Abstract
Cytokinesis is the final step in cell division. The process begins during chromosome segregation, when the ingressing cleavage furrow begins to partition the cytoplasm between the nascent daughter cells. The process is not completed until much later, however, when the final cytoplasmic bridge connecting the two daughter cells is severed. Cytokinesis is a highly ordered process, requiring an intricate interplay between cytoskeletal, chromosomal, and cell cycle regulatory pathways. A surprisingly broad range of additional cellular processes are also important for cytokinesis, including protein and membrane trafficking, lipid metabolism, protein synthesis and signaling pathways. As a highly regulated, complex process, it is not surprising that cytokinesis can sometimes fail. Cytokinesis failure leads to both centrosome amplification and production of tetraploid cells, which may set the stage for the development of tumor cells. However, tetraploid cells are abundant components of some normal tissues including liver and heart, indicating that cytokinesis is physiologically regulated. In this chapter, we summarize our current understanding of the mechanisms of cytokinesis, emphasizing steps in the pathway that may be regulated or prone to failure. Our discussion emphasizes findings in vertebrate cells although we have attempted to highlight important contributions from other model systems.
Cytokinesis Occurs in Multiple Stages
The process of cytokinesis can be divided into four stages including specification of the cleavage plane, ingression of the cleavage furrow, formation of the midbody, and abscission (Fig. 1). Each stage is dependent on the proper execution of the prior stage, and thus interference with any stage may result in cytokinesis failure. The first stage of cytokinesis specifies the cleavage plane by recruiting a central regulator of cytokinesis, RhoA, to the site of cleavage. If this step is perturbed, cytokinesis will not initiate properly. In the second stage of cytokinesis, the cleavage furrow ingresses through formation of an actomyosin ring and myosin-dependent motor activity. Failure at this step may lead to a lack of furrow initiation or partial ingression of the furrow followed by regression. The third stage of cytokinesis is characterized by formation of the midbody and stabilization of the cytokinetic furrow. This stage requires proper function of proteins located in the central spindle, a microtubule-based structure that separates segregated chromosomes during anaphase, and on proteins that stabilize interactions between the actomyosin ring and the central spindle. A failure at this stage will lead to regression of the cleavage furrow. The final stage in cytokinesis, abscission, is the step in which the cytoplasmic contents are finally separated from one another. This event requires the presence of a functional midbody, but also additional proteins involved in vesicle trafficking and fusion. Failure at this stage may lead to regression of the cleavage furrow or to formation of a persistent connection between the two daughter cells. Cytokinesis is thus a series of linked processes and a problem at any step of this cascade may be sufficient to induce failure. Some proteins participate in multiple steps in cytokinesis, and thus perturbation of their abundance or activity may be especially prone to induce cytokinesis failure.
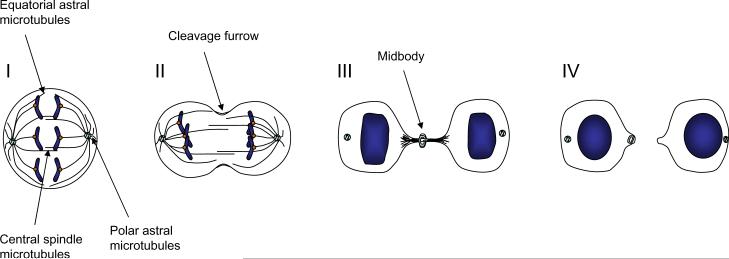
Figure 1. Multiple stages of cytokinesis.
Three populations of microtubules first specify the site of cleavage by activating RhoA in a narrow zone between segregating chromosomes (I). Formation and activation of the actomyosin ring next leads to furrow ingression (II). The constricting furrow compacts the central spindle microtubules leading to midbody formation (III). Abscission of the furrow occurs by physically separating the cytoplasm of the daughter cells (IV).
Stage I: Positioning the Division Plane and Initiating Cytokinesis
The Importance of Microtubules
Classic micromanipulation experiments determined that the mitotic spindle dictates the position of the cleavage furrow.1, 2 However, a bipolar spindle is not necessary for induction of a cleavage furrow,3, 4 suggesting that microtubules themselves play an essential role in initiating cleavage. Three separate populations of microtubules have been implicated in the regulation of cytokinesis (Fig. 1; reviewed by ref. 5). First, equatorial astral microtubules, which emanate from the spindle pole to the site of cleavage, may be stabilized in the equatorial cortical region3 and deliver positive signals that stimulate formation and contraction of the cleavage furrow.2 In contrast, polar astral microtubules, which emanate from the spindle pole to sites away from the site of the furrow, may help position the cleavage furrow by inhibiting cortical contractility,6–8 perhaps by spatially biasing the pattern of myosin recruitment.9, 10 Finally, central spindle microtubules, which form an overlapping network between the spindle poles following anaphase, send positive signals that become especially important during later steps of cytokinesis. The signals sent by these distinct microtubule populations are partially redundant, ensuring that selection of the division plane is robust.11, 12
The RhoA Pathway Plays an Essential Role in Furrow Initiation
What are the positive signals delivered by microtubules that initiate furrowing at the correct place in the cell? A central event is the localized activation of the small GTPase RhoA at the site of the future furrow (Fig. 2; reviewed by Ref. 13). RhoA is essential for furrow formation in animal cells,14–17 and activated RhoA localizes to a narrow zone within the furrow.18–22 Localized activation of RhoA within this narrow zone is thought to be important for efficient furrowing, as perturbations that broaden the zone of RhoA activation often lead to a failure of the furrow to form or to ingress.19 A narrow zone of activation is established by tethering RhoA activators to the central spindle, delivering a strong yet spatially restricted signal for cytokinesis initiation.
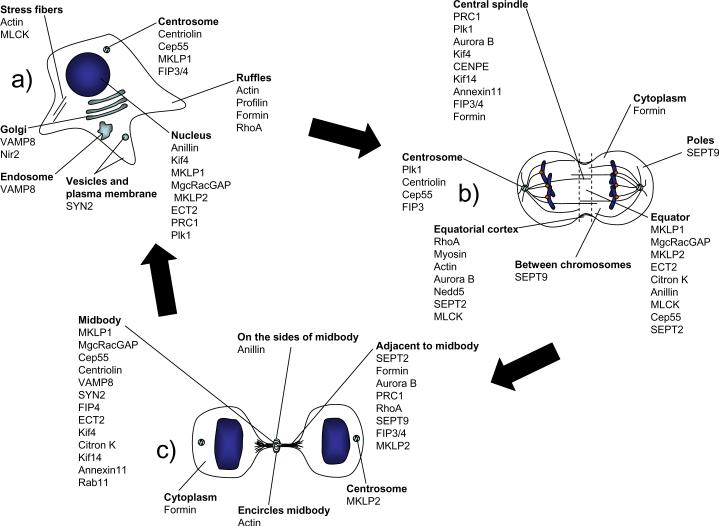
Figure 2. Localization of cytokinesis components.
Interphase (a), anaphase (b), and late cytokinesis (c).
An essential activator of RhoA is the guanine nucleotide exchange factor ECT2,17, 19, 23–26 originally identified as a protooncogene.27 ECT2 is sequestered in the nucleus during interphase (Fig. 2) and released following nuclear envelope breakdown in mitosis, but the protein remains inactive because it exists in an autoinhibited conformation.24, 28 In late anaphase, ECT2 localizes to the central spindle and associates with the centralspindlin complex, composed of the kinesin protein MKLP1 and the GTPase activating protein (GAP) MgcRacGAP.17, 19, 29, 30 MgcRacGAP binds to ECT2 and stabilizes it in an active conformation that permits it to interact with RhoA.19 Tethering of the centralspindlin complex to the central spindle is thought to restrict activated ECT2 within a narrow zone, resulting in a narrow zone of RhoA activation.19, 23, 25, 29 Depletion of MKLP119 or disruption of the central spindle31, 32 leads to delocalization of ECT2 and MgcRacGAP from the central spindle, broadening the region of RhoA activation.19 Cells containing a broad zone of RhoA activation fail to form a furrow.19 Therefore, tethering of MgcRacGAP and ECT2 to the central spindle is not essential for RhoA activation, but is instead important for efficient furrowing by restricting the zone of RhoA to within a narrow zone at the equator of the cell.
These findings suggest that cytokinesis failure could result from failure to properly deliver RhoA activators to the cortex, causing insufficient activation of RhoA. Alternatively, cytokinesis could fail if RhoA is activated too broadly, in regions outside the cleavage furrow. Interestingly, ECT2 deregulation can lead to oncogenic transformation27, 28, 33 although it is not clear whether perturbation of cytokinesis is an important component of this phenomenon, as ECT2 may participate in other processes such as spindle assembly34 and regulation of the Ras/MAP kinase pathway.35 Like many genes involved in cell division, ECT2 expression is induced by growth factors36 in a manner that depends on the Rb/E2F pathway.37 ECT2 is overexpressed in some tumors38, 39 where it could broaden the region of RhoA activation, perturbing proper initiation of cytokinesis. Alternatively, elevated ECT2 could perturb late stages of cytokinesis, as RhoA may need to be inactivated for cytokinesis to be completed.25. In fact, overexpression of some fragments of ECT2 has no effect on cytokinesis initiation, but specifically blocks later stages of cytokinesis.24, 25
Other proteins may regulate RhoA activity during cytokinesis.40 These include additional Rho GEFs such as GEF-H141 and MyoGEF,42 both of which are essential for cytokinesis in mammalian cells. Additional proteins may influence the location and timing of RhoA activation, including the armadillo protein p007143 and the Rho effector mDia1, which may sustain RhoA activation in a positive-feedback loop.44 In contrast, the protein HEF1, which is upregulated in tumor cells, may impair the RhoA activation cycle.45
GAP proteins are also important for controlling RhoA activation and inactivation. As stated earlier, RhoA may need to be inactivated during late cytokinesis to disassemble the cleavage furrow, and thus hyperactivation of RhoA could block cytokinesis completion. Two GAP proteins that may inactivate RhoA during cytokinesis are MgcRacGAP and p190 RhoGAP.46 Although MgcRacGAP plays a critical role in activation of RhoA by recruiting and activating ECT2, phosphorylation of MgcRacGAP by Aurora kinases may stimulate its ability to serve as a RhoGAP,47 contributing to RhoA inactivation. As its name suggests, MgcRacGAP may also inhibit the GTPase Rac. The activity of Rac is suppressed in the spindle midzone,21 and constitutively activated Rac induces a mutlinucleation.48 Thus in addition to activating RhoA by recruiting ECT2, MgcRacGAP may inactivate Rac in the furrow to support cytokinesis.48–51
Failure of Cytokinesis During Stage I
Together these studies emphasize the importance of microtubules in delivering signals that lead to localized activation of RhoA, and possibly suppression of Rac, in the furrow. Recent studies suggest that cytokinesis failure may occur in cells in which spindle elongation or spindle positioning is perturbed, disrupting delivery of activation signals to the cortex. The first example is binucleation of cells in the liver, which may be regulated physiologically.52, 53 In humans, the number of polyploid cells averages 30–40% in the adult liver.54, 55 Studies in rat hepatocytes indicate that tetraploid cells arise from cytokinesis failure in which diploid, mononucleated cells undergo mitosis but do not form a contractile ring.52 Cells do not undergo anaphase spindle elongation, perhaps because reorganization of the actin cytoskeleton is impaired.53 Furthermore, astral microtubules fail to contact the equatorial cortex in cells that fail cytokinesis,53 suggesting that the delivery of RhoA activators to the cortex is impaired. In rat liver, the number of binucleated cells increases following weaning, suggesting there may be important connections between liver physiology and cytokinesis regulation,53 but how these pathways might impact microtubule organization remains unclear.
The second example is cytokinesis failure that occurs in cells that contain mutations the APC (Adenomatous Polyposis Coli) tumor suppressor. Some APC mutations may induce cytokinesis failure by interfering with microtubule-dependent anchoring of the mitotic spindle.56 Although APC has important roles in formation of the mitotic spindle and the spindle checkpoint,57–60 cells expressing APC mutants become polyploid over time,56, 59, 61 indicating that the protein is important for proper cytokinesis. Different APC alleles may have distinct effects on mitosis.56 For example, in cells expressing a particular C-terminal truncation mutant of APC, microtubules make less contact with the cell cortex, spindles undergo considerable rotation during mitosis, and cells do not efficiently initiate cytokinesis.56 The physiological relevance of these findings was confirmed by the finding that the Min allele of APC gives rise to similar mitotic defects and that the frequency of tetraploid cells is greatly increased in Min56 and APC knockout mice.60 Although it is likely that tetraploidy can arise through multiple mechanisms in tumors carrying different APC alleles, these findings suggest that failure to properly anchor the mitotic spindle can be an important source of tetraploidy.
Stage II. Ingression of the Cleavage Furrow
In the second stage of cytokinesis, activated RhoA leads to recruitment and activation of effector proteins that organize the furrow and stimulate ingression (Fig. 3). RhoA stimulates actin polymerization through activation of formins, and stimulates myosin activity by activating kinases such as Rho kinase (ROCK) and Citron kinase. Scaffolding proteins such as anillin and septins also play important roles in organizing the cleavage furrow and promoting cytokinesis. Here we discuss each of these processes and how they might be perturbed to result in cytokinesis failure.
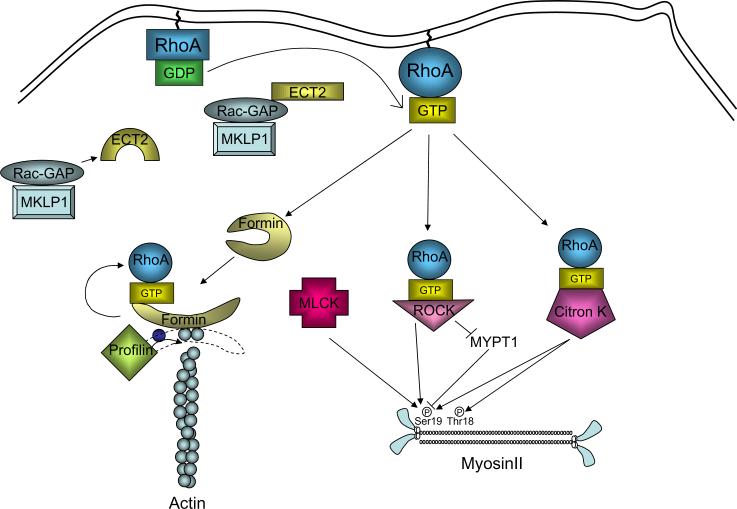
Figure 3. Role of the RhoA pathway in furrow initiation.
