Abstract
The aim of this study was to assess the cytotoxic effects of various concentrations of miltefosine on Leishmania major (MRHO/IR/75/ER) and L. tropica (MHOM/IR/02/Mash10) promastigotes and to observe the programmed cell death features. The colorimetric MTT assay was used to find L. major and L. tropica viability and the obtained results were expressed as 50% inhibitory concentration (IC50). Also, 50% effective doses (ED50) for L. major and L. tropica amastigotes were also determined. Annexin-V FLUOS staining was performed to study the cell death properties of miltefosine using FACS analysis. Qualitative analysis of the total genomic DNA fragmentation was performed by agarose gel electrophoresis. Furthermore, to observe changes in cell morphology, promastigotes were examined using light microscopy. In both strains of L. major and L. tropica, miltefosine induced dose-dependent death with features of apoptosis, including cell shrinkage, DNA laddering, and externalization of phosphatidylserine. The IC50 was achieved at 22 µM and 11 µM for L. major and L. tropica after 48 hr of incubation, respectively. ED50 of L. major and L. tropica amastigotes were 5.7 µM and 4.2 µM, respectively. Our results indicate that miltefosine induces apoptosis of the causative agent of cutaneous leishmaniasis in a dose-dependent manner. Interestingly, L. major did not display any apoptotic changes when it was exposed to miltefosine in concentrations sufficient to kill L. tropica.
Keywords: Leishmania major, Leishmania tropica, miltefosine, apoptosis, IC50, ED50
INTRODUCTION
Programmed cell death or apoptosis in all invertebrate and vertebrate multicellular organisms has been studied to include nematodes, insects, amphibians, and mammal [1-4]. Apoptosis is associated with characteristic morphological and biochemical changes, including cell shrinkage, membrane blebbing, cell surface changes, and loss of mitochondrial function [5]. These cell membrane alterations include exposure of phosphatidylserine residues on the outer leaflet of the plasma membrane [6]. Apoptosis also displays characteristic nuclear changes. The chromatin undergoes condensation as endonucleases are activated and the DNA begins to fragment [5].
It was initially believed that apoptosis does not occur in unicellular organisms but, to date, there is enough evidence to confirm that this phenomenon also occurs in single-cell organisms [7,8]. The mechanisms and pathways that lead to induction or inhibition of apoptosis in Leishmania spp. are of particular interest as they will be potential targets for development of anti-Leishmania medications. Leishmaniasis is one of the significant causes of morbidity and mortality in several countries. This disease affects 12 million people and threatens additional 350 million persons worldwide [9]. Leishmaniasis is manifested in several forms, including visceral, mucocutaneous, or cutaneous. Leishmania major and L. tropica are causative agents of old world cutaneous leishmaniasis (CL) in Middle East and Iran [10,11].
Antimonial compounds, particularly meglumine antimoniate are the first line drugs for the treatment of all forms of leishmaniasis in Iran [12]. Recent circumstantial evidence suggested that an increasing number of Iranian patients with CL is unresponsive to meglumine antimoniate [13]. Although pentavalent antimonials, paromomycin and fluconazole have been used in the treatment of CL, these medications have several limitations, including resistance to pentavalent antimonial drugs, parenteral route of administration, long duration of treatment, and unwanted side effects [14]. Oral miltefosine (hexadecylphosphocholine: HePC), an antitumor agent, has been used for treatment of visceral leishmaniasis in India [15]. A number of studies have been performed to elucidate the mechanism of action of HePC. The antineoplastic activity of HePC has been attributed to its apoptosis-inducing potential [16]. Apoptosis has also been proposed as the mechanism of antiprotozoal activity of this medication [17].
In the present study, we evaluated the dose-dependent leishmanicidal activity of miltefosine on different causative agents of CL in Iran.
MATERIALS AND METHODS
Miltefosine (1-O-hexadecylphosphocholine), with the structural formula of C21H46NO4P and molecular weight of 407.57, were prepared from Zentaris GmbH (Zentaris, GmbH, and Frankfurt, Germany). MTT colorimetric assay kit was purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). Furthermore, the Annexin-V FLUOS Staining Kit was purchased from Roche-applied-science (Berlin, Germany). J774 A.1 mouse macrophage cell line was purchased from Pasteur Institute (Tehran, Iran). L. major (MRHO/IR/75/ER) and L. tropica (MHOM/IR/02/Mash 10) were kindly provided by Dr. Mohebali (Tehran University of Medical Sciences, Tehran, Iran).
