language were included.
Inclusion and exclusion criteria
For inclusion, studies had to fulfill the following criteria: have a prospective cohort design; report relative risks (RRs) or hazard ratios and their corresponding 95% CIs (or data to calculate them) of pancreatic cancer relating to every category of coffee intake; and provide the frequency of coffee consumption. Studies were excluded if: case-control design was used; mixed beverage was reported, in which the effect of coffee could not be separated; only surrogate nutrients of coffee were reported; no categories of coffee intake were reported that could not allow for adequate classification of intake. If multiple published reports from the same study cohort were available, we included only the one with the most detailed information for both outcome and coffee consumption.
Data extraction
Data were extracted independently by two investigators (Yu and Dong) according to the meta-analysis of observation studies in epidemiology guidelines[9], and discrepancies were resolved by discussion with a third investigator (Zou). For each study, the following information was extracted: first author’s last name; year of publication; country of origin; follow-up period; number of subjects and cases; age at baseline; category amounts of coffee intake; outcome assessment; RRs or hazard ratios of pancreatic cancer and corresponding 95% CIs for every category of coffee intake; and covariates adjusted in the statistical analysis.
Statistical analysis
The measures of interest were the RR and the corresponding 95% CIs for included cohort studies. When RRs were not available in the published article, they were computed from the exposure distributions. Because various studies used different measurement units for coffee consumption, we converted these into cups per day as a standard measure. If coffee consumption was indicated by milliliter, we assumed 125 mL as approximately equivalent to 1 cup.
We computed the summary RR for coffee drinkers vs nondrinkers and for different levels of consumption by giving each study-specific RR a weight that was proportional to its precision (i.e. the inverse of the variance derived, when necessary, from the reported 95% CIs). To estimate the summary RR for various levels of coffee consumption, we first calculated the study-specific estimate separately for low to moderate consumption and high consumption.
Statistical heterogeneity among studies was estimated using Q and I2 statistics. For the Q statistic, heterogeneity was considered present when P < 0.1. We pooled the study-specific estimates using both the fixed effect model and the random effect model proposed by DerSimonian and Laird; when a significant heterogeneity was found, the random effect model results were presented. A sensitivity analysis was also conducted, in which one study at a time was removed and the rest analyzed to estimate whether the results could have been affected markedly by a single study.
For dose-response analysis, we used the method proposed by Greenland et al[10] to estimate study-specific slopes from the correlated natural logarithm of the RR across categories of coffee consumption, assigning to each class the dose corresponding to the midpoint of upper and lower boundaries. The highest, open-ended category was assumed to have the same amplitude of consumption as the preceding category[11]. Then the summary RR for pancreatic cancer risk with a 1 cup/d increment in coffee consumption was obtained by pooling the study-specific slopes, using the inverse of the corresponding variances as weights.
Finally, publication bias was evaluated through funnel plot visual analysis and with the Begg’s and Egger’s tests. P < 0.05 was considered statistically significant. All statistical analyses were performed with STATA (version 9.0; Stata Co., College Station, TX, USA).
RESULTS
Using the predefined search strategy, we identified 14 prospective cohort studies (Figure 1), including 671 080 participants and 1496 incident cases of pancreatic cancer with an average follow-up of 14.9 years, which were eligible for inclusion in the meta-analysis[12-25]. The characteristics of the included studies are summarized in Table 1. Initial agreement between the two reviewers on whether a study was eligible for inclusion occurred in 48/50 manuscripts (96%; κ = 0.92). Of the 14 cohorts included in the meta-analysis, 4 were conducted in Europe (Norway, Sweden and Finland), 6 in North America (the United States), and 4 in Asia (Japan).
Figure 1.
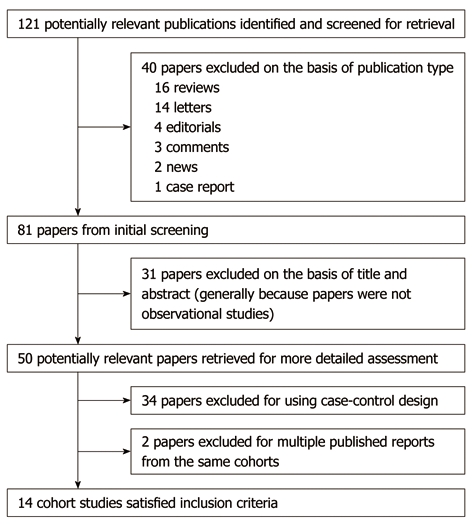
Flow diagram of search strategy and study selection.