Autoinhibition of ECT2 is suppressed by association of ECT2 with MgcRacGAP. ECT2 then activates RhoA by stimulating exchange of GDP for GTP. Active RhoA then activates formins to stimulate actin nucleation, and binds to ROCK and Citron kinases, stimulating phosphorylation and activation of myosin.
Stimulation of Actin Filament Assembly
Formins are proteins that nucleate formation of unbranched actin filaments in response to stimulation by RhoA (for review, see ref. 62). In the absence of active RhoA, most of the formins that regulate cytokinesis are autoinhibited.63 RhoA binding relieves autoinhibition to promote actin polymerization.64, 65 The mammalian formin mDia1 is activated downstream of Rho signaling,66 and cytokinesis is blocked if mDia1 is inhibited by antibody injection.67 However, deletion of mDia1 does not perturb cytokinesis in mouse embryonic fibroblasts,68 suggesting redundant pathways for actin nucleation. Cytokinesis may also depend on the use of preexisting actin filaments that are nucleated outside the furrow.69, 70 Formins may be important in later stages of cytokinesis, as they have been implicated in regulation of Src activity, which has been shown to be important for completion of cytokinesis.67, 71
Localization and Activation of Myosin
Myosin II (hereafter simply referred to as myosin) is the principle motor protein required for cytokinesis (for review, see ref. 72). Myosin is recruited to the cleavage furrow at early stages of cytokinesis in a RhoA-dependent fashion. Myosin activity and localization are regulated by phosphorylation of its regulatory light chain (myosin light chain or MLC). Because myosin motor activity is directly required for furrow ingression,73 perturbation of myosin localization or its activity could result in cytokinesis failure.
Phosphorylation of serine 19 of MLC simulates actin-activated ATPase activity of myosin,74, 75 whereas phosphorylation at threonine 18 promotes myosin assembly. Phosphorylation of myosin at these positions is important for proper localization of myosin to the furrow and for ingression.76–79 In contrast, phosphorylation at serines 1, 2 and 9 of MLC inhibits myosin ATPase activity80, 81 During mitosis, MLC is phosphorylated at these positions by CDK1.76, 82, 83 At anaphase, inactivation of CDK1, controlled by the degradation of mitotic cyclins by the Anaphase-Promoting Complex/Cyclosome (APC/C), is important for MLC dephosphorylation and myosin activation during cytokinesis. Therefore, failure to degrade mitotic cyclins, or to fully inactivate CDK1, could therefore perturb myosin activation and disrupt cytokinesis.
Three kinases contribute to myosin activation by phosphorylating positions 18 and 19 of MLC (Fig. 3). Two of these kinases, ROCK and Citron kinase, are activated by RhoA. ROCK localizes to cleavage furrows,84, 85 and a small molecule inhibitor of ROCK slows cleavage.84 Citron kinase also localizes to the cleavage furrow, and is required for cytokinesis in several systems.25, 50, 86–92 Citron kinase can phosphorylate MLC at both ser19 and thr18,93 and its overexpression causes unregulated contraction of the cortex, supporting its role as a positive regulator of myosin activity.87 Mouse knockout studies suggest that Citron kinase may play an especially important role in neurogeneic and spermatogenic cytokinesis.94–97 It is likely that ROCK and Citron kinase play partially overlapping roles, explaining why each protein is not essential for cytokinesis in all systems. There is no evidence to suggest that ROCK or Citron kinase is overexpressed or mutated in human tumors, but Citron kinase interacts with the kinesin protein KIF14,89 which is overexpressed in several tumor types.98–101 Whether overexpression of KIF14 perturbs Citron kinase function in cytokinesis remains unknown. Knockdown of KIF14 induces cytokinesis failure,102 perhaps as a result of a failure to recruit Citron kinase to the cleavage furrow.
Myosin light chain kinase (MLCK) is the third and final kinase that has been implicated in direct phosphorylation of myosin light chain. This kinase is activated by calcium/calmodulin, and some isoforms of MLCK and calmodulin localize to the cleavage furrow.103–106 Inhibition of calmodulin or MLCK can disrupt cytokinesis in cultured cells,106–108 but mice lacking MLCK develop normally, but die after birth, suggesting the kinase is not essential for cytokinesis in all tissues.109 How myosin light chain kinase is regulated in the cleavage furrow is unclear, but hydrolysis of PIP2 may be important for IP3-induced calcium release, which could stimulate MLCK activity.110 The mild and varied phenotypes associated with MLCK inhibition again suggest functional redundancy in MLC phosphorylation during cytokinesis.
The overall degree of MLC phosphorylation is also affected by the activity level of myosin phosphatase. This enzyme is inhibited by several mechanisms during cytokinesis to favor MLC phosphorylation. Myosin phosphatase is a heterotrimeric enzyme consisting of a targeting subunit that bind myosin (MYPT1 or MBS), a catalytic subunit (the delta isoform of PP1c), and an additional small subunit. Both ROCK and Aurora B may phosphorylate MYPT1 in the furrow to inactivate the phosphatase.111–113 In addition, a number of other kinases including Raf-1114 may negatively regulate myosin phosphatase (reviewed in ref. 72). Thus a number of signaling pathways could converge on myosin phosphatase to regulate cytokinesis.
Organization of Actin and Myosin in the Furrow
How actin and myosin are organized within the cleavage furrow, and how contraction occurs, is not well understood (for a more detailed discussion, see refs. 115 and 116). Phosphorylated myosin localizes to the furrow in early anaphase79 and localization of myosin requires RhoA activation13, 117 but not myosin ATPase activity.73, 118, 119 Recruitment may require phosphorylation of MLC, as mutations or inactivation of kinases that perturb MLC phosphorylation also disrupt myosin localization. Other scaffolding components, such as anillin, may be important for maintaining myosin within the furrow, as discussed below.
Both actin and myosin are highly dynamic in furrows, and dynamic actin is important for cytokinesis.108, 120 For example, the actin disassembly factor cofilin is necessary for cytokinesis.121 Cofilin is negatively regulated by the kinase LIMK1, and thus upregulation of LIMK1, or loss of its negative regulator LATS1, is sufficient to enhance actin polymerization and induce cytokinesis failure.122 Interestingly, inhibition of myosin slows disassembly of actin filaments of the furrow,123 suggesting that myosin motor activity may help drive actin disassembly.
Scaffolding Proteins in the Furrow
Anillin
Another conserved furrow component is anillin, which may act as a scaffold protein that binds F-actin, myosin, septins, and activated RhoA.124–129 Although anillin localizes to the furrow at early stages of cytokinesis, it is not essential for ingression. Instead, it may stabilize the furrow and be important for later stages of cytokinesis including midbody formation and abscission.92, 127, 130–132 However, anillin becomes essential for ingression if the central spindle is disrupted, suggesting it may make early steps of cytokinesis more robust.128 Anillin interacts with RhoA128 and its localization to the furrow requires activation of RhoA.128, 129, 132, 133 MgcRacGAP may also be directly involved in targeting anillin to the furrow,134 providing a link between the centralspindlin complex and anillin localization.
Anillin contains domains that permit it to interact with phosphorylated myosin,127 actin filaments, and septins.124, 125, 135 These features make anillin ideally suited to crosslink the actomyosin and septin cytoskeletons within the contractile ring. Anillin may enhance the robustness of early stages of cytokinesis by promoting the anchoring of myosin in the vicinity of activated RhoA, favoring myosin phosphorylation. Anillin may be essential for asymmetric ingression of the cytokinetic furrow, which occurs when the furrow ingresses from only one side of the cell, rather than circumferentially.136 Asymmetric ingression may be important in epithelia137 and embryos136, 138 where it may serve a mechanical function, enhancing the robustness of cytokinesis. Anillin is also important for completion of cytokinesis, as anillin remains in the cytoplasmic bridge even after myosin and actin have dissociated.127 Interestingly, whereas overexpression of anillin seems to have little phenotype in Drosophila S2 cells,129 overexpression of anillin in mammalian cells is very toxic,132 suggesting anillin could have important functions independent of cytokinesis. Levels of anillin appear to be controlled by the ubiquitin-proteasome pathway, as anillin is targeted for ubiquitination and degradation during G1 by the APC/C.132
Like the liver, the heart also contains a large number of tetraploid cells that arise through cytokinesis failure. Although it was originally proposed that cytokinesis failure might be a consequence of failure to disassemble myofibrils within cardiomyocytes, recent work suggests this is unlikely to be the case.139 Instead, cells that fail cytokinesis show complete disassembly of the myofibril, but show abnormal localization of anillin, and failure of anillin to concentrate at the midbody.139 However, these cells also show delays in furrow ingression, suggesting that earlier steps in cytokinesis may also be affected in these cells.
Septins
Septins represent a second class of scaffolding protein that may help to organize proteins within the cleavage furrow. Septins are GTP-binding proteins that can form filaments and localize to the cytokinetic ring.140–144 Several human septins have been implicated in cytokinesis, including SEPT2 (Nedd5), SEPT9 (MSF), and SEPT12. SEPT12 localizes to the central spindle and midbody during anaphase and cytokinesis, respectively.145 SEPT2 accumulates in the contractile ring and midbody,146–148 and microinjection of antibodies146 or antisense downregulation147 of SEPT2 interferes with cytokinesis. Inactivation of SEPT9 by antibody microinjection or siRNA also induces cytokinetic defects.149, 150
Septins may participate in several aspects of cytokinesis, including regulation of actin and microtubule dynamics. SEPT2 associates with actin, forming filaments in association with actin bundles and focal adhesions,146 whereas SEPT9 associates with the microtubule network.150, 151 Septins may also play a direct role in cytokinesis by interacting with anilllin.124, 125, 135 Furthermore, SEPT2-containing filaments may provide a molecular platform for myosin and its kinases to ensure the full activation of myosin that is necessary for cytokinesis.152 Finally, septins may form a barrier that restricts the diffusion of membrane proteins in the furrow,153, 154 thus helping retain activated RhoA within the narrow zone required for efficient initiation of cytokinesis. In mammalian cells, the p85 subunit of PI3 kinase may regulate SEPT2 in cytokinesis,155 linking cellular signaling pathways with steps in cytokinesis. Septins may be deregulated in tumors, either through gene fusions156–158 or by overexpression.159 The SEPT9 gene is amplified and overexpressed in mouse mammary tumors and human breast cancer cell lines,158 and high SEPT9 expression in human breast cancer cells is associated with oncogenic phenotypes and cytokinesis defects.160
Stage III. Formation of the Midbody
The central spindle, also referred to as the spindle midzone, plays an important role in keeping separated chromosomes apart prior to cytokinesis completion, because when microtubules are depolymerized in late anaphase, the nuclei collapse back together.73 Microtubules in the midzone may be locally nucleated, as the minus ends of the midzone microtubules are decorated with gamma-tubulin.161, 162 As cytokinesis progresses, the constricting furrow compacts the midzone microtubule array. The furrow ingresses until a cytoplasmic bridge is formed that is 1–1.5 microns in diameter. Several kinesin-like motor proteins and chromosomal passenger proteins move along the midzone spindle towards the plus ends and accumulate in the overlapping region, forming a phase-dense structure referred to as the Flemming body, stembody, telophase disc, or midbody (reviewed in ref. 163). Disassembly of the actomyosin ring may be an important step at this stage of cytokinesis, as loss of F-actin accompanies and may trigger midbody formation.164 Once the cytoplasmic bridge matures and abscission begins, the bridge becomes insensitive to the actin inhibitor latrunculin,92 implying that the plasma membrane is linked to the midbody by a connection that does not involve dynamic f-actin. Scaffolding proteins such as anillin and septins may stabilize the bridge structure. In almost all systems, central spindle formation is essential for midbody formation, which in turn is necessary for abscission.16, 32, 165 In this section, we discuss the components that are required for formation of the central spindle and midbody.
PRC1 is a microtubule bundling protein that is critical for midzone formation in mammalian cells.32, 166 PRC1 accumulates on the central spindle in anaphase, and suppression of PRC1 expression causes failure of microtubule interdigitation.32 In the absence of PRC1, astral microtubules can guide the equatorial accumulation of anillin, actin, and chromosome passenger proteins, enabling cleavage furrow ingression, but abscission fails.167 PRC1 has separate domains that independently target the protein to the midzone and bundle microtubules.32 PRC1 is targeted to the midzone by the kinesin protein KIF4, which transports PRC1 to the ends of microtubules. Absence of KIF4 leads to a failure to accumulate PRC1 in the central spindle, and abolishes central spindle formation.168 PRC1 in turn recruits the centralspindlin complex, and additional mitotic kinesins including CENP-E, MCAK169 and KIF14.89 PRC1 also serves as an important docking site for the kinase Plk1 in the central spindle.170 PRC1 expression may be perturbed in cancer cells or in response to checkpoint signaling pathways. PRC1 upregulation in tumors169, 171 may be a consequence of p53 inactivation, as induction of p53 can inhibit PRC1 expression and interfere with cytokinesis completion.172, 173
Although the centralspindlin complex (MKLP1 and MgcRacGAP) is important for cytokinesis initiation as described earlier, centralspindlin is also necessary for spindle midzone and midbody formation, and ultimately for abscission.31, 165 Centralspindlin is recruited to the midzone by PRC1 (reviewed in ref. 174), and proper localization requires the presence of both members of the centralspindlin complex.16 A splice variant of MKLP1, called CHO1, includes an additional domain that can interact with F-actin,175 suggesting that CHO1 could link the actin and microtubule cytoskeletons. Injection of antibodies that target this domain induce failure in late steps of cytokinesis,175 suggesting CHO1 may stabilize interactions between midbody microtubules and the ingressing cleavage furrow in late steps of cytokinesis. Centralspindlin is also important for recruiting additional proteins to the midbody that are required for abscission, and both components of the complex appear to be regulated by phosphorylation. Each of these topics will be discussed in more detail below.
Proteomic approaches have identified a large number of proteins that concentrate at the midbody (Fig. 2),176 and a functional role for some of these proteins in abscission has been supported by results of RNAi experiments.92, 162, 177 Several of these proteins localize to the Golgi apparatus during interphase, and are released from the Golgi during mitosis by phosphorylation.178–180 Inhibition of Golgi disassembly during mitosis perturbs cytokinesis,181 perhaps by interfering with release of components that are essential for cytokinesis. Precisely how these proteins function in cytokinesis remains unclear, but one potential function is to recruit mitotic regulators such as Plk1 to the midbody.179
Additional proteins that localize to the midbody and are required for cytokinesis include LAPSER1, which may recruit the microtubule severing protein katanin to the midbody,182 and annexin 11.183 Annexins are Ca(2+)-binding, membrane-fusogenic proteins with diverse but poorly understood functions. Cells lacking annexin 11 fail to establish a functional midbody, and instead remain connected by intercellular bridges that contain bundled microtubules but exclude normal midbody components such as MKLP1 and Aurora B.183 These data suggest that despite its potential role in membrane fusion, annexin 11 seems to be required at an earlier step for recruitment of MKLP1 and Aurora B to the midbody.