Culture of L. major and L. tropica
Briefly, 5×105 cells/ml were cultured in the RPMI 1640 media (pH 7.2, containing 25 mM HEPES) (Sigma) supplemented with 10% heat inactivated fetal bovine serum and antibiotics at 24℃ for 96 hr and subcultured at cell densities of 2×107 to 2.5×107 cells/ml. After subculture, promastigotes were seeded in 96-well culture plates at a density of 2×106 cells/ml and treated with HePC in final concentrations ranging from 1-100 µM. HePC was added in triplicate at final dilutions ranging from 1 to 100 µM. The plates were incubated at 25℃ for 48 hr before MTT assay. All tests were performed in triplicates.
J774 cell line culture and inoculation with L. tropica and L. major for finding ED50
To evaluate half maximal effective dose (ED50) of miltefosine on the amastigotes of Leishmania, J774 cell line, cultured in RPMI medium (containing 10% FCS and 100 µg/ml penicillin-streptomycin) at 37℃ with 5% CO2. Flasks of monolayer J774 cell line were prepared and inoculated with L. major and L. tropica strains, in a ratio of 5 parasites per macrophage. After 4 hr incubation at 32℃, to remove all free parasites from the flasks, the cells were washed 2 times. Different concentrations of miltefosine (1, 2.5, 5, 10, 20, and 30 µM) were added and flasks were incubated for 48 hr in 32℃ with 5% CO2. Each test was done triplicate. Microscopic slides were prepared from each cell suspension and stained by Giemsa (100 macrophages per treatment) to determine percentage of infected cells and the number of parasites per infected macrophage. The ED50 is defined for each strain as the effective dose of miltefosine that reduces the survival of Leishmania parasites by 50%.
Cell viability measurements by MTT assay
MTT [3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] colorimetric assay is to measure reduction of MTT dyes (tetrazolium) into formazan by mitochondrial enzymes in viable cells. Relative numbers of live cells were determined based on the optical absorbance of the treated and untreated samples and blank wells using the following formula:
Viable cells (%)=(AT-AB)/(AC-AB)×100
where AC is the absorbance of the untreated samples, AT is the absorbance of the treated samples, and AB is the absorbance of the blank. All values are means of triplicate wells. Results were expressed as the concentration that inhibited parasite growth by 50% (IC50: half maximal inhibitory concentration).
Phosphatidylserine externalization analysis of promastigotes
The percentages of viable, necrotic, and apoptotic cells were determined following treatment for various time points [4, 12, 18, 24, 36, and 48 hr]. The Annexin-V FLUOS Staining Kit was used for the detection of apoptotic and necrotic cells according to the manufacturer's protocol. Briefly, promastigotes were washed in cold PBS (×2) and centrifuged at 1,400 g for 10 min. Then, they were incubated for 15 min in the dark and at room temperature in 100 µl of Annexin-V FLUOS in the presence of PI (Propidium iodide). Afterwards, the samples were analyzed with FACSCalibur flow cytometer (Becton Dickinson and CellQuest software), and the percentage of positive cells was determined for each sample.
DNA fragmentation assay in the presence and absence of miltefosine
Qualitative analysis of total gDNA fragmentation was performed by agarose gel electrophoresis. In short, promastigotes (5×106 cells) were incubated and harvested in different time points. An apoptotic DNA ladder kit was used to extract DNA from apoptosis-induced and uninduced cells according to the manufacturer's instructions. DNA (10 µg DNA samples) was electrophoresed in 1.5% agarose gels at 100 V for 2 hr, visualized by using a UV transilluminator and photographed.
Promastigote morphology after treatment with HePC
To observe changes in cell morphology, promastigotes treated with or without miltefosine (IC50), were examined. Cells were centrifuged at a low speed (1,000 g) and the pellets suspended in PBS. Changes in morphology were observed under ×100 objectives on a light microscope. Alteration of cellular morphology was studied at different time points, and for each sample, at least 10 microscopic fields were observed under ×100 objectives.