Table 1.
Summary characteristics of studies included in the meta-analysis
| Study | Country | Follow-up period | Study subjects | No. of cases | Coffee consumption | Relative risk (95% CI) | Adjustments |
| Snowdon et al[12] | United States | 1960-1980 | 23 912 | 71 | < 1 cup/d | 1.00 (reference) | Age, sex |
| Aged ≥ 30 yr | 1 cup/d | 1.7 (0.9-3.3) | |||||
| 1984 | ≥ 2 cups/d | 0.8 (0.4-1.6) | |||||
| Jacobsen et al[13] | Norway | 1967-1978 | 16 555 | 63 | ≤ 2 cups/d | 1.00 (reference) | Sex, age and residence (men for cigarette smoking) |
| 13 664 male | 3-4 cups/d | 1.22 (0.66-2.35) | |||||
| 1986 | 2891 female | 5-6 cups/d | 0.53 (0.21-1.26) | ||||
| ≥ 7 cups/d | 0.62 (0.18-1.75) | ||||||
| Nomura et al[14] | Japan | 1965-1983 | 7355 male | 21 | 0 cup/d | 1.00 (reference) | Age, years of smoking, number of cigarettes smoked per day, smoking status at exam, and past smoking status |
| 1-2 cups/d | 0.83 (0.16-5.34) | ||||||
| 1986 | 3-4 cups/d | 1.39 (0.32-8.31) | |||||
| ≥ 5 cups/d | 1.27 (0.27-7.84) | ||||||
| Hiatt et al[15] | United States | 1978-1984 | 122 894 | 49 | < 1 cup/d | 0.4 (0.1-4.3) | Age, sex, ethnic origin, blood glucose levels, consumption of alcohol, tea |
| 1988 | 1-3 cups/d | 1.2 (0.3-4.4) | |||||
| > 4 cups/d | 0.8 (0.2-4.6) | ||||||
| Zheng et al[16] | United States | 1966-1986 | 17 633 male | 57 | < 3 cups/d | 1.00 (reference) | Age, smoking index, alcohol index |
| Aged ≥ 35 yr | 3-4 cups/d | 0.6 (0.3-1.2) | |||||
| 1993 | 5-6 cups/d | 0.7 (0.4-1.6) | |||||
| ≥ 7 cups/d | 0.9 (0.3-2.4) | ||||||
| Shibata et al[17] | United States | 1981-1990 | 13 979 | 63 | < 1 cup/d | 1.00 (reference) | Sex, age and cigarette smoking |
| 1 cup/d | 1.82 (0.75-4.43) | ||||||
| 1994 | 2-3 cups/d | 1.67 (0.74-3.77) | |||||
| ≥ 4 cups/d | 0.88 (0.28-2.80) | ||||||
| Stensvold et al[18] | Norway | 1977-1990 | 42 973 | 41 | ≤ 2 cups/d | 1.00 (reference) | Age, cigarette smoking, county of residence |
| 21 735 male | 26 M | 3-4 cups/d | 2.76 (0.63-25.21) | ||||
| 1994 | 21 238 female | 15 F | 5-6 cups/d | 3.09 (0.72-27.87) | |||
| Aged 35-54 yr | ≥ 7 cups/d | 2.71 (0.59-25.15) | |||||
| Zheng et al[19] | United States | 1986-1993 | 35 369 female | 66 | Never/monthly | 1.00 (reference) | Age, education, smoking status, pack-years of smoking, physical activity, all fruit and vegetable intake, total energy intake, waist/hip ratio, family history of cancer, prior history of blood transfusion |
| Aged 55-69 yr | Weekly-3 cups/d | 1.82 (0.87-3.82) | |||||
| 1996 | ≥ 4 cups/d | 2.15 (1.01-4.07) | |||||
| Michaud et al[20] | United | 1986-1998 | 136 593 | 288 | None | 1.00 (reference) | Age in 5-yr categories, pack-years of smoking, bidy mass index, history of diabetes mellitus, history of cholecystectomy, energy intake, and period |