Stage IV. Abscission
Once the midbody is formed, it subsequently organizes the final event of cytokinesis, termed abscission. By the time of abscission, the cytoplasmic bridge has narrowed to 0.2 microns in diameter. At these late stages, microtubule bundles become compacted and begin to disappear.92, 184 In this process, the cytoplasmic bridge is reorganized to permit separation of the daughter cells. A wide variety of proteins involved in vesicle and protein trafficking, membrane fusion, and other processes are required for abscission, suggesting the final stage of cytokinesis is just as complex as earlier stages. Human cultured cells may remain connected by the cytoplasmic bridge for many hours before undergoing abscission.185 In some systems, such as embryos, blastomeres often remain connected by intracellular bridges for many cell cycles. In spermatocytes, the cytoplasmic bridge is in fact stabilized,186 and cytokinesis completion does not occur, enabling communication between the cytoplasm of adjacent cells. Thus in certain circumstances abscission may be the target of physiological regulation.
Membrane Trafficking and Cytokinesis
Membrane trafficking plays a critical role in the process of cytokinesis (Fig. 4). Three pathways have been implicated in the process of cytokinesis. First, the secretory pathway, including Golgi-derived components, may contribute new membranes and proteins to the ingressing furrow, and also participate in late steps of cytokinesis completion. Second, the endocytic pathway and recycling endosomes may remodel membranes in the cleavage furrow and also contribute vesicles that may participate in the final steps of cytokinesis. Finally, recent evidence suggests that components of the ESCRT machinery, best characterized for its role in multivesicular body formation, may also be essential for the final stages of cytokinesis. The relative contributions of each of these pathways in the process of cytokinesis is likely to be dependent on cell type.
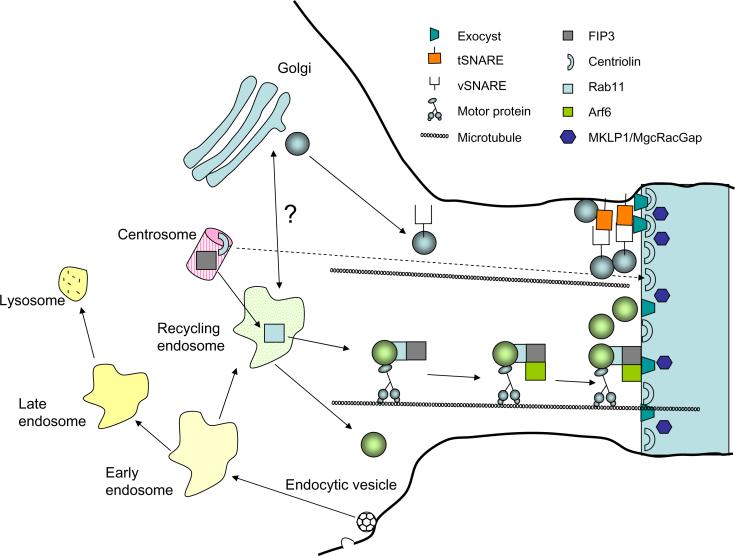
Figure 4. Membrane trafficking in cytokinesis.
Secretory vesicles accumulate at the intercellular bridge in a centriolin-dependent manner by SNARE interaction and vesicle tethering by the exocyst complex. Vesicles originating from the recycling endosome and containing the complex Rab11/FIP3 move along microtubules. Interaction of FIP3 with both ARF6 and the exocyst permits vesicle targeting to the midbody. Vesicles then fuse with each other and the plasma membrane, physically separating the daughter cells.
The Role of the Secretory Pathway
In large embryos, such as Xenopus and Sea Urchins, furrow ingression is coupled to insertion of new membrane via microtubule-dependent exocytosis.187–190 In smaller cells, such as mammalian tissue culture cells, it is less clear whether new membrane insertion is required. Brefeldin A (BFA), which disrupts ER-Golgi-dependent trafficking, blocks cytokinesis completion in some studies191, 192 but not others.193–196 Further evidence for a role of Golgi-derived vesicles in cytokinesis completion has emerged from studies of the protein centriolin, which may help recruit secretory vesicles to the site of abscission at the midbody.191 Centriolin was initially identified as protein that localizes to the maternal centriole during interphase and accumulates on mature centrioles during metaphase.197 Knockdown of centriolin in mammalian cells causes cytokinesis failure, with the two cells remaining connected by a cytoplasmic bridge.197 Centriolin localizes to a ring-like structure within the midbody,191 that also contains gamma-tubulin, GAP-CenA, and the centralspindlin complex. Recruitment of centriolin to the midbody ring is dependent on the centralspindlin complex, explaining why centralspindlin may be essential for cytokinesis completion.191
Centriolin may facilitate cytokinesis completion by recruiting to the midbody proteins involved in vesicle tethering and fusion. Centriolin interacts with components of the exocyst,191 a protein complex that tethers secretory vesicles to the plasma membrane (reviewed in ref. 198). Several components of the exocyst localize to the midbody ring in a centriolin-dependent manner, and depletion of exocyst components by siRNA interferes with cytokinesis completion.191 Centriolin also interacts with snapin, a snare-associated protein, and centriolin is required for the recruitment of snapin and SNARE proteins to the midbody.191
Secretory vesicles, derived from the Golgi apparatus, accumulate at the intercellular bridge during late steps in cytokinesis.191 At the time of abscission, the vesicles disappear, suggesting they undergo homotypic fusion with each other, and also heterotypic fusion with the plasma membrane, releasing their contents.191 Interestingly, vesicles seem to accumulate only on one side of the midbody, suggesting that delivery is asymmetric.191 This finding is consistent with the fact that following abscission, the midbody remains attached to one of the two daughter cells where it may play additional roles in signaling or marking the age of the cell.191 Why abscission typically occurs on only one side of the midbody remains unclear. It has been suggested that abscission may be triggered by arrival of the maternal centriole from one daughter cell,184 but this event has not been observed consistently.191 Asymmetric abscission may be important to enable the midbody to remain attached to a daughter cell, or it may provide an opportunity to regulate the timing of abscission. However, asymmetric abscission may be inherently more prone to failure than if abscission were to occur on both sides of the midbody. The mechanism and significance of asymmetric abscission is an interesting topic for future investigation.
The Role of Endocytosis and the Recycling Endosome Pathway
Several lines of evidence suggest that the endocytic and the recycling endosome pathways play critical roles in cytokinesis completion. Endocytosis within the furrow may be important for remodeling the plasma membrane during ingression. In addition, endocytosis from other regions of the cell may serve as a source of vesicles destined for delivery to the cleavage furrow either directly or through the recycling endosome. For example, some endocytic vesicles internalized from the polar region are subsequently trafficked to the midbody area during later stages of cytokinesis.199 Inhibition of proteins essential for endocytosis, including clathrin, dynamin, and alpha-adaptin, perturb cytokinesis in several systems,200–204 and inhibitors of clathrin-dependent endocytosis block cytokinesis completion in mammalian cells.195, 199 In addition, there may be direct interactions between the endocytic machinery and proteins required for cytokinesis such as anillin.205
Small GTPases that regulate membrane trafficking have been directly implicated in cytokinesis completion. Arf GTPases initiate the budding of coated carrier vesicles by recruiting coat protein complexes onto donor membranes, whereas Rab GTPases regulate the targeting and docking/fusion of vesicles with acceptor membranes.206 Two different GTPases, Arf6 and Rab11, have been implicated in regulation of cytokinesis. Rab11 localizes preferentially to the recycling endosome (RE), and is required for proper RE organization and the recycling of vesicles to the plasma membrane. Both Arf6 and Rab11 concentrate near the cleavage furrow and are required for late steps of cytokinesis in mammalian cells.199, 207–210 Both GTPases interact with a common set of effector proteins that assist in delivery of endosomal vesicles to the cleavage furrow, termed FIP3 (Arfophilin-1) and FIP4 (Arfophilin-2).207–209, 211–214 FIP3-containing endosomes accumulate near the cleavage furrow and are required for successful completion of cytokinesis.209 Recruitment of FIP3 to the midbody requires ARF6, and recruitment of ARF6 to the midbody requires FIP3.215 Other studies show that Arf6 interacts with MKLP1, suggesting the centralspindlin complex is important for targeting Arf6 to the cleavage furrow.207, 216 The exocyst has also been implicated in targeting of vesicles derived from the recycling endosome. For example, Exo70, a component of the exocyst complex, colocalizes with Arf6 in Rab11-positive endosomes.208 Exo70 interacts with FIP3 and FIP4 biochemically, and depletion of Exo70 impairs FIP3 and Rab11 localization to the furrow and midbody.208 Together these studies suggest the following model of delivery of endosomal vesicles to the midbody (Fig. 4). Rab11 first recruits FIP3 to endosomes. FIP3 in turn associates with ARF6, and together this complex localizes to the midbody via interactions with the exocyst and MKLP1.
Membrane Fusion During Abscission
Following vesicle targeting to the site of abscission, membrane fusion is necessary to complete cytokinesis. SNARE proteins are critical components required for membrane fusion (reviewed in ref. 217). Several SNARE proteins or associated components have been implicated in cytokinesis completion in different organisms.92, 218–220 In mammalian cells, two SNARE proteins, syntaxin 2 and endobrevin/VAMP-8, localize to the midbody during cytokinesis.191, 221, 222 Expression of dominant negative mutants or depletion of SNAREs impairs abscission, but has no effect on ingression of the cleavage furrow, suggesting that SNARE-mediated fusion is required only in the latest steps of cytokinesis.191, 221 Septin proteins may assist in membrane fusion by restricting the diffusion of membrane-associated components such as the exocyst to the region of abscision.153 Furthermore, septins may assist in abscission by directly recruiting the exocyst223 and SNARE proteins.224
Role of the ESCRT Machinery
Recently, protein subunits of the Endosomal Sorting Complex Required for Transport (ESCRT) that are normally involved in late endosome to lysosome trafficking have also been implicated in abscission. These proteins are best known for their roles in multivesicular body formation (reviewed in ref. 225), where they are important for membrane invagination. ESCRT complexes also play important roles in the topologically equivalent process of viral budding. Because abscission likely requires changes in membrane organization, a role for the ESCRT complex in cytokinesis is very intriguing. However, the precise mechanism of membrane invagination mediated by the ESCRT complex remains unknown, and it is unclear whether the ESCRT pathway functions independently in abscission or whether it assists in secretory- or endosomal vesicle-mediated cytokinesis completion.
Components of the ESCRT machinery localize to the midbody, and inhibition of some ESCRT complexes blocks late steps in cytokinesis. For example, CHMP3, a subunit of the ESCRT-III complex, localizes to the midbody, and deletion of a C-terminal autoinhibitory domain of CHMP3 inhibits cytokinesis.226 Other subunits of the ESCRT machinery implicated in abscission include tumor-susceptibility gene 101 (Tsg101), a subunit of the ESCRT-I complex, and Alix, an ESCRT-associated protein.222, 227 Alix may interact with actin and microtubules,228, 229 establishing a link between the ESCRT machinery and cytoskeletal components that are present at the midbody. Alix and Tsg101 are recruited to the midbody by interaction with centrosome protein 55 (Cep55), a centrosome and midbody protein essential for abscission.222, 230, 231 Interestingly, Tsg101 has been implicated in cancer, and may have additional functional roles in cell cycle and transcriptional regulation (reviewed in Ref 225).
Regulation of Cytokinesis
Thus far we have outlined the core pathways and components essential for each stage of cytokinesis. In the remaining part of the chapter, we discuss how these components are regulated to ensure that cytokinesis occurs at the proper place and time. Many regulatory pathways impinge upon the cytokinesis machinery, suggesting that cytokinesis may be responsive to a variety of different cues within the cell. The complexity of cytokinesis regulation suggests that cytokinesis failure could result from alterations in the activity of these regulatory pathways.
Regulation of Cytokinesis by Protein Kinases
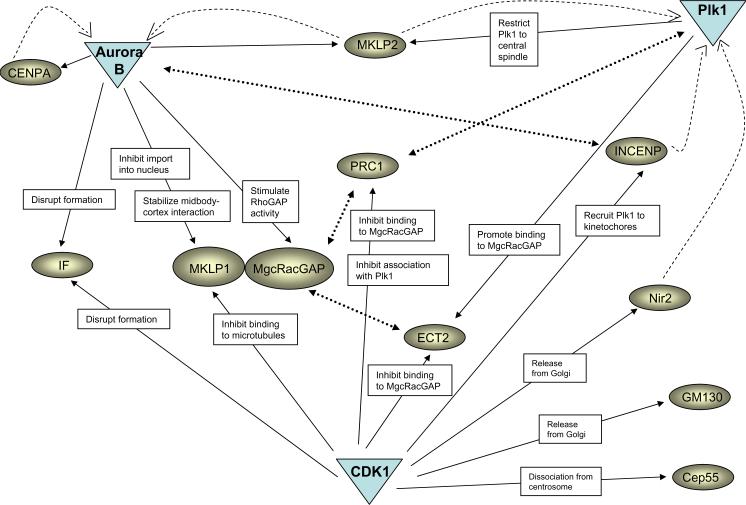
Cytokinesis is regulated by mitotic protein kinases, including cyclin-dependent kinases (CDKs), Polo kinase (Plk1), and the Aurora B kinase complex (Fig. 5). Mitotic CDK activity prevents cytokinesis onset until anaphase by phosphorylating cytokinesis components in a manner that inhibits their activity. For this reason, CDK1 must be inactivated for cytokinesis to proceed.232 In fact, inhibition of CDK1 with a small molecule is sufficient to induce the initial events of cytokinesis,233–235 suggesting that CDK1 inactivation is the trigger for cytokinesis initiation.
Figure 5. Regulation of cytokinesis by mitotic kinases.
A major function of CDK1 is to prevent precocious cytokinesis before proper chromosome segregation. CDK1 thus negatively regulates some of the main players of cytokinesis. At the same time, CDK1 plays a positive role in cytokinesis by releasing cytokinesis proteins from the Golgi apparatus, and by facilitating binding of Plk1 to its substrates. Plk1 and Aurora B phosphorylate substrates that are important for both early and late steps of cytokinesis. Solid arrows indicate phosphorylation; dashed arrows indicate changed protein localization; dotted arrows indicate protein interactions. IF, intermediate filaments.
