Abstract
Objective
The consequences of macrophage triglyceride (TG) accumulation on atherosclerosis have not been studied in detail so far. Adipose triglyceride lipase (ATGL) is the rate-limiting enzyme for the initial step in TG hydrolysis. Because ATGL knockout (KO) mice exhibit massive TG accumulation in macrophages, we used ATGL KO mice to study the effects of macrophage TG accumulation on atherogenesis.
Methods and Results
Low-density lipoprotein receptor (LDLr) KO mice were transplanted with bone marrow from ATGL KO (ATGL KO→LDLr KO) or wild-type (wild-type→LDLr KO) mice and challenged with a Western-type diet for 9 weeks. Despite TG accumulation in ATGL KO macrophages, atherosclerosis in ATGL KO→LDLr KO mice was 43% reduced associated with decreased plasma MCP-1 and macrophage interleukin-6 concentrations. This coincided with a reduced amount of macrophages, possibly because of a 39% increase in intraplaque apoptosis and a decreased migratory capacity of ATGL KO macrophages. The reduced number of white blood cells might be due to a 36% decreased Lin−Sca-1+cKit+ hematopoietic stem cell population.
Conclusion
We conclude that the attenuation of atherogenesis in ATGL KO→LDLr KO mice is due to decreased infiltration of less inflammatory macrophages into the arterial wall and increased macrophage apoptosis.
Keywords: apoptosis, atherosclerosis, leukocytes, lipids, macrophages
In most mammalian cells, excess neutral lipids form lipid droplets as storage organelles. Macrophage-derived foam cells are a hallmark of atherogenesis. Generally, foam cells in early atherosclerotic lesions accumulate cholesteryl esters (CE), whereas advanced lesions have increased concentrations of free cholesterol (FC), leading to macrophage apoptosis and an increased risk for plaque rupture.1-4 Macrophages may also transform into TG-rich foam cells on incubation with very-low-density lipoproteins.5-7 However, the (patho) physiological consequences of triglyceride (TG) accumulation in macrophages on atherogenesis have not been studied in detail so far. Unesterified fatty acids (NEFA) in macrophages are taken up from the plasma after hydrolysis of TG-rich lipoproteins by lipoprotein lipase or by absorption of albumin-bound fatty acids (FA). Inside the cells, FA are esterified within TG and CE, which are less harmful to the cells than NEFA,8,9 or routed to mitochondrial FA β-oxidation. Thus, CE and TG accumulation within lipid droplets might be seen as a buffer to detoxify excess NEFA and FC.
Both FA and FC are released from lipid droplets by intracellular lipases. Adipose triglyceride lipase (ATGL) (also named patatin-like phospholipase domain–containing protein-2, PNPLA2, desnutrin, and phospholipase A2ζ) plays a critical role in cellular lipid homeostasis because it specifically catalyzes the initial step in TG hydrolysis.10-12 Mice lacking ATGL exhibit massive TG accumulation in many tissues and cells as a consequence of defective TG hydrolysis and die from heart failure at an early age.13 The importance of ATGL in macrophage TG metabolism and whether its activity contributes to atherogenesis in mice has not been investigated so far. Consistent with the hypothesis that lipase-mediated modulation of FA concentrations affects atherogenesis, it was reported that atherosclerosis susceptibility is increased in mice with high expression levels of lipoprotein lipase in macrophages and reduced in mice that lack lipoprotein lipase.14-16
The present study was designed to address the role of ATGL in macrophages and to examine the consequences of macrophage TG accumulation for atherogenesis. We used bone marrow transfer to investigate mechanisms by which the absence of macrophage ATGL may modulate atherosclerotic susceptibility. Evidence is provided that ATGL deficiency in macrophages and the concomitant TG accumulation resulted in reduced atherosclerosis in low-density lipoprotein receptor (LDLr) knockout (KO) mice. Downregulation of ATGL in macrophages might thus be an interesting and prospective strategy to attenuate early atherogenesis.
Materials and Methods
For detailed methodology, please see the supplemental data, available online at http://atvb.ahajournals.org.