| States | 1980-1996 | 47 794 male | 130 M | < 1 cup/d | 0.94 (0.65-1.36) | ||
| 2001 | Aged 40-75 yr | 158 F | 1 cup/d | 0.60 (0.38-0.94) | |||
| 88 799 female | 2-3 cups/d | 0.88 (0.65-1.21) | |||||
| Aged 30-55 yr | > 3 cups/d | 0.62 (0.27-1.43) | |||||
| Isaksson et al[21] | Sweden | 1961-1997 | 21 884 | 131 | 0-2 cups/d | 1.00 (reference) | Sex, age, cigarette smoking |
| 9680 male | 3-6 cups/d | 0.91 (0.60-1.38) | |||||
| 2002 | 12 204 female | ≥ 7 cups/d | 0.39 (0.17-0.89) | ||||
| Aged 36-75 yr | |||||||
| Lin et al[22] | Japan | 1988-1997 | 99 527 | 225 | Nondrinkers | Age, cigarette smoking in pack-years | |
| 2002 | 44 646 male | 1-2 cups/mo | |||||
| 54 881 female | 1-4 cups/wk | ||||||
| Aged 40-79 yr | 1 cup/d | ||||||
| 2-3 cups/d | |||||||
| ≥ 4 cups/d | |||||||
| Stolzenberg-Solomon et al[23] | Finland | 1985-1997 | 27 111 male | 163 | ≤ 321.4 g/d | 1.00 (reference) | Age, years of smoking |
| Aged 50-69 yr | > 321.4-≤ 450.0 g/d | 1.48 (0.89-2.46) | |||||
| > 450.0-≤ 624.9 g/d | 1.12 (0.61-2.03) | ||||||
| 2002 | > 624.9-≤ 878.6 g/d | 1.72 (1.01-2.86) | |||||
| > 878.6 g/d | 0.95 (0.54-1.68) | ||||||
| Khan et al[24] | Japan | 1984-2002 | 3158 | 25 | ≤ several times/mo | 1.00 (reference) | Age, sex, health education, health examination, health status, moking |
| 2004 | 1524 male | 12 M | ≥ several times/wk | 0.38 (0.01-1.05) | |||
| 1634 female | 13 F | ||||||
| Aged ≥ 40 yr | |||||||
| Luo et al[25] | Japan | 1990-2003 | 102 137 | 233 | Rarely | 1.00 (reference) | Body mass index, frequency of sports, smoking status, alcohol intake, history of diabetes, history of cholelithiasis, study area, age and tea consumption |
| 2007 | 48 783 male | 135 M | 1-2 cups/wk | 1.0 (0.7-1.4) | |||
| 53 354 female | 98 F | 3-4 cups/wk | 1.1 (0.7-1.7) | ||||
| Aged 40-69 yr | 1-2 cups/d | 0.9 (0.6-1.3) | |||||
| ≥ 3 cups/d | 0.8 (0.4-1.3) |
Figure 2A shows the estimated RRs for coffee drinkers vs non/lowest drinkers from the cohort studies. The summary RR of pancreatic cancer from all combined studies was 0.82 (95% CI: 0.69-0.95). There was significant heterogeneity across the studies (Q = 21.88, P = 0.057, I2 = 40.6%). Figure 2B and C give the RRs according to low to moderate and high coffee consumption from various studies. The summary RR was 0.86 (95% CI: 0.76-0.96) for low to moderate coffee consumption, with no heterogeneity between studies (Q = 16.12, P = 0.186, I2 = 25.6%). The summary RR for high consumption of coffee was 0.68 (95% CI: 0.51-0.84), also with no heterogeneity between studies (Q = 7.82, P = 0.729, I2 = 0%). The summary RR for an increment of 1 cup of coffee per day was 0.96 (95% CI: 0.90-1.02) for all studies combined, but was statistically insignificant.
Figure 2.