In contrast, Polo kinase and Aurora B kinase positively regulate the events of cytokinesis, and must remain active for a period of time following CDK inactivation to promote cytokinesis. This period of the cell cycle, which lasts for about an hour in HeLa cells, has been referred to as “C-phase”.3, 236 C-phase is initiated by inactivation of CDK1, mediated by cyclin destruction catalyzed by the APC/C. At later times following anaphase, the APC/C also ubiquitinates other proteins that are essential for cytokinesis, including anillin,132 Polo kinase,237 and Aurora B.238 Thus the APC/C may also be responsible for terminating C-phase, an idea that is consistent with the finding that treatment of cells with proteasome inhibitors doubles the duration of C-phase.73
Regulation of Cytokinesis by CDK Activity
Because CDK1 is a central negative regulator of cytokinesis, it is possible that failure to fully inactivate CDK1, perhaps as a consequence of failure to fully degrade mitotic cyclins, could inhibit cytokinesis at some step. One setting in which this might occur is in cells that become arrested in mitosis due to persistent activation of the spindle checkpoint, which normally inhibits the APC/C until chromosomes become properly aligned and attached at the metaphase plate.239 Prolonged activation of the checkpoint may result in abnormal mitotic exit, resulting in incomplete activation of APC/C, or improper timing of degradation of different substrates, leading to cytokinesis failure. There is also evidence that APC/C activity may be spatially regulated within the cell, with the subpopulation of APC/C that is associated with the spindle poles remaining inhibited until later stages of mitosis.240 It is therefore possible that perturbation of spindle organization could interfere with the timing of degradation of mitotic regulators, thus perturbing cytokinesis.
CDK1 activity restrains multiple steps in cytokinesis. Cytokinesis initiation is inhibited because the RhoA pathway is kept inactive. This is a consequence of phosphorylation of ECT2 by CDK1 at a site that blocks its association with MgcRacGAP.19, 26 In addition, myosin light chain is phosphorylated by CDK1 at sites that inhibit myosin activation,82 and high CDK1 activity also inhibits cortical recruitment of myosin.241 Central spindle formation is also inhibited by CDK1-dependent phosphorylation. Phosphorylation of PRC1 by CDK1 inhibits its ability to bundle microtubules32, 166, 242 and its ability to interact with Plk1.170 CDK1 also phosphorylates MKLP1, inhibiting its motor activity by reducing its affinity for microtubules.243 Thus CDK1-dependent phosphorylation acts at many steps to block cytokinesis.
Though CDK1 restrains cytokinesis onset, CDK1-dependent phosphorylation is also essential for cytokinesis because it primes Plk1-dependent phosphorylation that occurs during early stages of cytokinesis. CDK1 activity is also important during mitosis to promote dissociation of cytokinesis proteins from cellular organelles that would otherwise sequester the protein. For example, the protein Nir2 is required for cytokinesis, and must dissociate from the Golgi apparatus in order to participate in cytokinesis; dissociation is mediated by CDK1-dependent phosphorylation.179 CDK1-dependent phosphorylation is also important for dissociation of Cep55 from the centrosome, and its subsequent phosphorylation by Plk1.231
Regulation by Polo Kinase
Polo kinase is an essential positive regulator of cytokinesis in multiple organisms. In mammalian cells, Plk1 localizes to the midzone during anaphase and to the midbody during telophase and cytokinesis,244 and plays an essential role in the initiation of cytokinesis.245–248 Plk1 activity is required for recruitment of itself and ECT2 to the central spindle, and inhibition of Plk1 with small molecule inhibitors abolishes RhoA GTPase localization to the equatorial cortex, suppressing cleavage furrow formation.246–248 Plk1 also appears to be important for the interaction between ECT2 and MgcRacGAP.247 Other evidence suggests that Plk1 may bind to ECT2 in a CDK1-dependent manner.249 Another study using a distinct Plk1 inhibitor demonstrated that when Plk1 is inhibited, it spreads over the arms of chromosomes, resembling the localization of its binding partner PICH.245 Therefore, Plk1 activity is required for its own proper localization during cytokinesis, and also for recruitment and activation of RhoA.
Plk1 activity may be required for later steps in cytokinesis as well, as Plk1 is targeted to the central spindle by the motor protein MKLP2, and phosphorylation of MKLP2 by Plk1 is required for cytokinesis.250 Phosphorylation of MKLP2 by Plk1 may be necessary for the spatial restriction of Plk1 to the central spindle during anaphase and telophase, although interaction with PRC1 also appears to be important for docking of Plk1 to the central spindle.170 Plk1 may also interact with and phosphorylate MKLP1 during cytokinesis,244, 251 although others suggest that Aurora B may be the relevant kinase.252 Cep55 also appears to be a Plk1 substrate whose phosphorylation is primed by CDK1 but the consequences of this phosphorylation remains unknown.231 A recent proteomic screen identified a large number of proteins that bind to the Polo-box domain of Plk1, including the Rho kinase ROCK2,253 where Plk1 and RhoA may function together to enhance ROCK2 activity. Another substrate of Plk1 that may be involved in regulation of mitosis and cytokinesis is NudC,254, 255 a dynein/dynactin associated protein that is essential for midzone formation and cytokinesis completion in C. elegans and mammalian cells.254
Plk1 is overexpressed in a broad range of human tumors (for review see Ref. 256). Overexpression of Plk1 in HeLa cells leads to an increase of cells with large, often fragmented nuclei or multiple nuclei257 as well as centrosome amplification,258 suggesting that increased expression of Plk1 observed in some tumors may have an effect on cytokinesis completion as well as chromosome segregation. This finding has been corroborated in human primary cells.259
Regulation by Aurora B and the Chromosome Passenger Complex
The chromosome passenger complex (CPC) consists of the proteins Aurora B, INCENP, survivin, and borealin. The complex plays many important roles throughout mitosis, and has been implicated in the regulation of cytokinesis (see ref. 260 for review). At the metaphaseanaphase transition, the CPC relocalizes from centromeres to the spindle midzone and the equatorial cortex,261–263 and ultimately concentrates near the midbody, adjacent to the centriolin ring.191 MKLP2, a kinesin-6 family motor protein, is required for relocalization of Aurora B, and also Plk1, to the central spindle in human cells.250, 252, 264, 265
Aurora B activity is necessary for several events in cytokinesis (Fig. 5). First, Aurora B is required for proper localization and function MKLP1. Treatment of human cells with a small molecule inhibitor of Aurora B in early mitosis inhibits localization of MKLP1 (and its binding partner MgcRacGAP) to the central spindle.266 However, addition of an Aurora inhibitor at later stages of mitosis inhibits phosphorylation of MKLP1 without disrupting its localization,252, 267 yet perturbs cytokinesis completion, indicating that MKLP1 must remain phosphorylated to permit abscission. How phosphorylation regulates MKLP1 is not completely clear, as MKLP1 is phosphorylated at multiple sites that may have distinct effects.252, 267 Phosphorylation of MKLP1 could be important for stabilizing interactions between the cortex and midbody, or be important for recruiting proteins such as centriolin that are necessary for abscission. In addition, phosphorylation of MKLP1 by Aurora B is important to prevent the protein from being sequestered back in the nucleus as cells enter interphase.252 It is interesting to note that many components required for cytokinesis are located in the nucleus or associated with Golgi apparatus during interphase (Fig. 2), and thus localization of cytokinesis components to the midbody could require sustained phosphorylation that prevents the proteins from being resequestered by these structures as cells exit mitosis.
Another important substrate of Aurora B is MgcRacGAP, whose phosphorylation appears important for completion of cytokinesis.47, 268, 269 Phosphorylation of MgcRacGAP has been proposed to stimulate its activity as a GAP for RhoA, which could be important for terminating RhoA activity in late stages of cytokinesis.47 Another study indicates that phosphorylation of MgcRacGAP by Aurora B at a different site might activate the protein by stimulating release of the GAP domain from an inhibitory interaction with PRC1.268
There are several other important Aurora substrates include vimentin, an abundant intermediate filament protein. Intermediate filaments must be disassembled during mitosis to allow cell division, and mitotic phosphorylation is important for filament dissociation, as expression of nonphosphorylatable mutants of vimentin leads to cells that show a persistent filamentous bridge.270, 271 Following mitotic exit and during later stages of cytokinesis, ROCK272 and Aurora B112, 270, 271, 273 maintain vimentin phosphorylation after CDK1 is inactivated. Aurora B may also promote cytokinesis by inhibiting myosin light chain phosphatase.112 Aurora B also phosphorylates CENPA, which appears to play an important role in cytokinesis.274 Cells expressing mutants of CENPA that cannot be phosphorylated result in mislocalization of the passenger complex, and cause a delay in the final stages of cytokinesis.
Although Aurora B is a critical positive regulator of cytokinesis in vertebrate cells, this role does not seem conserved in yeast as the budding yeast ortholog Ipl1 and the fission yeast ortholog Ark1 are not essential for cytokinesis. However, in budding yeast, Ipl1 may negatively regulate late steps of cytokinesis in cells with spindle defects,275, 276 perhaps by regulating the localization of anillin-like proteins. This pathway may prevent abscission until segregating chromosomes have cleared the midzone. Whether a similar pathway operates in mammalian cells is not yet clear.
We are just beginning to learn about the mechanisms that regulate Aurora B activation. New work suggests that the TD60 protein may play an important role in activating Aurora B at centromeres.277 In multiple organisms, components of the mitotic exit network, including the phosphatase CDC14, play important roles in regulating cytokinesis,278–280 in part through regulation of targeting of the Aurora B complex.281 Aurora B is also regulated by a Cul3-containing ubiquitin ligase, which is important for removing Aurora B from mitotic chromosomes and allowing its accumulation on the central spindle during anaphase.282
In vertebrate cells, Aurora B is expressed in a cell-cycle dependent manner, peaking in the G2/M phase of the cell cycle,283, 284 and is highly expressed in a number of cancers.263, 284–291 However, in these tumors, expression of other proliferative markers, such as Ki-67, MCM2, geminin, and Aurora A is also increased,291, 292 suggesting that Aurora B upregulation may be part of a broader upregulation of mitotic components in tumor cells. Because Aurora B is a positive regulator of cytokinesis, it is unclear whether its overexpression would perturb cytokinesis. Elevated levels of Aurora B may promote cytokinesis completion in cells that would otherwise undergo cytokinesis failure due to other abnormalities in the mitotic machinery. However, it is possible that perturbing Aurora B expression, or other components of the CPC, could alter the stoichiometry of the complex, perturbing cytokinesis.293
Regulation of Cytokinesis by Tyrosine Kinases
Tyrosine kinase signaling pathways may also regulate cytokinesis completion. Small molecule inhibitors of Src, including PP2 and SU6656, inhibit abscission in HeLa cells.294 Src activity appears to be required in early mitosis, followed by delivery of tyrosine-phosphorylated proteins to the midbody via Rab11-driven vesicle transport.294 Src co-localizes with the diaphanous-related formins mDia1 and mDia2 in endosomes and midbodies of dividing cells, and inhibition of Src blocks cytokinesis.67 Other tyrosine kinases, such as Fyn and its associated proteins are required for cytokinesis in lymphocytes,295 through mechanisms that remain obscure.
Regulation of Cytokinesis by Lipids
Several studies indicate that phosphoinositide-containing lipids may be important for cytokinesis, with phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) playing a central role. In mammalian cells, PtdIns(4,5)P2 accumulates at the cleavage furrow, and overexpression of proteins that bind to PtdIns(4,5)P2 perturbs cytokinesis completion but not ingression of the cleavage furrow.296 Overexpression of dominant negative kinases required for PtdIns(4,5)P2 generation also inhibits cytokinesis completion in mammalian cells.296
Hydrolysis of PtdIns(4,5)P2 by PLC yields inositol trisphosphate (IP3), which stimulates calcium release from internal stores. Inhibitors of PLC can interfere with cytokinesis,297 which can in some cases be rescued by addition of calcium.298 Alternatively, PtdIns(4,5)P2 may play a direct role in recruiting membrane proteins required for stability of the furrow. Several proteins required for cytokinesis, such as septin, profilin, and anillin can bind to PtdIns(4,5)P2, and overexpression of a protein containing a PtdIns(4,5)P2-binding domain blocks cytokinesis completion by interfering with adhesion of the plasma membrane to the contractile ring at the furrow.296
Other studies have shown that the membrane lipid phosphatidylethanolamine (PE) is exposed on the cell surface of the cleavage furrow during late cytokinesis.299 Addition of a cyclic peptide that binds tightly to PE inhibits cytokinesis completion,299 perhaps by interfering with contractile ring disassembly.300 Mutant cell lines that fail to synthesize adequate PE also show defects in cytokinesis completion that can be rescued by PE addition.300 Proper PE organization may be essential for RhoA inactivation at late stages of cytokinesis, which may in turn be necessary for actin disassembly.301
Coupling of Cytokinesis to Other Cellular Pathways
The complexity of cytokinesis regulation provides opportunities for linking cytokinesis to other cellular pathways. Emerging evidence suggests interesting new connections between cytokinesis and the pathways involved in regulation of protein synthesis, DNA replication, and DNA damage.
Cytokinesis and Protein Synthesis
Recent work suggests that proper regulation of protein synthesis may be essential for cytokinesis to proceed with high efficiency. Interestingly, of 214 genes identified in a genome-wide RNAi screen in Drosophila S2 cells, 22% were ribosomal proteins, and another 5% were involved in translation.177 Recent work suggests that the protein 14-3-3σ may play an important role in regulating protein synthesis during mitosis.302 In normal cells, cap-dependent translation is suppressed during mitosis, whereas cap-independent translation is increased. Cells lacking 14-3-3σ do not make this switch, perturbing the pattern of proteins that are synthesized.302 Downregulation of 14-3-3σ perturbs localization of Plk1 to the midbody, and leads to cytokinesis failure.302 These effects may be a consequence of failure to properly synthesize proteins containing an internal ribosomal entry site during mitosis, such as Cdk11.302 Interestingly, 14-3-3σ expression is often reduced in tumor cells by targeted degradation or promoter hypermethylation. Loss of 14-3-3σ may in turn result in defective cytokinesis as a consequence of alterations in protein synthesis.