Bone marrow transplantation (BMT) was used to selectively disrupt ATGL in hematopoietic cells, including macrophages. Briefly, female LDLr KO mice were exposed to a single dose of 9 Gy (0.19 Gy/min, 200 kV, 4 mA) x-ray total body irradiation, using an Andrex Smart 225 Röntgen source (Yxlon International, Copenhagen, Denmark) 1 day before transplantation. Irradiated recipients were transplanted by intravenous injection of 5×106 bone marrow cells, isolated from male ATGL KO mice or from wild-type (WT) littermates. To induce the development of atherosclerosis, mice were fed a Western-type diet (WTD), containing 15% (w/w) cocoa butter and 0.25% (w/w) cholesterol (Diet W, Special Diet Services, Witham, United Kingdom) for 9 weeks, starting at 8 weeks after transplantation, after which the mice were euthanized and atherosclerotic lesion development and lesion composition was quantified. For determination of white blood cell (WBC) counts, serum lipids, and monocyte chemoattractant protein 1 levels, blood was drawn after an overnight fasting period. Upon euthanization, bone marrow was isolated to analyze the Lin−Sca-1+cKit+ (LSK) population by fluorescence-activated cell sorting.
BMT experiments were performed at the Gorlaeus Laboratories of the Leiden/Amsterdam Center for Drug Research in accordance with national laws. All experimental protocols were approved by the Ethics Committee for Animal Experiments of Leiden University, the Netherlands, and by the Austrian Federal Ministry of Science and Research, Division of Genetic Engineering and Animal Experiments.
Results
Increased Lipid Accumulation in ATGL KO Macrophages
First, we determined the presence of ATGL in bone marrow–derived macrophages (BMDM) and foam cells by real-time polymerase chain reaction. ATGL was highly expressed in both BMDM and β-very-low-density lipoprotein–loaded macrophage foam cells (ATGL Ct: 22.1±0.31 and 22.5±0.51 respectively; β-actin Ct: 17.0±0.55 and 17.0±0.23 respectively). No significant difference in ATGL expression was found between nonloaded BMDM and β-very-low-density lipoprotein–loaded foam cells, indicating that ATGL expression was not regulated by lipid loading.
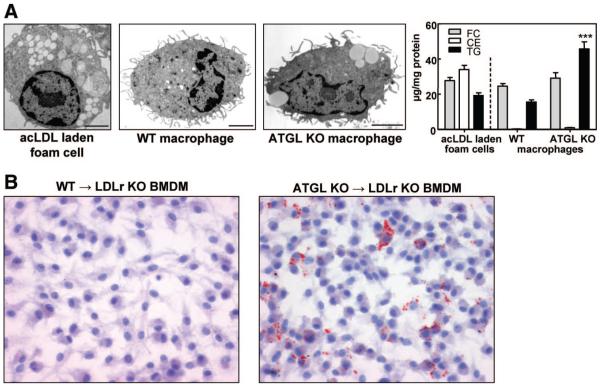
Next, we visualized lipid droplets in peritoneal macrophages of WT and ATGL KO mice by electron microscopy. For comparison, acetylated low-density lipoprotein (ac-LDL)-laden macrophage foam cells were included. As expected, ac-LDL-laden macrophage foam cells showed extensive lipid droplet accumulation and a drastic increase in cellular CE concentrations (Figure 1A). ATGL KO BMDM accumulated lipid droplets even in the absence of exogenous lipid loading, whereas this was not observed in the majority of the WT macrophages (Figure 1A). Lipid droplet formation in the ATGL KO macrophages was the consequence of a 3-fold (P<0.001) increase in cellular TG concentrations compared with WT macrophages (Figure 1A). FC and CE concentrations were comparable in macrophages from both groups.
Figure 1.
ATGL deficiency induces lipid droplet accumulation in macrophages. A, Electron micrographs (scale bar, 2 μm) and lipid parameters of isolated peritoneal macrophages. B, BMDM from transplanted mice. Images show representative Oil Red O–stained BMDM. Magnification, ×40. ***P<0.001.