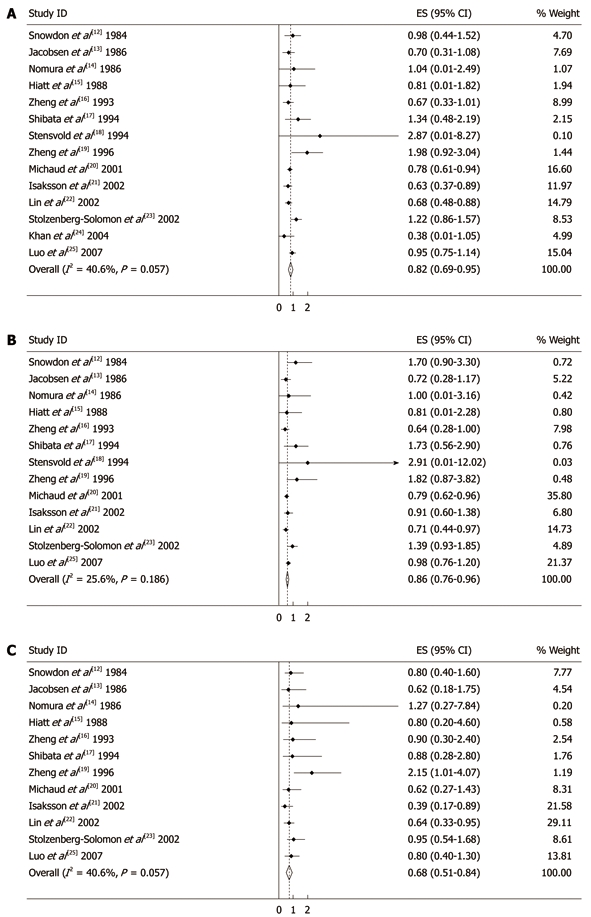
Summary relative risks of pancreatic cancer for coffee drinkers vs non/lowest drinkers from included studies (A), low to moderate coffee drinkers vs non/lowest drinkers from included studies (B) and high coffee drinkers vs non/lowest drinkers from included studies (C). Weights are from random effect analysis. Squares represent study-specific relative risk estimates (size of the square reflects the study-specific statistical weight, that is, the inverse of the variance); horizontal lines represent 95% CIs; diamonds represent summary relative risk estimates with corresponding 95% CIs.
Various sources of heterogeneity likely exist due to international differences in coffee consumption (e.g. coffee type, serving size, or brewing method) in this analysis. To examine the magnitude of the combined RR in each stratum and its respective test of heterogeneity, we conducted subgroup analyses by gender and geographic regions. The summary RR was 0.73 (95% CI: 0.63-0.84) for men and 0.82 (95% CI: 0.52-1.11) for women when combining all studies. There was no heterogeneity for men (Q = 9.01, P = 0.252, I2 = 22.3%) and a significant heterogeneity for women (Q = 12.42, P = 0.029, I2 = 59.7%).
Associations were also similar in studies from North America, Europe and the Asia-Pacific region. The RR was 0.81 (95% CI: 0.67-0.95) when considering the 6 studies conducted in North America, 0.86 (95% CI: 0.50-1.22) for the 4 studies from Europe, and 0.76 (95% CI: 0.52-0.99) for the 4 Asian studies. No significant differences by sex were found.
There was no indication of publication bias from either visualization of the funnel plot or Egger’s (P = 0.735) and Begg’s (P = 0.381) (Figure 3) tests. A sensitivity analysis, in which one study was removed at a time, was performed to evaluate the stability of the results. This analysis confirmed the stability of our results.
Figure 3.
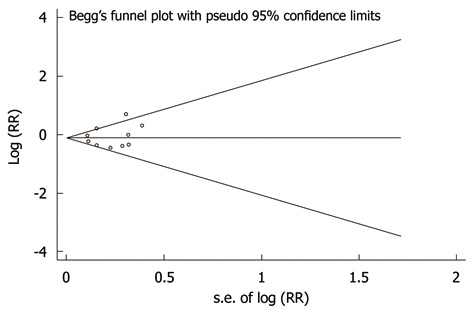
Publication bias in the studies. Begg’s funnel plot indicating no publication bias in the studies included in this meta-analysis. No indication of publication bias was noted from both visualization of funnel plot and Egger’s test. RR: Relative risk.