Cytokinesis and DNA Replication
Interestingly, one of the components of the Origin Recognition Complex (ORC), which is required for initiation of DNA replication, may also play a role in cytokinesis in metazoans. In vertebrate cells, Orc6 localizes to kinetochores and to a reticular-like structure around the cell periphery, and ultimately to the cleavage furrow and midbody.303 Elimination of Orc6 induces mutlipolar spindles and formation of multinucleated cells in both human cells303 and Drosophila,304 suggesting this function is conserved. In Drosophila, Orc6 interacts with a septin protein that may be important for cytokinesis. Domains of Orc6 required for DNA replication and cytokinesis appear separable, suggesting that Orc6 has evolved a domain that participates specifically in cytokinesis.304 How Orc6 might couple the processes of DNA replication and cytokinesis completion remains unclear.
Cytokinesis and DNA Damage
Several lines of evidence suggest that cytokinesis may be regulated in response to DNA damage. Components required for DNA repair, such as BRCA2, may be directly involved in cytokinesis. Other evidence suggests that DNA damage pathways may regulate the expression of cytokinesis proteins, or regulate their activity by post-translational modification. Coupling of DNA damage pathways to cytokinesis regulation could be important for preventing the cleavage furrow from cutting damaged DNA that cannot be accurately segregated during mitosis. The existence of such pathways may explain why spontaneous chromosome missegregation is tightly coupled to cytokinesis failure in human cells.185 In this model, DNA damage, or perhaps incompletely replicated DNA, may trigger pathways that prevent segregation of unreplicated or damaged sister chromatids, and at the same time activate pathways that block cytokinesis completion.
BRCA2 is an example of a protein that may play direct roles in both DNA repair and cytokinesis. BRCA2 is required for recombination-based repair of DNA double-strand breaks.305 However, BRCA2-deficient cells also show centrosome amplification that may be a consequence of defective cytokinesis.306 BRCA2 localizes to the midbody, and inactivation of BRCA2 in murine embryonic fibroblasts and HeLa cells interferes with cytokinesis.307 BRCA2 may be regulated during mitosis, as it is a Plk1 substrate whose phosphorylation is inhibited in the presence of DNA damage.308 Interestingly, downregulation of a BRCA2-interacting protein (BCCIP) also leads to defective cytokinesis.309 Other proteins involved in DNA damage responses may influence cytokinesis regulation by interacting with cytokinesis components. For example, the DNA damage checkpoint kinase Rad53 has been shown to associate with septins in budding yeast.310 In mammalian cells, Ku70, a DNA-binding protein required for DNA damage repair, forms a complex with ARF6 during mitosis,311 suggesting a possible link between the DNA damage pathway and completion of cytokinesis.
Transcriptional controls may provide another mechanism for inhibiting cytokinesis in response to DNA damage. The expression of several cytokinesis proteins, including Plk1, ECT2, anillin, and survivin, is repressed when DNA is damaged, in a manner that depends on an intact Rb pathway.312 Other studies suggest that expression of cytokinesis proteins may be inhibited by activation of the p53 pathway.313 For example, it has been shown that ECT2 expression is repressed by p53 via protein methyltransferases, suggesting that cytokinesis could be more likely to fail under conditions of p53 activation.314
Post-translational modifications may also regulate cytokinesis in response to DNA damage. For example, Aurora B becomes highly poly-ADP-ribosylated when DNA is damaged, a modification that inhibits its kinase activity.315 Poly(ADP-ribosyl)ation is an immediate cellular response to DNA strand breaks that is catalyzed by NAD+-dependent enzymes, poly(ADP-ribose) polymerases (PARPs).316 This effect is mediated by direct interaction between the BRCT domain of PARP1 and Aurora B.315 Because Aurora B activity is essential for chromosome segregation and cytokinesis, induction of DNA damage could lead to errors in chromosome segregation and failure of cytokinesis.
Conclusions
Cytokinesis is a surprisingly complex process that requires the interplay of many components and regulatory pathways. Cytokinesis failure can arise through defects in any of the four stages in cytokinesis, and as a consequence of inactivation or hyperactivation of any of a large number of different components (summarized in Fig. 6). Although many cytokinesis proteins have been identified, we are just beginning to understand how these proteins interact with one another, and how they are regulated. Understanding the causes of cytokinesis failure is important, as it may set the stage for genetically unstable tetraploid cells that give rise to tumors.317 However, cytokinesis failure also seems to occur physiologically in some tissues, even in those that are not tumor prone such as the heart. Understanding how cytokinesis is regulated physiologically in response to different signals, or under conditions of cell stress or damage, remains an important area for future research. Although cytokinesis failure may be accompany certain pathological states such as cancer, it is likely that pharmacologically-induced cytokinesis failure may be an important issue to consider as new medicines are developed. Inhibitors of Rho kinase are being developed for cardiovascular medicine,318 and inhibitors of Aurora kinase are under development as anticancer agents.319 Because these compounds are likely to induce cytokinesis failure in normal tissues, it will be important to determine how sensitive various tissues are to cytokinesis failure, and the consequences of production of tetraploid cells in different tissue types.
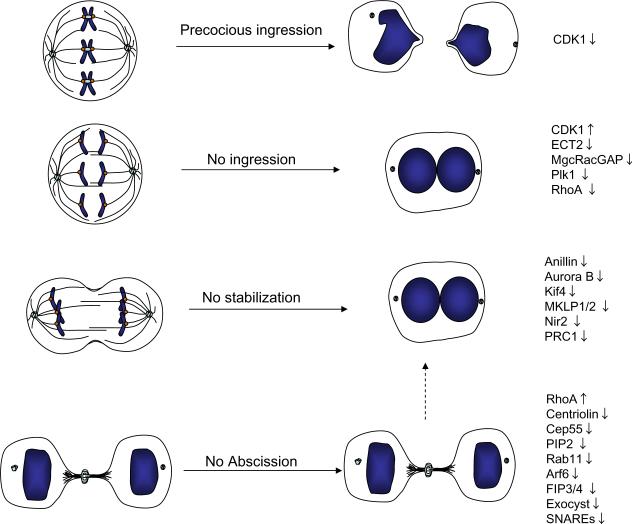
Figure 6. Summary of different phenotypes resulting from cytokinesis failure.
Inhibition (downward arrow) or excessive activation (upward arrow) of different cytokinesis components can give rise to distinct phenotypes, including precocious ingression before the chromosomes have been separated, regression of the furrow giving rise to binucleated cells, or stabilization of the cytoplasmic bridge where daughter cells remain connected. The list is not comprehensive; see text for additional examples.
Acknowledgments
Work in the author's laboratory is supported by grants from the National Instiutes of Health (GM 66492), the Dana-Farber/Harvard SPORE in Breast Cancer (CA089393) and the Susan G. Komen Foundation (BCTR131606).
References
- 1.Rappaport R. Cytokinesis in animal cells. Int Rev Cytol. 1971;31:169–213. doi: 10.1016/s0074-7696(08)60059-5. [DOI] [PubMed] [Google Scholar]
- 2.Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- 3.Canman JC, Cameron LA, Maddox PS, et al. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- 4.Hiramoto Y. Analysis of cleavage stimulus by means of micromanipulation of sea urchin eggs. Exp Cell Res. 1971;68:291–298. doi: 10.1016/0014-4827(71)90153-4. [DOI] [PubMed] [Google Scholar]
- 5.Burgess DR, Chang F. Site selection for the cleavage furrow at cytokinesis. Trends Cell Biol. 2005;15:156–162. doi: 10.1016/j.tcb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93(Pt 2):255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- 7.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pletjushkina OJ, Rajfur Z, Pomorski P, Oliver TN, Vasiliev JM, Jacobson KA. Induction of cortical oscillations in spreading cells by depolymerization of microtubules. Cell Motil Cytoskeleton. 2001;48:235–244. doi: 10.1002/cm.1012. [DOI] [PubMed] [Google Scholar]
- 9.Canman JC, Bement WM. Microtubules suppress actomyosin-based cortical flow in Xenopus oocytes. J Cell Sci. 1997;110(Pt 16):1907–1917. doi: 10.1242/jcs.110.16.1907. [DOI] [PubMed] [Google Scholar]
- 10.Werner M, Munro E, Glotzerl M. Astral signals spatially bias cortical myosin recruitment to break symmetry and promote cytokinesis. Current Biology. 2007;17:1286–1297. doi: 10.1016/j.cub.2007.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bringmann H, Hyman AA. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–734. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- 12.Dechant R, Glotzer M. Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell. 2003;4:333–344. doi: 10.1016/s1534-5807(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 13.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 16.Jantsch-Plunger V, Gonczy P, Romano A, et al. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J Cell Biol. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamijo K, Ohara N, Abe M, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J Cell Sci. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizaki H, Ohba Y, Kurokawa K, et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JE, Billadeau DD, Chen JJ. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J Biol Chem. 2005;280:5733–5739. doi: 10.1074/jbc.M409298200. [DOI] [PubMed] [Google Scholar]
- 25.Chalamalasetty RB, Hummer S, Nigg EA, Sillje HHW. Influence of human Ect2 depletion and overexpression on cleavage furrow formation and abscission. J Cell Sci. 2006;119:3008–3019. doi: 10.1242/jcs.03032. [DOI] [PubMed] [Google Scholar]
- 26.Hara T, Abe M, Inoue H, et al. Cytokinesis regulator ECT2 changes its conformation through phosphorylation at Thr-341 in G2/M phase. Oncogene. 2006;25:566–578. doi: 10.1038/sj.onc.1209078. [DOI] [PubMed] [Google Scholar]
- 27.Miki T, Smith CL, Long JE, Eva A, Fleming TP. Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature. 1993;362:462–465. doi: 10.1038/362462a0. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Liu XF, Kamijo K, et al. Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation. J Biol Chem. 2004;279:7169–7179. doi: 10.1074/jbc.M306725200. [DOI] [PubMed] [Google Scholar]
- 29.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 30.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 31.Matuliene J, Kuriyama R. Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1. Mol Biol Cell. 2004;15:3083–3094. doi: 10.1091/mbc.E03-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollinari C, Kleman JP, Jiang W, Schoehn G, Hunter T, Margolis RL. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J Cell Biol. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemens F, Verma R, Ramnath J, Landolph JR. Amplification of the Ect2 protooncogene and over-expression of Ect2 mRNA and protein in nickel compound and methylcholanthrene-transformed 10T1/2 mouse fibroblast cell lines. Toxicol Appl Pharmacol. 2005;206:138–149. doi: 10.1016/j.taap.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Tatsumoto T, Sakata H, Dasso M, Miki T. Potential roles of the nucleotide exchange factor ECT2 and Cdc42 GTPase in spindle assembly in Xenopus egg cell-free extracts. J Cell Biochem. 2003;90:892–900. doi: 10.1002/jcb.10750. [DOI] [PubMed] [Google Scholar]
- 35.Canevascini S, Marti M, Frohli E, Hajnal A. The Caenorhabditis elegans homologue of the proto-oncogene ect-2 positively regulates RAS signalling during vulval development. EMBO Rep. 2005;6:1169–1175. doi: 10.1038/sj.embor.7400574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito S, Tatsumoto T, Lorenzi MV, et al. Rho exchange factor ECT2 is induced by growth factors and regulates cytokinesis through the N-terminal cell cycle regulator-related domains. J Cell Biochem. 2003;90:819–836. doi: 10.1002/jcb.10688. [DOI] [PubMed] [Google Scholar]
- 37.Eguchi T, Takaki T, Itadani H, Kotani H. RB silencing compromises the DNA damage-induced G2/M checkpoint and causes deregulated expression of the ECT2 oncogene. Oncogene. 2007;26:509–520. doi: 10.1038/sj.onc.1209810. [DOI] [PubMed] [Google Scholar]
- 38.Roversi G, Pfundt R, Moroni RF, et al. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene. 2006;25:1571–1583. doi: 10.1038/sj.onc.1209177. [DOI] [PubMed] [Google Scholar]
- 39.Sano M, Genkai N, Yajima N, et al. Expression level of ECT2 proto-oncogene correlates with prognosis in glioma patients. Oncol Rep. 2006;16:1093–1098. [PubMed] [Google Scholar]
- 40.Kanada M, Nagasaki A, Uyeda TQ. Novel functions of Ect2 in polar lamellipodia formation and polarity maintenance during “Contractile Ring-Independent” cytokinesis in adherent cells. Mol Biol Cell. 2008;19:8–16. doi: 10.1091/mbc.E07-04-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D, Asiedu M, Adelstein RS, Wei Q. A novel guanine nucleotide exchange factor MyoGEF is required for cytokinesis. Cell Cycle. 2006;5:1234–1239. doi: 10.4161/cc.5.11.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf A, Keil R, Gotzl O, et al. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nature Cell Biol. 2006;8:1432–U1478. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 44.Kitzing TM, Sahadevan AS, Brandt DT, et al. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dadke D, Jarnik M, Pugacheva EN, Singh MK, Golemis EA. Deregulation of HEF1 impairs M-phase progression by disrupting the RhoA activation cycle. Mol Biol Cell. 2006;17:1204–1217. doi: 10.1091/mbc.E05-03-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J Cell Biol. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minoshima Y, Kawashima T, Hirose K, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549–560. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 48.Yoshizaki H, Ohba Y, Parrini MC, et al. Cell type-specific regulation of RhoA activity during cytokinesis. J Biol Chem. 2004;279:44756–44762. doi: 10.1074/jbc.M402292200. [DOI] [PubMed] [Google Scholar]
- 49.D'Avino PP, Savoian MS, Capalbo L, Glover DM. RacGAP50C is sufficient to signal cleavage furrow formation during cytokinesis. J Cell Sci. 2006;119:4402–4408. doi: 10.1242/jcs.03210. [DOI] [PubMed] [Google Scholar]
- 50.D'Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol. 2004;166:61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oceguera-Yanez F, Kimura K, Yasuda S, et al. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J Cell Biol. 2005;168:221–232. doi: 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guidotti JE, Bregerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: A pivotal role for binuclear hepatocytes. J Biol Chem. 2003;278:19095–19101. doi: 10.1074/jbc.M300982200. [DOI] [PubMed] [Google Scholar]
- 53.Margall-Ducos G, Celton-Morizur S, Couton D, Bregerie O, Desdouets C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J Cell Sci. 2007;120:3633–3639. doi: 10.1242/jcs.016907. [DOI] [PubMed] [Google Scholar]
- 54.Kudryavtsev BN, Kudryavtseva MV, Sakuta GA, Stein GI. Human hepatocyte polyploidization kinetics in the course of life cycle. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:387–393. doi: 10.1007/BF02915139. [DOI] [PubMed] [Google Scholar]