To investigate the consequences of macrophage ATGL deficiency on atherosclerotic lesion formation, we performed a transplantation of ATGL KO and WT bone marrow into LDLr KO mice. Successful hematologic chimerism of transplanted LDLr KO mice was confirmed by polymerase chain reaction analysis of genomic DNA from bone marrow isolated 17 weeks after BMT (Supplemental Figure I). As expected, BMDM from LDLr KO mice transplanted with WT bone marrow (WT→LDLr KO mice) exhibited no lipid accumulation. In contrast, BMDM from ATGL KO→LDLr KO mice accumulated cytoplasmic lipid droplets (Figure 1B), indicative of the reduced TG hydrolytic capacity and TG accumulation in ATGL KO macrophages.
Serum Lipid Levels Are Comparable in ATGL KO→LDLr KO and WT→LDLr KO Mice
After BMT, mice were first fed a chow diet for 8 weeks followed by WTD feeding for another 9 weeks. Lipid parameters in WT→LDLr KO and ATGL KO→LDLr KO mice were measured at the end of each diet regimen. No significant differences in plasma TG (chow and WTD) and NEFA (WTD) concentrations were observed (Table). WTD feeding resulted in a general ≈5-fold increase in cholesterol levels in both groups.
Table.
Plasma Lipid Parameters of Overnight Fasted ATGL KO→LDLr KO and WT→LDLr KO Mice. After BMT, Mice Were First Fed Chow Diet for 8 Weeks (wks) Followed by WTD Feeding for Another 9 Weeks. The Data Represent Mean±SEM of ≥9 Mice
| Donor Bone Marrow |
Time (Weeks After BMT) |
Diet |
Free Cholesterol (mg/dL) |
Total Cholesterol (mg/dL) |
Triglycerides (mg/dL) |
NEFA (mM) |
|---|---|---|---|---|---|---|
| WT | 8 | Chow | 63±2 | 314±9 | 131±9 | ND |
| 17 | WTD | 274±16 | 1516±96 | 104±10 | 1.07±0.03 | |
| ATGL KO | 8 | Chow | 78±5a | 357±22 | 161±16 | ND |
| 17 | WTD | 315±15 | 1711±87 | 133±18 | 1.06±0.03 |
ND indicates not determined.
P<0.05.
The distribution of TC and TG within serum lipoproteins was analyzed by fast protein liquid chromatography. Lipoprotein profiles of ATGL KO→LDLr KO and WT→LDLr KO mice were similar on both chow diet (data not shown) and WTD (Supplemental Figure II). WTD feeding led to ≈6-fold increased non–high-density lipoprotein cholesterol (very-low-density lipoprotein and low-density lipoprotein cholesterol) in both groups, whereas no significant changes in high-density lipoprotein cholesterol concentrations were observed. In addition, lipoprotein-associated TG distribution was similar in both groups.
Decreased Atherosclerosis in ATGL KO→LDLr KO Mice
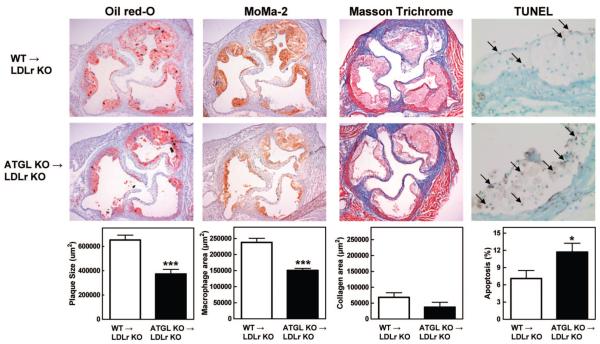
Visual inspection of Oil Red O–stained aortic root sections showed a clear difference in lesion formation between WT→LDLr KO and ATGL KO→LDLr KO animals (Figure 2). Quantitative analysis of lesion areas revealed a 43% reduction in the mean atherosclerotic lesion size in ATGL KO→LDLr KO mice compared with WT controls (373×103±37×103 and 652×103±40×103 μm2, respectively; P<0.001).