Effects of miltefosine on the cell cycle
Flow cytometric analysis after cell permeabilization and labeling with PI was used to quantify the percentage of pseudo-hypodiploid cells. Parasites (1×106 cells) were treated with an IC50 dose of 24, 36, and 48 hr at 24℃; at each time point, cells were fixed in chilled 70% ethanol and kept at -20℃ until analysis. After washing the cells in PBS, the resultant pellet was resuspended in 500 ml DNase-free RNase (200 µg) and incubated for 1 hr at 37℃. Cells were then stained with PI (40 µg) and incubated in the dark for 20 min at 20-25℃. Data were obained using a FACS Calibur and analyzed using the CELLQUEST PRO software.
Statistical analysis
In vitro anti-leishmanial activity was expressed as IC50 (50% inhibitory concentration), which was determined by linear regression analysis.
RESULTS
Cytotoxic activity of miltefosine in L. major and L. tropica promastigotes
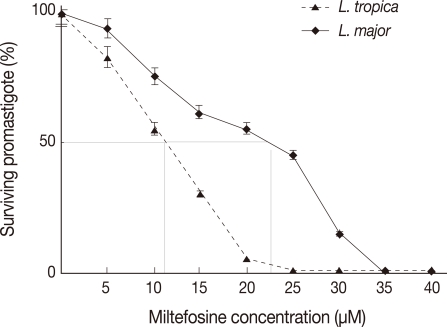
Cytotoxic potential of miltefosine on L. major and L. tropica promastigotes was tested using the MTT assay in order to determine 50% inhibitory concentration (IC50) of this drug. The viability of treated L. major and L. tropica promastigotes was different (Fig. 1). Miltefosine showed a dose-dependent cytotoxic effects with almost 100% death at a concentration of about 24 µM for L. tropica promastigotes. However, for L. major, 100% death occurred at a concentration of 32 µM.
Fig. 1.
The viability of L. major and L. tropica promastigotes, in the presence of various concentrations of HePC, assessed by MTT. Each point represents the means of 3 independent tests. Dotted line: L. tropica promastigotes, solid line: L. major.
In vitro effects of miltefosine in L. major and L. tropica amastigotes
ED50 for L. major and L. tropica amastigotes was determined after 48 hr of treatment to different concentrations of miltefosine. The data represent the means±standard deviations (SDs) of 3 independent experiments.
The observed ED50 of miltefosine was 5.7 µM and 4.2 µM for L. major and L. tropica, respectively. In L. tropica amastigote-infected macrophages, more than 95% of cells (parasites and macrophage) were killed after a 48-hr incubation with 20 µM miltefosine. In the case of L. major, after 2 days incubation 80% of macrophages and amastigotes were killed.
Morphological changes of treated promastigotes with HePc
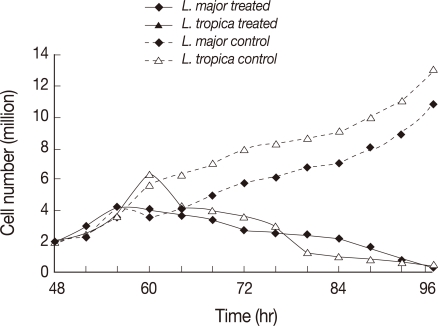
All tests were performed with Leishmania cultures in the logarithmic phase of growth. L. major and L. tropica promastigotes were treated with 22 µM and 11 µM miltefosine, respectively. The treated and untreated (control) cells were observed with a light microscope at 4 hr intervals up to 48 hr. The number of promastigotes at the time of drug treatment was 2×106/ml. The untreated L. tropica control cells continuously grew up to 11-13×106/ml in 48 hr. L. tropica treated cells showed proliferative activity up to 12 hr post-treatment during which they have grown up to 6.0-6.5×106/ml in the number. The numbers of live promastigotes decreased from 18-48 hr post-treatment contentiously. L. major treated promastigotes showed proliferative activity up to 8 hr post-treatment and the numbers of live promastigotes decrease from 8-48 hr post-treatment contentiously (Fig. 2).