- 55.Toyoda H, Bregerie O, Vallet A, et al. Changes to hepatocyte ploidy and binuclearity profiles during human chronic viral hepatitis. Gut. 2005;54:297–302. doi: 10.1136/gut.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. J Cell Biol. 2007;178:1109–1120. doi: 10.1083/jcb.200703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 58.Green RA, Kaplan KB. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci. 2004;117:6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- 60.Dikovskaya D, Schiffmann D, Newton IP, et al. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fodde R, Kuipers J, Rosenberg C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 62.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 63.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–2830. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 64.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–281. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–518. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe N, Madaule P, Reid T, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 68.Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol. 2003;13:534–545. doi: 10.1016/s0960-9822(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 69.Cao LG, Wang YL. Mechanism of the formation of contractile ring in dividing cultured animal cells. II. Cortical movement of microinjected actin filaments. J Cell Biol. 1990;111:1905–1911. doi: 10.1083/jcb.111.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao LG, Wang YL. Mechanism of the formation of contractile ring in dividing cultured animal cells. I. Recruitment of preexisting actin filaments into the cleavage furrow. J Cell Biol. 1990;110:1089–1095. doi: 10.1083/jcb.110.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Satoh S, Tominaga T. mDia-interacting protein acts downstream of Rho-mDia and modifies Src activation and stress fiber formation. J Biol Chem. 2001;276:39290–39294. doi: 10.1074/jbc.M107026200. [DOI] [PubMed] [Google Scholar]
- 72.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Straight AF, Cheung A, Limouze J, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 74.Sellers JR. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991;3:98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- 75.Trybus KM, Warshaw DM. Regulation of the interaction between smooth muscle myosin and actin. J Cell Sci Suppl. 1991;14:87–89. doi: 10.1242/jcs.1991.supplement_14.18. [DOI] [PubMed] [Google Scholar]
- 76.Yamakita Y, Yamashiro S, Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeBiasio RL, LaRocca GM, Post PL, Taylor DL. Myosin II transport, organization, and phosphorylation: evidence for cortical flow/solation-contraction coupling during cytokinesis and cell locomotion. Mol Biol Cell. 1996;7:1259–1282. doi: 10.1091/mbc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jordan P, Karess R. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J Cell Biol. 1997;139:1805–1819. doi: 10.1083/jcb.139.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsumura F, Ono S, Yamakita Y, Totsukawa G, Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bengur AR, Robinson EA, Appella E, Sellers JR. Sequence of the sites phosphorylated by protein kinase C in the smooth muscle myosin light chain. J Biol Chem. 1987;262:7613–7617. [PubMed] [Google Scholar]
- 81.Ikebe M, Reardon S. Phosphorylation of bovine platelet myosin by protein kinase C. Biochemistry. 1990;29:2713–2720. doi: 10.1021/bi00463a014. [DOI] [PubMed] [Google Scholar]
- 82.Satterwhite LL, Lohka MJ, Wilson KL, et al. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Totsukawa G, Himi-Nakamura E, Komatsu S, et al. Mitosis-specific phosphorylation of smooth muscle regulatory light chain of myosin II at Ser-1 and/or -2 and Thr-9 in sea urchin egg extract. Cell Struct Funct. 1996;21:475–482. doi: 10.1247/csf.21.475. [DOI] [PubMed] [Google Scholar]
- 84.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 85.Ueda K, Murata-Hori M, Tatsuka M, Hosoya H. Rho-kinase contributes to diphosphorylation of myosin II regulatory light chain in nonmuscle cells. Oncogene. 2002;21:5852–5860. doi: 10.1038/sj.onc.1205747. [DOI] [PubMed] [Google Scholar]
- 86.Eda M, Yonemura S, Kato T, et al. Rho-dependent transfer of Citron-kinase to the cleavage furrow of dividing cells. J Cell Sci. 2001;114:3273–3284. doi: 10.1242/jcs.114.18.3273. [DOI] [PubMed] [Google Scholar]
- 87.Madaule P, Eda M, Watanabe N, et al. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 88.Paramasivam M, Chang YJ, LoTurco JJ. ASPM and citron kinase co-localize to the midbody ring during cytokinesis. Cell Cycle. 2007;6:1605–1612. doi: 10.4161/cc.6.13.4356. [DOI] [PubMed] [Google Scholar]
- 89.Gruneberg U, Neef R, Li X, et al. KIF14 and citron kinase act together to promote efficient cytokinesis. J Cell Biol. 2006;172:363–372. doi: 10.1083/jcb.200511061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shandala T, Gregory SL, Dalton HE, Smallhorn M, Saint R. Citron kinase is an essential effector of the Pbl-activated Rho signalling pathway in Drosophila melanogaster. Development. 2004;131:5053–5063. doi: 10.1242/dev.01382. [DOI] [PubMed] [Google Scholar]
- 91.Naim V, Imarisio S, Di Cunto F, Gatti M, Bonaccorsi S. Drosophila citron kinase is required for the final steps of cytokinesis. Mol Biol Cell. 2004;15:5053–5063. doi: 10.1091/mbc.E04-06-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Echard A, Hickson GR, Foley E, O'Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamashiro S, Totsukawa G, Yamakita Y, et al. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol Biol Cell. 2003;14:1745–1756. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Cunto F, Imarisio S, Hirsch E, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 95.LoTurco JJ, Sarkisian MR, Cosker L, Bai J. Citron kinase is a regulator of mitosis and neurogenic cytokinesis in the neocortical ventricular zone. Cereb Cortex. 2003;13:588–591. doi: 10.1093/cercor/13.6.588. [DOI] [PubMed] [Google Scholar]
- 96.Sarkisian MR, Li WW, Di Cunto F, D'Mello SR, LoTurco JJ. Citron-kinase, a protein essential to cytokinesis in neuronal progenitors, is deleted in the flathead mutant rat. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-08-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Di Cunto F, Imarisio S, Camera P, Boitani C, Altruda F, Silengo L. Essential role of citron kinase in cytokinesis of spermatogenic precursors. J Cell Sci. 2002;115:4819–4826. doi: 10.1242/jcs.00163. [DOI] [PubMed] [Google Scholar]
- 98.Madhavan J, Coral K, Mallikarjuna K, et al. High expression of KIF14 in retinoblastoma: Association with older age at diagnosis. Investigative Ophthalmology & Visual Science. 2007;48:4901–4906. doi: 10.1167/iovs.07-0063. [DOI] [PubMed] [Google Scholar]
- 99.Corson TW, Huang A, Tsao MS, Gallie BL. KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene. 2005;24:4741–4753. doi: 10.1038/sj.onc.1208641. [DOI] [PubMed] [Google Scholar]
- 100.Corson TW, Gallie BL. KIF14 mRNA expression is a predictor of grade and outcome in breast cancer. Int J Cancer. 2006;119:1088–1094. doi: 10.1002/ijc.21954. [DOI] [PubMed] [Google Scholar]
- 101.Corson TW, Zhu CQ, Lau SK, Shepherd FA, Tsao MS, Gallie BL. KIF14 messenger RNA expression is independently prognostic for outcome in lung cancer. Clin Cancer Res. 2007;13:3229–3234. doi: 10.1158/1078-0432.CCR-07-0393. [DOI] [PubMed] [Google Scholar]
- 102.Carleton M, Mao M, Biery M, et al. RNA interference-mediated silencing of mitotic kinesin KIF14 disrupts cell cycle progression and induces cytokinesis failure. Mol Cell Biol. 2006;26:3853–3863. doi: 10.1128/MCB.26.10.3853-3863.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL. A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J Cell Biol. 2002;156:543–553. doi: 10.1083/jcb.200110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Poperechnaya A, Varlamova O, Lin PJ, Stull JT, Bresnick AR. Localization and activity of myosin light chain kinase isoforms during the cell cycle. J Cell Biol. 2000;151:697–708. doi: 10.1083/jcb.151.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erent M, Pagakis S, Browne JP, Bayley P. Association of calmodulin with cytoskeletal structures at different stages of HeLa cell division, visualized by a calmodulin-EGFP fusion protein. Mol Cell Biol Res Commun. 1999;1:209–215. doi: 10.1006/mcbr.1999.0137. [DOI] [PubMed] [Google Scholar]
- 106.Yu YY, Chen Y, Dai G, et al. The association of calmodulin with central spindle regulates the initiation of cytokinesis in HeLa cells. Int J Biochem Cell Biol. 2004;36:1562–1572. doi: 10.1016/j.biocel.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 107.Li CJ, Heim R, Lu P, Pu Y, Tsien RY, Chang DC. Dynamic redistribution of calmodulin in HeLa cells during cell division as revealed by a GFP-calmodulin fusion protein technique. J Cell Sci. 1999;112(Pt 10):1567–1577. doi: 10.1242/jcs.112.10.1567. [DOI] [PubMed] [Google Scholar]
- 108.Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 109.Somlyo AV, Wang H, Choudhury N, et al. Myosin light chain kinase knockout. J Muscle Res Cell Motil. 2004;25:241–242. doi: 10.1023/b:jure.0000038362.84697.c0. [DOI] [PubMed] [Google Scholar]
- 110.Wong R, Fabian L, Forer A, Brill JA. Phospholipase C and myosin light chain kinase inhibition define a common step in actin regulation during cytokinesis. BMC Cell Biol. 2007;8 doi: 10.1186/1471-2121-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yokoyama T, Goto H, Izawa I, Mizutani H, Inagaki M. Aurora-B and Rhokinase/ROCK, the two cleavage furrow kinases, independently regulate the progression of cytokinesis: possible existence of a novel cleavage furrow kinase phosphorylates ezrin/radixin/moesin (ERM) Genes Cells. 2005;10:127–137. doi: 10.1111/j.1365-2443.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 113.Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. J Cell Sci. 2002;115:2271–2282. doi: 10.1242/jcs.115.11.2271. [DOI] [PubMed] [Google Scholar]
- 114.Broustas CG, Grammatikakis N, Eto M, Dent P, Brautigan DL, Kasid U. Phosphorylation of the myosin-binding subunit of myosin phosphatase by Raf-1 and inhibition of phosphatase activity. J Biol Chem. 2002;277:3053–3059. doi: 10.1074/jbc.M106343200. [DOI] [PubMed] [Google Scholar]
- 115.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: From parts list to mechanisms. Ann Rev Biochem. 2006;75:543–566. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 116.Wang YL. The mechanism of cortical ingression during early cytokinesis: thinking beyond the contractile ring hypothesis. Trends Cell Biol. 2005;15:581–588. doi: 10.1016/j.tcb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 117.Dean SO, Rogers SL, Stuurman N, Vale RD, Spudich JA. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc Natl Acad Sci U S A. 2005;102:13473–13478. doi: 10.1073/pnas.0506810102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guha M, Zhou M, Wang YL. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 119.Zang JH, Spudich JA. Myosin II localization during cytokinesis occurs by a mechanism that does not require its motor domain. Proc Natl Acad Sci U S A. 1998;95:13652–13657. doi: 10.1073/pnas.95.23.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yumura S. Myosin II dynamics and cortical flow during contractile ring formation in Dictyostelium cells. J Cell Biol. 2001;154:137–146. doi: 10.1083/jcb.200011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gunsalus KC, Bonaccorsi S, Williams E, Verni F, Gatti M, Goldberg ML. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang X, Yu K, Hao Y, et al. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol. 2004;6:609–617. doi: 10.1038/ncb1140. [DOI] [PubMed] [Google Scholar]
- 123.Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol. 2008;375:325–330. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 124.Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150:539–552. doi: 10.1083/jcb.150.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paoletti A, Chang F. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol Biol Cell. 2000;11:2757–2773. doi: 10.1091/mbc.11.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Straight AF, Field CM, Mitchison TJ. Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol Biol Cell. 2005;16:193–201. doi: 10.1091/mbc.E04-08-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Piekny AJ, Glotzer M. Anillin Is a Scaffold Protein That Links RhoA, Actin, and Myosin during Cytokinesis. Curr Biol. 2008;18:30–36. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 129.Hickson GR, O'Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008 doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Somma MP, Fasulo B, Cenci G, Cundari E, Gatti M. Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol Biol Cell. 2002;13:2448–2460. doi: 10.1091/mbc.01-12-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–2860. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- 132.Zhao WM, Fang G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem. 2005;280:33516–33524. doi: 10.1074/jbc.M504657200. [DOI] [PubMed] [Google Scholar]
- 133.Prokopenko SN, Brumby A, O'Keefe L, et al. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell Division Requires a Direct Link between Microtubule-Bound RacGAP and Anillin in the Contractile Ring. Curr Biol. 2008;18:25–29. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 135.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actintemplated assembly of Mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 136.Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Developmental Cell. 2007;12:827–835. doi: 10.1016/j.devcel.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 137.Reinsch S, Karsenti E. Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol. 1994;126:1509–1526. doi: 10.1083/jcb.126.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rappaport R. Cytokinesis in Animal Cells. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- 139.Engel FB, Schebesta M, Keating MT. Anillin localization defect in cardiomyocyte binucleation. J Mol Cell Cardiol. 2006;41:601–612. doi: 10.1016/j.yjmcc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 140.Berlin A, Paoletti A, Chang F. Mid2p stabilizes septin rings during cytokinesis in fission yeast. J Cell Biol. 2003;160:1083–1092. doi: 10.1083/jcb.200212016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tasto JJ, Morrell JL, Gould KL. An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J Cell Biol. 2003;160:1093–1103. doi: 10.1083/jcb.200211126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–409. doi: 10.1016/s0962-8924(03)00151-x. [DOI] [PubMed] [Google Scholar]
- 143.Kinoshita M. Diversity of septin scaffolds. Curr Opin Cell Biol. 2006;18:54–60. doi: 10.1016/j.ceb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 144.Martin-Cuadrado AB, Morrell JL, Konomi M, et al. Role of septins and the exocyst complex in the function of hydrolytic enzymes responsible for fission yeast cell separation. Mol Biol Cell. 2005;16:4867–4881. doi: 10.1091/mbc.E04-12-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ding XM, Yu WB, Liu M, et al. SEPT12 interacts with SEPT6 and this interaction alters the filament structure of SEPT6 in Hela cells. J Biochem Mol Biol. 2007;40:973–978. doi: 10.5483/bmbrep.2007.40.6.973. [DOI] [PubMed] [Google Scholar]