Figure 2.
Decreased lesion formation and increased apoptosis in aortic root sections of ATGL KO→LDLr KO mice. Representative slides were stained with Oil Red O, MoMa-2, Masson’s trichrome, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) for the detection of lipids, macrophages, collagen, and apoptosis, respectively. Arrows indicate apoptotic nuclei. Oil Red O and lesion composition data represent the means of 10 or 5 aortic root sections of n≥9 or n≥5 animals±SEM, respectively. Magnification, ×5 (apoptosis: ×40). *P<0.05; ***P<0.001.
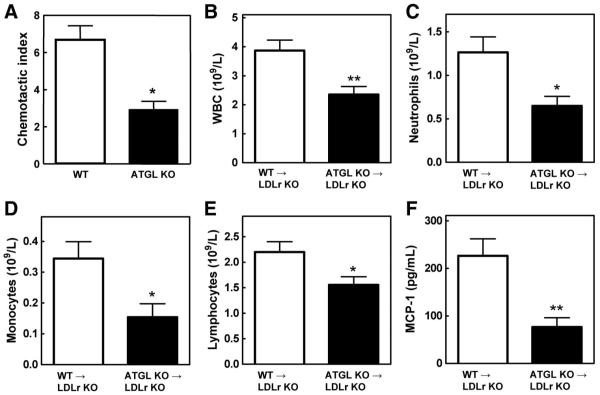
Masson’s Trichrome staining, which was used to identify collagen in the lesion, showed no differences between WT→LDLr KO and ATGL KO→LDLr KO mice (68±15×103 μm2 and 37±16×103 μm2, respectively; Figure 2). To visualize macrophages inside the lesions, sections were stained with the monocyte/macrophage-specific MoMa-2 antibody (Figure 2). In agreement with the observed attenuation in lesion size, quantification of the macrophage area revealed a 37% decrease in macrophage content between lesions of WT→LDLr KO (238±36×103 μm2) and ATGL KO→LDLr KO mice (151±17×103 μm2; P<0.001; Figure 2). Next, we examined whether the migration ability of the macrophages might be influenced by ATGL deficiency. As shown in Figure 3A, ATGL KO macrophages had a 56% (P<0.05) reduced migration rate compared with WT macrophages. In addition, terminal deoxynucleotidyl transferase dUTP nick-end labeling staining showed a marked 39% increase in intraplaque apoptosis in ATGL KO→LDLr KO compared with WT→LDLr KO animals (11.7±1.5% and 7.1±1.4%, respectively; P<0.05; Figure 2).
Figure 3.
Reduced WBC and MCP-1 concentration in ATGL KO→LDLr KO mice. A, Macrophage migration assays were performed using transwell plates. Values represent the means of n=3±SEM. WBC (B), neutrophils (C), monocytes (D), and lymphocytes (E) in plasma were determined with a hematology analyzer. F, MCP-1 concentrations were determined by ELISA. Values represent the means of n≥9±SEM. *P<0.05; **P<0.01.
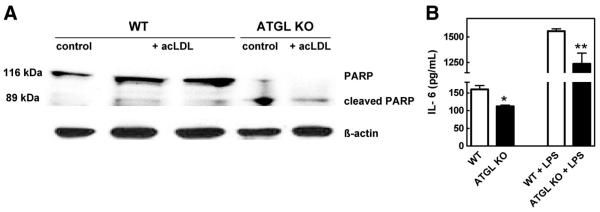
To further investigate the observed increase in intraplaque apoptosis, we measured poly(ADP-ribose) polymerase (PARP) protein expression in ATGL KO macrophages. During apoptosis, full-length PARP (116 kDa) is cleaved by caspase-3, and possibly other caspases, into an 89-kDa fragment.17 In agreement with the observed increase in intraplaque apoptosis, a clear shift toward cleaved PARP was detectable in ATGL KO macrophages, which is not the case in WT macrophages (Figure 4A). This finding indicates that macrophages deficient for ATGL are more prone to apoptosis.
Figure 4.