Fig. 2.
Number of promastigotes treated with or without miltefosine at different time points after 48 hr. Dotted line: L. major (MRHO/IR/75/ER) promastigotes and L. tropica (MHOM/IR/02/Mash10) control group, solid line: L. tropica and L. major-treated cells.
Microscopic examination of the treated cells showed that cell shrinkage started around 4 hr after the drug treatment in both L. major and L. tropica promastigotes. Almost all the treated cells showed cytoplasmic condensation, shrinkage, and reduction in size in comparison to the control samples at the end of 48 hr after the treatment (Fig. 3).
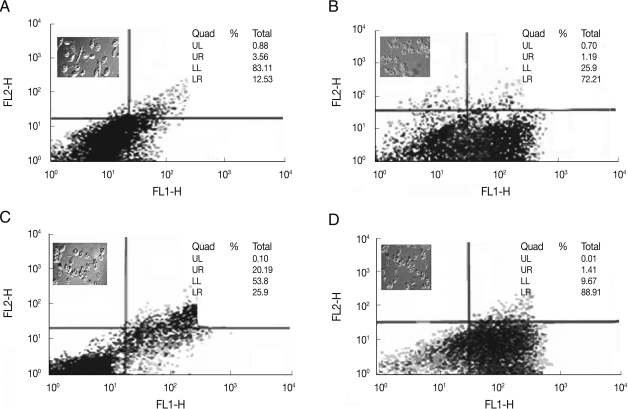
Fig. 3.
Analysis of morphology in light microscopy (magnification, ×100) and flow cytometry analysis of L. major and L. tropica promastigotes following treatment with 22 µM and 11 µM HePC, respectively, at different time points after miltefosine treatment. (A) L. major 24 hr after treatment. (B) L. major 48 hr after treatment. (C) L. tropica 24 hr after treatment. (D) L. tropica 48 hr after treatment. Lower right region (LR) belongs to apoptotic cells (annexin positive) and upper left region (UL) belongs to necrotic cells (PI positive).
Phosphatidylserine translocation analysis of treated cells with HePC
Flow cytometric analysis of treated L. tropica and L. major promastigotes was performed with 24 and 32 µM HePC, respectively, after labeling with Annexin-V FLUOS. In early stages of metazoan apoptosis, several alterations happen in the plasma membrane. Translocation of phosphatidylserine from the inner side of the cell membrane to the outer side performs one of these changes. Fluorescein-conjugated Annexin-V is used to detect the externalized phosphatidylserine as it has a high binding affinity to this phospholipid component. Additionally, annexin-V FLUOS allows distinguishing between apoptotic cells (annexin V positive, PI negative), necrotic cells (annexin positive, PI positive), and surviving cells (annexinV negative, PI negative). In the case of L. major promastigotes, after 18 hr of incubation 3.2% of the treated cells and 3.0% of the control cells were annexin-VFLUOS positive. While, after 24 hr of incubation, annexine-V FLUOS positivity was 16.1% for treated L. major promastigotes and 3.5% for the control cells.
After 36 and 48 hr of treatment, the percent of annexin-positive cells were 37.9% and 73.4%, respectively, whereas the control group just showed 3.9% for both time points (Fig. 3A). In L. tropica promastigotes treated with miltefosine after 18 hr of incubation, 14.7% of the treated cells and 3% of the untreated cells were Annexin-VFLUOS positive. In the next several time points (24, 36, and 48 hr), Annexin-VFLUOS positive cells were 46.9%, 63.4%, and 90.1%, respectively. In L. tropica, similar to L. major in several time points, miltefosine did not show necrosis even after a prolonged incubation, as all the cells remained negative on PI. In the untreated L. tropica promastigots with HePC, Annexin-VFLUOS positive cells were just 3.8% for both time points (Fig. 3B).