- 146.Kinoshita M, Kumar S, Mizoguchi A, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 147.Sakai K, Kurimoto M, Tsugu A, Hubbard SL, Trimble WS, Rutka JT. Expression of Nedd5, a mammalian septin, in human brain tumors. J Neurooncol. 2002;57:169–177. doi: 10.1023/a:1015721801075. [DOI] [PubMed] [Google Scholar]
- 148.Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for mammalian chromosome congression and segregation. Science. 2005;307:1781–1785. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagata K, Kawajiri A, Matsui S, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- 150.Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robertson C, Church SW, Nagar HA, Price J, Hall PA, Russell SE. Properties of SEPT9 isoforms and the requirement for GTP binding. J Pathol. 2004;203:519–527. doi: 10.1002/path.1551. [DOI] [PubMed] [Google Scholar]
- 152.Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Devel Cell. 2007;13:677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 154.Schmidt K, Nichols BJ. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr Biol. 2004;14:1002–1006. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 155.Silio V, Marques M, Cortes I, Zuluaga S, Carrera AC. A cascade involving p85, Cdc42 and septin 2 regulates cytokinesis. Biochem Soc Trans. 2007;35:222–224. doi: 10.1042/BST0350222. [DOI] [PubMed] [Google Scholar]
- 156.Osaka M, Rowley JD, Zeleznik-Le NJ. MSF (MLL septin-like fusion), a fusion partner gene of MLL, in a therapy-related acute myeloid leukemia with a t(11;17)(q23;q25) Proc Natl Acad Sci U S A. 1999;96:6428–6433. doi: 10.1073/pnas.96.11.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Russell SE, McIlhatton MA, Burrows JF, et al. Isolation and mapping of a human septin gene to a region on chromosome 17q, commonly deleted in sporadic epithelial ovarian tumors. Cancer Res. 2000;60:4729–4734. [PubMed] [Google Scholar]
- 158.Montagna C, Lyu MS, Hunter K, et al. The Septin 9 (MSF) gene is amplified and overexpressed in mouse mammary gland adenocarcinomas and human breast cancer cell lines. Cancer Res. 2003;63:2179–2187. [PubMed] [Google Scholar]
- 159.Scott M, Hyland PL, McGregor G, Hillan KJ, Russell SE, Hall PA. Multimodality expression profiling shows SEPT9 to be overexpressed in a wide range of human tumours. Oncogene. 2005;24:4688–4700. doi: 10.1038/sj.onc.1208574. [DOI] [PubMed] [Google Scholar]
- 160.Gonzalez ME, Peterson EA, Privette LM, Loffreda-Wren JL, Kalikin LM, Petty EM. High SEPT9_v1 expression in human breast cancer cells is associated with oncogenic phenotypes. Cancer Research. 2007;67:8554–8564. doi: 10.1158/0008-5472.CAN-07-1474. [DOI] [PubMed] [Google Scholar]
- 161.Julian M, Tollon Y, Lajoie-Mazenc I, et al. gamma-Tubulin participates in the formation of the midbody during cytokinesis in mammalian cells. J Cell Sci. 1993;105(Pt 1):145–156. doi: 10.1242/jcs.105.1.145. [DOI] [PubMed] [Google Scholar]
- 162.Shu HB, Li ZQ, Palacios MJ, Li QQ, Joshi HC. A transient association of gamma-tubulin at the midbody is required for the completion of cytokinesis during the mammalian cell division. J Cell Sci. 1995;108:2955–2962. doi: 10.1242/jcs.108.9.2955. [DOI] [PubMed] [Google Scholar]
- 163.Margolis RL, Andreassen PR. The telophase disc: its possible role in mammalian cell cleavage. Bioessays. 1993;15:201–207. doi: 10.1002/bies.950150310. [DOI] [PubMed] [Google Scholar]
- 164.Schroeder TE. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972;53:419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matuliene J, Kuriyama R. Kinesin-like protein CHO1 is required for the formation of midbody matrix and the completion of cytokinesis in mammalian cells. Mol Biol Cell. 2002;13:1832–1845. doi: 10.1091/mbc.01-10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jiang W, Jimenez G, Wells NJ, et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol Cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- 167.Mollinari C, Kleman JP, Saoudi Y, et al. Ablation of PRC1 by small interfering RNA demonstrates that cytokinetic abscission requires a central spindle bundle in mammalian cells, whereas completion of furrowing does not. Mol Biol Cell. 2005;16:1043–1055. doi: 10.1091/mbc.E04-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu CJ, Jiang W. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc Natl Acad Sci. 2005;102:343–348. doi: 10.1073/pnas.0408438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shimo A, Nishidate T, Ohta T, Fukuda M, Nakamura Y, Katagiri T. Elevated expression of protein regulator of cytokinesis 1, involved in the growth of breast cancer cells. Cancer Science. 2007;98:174–181. doi: 10.1111/j.1349-7006.2006.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Neef R, Gruneberg U, Kopajtich R, et al. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nature Cell Biol. 2007;9:436–U132. doi: 10.1038/ncb1557. [DOI] [PubMed] [Google Scholar]
- 171.Kanehira M, Katagiri T, Shimo A, et al. Oncogenic role of MPHOSPH1, a cancer-testis antigen specific to human bladder cancer. Cancer Research. 2007;67:3276–3285. doi: 10.1158/0008-5472.CAN-06-3748. [DOI] [PubMed] [Google Scholar]
- 172.Li C, Shridhar K, Liu J. Molecular characterization of oncostatin M-induced growth arrest of MCF-7 cells expressing a temperature-sensitive mutant of p53. Breast Cancer Res Treat. 2003;80:23–37. doi: 10.1023/A:1024483017549. [DOI] [PubMed] [Google Scholar]
- 173.Li C, Lin M, Liu J. Identification of PRC1 as the p53 target gene uncovers a novel function of p53 in the regulation of cytokinesis. Oncogene. 2004;23:9336–9347. doi: 10.1038/sj.onc.1208114. [DOI] [PubMed] [Google Scholar]
- 174.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 175.Kuriyama R, Gustus C, Terada Y, Uetake Y, Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. J Cell Biol. 2002;156:783–790. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Skop AR, Liu H, Yates J, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eggert US, Kiger AA, Richter C, et al. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS Biol. 2004;2:e379. doi: 10.1371/journal.pbio.0020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Litvak V, Argov R, Dahan N, et al. Mitotic phosphorylation of the peripheral Golgi protein Nir2 by Cdk1 provides a docking mechanism for Plk1 and affects cytokinesis completion. Mol Cell. 2004;14:319–330. doi: 10.1016/s1097-2765(04)00214-x. [DOI] [PubMed] [Google Scholar]
- 180.Litvak V, Tian D, Carmon S, Lev S. Nir2, a human homolog of Drosophila melanogaster retinal degeneration B protein, is essential for cytokinesis. Mol Cell Biol. 2002;22:5064–5075. doi: 10.1128/MCB.22.14.5064-5075.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Altan-Bonnet N, Phair RD, Polishchuk RS, Weigert R, Lippincott-Schwartz J. A role for Arf1 in mitotic Golgi disassembly, chromosome segregation, and cytokinesis. Proc Natl Acad Sci. 2003;100:13314–13319. doi: 10.1073/pnas.2234055100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sudo H, Maru Y. LAPSER1 is a putative cytokinetic tumor suppressor that shows the same centrosome and midbody subcellular localization pattern as p80 katanin. Faseb J. 2007;21:2086–2100. doi: 10.1096/fj.06-7254com. [DOI] [PubMed] [Google Scholar]
- 183.Tomas A, Futter C, Moss SE. Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J Cell Biol. 2004;165:813–822. doi: 10.1083/jcb.200311054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Piel M, Nordberg J, Euteneuer U, Bornens M. Centrosome-dependent exit of cytokinesis in animal cells. Science. 2001;291:1550–1553. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- 185.Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- 186.Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev Biol. 2007;305:389–396. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Danilchik MV, Bedrick SD, Brown EE, Ray K. Furrow microtubules and localized exocytosis in cleaving Xenopus laevis embryos. J Cell Sci. 2003;116:273–283. doi: 10.1242/jcs.00217. [DOI] [PubMed] [Google Scholar]
- 188.Danilchik MV, Funk WC, Brown EE, Larkin K. Requirement for microtubules in new membrane formation during cytokinesis of Xenopus embryos. Dev Biol. 1998;194:47–60. doi: 10.1006/dbio.1997.8815. [DOI] [PubMed] [Google Scholar]
- 189.Bluemink JG, de Laat SW. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis. I. Electron microscope observations. J Cell Biol. 1973;59:89–108. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shuster CB, Burgess DR. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc Natl Acad Sci U S A. 2002;99:3633–3638. doi: 10.1073/pnas.052342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gromley A, Yeaman C, Rosa J, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 192.Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–746. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moskalewski S, Popowicz P, Thyberg J. Functions of the Golgi complex in cell division: formation of cell-matrix contacts and cell-cell communication channels in the terminal phase of cytokinesis. J Submicrosc Cytol Pathol. 1994;26:9–20. [PubMed] [Google Scholar]
- 194.Thyberg J, Moskalewski S. Reorganization of the Golgi complex in association with mitosis: redistribution of mannosidase II to the endoplasmic reticulum and effects of brefeldin A. J Submicrosc Cytol Pathol. 1992;24:495–508. [PubMed] [Google Scholar]
- 195.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc of the Natl Acad Sci. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Payne C, Schatten G. Golgi dynamics during meiosis are distinct from mitosis and are coupled to endoplasmic reticulum dynamics until fertilization. Dev Biol. 2003;264:50–63. doi: 10.1016/j.ydbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 197.Gromley A, Jurczyk A, Sillibourne J, et al. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–545. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 199.Schweitzer JK, Burke EE, Goodson HV, D'Souza-Schorey C. Endocytosis resumes during late mitosis and is required for cytokinesis. J Biol Chem. 2005;280:41628–41635. doi: 10.1074/jbc.M504497200. [DOI] [PubMed] [Google Scholar]
- 200.Dornan S, Jackson AP, Gay NJ. Alpha-adaptin, a marker for endocytosis, is expressed in complex patterns during Drosophila development. Mol Biol Cell. 1997;8:1391–1403. doi: 10.1091/mbc.8.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gerald NJ, Damer CK, O'Halloran TJ, De Lozanne A. Cytokinesis failure in clathrin-minus cells is caused by cleavage furrow instability. Cell Motil Cytoskel. 2001;48:213–223. doi: 10.1002/1097-0169(200103)48:3<213::AID-CM1010>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 202.Feng B, Schwarz H, Jesuthasan S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp Cell Res. 2002;279:14–20. doi: 10.1006/excr.2002.5579. [DOI] [PubMed] [Google Scholar]
- 203.Thompson HM, Skop AR, Euteneuer U, Meyer BJ, McNiven MA. The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis. Curr Biol. 2002;12:2111–2117. doi: 10.1016/s0960-9822(02)01390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Konopka CA, Schleede JB, Skop AR, Bednarek SY. Dynamin and cytokinesis. Traffic. 2006;7:239–247. doi: 10.1111/j.1600-0854.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Monzo P, Gauthier NC, Keslair F, et al. Clues to CD2-associated protein involvement in cytokinesis. Mol Biol Cell. 2005;16:2891–2902. doi: 10.1091/mbc.E04-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chavrier P, Goud B. The role of ARF and Rab GTPases in membrane transport. Curr Opin Cell Biol. 1999;11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- 207.Schweitzer JK, D'Souza-Schorey C. Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J Biol Chem. 2002;277:27210–27216. doi: 10.1074/jbc.M201569200. [DOI] [PubMed] [Google Scholar]
- 208.Fielding AB, Schonteich E, Matheson J, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wilson GM, Fielding AB, Simon GC, et al. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yu XZ, Prekeris R, Gould GW. Role of endosomal Rab GTPases in cytokinesis. Eur J Cell Biol. 2007;86:25–35. doi: 10.1016/j.ejcb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 211.Hales CM, Griner R, Hobdy-Henderson KC, et al. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 212.Prekeris R, Davies JM, Scheller RH. Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J Biol Chem. 2001;276:38966–38970. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- 213.Hickson GR, Matheson J, Riggs B, et al. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Horgan CP, Walsh M, Zurawski TH, McCaffrey MW. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem Biophys Res Commun. 2004;319:83–94. doi: 10.1016/j.bbrc.2004.04.157. [DOI] [PubMed] [Google Scholar]
- 215.Schonteich E, Pilli M, Simon GC, et al. Molecular characterization of Rab11-FIP3 binding to ARF GTPases. Eur J Cell Biol. 2007;86:417–431. doi: 10.1016/j.ejcb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 216.Boman AL, Kuai J, Zhu X, Chen J, Kuriyama R, Kahn RA. Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Motil Cytoskeleton. 1999;44:119–132. doi: 10.1002/(SICI)1097-0169(199910)44:2<119::AID-CM4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 217.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 218.Conner SD, Wessel GM. Syntaxin is required for cell division. Mol Biol Cell. 1999;10:2735–2743. doi: 10.1091/mbc.10.8.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jantsch-Plunger V, Glotzer M. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr Biol. 1999;9:738–745. doi: 10.1016/s0960-9822(99)80333-9. [DOI] [PubMed] [Google Scholar]
- 220.Li WM, Webb SE, Lee KW, Miller AL. Recruitment and SNARE-mediated fusion of vesicles in furrow membrane remodeling during cytokinesis in zebrafish embryos. Exp Cell Res. 2006;312:3260–3275. doi: 10.1016/j.yexcr.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 221.Low SH, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Devel Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 222.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 223.Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 224.Vega IE, Hsu SC. The septin protein Nedd5 associates with both the exocyst complex and microtubules and disruption of its GTPase activity promotes aberrant neurite sprouting in PC12 cells. Neuroreport. 2003;14:31–37. doi: 10.1097/00001756-200301200-00006. [DOI] [PubMed] [Google Scholar]
- 225.Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 226.Dukes JD, Richardson JD, Simmons R, Whitley P. A dominant negative ESCRT-III protein perturbs cytokinesis and trafficking to lysosomes. Biochem J. 2007 doi: 10.1042/BJ20071296. [DOI] [PubMed] [Google Scholar]
- 227.Morita E, Sandrin V, Chung HY, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. Embo Journal. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schmidt MHH, Chen B, Randazzo LM, Bogler O. SETA/CIN85/Ruk and its binding partner AIP1 associate with diverse cytoskeletal elements, including FAKs, and modulate cell adhesion. J Cell Sci. 2003;116:2845–2855. doi: 10.1242/jcs.00522. [DOI] [PubMed] [Google Scholar]
- 229.Cabezas A, Bache KG, Brech A, Stenmark H. Alix regulates cortical actin and the spatial distribution of endosomes. J Cell Sci. 2005;118:2625–2635. doi: 10.1242/jcs.02382. [DOI] [PubMed] [Google Scholar]