Decreased IL-6 production in ATGL KO macrophages. A, PARP protein expression in peritoneal macrophages of ATGL KO and WT mice. B, IL-6 concentrations were determined by ELISA in supernatants of peritoneal macrophages. Values represent the means of n≥3±SEM. *P<0.05; **P<0.01.
Cellular Cholesterol Efflux and mRNA Levels of Genes Implicated in Lipid Homeostasis in ATGL KO Macrophages
To study the effect of ATGL deficiency on the cholesterol transport from macrophages to exogenous lipid acceptors, we measured cholesterol efflux to apolipoprotein AI and high-density lipoprotein. Cholesterol efflux toward apolipoprotein AI and high-density lipoprotein was comparable in BMDM isolated from both genotypes (Supplemental Figure III). Next, we performed real-time polymerase chain reaction to investigate whether lack of ATGL in macrophages resulted in compensatory upregulation of genes involved in TG hydrolysis (HSL, LPL, LAL) and biosynthesis (DGAT-1) or cholesterol uptake (SR-A, SR-BI, CD36, LDLr), esterification (ACAT-1), and efflux (ABCA1, ABCG1, SR-BI, ApoE). We found a 1.6-fold upregulation of CD36 (P<0.05) but no significant differences in mRNA expression of other genes (data not shown), indicating that ATGL deficiency does not affect macrophage cholesterol homeostasis, TG synthesis, and expression of other lipases.
Attenuated Production of the Proinflammatory Cytokine Interleukin-6 in ATGL KO Macrophages
Because inflammation is a critical process in atherogenesis, we measured interleukin-6 (IL-6) concentrations in the supernatant of peritoneal macrophages cultured in the absence or presence of lipopolysaccharide (Figure 4B). Basal and lipopolysaccharide-stimulated IL-6 concentrations were significantly lower in ATGL KO macrophages compared with WT macrophages (30% [P<0.05] and 20% [P<0.01], respectively).
Reduced Circulating Number of WBC and Plasma MCP-1 Levels in ATGL KO→LDLr KO Mice Challenged With WTD
On a regular chow diet, no differences in WBC counts were observed. After 9 weeks of WTD feeding, the amount of circulating WBC, however, was 39% lower in ATGL KO→LDLr KO mice (2.35±0.24×109/L) compared with WT→LDLr KO animals (3.87±0.33×109/L; P<0.01; Figure 3B). Challenging the mice with WTD is thus essential for the observed reduction in WBC counts on disruption of ATGL in bone marrow-derived cells. This decrease was caused by a 49% decrease in neutrophils (WT, 1.26±0.18×109/L; ATGL KO, 0.65±0.11×109/L; P<0.05; Figure 3C) and a 55% decrease in monocytes (WT, 0.34±0.05×109/L; ATGL KO, 0.15±0.04×109/L; P<0.05; Figure 3D). We also observed a significant 29% reduction in lymphocytes (WT, 2.20±0.20×109/L; ATGL KO, 1.56±0.16×109/L; P<0.05; Figure 3E). In addition, MCP-1 concentrations were 66% lower in ATGL KO→LDLr KO mice (74±19 pg/mL) compared with WT→LDLr KO mice (220±35 pg/mL; P<0.01; Figure 3F).
Unchanged WBC Half-Life in the Circulation and Decreased LSK Population in Bone Marrow of ATGL KO→LDLr KO Bone Marrow
To further investigate the apparent decrease in WBC in ATGL KO→LDLr KO mice, circulating WBC were biotinylated to measure their half-life. WBC of ATGL KO→LDLr KO mice had a half-life of 96±2.2 hours compared with 99±3.9 hours for WT→LDLr KO animals. These data indicate that the WBC half-life cannot account for the observed decrease in the total number of WBC in ATGL KO→LDLr KO mice.