DNA fragmentation in L. major and L. tropica promastigotes
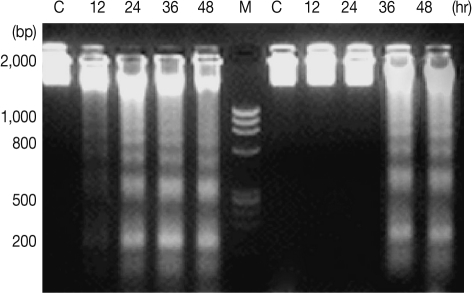
DNA fragmentation in promastigotes of L. major and L. tropica was confirmed by the presence of fragmented DNA in agarose gel electrophoresis. The fragments were in oligonucleosome size (in approximate multiples of 180-200 bp) in promastigotes treated with 11 µM HePC for 24 hr in L. tropica, whereas untreated cells did not show DNA fragmentation (Fig. 4). No DNA fragmentation was observed in L. major promastigotes treated with 22 µM concentrations of HePC post-24 hr incubation. After 36 hr, DNA fragmentation was observed in L. major promastigotes. Study of DNA fragmentation in promastigotes of L. tropica promastigotes did not show any difference in comparison with L. major after 48 hr.
Fig. 4.
DNA fragmentation detected with agarose gel electrophoresis of L. major (right) and L. tropica (left). C, Not treated; Lanes 12, 24, 36, and 48 hr incubation periods after treatment with miltefosine. Lane M, size marker.
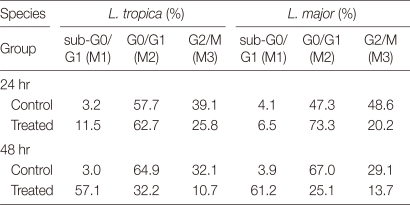
Miltefosine-induced sub-G0/G1 phase cell-division arrest
In a given cell, the amount of bound dyes is correlated with the DNA content and thus DNA fragmentation in apoptotic cells translates into fluorescence intensity lower than that of G0/G1 cells, i.e., a sub-G0/G1 peak. In L. tropica promastigotes incubated with 24 µM miltefosine for 24 hr, the proportion of cells in the sub-G0/G1 phase increased to compare to controls (11.5% vs. 3.2%) (Table 1).
Table 1.
The cell cyclea of L. tropica and L. major after treatment with 24 µM and 32 µM miltefosine for 24 hr and 48 hr, respectively
aMethods for cell-cycle analysis are described in Materials and Methods.
Following 48 hr of HePc treatment, the ratio of promastigotes in the sub-G0/G1 phase increased in comparison to control cells. In contrast, increase in the number of cells in the sub-G0/G1 phase led to a decrease in the cell number in the G2/M phase compared with untreated cells. At 24 hr after L. major promastigotes were incubated with 32 µM miltefosine, the proportion of cells in the sub-G0/G1 phase did not show any significant increase in the ratio in comparison to controls. Following 48 hr of drug treatment, the ratio of cells in the sub-G0/G1 phase increased further in comparison to controls. Similar to L. tropica, an increase in sub-G0/G1 phase was accompanied by a decrease in the number of cells in the G2/M phase compared with untreated cells at 48 hr (Table 1). The increased ratio of cells in the sub-G0/G1 phase, especially after 48 hr, showed that miltefosine apoptosis in L. major and L. tropica promastigotes resulted in DNA degradation.
DISCUSSION
CL, due to L. major and L. tropica, is endemic in different parts of Iran [10,11]. Different Leishmania species may need to various clinical and therapeutic managements in Iran [18,19]. Antimonial drug compounds are the first line of treatment of CL, but due to side effects, treatment failures and drug resistance, the value of antimony therapy is limited [14,19]. Miltefosine is the only oral drug that recently has been developed for treatment of leishmaniasis, which is undergoing clinical trials in several countries [15].
Complications and side effects of miltefosine include nausea and vomiting but not diarrhea, which is considerably less than in antimonial drugs [16]. Hadighi et al. [20] showed that glucantime-resistant L. tropica isolated from Iranian patients with CL are sensitive to alternative anti-leishmania drug. Esmaeili et al. [21] showed that miltefosine is effective on L. major [21]. Mohebali et al. [16], in a randomized clinical trial, showed that 2 weeks, 3 months, and 6 months after the end of treatment by miltefosine, the clinical outcome is apparently at least as good as using meglumine antimoniate for CL caused by L. major. Induction of programmed cell death is one of the advantages of miltefosine against the other currently used drugs, including antimonials [22]. Promastigotes of L. donovani were particularly studied for induction of apoptosis. It has been shown that they undergo apoptosis after exposure to some agents and nutrient deficiencies that cause programmed cell death in higher eukaryotes [17,23]. The process of apoptosis shares many similar features in both metazoan and protozoan organisms [7,24]. Two prominent features observed in both of these eukaryotes are DNA condensation and fragmentation.