- 230.Zhao WM, Seki A, Fang G. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell. 2006;17:3881–3896. doi: 10.1091/mbc.E06-01-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fabbro M, Zhou BB, Takahashi M, et al. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell. 2005;9:477–488. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 232.Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Niiya F, Xie X, Lee KS, Inoue H, Miki T. Inhibition of cyclin-dependent kinase 1 induces cytokinesis without chromosome segregation in an ECT2 and MgcRacGAP-dependent manner. J Biol Chem. 2005;280:36502–36509. doi: 10.1074/jbc.M508007200. [DOI] [PubMed] [Google Scholar]
- 234.Potapova TA, Daum JR, Pittman BD, et al. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440:954–958. doi: 10.1038/nature04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vassilev LT, Tovar C, Chen S, et al. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Martineau SN, Andreassen PR, Margolis RL. Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J Cell Biol. 1995;131:191–205. doi: 10.1083/jcb.131.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stewart S, Fang G. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 2005;65:8730–8735. doi: 10.1158/0008-5472.CAN-05-1500. [DOI] [PubMed] [Google Scholar]
- 239.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 240.Ban KH, Torres JZ, Miller JJ, et al. The END network couples spindle pole assembly to inhibition of the anaphase-promoting complex/cyclosome in early mitosis. Dev Cell. 2007;13:29–42. doi: 10.1016/j.devcel.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 241.Royou A, Sullivan W, Karess R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J Cell Biol. 2002;158:127–137. doi: 10.1083/jcb.200203148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhu C, Lau E, Schwarzenbacher R, Bossy-Wetzel E, Jiang W. Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc Natl Acad Sci. 2006;103:6196–6201. doi: 10.1073/pnas.0506926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mishima M, Pavicic V, Gruneberg U, Nigg EA, Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 244.Lee KS, Yuan YL, Kuriyama R, Erikson RL. Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Santamaria A, Neef R, Eberspacher U, et al. Use of the novel Plk1 inhibitor ZK-Thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell. 2007;18:4024–4036. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Burkard ME, Randall CL, Larochelle S, et al. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 248.Brennan IM, Peters U, Kapoor TM, Straight AF. Polo-like kinase controls vertebrate spindle elongation and cytokinesis. PLoS ONE. 2007;2:e409. doi: 10.1371/journal.pone.0000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Niiya F, Tatsumoto T, Lee KS, Miki T. Phosphorylation of the cytokinesis regulator ECT2 at G2/M phase stimulates association of the mitotic kinase Plk1 and accumulation of GTP-bound RhoA. Oncogene. 2006;25:827–837. doi: 10.1038/sj.onc.1209124. [DOI] [PubMed] [Google Scholar]
- 250.Neef R, Preisinger C, Sutcliffe J, et al. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863–875. doi: 10.1083/jcb.200306009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu X, Zhou T, Kuriyama R, Erikson RL. Molecular interactions of Polo-like-kinase 1 with the mitotic kinesin-like protein CHO1/MKLP-1. J Cell Sci. 2004;117:3233–3246. doi: 10.1242/jcs.01173. [DOI] [PubMed] [Google Scholar]
- 252.Neef R, Klein UR, Kopajtich R, Barr FA. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr Biol. 2006;16:301–307. doi: 10.1016/j.cub.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 253.Lowery DM, Clauser KR, Hjerrild M, et al. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–2273. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Aumais JP, Williams SN, Luo W, et al. Role for NudC, a dynein-associated nuclear movement protein, in mitosis and cytokinesis. J Cell Sci. 2003;116:1991–2003. doi: 10.1242/jcs.00412. [DOI] [PubMed] [Google Scholar]
- 255.Nishino M, Kurasawa Y, Evans R, Lin SH, Brinkley BR, Yu-Lee LY. NudC is required for PIk1 targeting to the kinetochore and chromosome congression. Curr Biol. 2006;16:1414–1421. doi: 10.1016/j.cub.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 256.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 257.Mundt KE, Golsteyn RM, Lane HA, Nigg EA. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 258.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Incassati A, Patel D, McCance DJ. Induction of tetraploidy through loss of p53 and upregulation of Plk1 by human papillomavirus type-16 E6. Oncogene. 2006;25:2444–2451. doi: 10.1038/sj.onc.1209276. [DOI] [PubMed] [Google Scholar]
- 260.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 261.Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Earnshaw WC, Cooke CA. Analysis of the distribution of the INCENPs throughout mitosis reveals the existence of a pathway of structural changes in the chromosomes during metaphase and early events in cleavage furrow formation. J Cell Sci. 1991;98(Pt 4):443–461. doi: 10.1242/jcs.98.4.443. [DOI] [PubMed] [Google Scholar]
- 263.Adams RR, Eckley DM, Vagnarelli P, et al. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma. 2001;110:65–74. doi: 10.1007/s004120100130. [DOI] [PubMed] [Google Scholar]
- 264.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cesario JM, Jang JK, Redding B, Shah N, Rahman T, McKim KS. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. J Cell Sci. 2006;119:4770–4780. doi: 10.1242/jcs.03235. [DOI] [PubMed] [Google Scholar]
- 266.Hauf S, Cole RW, LaTerra S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15:778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 268.Ban R, Irino Y, Fukami K, Tanaka H. Human mitotic spindle-associated protein PRC1 inhibits MgcRacGAP activity toward Cdc42 during the metaphase. J Biol Chem. 2004;279:16394–16402. doi: 10.1074/jbc.M313257200. [DOI] [PubMed] [Google Scholar]
- 269.Toure A, Mzali R, Liot C, et al. Phosphoregulation of MgcRacGAP in mitosis involves Aurora B and Cdk1 protein kinases and the PP2A phosphatase. FEBS Lett. 2008 doi: 10.1016/j.febslet.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 270.Yasui Y, Goto H, Matsui S, et al. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene. 2001;20:2868–2876. doi: 10.1038/sj.onc.1204407. [DOI] [PubMed] [Google Scholar]
- 271.Goto H, Yasui Y, Kawajiri A, et al. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278:8526–8530. doi: 10.1074/jbc.M210892200. [DOI] [PubMed] [Google Scholar]
- 272.Goto H, Kosako H, Tanabe K, et al. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 273.Izawa I, Inagaki M. Regulatory mechanisms and functions of intermediate filaments: a study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006;97:167–174. doi: 10.1111/j.1349-7006.2006.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155:1147–1157. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bloom K. NoCut: cytokinesis in check. Cell. 2006;125:17–18. doi: 10.1016/j.cell.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 276.Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 277.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 278.Gruneberg U, Glotzer M, Gartner A, Nigg EA. The CeCDC-14 phosphatase is required for cytokinesis in the Caenorhabditis elegans embryo. J Cell Biol. 2002;158:901–914. doi: 10.1083/jcb.200202054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Krasinska L, de Bettignies G, Fisher D, Abrieu A, Fesquet D, Morin N. Regulation of multiple cell cycle events by Cdc14 homologues in vertebrates. Exp Cell Res. 2007;313:1225–1239. doi: 10.1016/j.yexcr.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 280.Mailand N, Lukas C, Kaiser BK, Jackson PK, Bartek J, Lukas J. Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol. 2002;4:317–322. doi: 10.1038/ncb777. [DOI] [PubMed] [Google Scholar]
- 281.Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 282.Sumara I, Quadroni M, Frei C, et al. A Cul3-based E3 ligase removes aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell. 2007;12:887–900. doi: 10.1016/j.devcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 283.Terada Y, Tatsuka M, Suzuki F, Yasuda Y, Fujita S, Otsu M. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 1998;17:667–676. doi: 10.1093/emboj/17.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tatsuka M, Katayama H, Ota T, et al. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–4816. [PubMed] [Google Scholar]
- 285.Katayama H, Ota T, Jisaki F, et al. Mitotic kinase expression and colorectal cancer progression. J Natl Cancer Inst. 1999;91:1160–1162. doi: 10.1093/jnci/91.13.1160. [DOI] [PubMed] [Google Scholar]
- 286.Chieffi P, Cozzolino L, Kisslinger A, et al. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate. 2006;66:326–333. doi: 10.1002/pros.20345. [DOI] [PubMed] [Google Scholar]
- 287.Smith SL, Bowers NL, Betticher DC, et al. Overexpression of aurora B kinase in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer. 2005;93:719–729. doi: 10.1038/sj.bjc.6602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5:2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 289.Araki K, Nozaki K, Ueba T, Tatsuka M, Hashimoto N. High expression of Aurora-B/Aurora and Ipll-like midbody-associated protein (AIM-1) in astrocytomas. J Neurooncol. 2004;67:53–64. doi: 10.1023/b:neon.0000021784.33421.05. [DOI] [PubMed] [Google Scholar]
- 290.Chieffi P, Troncone G, Caleo A, et al. Aurora B expression in normal testis and seminomas. J Endocrinol. 2004;181:263–270. doi: 10.1677/joe.0.1810263. [DOI] [PubMed] [Google Scholar]
- 291.Sorrentino R, Libertini S, Pallante PL, et al. Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J Clin Endocrinol Metab. 2005;90:928–935. doi: 10.1210/jc.2004-1518. [DOI] [PubMed] [Google Scholar]
- 292.Kulkarni AA, Loddo M, Leo E, et al. DNA Replication Licensing Factors and Aurora Kinases are Linked to Aneuploidy and Clinical Outcome in Epithelial Ovarian Carcinoma. Clin Cancer Res. 2007;13:6153–6161. doi: 10.1158/1078-0432.CCR-07-0671. [DOI] [PubMed] [Google Scholar]
- 293.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 294.Kasahara K, Nakayama Y, Nakazato Y, Ikeda K, Kuga T, Yamaguchi N. Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem. 2007;282:5327–5339. doi: 10.1074/jbc.M608396200. [DOI] [PubMed] [Google Scholar]
- 295.Campbell KS, Cooper S, Dessing M, Yates S, Buder A. Interaction of p59fyn kinase with the dynein light chain, Tctex-1, and colocalization during cytokinesis. J Immunol. 1998;161:1728–1737. [PubMed] [Google Scholar]
- 296.Field SJ, Madson N, Kerr ML, et al. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr Biol. 2005;15:1407–1412. doi: 10.1016/j.cub.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 297.Saul D, Fabian L, Forer A, Brill JA. Continuous phosphatidylinositol metabolism is required for cleavage of crane fly spermatocytes. J Cell Sci. 2004;117:3887–3896. doi: 10.1242/jcs.01236. [DOI] [PubMed] [Google Scholar]
- 298.Wong R, Hadjiyanni I, Wei HC, et al. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr Biol. 2005;15:1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 299.Emoto K, Kobayashi T, Yamaji A, et al. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc Natl Acad Sci. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol. 2000;149:1215–1224. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Emoto K, Inadome H, Kanaho Y, Narumiya S, Umeda M. Local Change in Phospholipid Composition at the Cleavage Furrow Is Essential for Completion of Cytokinesis. J. Biol. Chem. 2005;280:37901–37907. doi: 10.1074/jbc.M504282200. [DOI] [PubMed] [Google Scholar]
- 302.Wilker EW, van Vugt M, Artim SA, et al. 14-3-3 sigma controls mitotic translation to facilitate cytokinesis. Nature. 2007;446:329–332. doi: 10.1038/nature05584. [DOI] [PubMed] [Google Scholar]
- 303.Prasanth SG, Prasanth KV, Stillman B. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science. 2002;297:1026–1031. doi: 10.1126/science.1072802. [DOI] [PubMed] [Google Scholar]
- 304.Chesnokov IN, Chesnokova ON, Botchan M. A cytokinetic function of Drosophila ORC6 protein resides in a domain distinct from its replication activity. Proc Natl Acad Sci. 2003;100:9150–9155. doi: 10.1073/pnas.1633580100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Patel KJ, Yu VP, Lee H, et al. Involvement of Brca2 in DNA repair. Mol Cell. 1998;1:347–357. doi: 10.1016/s1097-2765(00)80035-0. [DOI] [PubMed] [Google Scholar]
- 306.Tutt A, Gabriel A, Bertwistle D, et al. Absence of Brca2 causes genome instability by chromosome breakage and loss associated with centrosome amplification. Curr Biol. 1999;9:1107–1110. doi: 10.1016/s0960-9822(99)80479-5. [DOI] [PubMed] [Google Scholar]
- 307.Daniels MJ, Wang Y, Lee M, Venkitaraman AR. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science. 2004;306:876–879. doi: 10.1126/science.1102574. [DOI] [PubMed] [Google Scholar]
- 308.Lin HR, Ting NS, Qin J, Lee WH. M phase-specific phosphorylation of BRCA2 by Polo-like kinase 1 correlates with the dissociation of the BRCA2-P/CAF complex. J Biol Chem. 2003;278:35979–35987. doi: 10.1074/jbc.M210659200. [DOI] [PubMed] [Google Scholar]
- 309.Meng X, Fan J, Shen Z. Roles of BCCIP in chromosome stability and cytokinesis. Oncogene. 2007;26:6253–6260. doi: 10.1038/sj.onc.1210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Smolka MB, Chen SH, Maddox PS, et al. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J Cell Biol. 2006;175:743–753. doi: 10.1083/jcb.200605081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Schweitzer JK, D'Souza-Schorey C. A requirement for ARF6 during the completion of cytokinesis. Exp Cell Res. 2005;311:74–83. doi: 10.1016/j.yexcr.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 312.Jackson MW, Agarwal MK, Yang J, et al. p130/p107/p105Rb-dependent transcriptional repression during DNA-damage-induced cell-cycle exit at G2. J Cell Sci. 2005;118:1821–1832. doi: 10.1242/jcs.02307. [DOI] [PubMed] [Google Scholar]
- 313.Date DA, Jacob CJ, Bekier ME, Stiff AC, Jackson MW, Taylor WR. Borealin is repressed in response to p53/Rb signaling. Cell Biol Int. 2007;31:1470–1481. doi: 10.1016/j.cellbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Scoumanne A, Chen X. The epithelial cell transforming sequence 2, a guanine nucleotide exchange factor for Rho GTPases, is repressed by p53 via protein methyltransferases and is required for G1-S transition. Cancer Res. 2006;66:6271–6279. doi: 10.1158/0008-5472.CAN-06-0121. [DOI] [PubMed] [Google Scholar]
- 315.Monaco L, Kolthur-Seetharam U, Loury R, Murcia JM-d, de Murcia G, Sassone-Corsi P. Inhibition of Aurora-B kinase activity by poly(ADP-ribosyl)ation in response to DNA damage. Proc Natl Acad Sci. 2005;102:14244–14248. doi: 10.1073/pnas.0506252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 317.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 318.Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharm Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 319.Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12:6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]