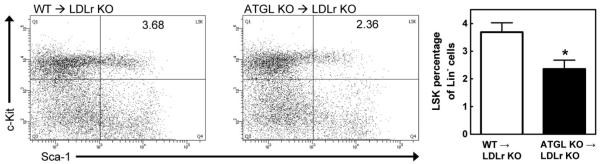
Therefore, next, we measured the LSK population representing hematopoietic stem and multipotential progenitor cells in the bone marrow. Hematopoietic stem and multipotential progenitor cells are capable of extensive self-renewal and are responsible for the production of WBC.18-20 No differences were observed in the Lin− population between groups. However, ATGL KO→LDLr KO mice exhibited a significant 36% decrease in LSK cells within the Lin− population as compared with WT→LDLr KO mice (P<0.05; Figure 5), indicating a decreased WBC production from the bone marrow.
Figure 5.
Decreased LSK population in ATGL KO→LDLr KO mice. Dot plots show representative examples of the LSK population within the bone marrow (Lin− cells, double positive for anti-LY6A/E [Sca-1] and anti-CD117 [cKit]). The right panel shows the percentage of LSK cells within the Lin− population. Values represent the means of n=5±SEM. *P<0.05.
Discussion
ATGL is expressed at high levels in macrophages and foam cells, raising the possibility that it might affect atherosclerosis. Similar to other tissues, the absence of ATGL in macrophages resulted in increased intracellular TG concentrations. Generally, macrophage-derived foam cells are filled with CE, which are hydrolyzed by the action of a neutral CE hydrolase resulting in the release of FC. ATGL KO macrophages, however, accumulated TG even in the absence of exogenous lipid loading, resulting in a cell morphology resembling macrophage foam cells.21 CE and FC concentrations were unchanged compared with control cells. Therefore, ATGL KO macrophages represent a perfect tool to elucidate the in vivo consequences of TG accumulation in macrophages on atherosclerosis. Thus, we created mice chimeric for macrophage ATGL expression by transplanting LDLr KO mice with bone marrow from ATGL KO and WT littermates. BMT results in the replacement of tissue macrophages, including those of the arterial wall that are involved in fatty streak formation.22
In contrast to total body ATGL KO mice,13 no differences were found in plasma TG, TC, or NEFA concentrations between ATGL KO→LDLr KO and WT→LDLr KO mice after feeding the WTD. Thus, lack of ATGL in bone marrow-derived cells has no significant influence on serum lipid levels. In line with biochemical data determined in peritoneal ATGL KO macrophages, distinct neutral lipid loading was observed in BMDM of ATGL KO→LDLr KO and WT→LDLr KO mice. Despite increased macrophage TG concentrations, atherosclerotic lesion formation in the aortic root was highly attenuated in ATGL KO→LDLr KO mice. The integrity of the fibrous cap overlying lipid-rich cores fundamentally determines the stability of an atherosclerotic lesion.23 Quantification of the collagen content in the lesions revealed no differences between the groups, indicating that the plaque stability was unchanged. However, the observed reduction in atherosclerotic lesion formation coincided with increased apoptosis, which might limit lesion cellularity and the progression of early lesions in these animals.1,24 During atherosclerotic lesion development, macrophages inside the lesion undergo a steady rate of apoptosis. In early lesions, apoptotic macrophages are rapidly cleared by other macrophages, thereby limiting lesion progression.25,26 In advanced atherosclerotic lesions, however, impaired clearance of apoptotic macrophages leads to secondary necrosis and the formation of a necrotic core.27 Depending on the stage of lesion development, increased apoptosis can thus either enhance or suppress lesion development. The increased apoptosis rate observed in lesions of mice lacking macrophage ATGL might have prevented the formation of an advanced lesion and, at least in part, might have contributed to the observed attenuation of lesion development. In isolated ATGL KO macrophages, a shift toward cleaved PARP (a marker for apoptosis) was evident, indicating that macrophages lacking ATGL are more susceptible to apoptosis, which explains the observed increase in intraplaque apoptosis.