In the present study, L. major and L. tropica promastigotes treated with HePC that both species showed programmed cell death features, include cell shrinkage, DNA fragmentation, and externalization of phosphatidylserine with preservation of integrity of the cell membrane. Similarly, a study was conducted previously that L. infantum promastigotes exposed to HePC, reported a cell death that shares most features associated with metazoan apoptosis [25].
The DNA content in cells had direct relations with the amount of fluorescence intensities and in apoptotic cell's DNA degradation translates into PI intensity lower than G1 cells (sub-G1 peak). L. tropica promastigotes exposed to 24 µM miltefosine after 24 hr showed that 11.5% of these cells were found in the sub-G1 peak region, while in the control group only 4% were found in this region (Table 1). Furthermore, L. major promastigotes treated with 32 µM HePC for 24 hr induced DNA degradation.
Arnoult et al. [22] showed some forms of DNA degradation in L. major promastigotes by staurosporine but oligonucleosomal fragmentation was not detected. The typical nuclear features, however, has been reported to occur in the amphotericin B-treated L. donovani promastigotes [26]. The IC50 is commonly used as a measure of drug potency in pharmacological research. In our study, IC50 of HePC on the L. major and L. tropica promastigotes was determined using MTT assay.
Our results showed that L. tropica promastigotes are more sensitive to miltefosine than L. major promastigotes (11 µM vs. 22 µM). The observed IC50 of HePC on L. tropica (MHOM/IR/02/Mash10) promastigotes was very close to that on L. infantum (strain MCAN/IR/96/LON49) as reported previously [26]. The reported IC50 for 2 different strains of L. donovani was different from that of the present study. Different IC50 among Leishmania species showed that each organism responds to various drug concentrations.
The macrophage-model amastigote cell line was suitable for study of the activity of anti-leishmania drugs, which is defined as ED50. The observed ED50 of miltefosine for L. major and L. tropica amastigotes showed different results, which demonstrated an intracellular form of L. tropica that is more sensitive than L. major to miltefosine. In agreement with this finding, Esmaeili et al. [21] reported that Miltefosine has lower ED50 in L. major infected macrophage than Glucantime (2.20 µM vs. 7.2 µM).
In conclusion, HePC may induce L. major and L. tropica apoptotic cell death. The results of the present study about the concentration and time-dependent miltefosine-induced DNA fragmentation were in agreement with other reports [17,27]. Interestingly, L. major did not show any apoptotic changes when it was exposed to HePC concentrations that is sufficient to kill L. tropica. As L. tropica is responsible for residual leishmaniasis after treatment with glucantime, use of miltefosine can be a promising treatment. Better understanding of the mechanisms of miltefosine action may help in finding new targets for the treatment of Leishmania parasites. Finally, our findings indicate that HePC may induce dose-dependent apoptosis on the causative agent of CL.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Mahdi Mohebali and Dr. Homa Hajjaran, Tehran University of Medical Sciences, Iran, for kind donation of the Iranian standard strains of Leishmania.