DISCUSSION
Coffee consumption, as a major and frequent dietary exposure in diverse cultures around the globe, has been shown to be associated with pancreatic cancer in epidemiological studies. However, there is no comprehensive, up-to-date overview of the entirety of the substantial body of epidemiologic evidence. To address this need, we quantitatively assessed the relationship between coffee intake and incidence of pancreatic cancer in a meta-analysis of cohort studies.
Coffee can potentially impact the etiology of cancer of various sites along multiple pathways, ranging from carcinogenesis to cellular apoptosis. A number of in vitro studies suggest that caffeine can influence carcinogenesis through inhibition of DNA repair, and induction of mitotic events before DNA replication is completed[26-28]. Porta et al[29] argues that in exocrine pancreatic cancer, caffeine, other coffee compounds, or other correlates of coffee drinking could modulate Ki-ras activation by interfering with DNA repair, cell-cycle checkpoints, and apoptosis. However, for most cancer sites, there is a significant amount of evidence to show that there is no detrimental effect following the consumption of up to 6 cups of coffee per day in relation to cancer occurrence. Through the meta-analysis of cohort studies, we found that compared with individuals who did not drink or seldom drank coffee per day, the pooled RR of pancreatic cancer was 0.82 (95% CI: 0.69-0.95) for regular coffee drinkers, 0.86 (0.76-0.96) for low to moderate coffee drinkers, and 0.68 (0.51-0.84) for high drinkers. Overall, an increase in consumption of 1 cup of coffee per day was associated with a 4% reduced risk of pancreatic cancer (RR, 0.96; 95% CI: 0.90-1.02). Thus, the evidence presented above suggests that coffee intake might prevent pancreatic cancer occurrence in humans.
Over the past two decades, many studies have been carried out on coffee and pancreatic cancer following the early warning in the early 1980s that coffee consumption was related to pancreatic cancer risk. Some ecological[30], case-control[31], and cohort[20,22] studies carried out in the USA, Canada, Europe and Asia investigated the relationship between coffee consumption and the risk of pancreatic cancer. In general, these investigations yielded inconsistent results, with a meta-analysis on 25 case-control studies giving a summary effect estimate of 1.04 (95% CI: 1.00-1.07) and a summary RR of 1.00 (0.94-1.07) per 1 cup/d for 10 cohort studies[5]. Since the WCRF report, Luo et al[25] studied the association between drinking coffee and the risk of pancreatic cancer in a large population-based cohort study in Japan. Among 102 137 participants followed for an average of 11 years in which 233 incident cases of pancreatic cancer were identified, there was no increased risk of pancreatic cancer with coffee intake. A reduced risk was apparent among men who drank at least 3 cups of coffee per day compared with those who did not drink any or only rarely drank coffee. After a pooled analysis of 14 cohort studies, we found that there was a reverse association between coffee consumption and the risk of pancreatic cancer.
Some limitations of this meta-analysis should be acknowledged. First, as in all observational studies of diet and disease, the possibility of bias and confounding can not be excluded. However, cohort studies, which are less susceptible to bias because of the prospective design, also showed an inverse association between coffee consumption and risk of pancreatic cancer, suggesting that the finding is not likely attributable to recall and selection bias. Individual studies may have failed to adjust for potential known or unknown confounders. Second, our results are likely to be affected by the misclassification of coffee consumption. Coffee exposure is mostly assessed in relation to the number of cups of coffee consumed daily, weekly or monthly. However, most of the studies included in our meta-analysis did not provide information on coffee type, serving size, or brewing method. Serving sizes and brewing methods for coffee can vary substantially within and between countries. Standard coffee cups are larger in the United States than in Europe or Japan, and the difference in the strength of the coffee brewed may compensate for the different serving size between countries[32]. Third, we extracted the risk estimates that reflected the greatest degree of the control potential confounders, because it was hard to obtain raw data from each study to conduct standardized adjustments. Therefore, it is probable that the results based on the adjustment for different confounders were different from those based on standardized adjustments. Finally, only published studies were included in our meta-analysis. Therefore, publication bias may have occurred although no publication bias was indicated from both visualization of the funnel plot and Egger’s test.