References
- 1.Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Ann Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 2.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 3.Vaux DL. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci USA. 1993;90:786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 5.Heussler VT, Küenzi P, Rottenberg S. Inhibition of apoptosis by intracellular protozoan parasites. Int J Parasitol. 2001;31:1166–1176. doi: 10.1016/s0020-7519(01)00271-5. [DOI] [PubMed] [Google Scholar]
- 6.McConkey DJ, Zhivotovsky B, Orrenius S. Apoptosis--molecular mechanisms and biomedical implications. Mol Aspects Med. 1996;17:1–110. doi: 10.1016/0098-2997(95)00006-2. [DOI] [PubMed] [Google Scholar]
- 7.Hamann A, Brust D, Osiewacz HD. Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol. 2008;16:276–283. doi: 10.1016/j.tim.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Wanderley JL, Benjamin A, Real F, Bonomo A, Moreira ME, Barcinski MA. Apoptotic mimicry: an altruistic behavior in host/Leishmania interplay. Braz J Med Biol Res. 2005;38:807–812. doi: 10.1590/s0100-879x2005000600001. [DOI] [PubMed] [Google Scholar]
- 9.Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Wasunna MK, Bryceson AD. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 10.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10:591–599. [PubMed] [Google Scholar]
- 11.Mohebali M, Edrissian GH, Nadim A, Hajjaran H, Akhoundi B, Hooshmand B, Zarei Z, Arshi A, Mirsamadi N, Naeini KM, Mamishi S, Sanati AA, Moshfe AA, Charehdar S, Fakhar M. Application of direct agglutination test (DAT) for the diagnosis and seroepidemiological studies of visceral leishmaniasis in the Islamic Republic of Iran. Iran J Parasitol. 2006;1:15–25. [Google Scholar]
- 12.Momeni AZ, Aminjavaheri M. Successful treatment of non-healing cases of cutaneous leishmaniasis, using a combination of meglumine antimoniate plus allopurinol. Eur J Dermatol. 2003;13:40–43. [PubMed] [Google Scholar]
- 13.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra BB, Kale RR, Singh RK, Tiwari VK. Alkaloids: future prospective to combat leishmaniasis. Fitoterapia. 2009;80:81–90. doi: 10.1016/j.fitote.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Richard JV, Werbovetz KA. New antileishmanial candidates and lead compounds. Curr Opin Chem Biol. 2010;14:447–455. doi: 10.1016/j.cbpa.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, Razaghian AR, Kabir MJ, Nadim A. Comparison of miltefosime and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103:33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Verma NK, Dey CS. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother. 2004;48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wortmann G, Hochberg L, Houng HH, Sweeney C, Zapor M, Aronson N, Weina P, Ockenhouse CF. Rapid identification of Leishmania complexes by a real-time PCR assay. Am J Trop Med Hyg. 2005;73:999–1004. [PubMed] [Google Scholar]
- 19.Ameen M. Cutaneous and mucocutaneous leishmaniasis: emerging therapies and progress in disease management. Expert Opin Pharmacother. 2010;11:557–569. doi: 10.1517/14656560903555219. [DOI] [PubMed] [Google Scholar]
- 20.Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, Ouellette M, Mohebali M. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res. 2007;101:1319–1322. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- 21.Esmaeili J, Mohebali M, Edrissian GH, Rezayat SM, Ghazi-Khansari M, Charehdar S. Evaluation of miltefosine against L. major(mrho/ir/75/er): in vitro and in vivo studies. Acta Med Iran. 2008;4:191–196. [Google Scholar]
- 22.Arnoult D, Akarid K, Grodet A, Petit PX, Estaquier J, Ameisen JC. On the evolution of programmed cell death: apoptosis of the unicellular eukaryote Leishmania major involves cysteine proteinase activation and mitochondrion permeabilization. Cell Death Differ. 2002;9:65–81. doi: 10.1038/sj.cdd.4400951. [DOI] [PubMed] [Google Scholar]
- 23.Paris C, Loiseau PM, Bories C, Bréard J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2004;48:852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanderley JL, Barcinski MA. Apoptosis and apoptotic mimicry: the Leishmania connection. Cell Mol Life Sci. 2010;67:1653–1659. doi: 10.1007/s00018-010-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khademvatan S, Gharavi MJ, Akhlaghi L, Samadikuchaksaraei A, Oormazdi H, Mousavizadeh K, Hadighi R, Saki J. Induction of apoptosis by miltefosine in Iranian strain of Leishmania infantum promastigotes. Iranian J Parasitol. 2009;4:23–31. [Google Scholar]
- 26.Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002;9:53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee SB, Das M, Sudhandiran G, Shaha C. Increase in cytosolic Ca2+ levels through the activation of non-selective cation channels induced by oxidative stress causes mitochondrial depolarization leading to apoptosis-like death in Leishmania donovani promastigotes. J Biol Chem. 2002;277:24717–24727. doi: 10.1074/jbc.M201961200. [DOI] [PubMed] [Google Scholar]