In summary, there is substantial evidence from both laboratory and animal studies on the favorable influence of coffee on the risk of pancreatic cancer. Although well designed studies, in particular randomized clinical studies among high risk populations, are needed to provide valuable insights into coffee consumption and the risk of pancreatic cancer, our meta-analysis which included 14 prospective cohort studies confirmed that coffee consumption is inversely associated with the risk of pancreatic cancer.
COMMENTS
Background
Coffee consumption, as a major and frequent dietary exposure in diverse cultures around the globe, has been shown to be associated with pancreatic cancer in epidemiological studies. However, there is no comprehensive, up-to-date overview of the entirety of the substantial body of epidemiologic evidence.
Research frontiers
Over the past two decades, many studies have been carried out on coffee and pancreatic cancer. Some ecological, case-control, and cohort studies carried out in the USA, Canada, Europe and Asia investigated the relationship between coffee consumption and the risk of pancreatic cancer. However, these investigations yielded inconsistent results, with a meta-analysis on 25 case-control studies giving a summary effect estimate of 1.04 and a summary relative risk (RR) of 1.00 per 1 cup/d for 10 cohort studies.
Innovations and breakthroughs
Findings from this meta-analysis suggested that, compared with individuals who did not drink or seldom drank coffee per day, the pooled RR of pancreatic cancer was 0.82 for regular coffee drinkers, 0.86 for low to moderate coffee drinkers, and 0.68 for high drinkers. Coffee drinking was associated with a reduced risk of pancreatic cancer for men, while this association was not seen in women.
Applications
Coffee consumption may reduce pancreatic cancer incidence and it has a consistent preventive effect on some type of cancers. These epidemiological observations should provide information for the exploration of biological mechanisms involved in the inverse relationship between coffee drinking and risk of pancreatic cancer.
Terminology
Roasted coffee is a complex mixture of more than one thousand chemicals. Many of these constituents could potentially alter cancer risk through several biological mechanisms. The anticarcinogenic components in coffee which have received attention are caffeine, cafestol, kahweol, polyphenols, caffeic acid and chlorogenic acid.
Peer review
Dr. Dong et al showed that coffee consumption may reduce the pancreatic cancer incidence, using meta-analysis. The results are very interesting.
Acknowledgments
We thank the authors who kindly provided the data necessary for our meta-analysis.
Footnotes
Peer reviewer: Mitsuyoshi Urashima, MD, PhD, MPH, Division of Molecular Epidemiology, Jikei University School of Medicine, 3-25-8 Nishi-shimbashi, Minato-ku, Tokyo 105-8461, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
References
- 1. Food and Agricultural Organization. Food balance sheets. Accessed on Apr 2004. Available from: http://www.fao.org/waicent/portal/statistics_en.asp.
- 2.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 3.Sääksjärvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Männistö S. Prospective study of coffee consumption and risk of Parkinson's disease. Eur J Clin Nutr. 2008;62:908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 4.Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201–209. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post SM, de Roos B, Vermeulen M, Afman L, Jong MC, Dahlmans VE, Havekes LM, Stellaard F, Katan MB, Princen HM. Cafestol increases serum cholesterol levels in apolipoprotein E*3-Leiden transgenic mice by suppression of bile acid synthesis. Arterioscler Thromb Vasc Biol. 2000;20:1551–1556. doi: 10.1161/01.atv.20.6.1551. [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006;27:269–277. doi: 10.1093/carcin/bgi206. [DOI] [PubMed] [Google Scholar]
- 7.Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007;247:26–39. doi: 10.1016/j.canlet.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito K, Nakazato T, Miyakawa Y, Yamato K, Ikeda Y, Kizaki M. Caffeine induces G2/M arrest and apoptosis via a novel p53-dependent pathway in NB4 promyelocytic leukemia cells. J Cell Physiol. 2003;196:276–283. doi: 10.1002/jcp.10289. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 11.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 12.Snowdon DA, Phillips RL. Coffee consumption and risk of fatal cancers. Am J Public Health. 1984;74:820–823. doi: 10.2105/ajph.74.8.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen BK, Bjelke E, Kvåle G, Heuch I. Coffee drinking, mortality, and cancer incidence: results from a Norwegian prospective study. J Natl Cancer Inst. 1986;76:823–831. [PubMed] [Google Scholar]
- 14.Nomura A, Heilbrun LK, Stemmermann GN. Prospective study of coffee consumption and the risk of cancer. J Natl Cancer Inst. 1986;76:587–590. doi: 10.1093/jnci/76.4.587. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt RA, Klatsky AL, Armstrong MA. Pancreatic cancer, blood glucose and beverage consumption. Int J Cancer. 1988;41:794–797. doi: 10.1002/ijc.2910410603. [DOI] [PubMed] [Google Scholar]
- 16.Zheng W, McLaughlin JK, Gridley G, Bjelke E, Schuman LM, Silverman DT, Wacholder S, Co-Chien HT, Blot WJ, Fraumeni JF Jr. A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States) Cancer Causes Control. 1993;4:477–482. doi: 10.1007/BF00050867. [DOI] [PubMed] [Google Scholar]
- 17.Shibata A, Mack TM, Paganini-Hill A, Ross RK, Henderson BE. A prospective study of pancreatic cancer in the elderly. Int J Cancer. 1994;58:46–49. doi: 10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- 18.Stensvold I, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 1994;5:401–408. doi: 10.1007/BF01694753. [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Doyle TJ, Kushi LH, Sellers TA, Hong CP, Folsom AR. Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1996;144:175–182. doi: 10.1093/oxfordjournals.aje.a008905. [DOI] [PubMed] [Google Scholar]
- 20.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomarkers Prev. 2001;10:429–437. [PubMed] [Google Scholar]
- 21.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin Registry. Int J Cancer. 2002;98:480–482. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Tamakoshi A, Kawamura T, Inaba Y, Kikuchi S, Motohashi Y, Kurosawa M, Ohno Y. Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Cancer. 2002;99:742–746. doi: 10.1002/ijc.10402. [DOI] [PubMed] [Google Scholar]
- 23.Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002;155:783–792. doi: 10.1093/aje/155.9.783. [DOI] [PubMed] [Google Scholar]
- 24.Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, Sakauchi F, Washio M, Mori M. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev. 2004;5:58–65. [PubMed] [Google Scholar]
- 25.Luo J, Inoue M, Iwasaki M, Sasazuki S, Otani T, Ye W, Tsugane S. Green tea and coffee intake and risk of pancreatic cancer in a large-scale, population-based cohort study in Japan (JPHC study) Eur J Cancer Prev. 2007;16:542–548. doi: 10.1097/CEJ.0b013e32809b4d30. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlmann W, Fromme HG, Heege EM, Ostertag W. The mutagenic action of caffeine in higher organisms. Cancer Res. 1968;28:2375–2389. [PubMed] [Google Scholar]
- 27.Selby CP, Sancar A. Molecular mechanisms of DNA repair inhibition by caffeine. Proc Natl Acad Sci USA. 1990;87:3522–3525. doi: 10.1073/pnas.87.9.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlegel R, Pardee AB. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science. 1986;232:1264–1266. doi: 10.1126/science.2422760. [DOI] [PubMed] [Google Scholar]
- 29.Porta M, Ayude D, Alguacil J, Jariod M. Exploring environmental causes of altered ras effects: fragmentation plus integration? Mol Carcinog. 2003;36:45–52. doi: 10.1002/mc.10093. [DOI] [PubMed] [Google Scholar]
- 30.Vioque J, González Sáez L, Cayuela Domínguez A. [Cancer of the pancreas: an ecologic study] Med Clin (Barc) 1990;95:121–125. [PubMed] [Google Scholar]
- 31.Ghadirian P, Simard A, Baillargeon J. Tobacco, alcohol, and coffee and cancer of the pancreas. A population-based, case-control study in Quebec, Canada. Cancer. 1991;67:2664–2670. doi: 10.1002/1097-0142(19910515)67:10<2664::aid-cncr2820671043>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP. Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology. 2002;13:165–171. doi: 10.1097/00001648-200203000-00011. [DOI] [PubMed] [Google Scholar]