Leukocytes play an essential role in all stages of atherosclerotic lesion development.28-30 In humans, increased levels of neutrophils and monocytes induce the progression of atherosclerosis.31,32 Conversely, a reduced number of circulating monocytes inhibits the initiation and progression of atherosclerotic lesions in mice lacking endogenous M-CSF production.33,34 Importantly, we found a greatly decreased number of circulating WBC in WTD-fed ATGL KO→LDLr KO mice. No differences were observed in the half-life of WBC of ATGL KO→LDLr KO mice compared with WT→LDLr KO controls, suggesting that the reduced number of circulating WBC is merely the consequence of impaired cellular recruitment from the bone marrow.
WBC are produced in the bone marrow by hematopoietic stem and multipotential progenitor cells. Virtually all hematopoietic stem and multipotential progenitor cell activity is contained within the LSK population in the bone marrow.18-20 Remarkably, the LSK population from ATGL KO→LDLr KO mice is decreased compared with WT→LDLr KO controls, indicating a reduction in WBC-producing cells. This phenomenon most likely contributes to the observed attenuation in circulating WBC.
Furthermore, the production of the proinflammatory cytokine IL-6 was markedly reduced in ATGL KO macrophages. IL-6 is one of the most important mediators of inflammation because it promotes migration as well as recruitment and activation of inflammatory cells. Although ATGL KO macrophages exhibited a decreased IL-6 production on lipopolysaccharide stimulation, basal IL-6 production was already mitigated in ATGL KO macrophages, indicating an impaired proinflammatory IL-6 signaling. It is generally accepted that inflammatory responses contribute to the development of atherosclerosis. Interestingly, recently Koliwad et al also found that overexpression of macrophage DGAT-1, which catalyzes the final step in TG biosynthesis, protects against free FA-induced inflammatory activation of the macrophages during diet-induced obesity.35 Thus, increasing macrophage TG accumulation by either ATGL deletion or DGAT-1 overexpression impairs macrophage proinflammatory signaling.
Moreover, plasma levels of MCP-1, an important chemoattractant for mononuclear cells, were drastically reduced in ATGL KO→LDLr KO mice. Deletion of chemokine (C-C motif) receptor 2, the receptor for MCP-1, inhibits the egress of monocytes from bone marrow into the blood circulation.36 Decreased concentrations of MCP-1 in the circulation might thus also have affected the amount of circulating mononuclear cells. Mice lacking MCP-1 or its receptor chemokine receptor 2 develop fewer and smaller atherosclerotic lesions than control mice, as a consequence of the reduced ability to recruit monocytes to sites in the arterial wall prone to atherosclerotic lesion development.37-39 In addition to the reduction in plasma MCP-1 levels, we also observed a reduced migration rate of ATGL KO macrophages compared with control cells in response to MCP-1. The highly attenuated lesion size of ATGL KO→LDLr KO mice coincided with a decrease in the macrophage area of the lesion, indicating that the total number of macrophages that had infiltrated into the arterial wall is decreased. These findings suggest that ATGL-dependent lipolysis of TG and the mobilization of NEFA as energy substrate is necessary for efficient migration.
In summary, our data indicate that macrophage ATGL plays a significant role in atherogenesis independent of its expression in other tissues. The absence of ATGL in macrophages resulted in reduced susceptibility to diet-induced atherosclerosis in LDLr KO mice because of decreased infiltration of less inflammatory macrophages. Therefore, we hypothesize that increasing intracellular TG content in macrophages is protective against lesion development and that macrophage ATGL is a novel target to attenuate early atherogenesis.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the Netherlands Heart Foundation (Grants 2005B002 [to B.L.], 2003B134 [to R.O.], and 2007T039 [to G.H.M.V.P.]), the Netherlands Organization for Scientific Research (VIDI Grant 917.66.301 [to I.M. and M.V.E.]), Top Institute Pharma (TIPharma project T2-110 [to R.O., T.J.C.V.B.]), the Austrian Science Fund (SFB-LIPOTOX F30 [to D.K., G.H., and R.Z.] and P19186 [to D.K.]), and the Austrian Federal Ministry of Science and Research (GEN-AU Project Genomics of Lipid-associated Disorders III-GOLDIII [to D.K. and R.Z.]). Dr Van Eck is an Established Investigator of the Netherlands Heart Foundation (Grant 2007T056).
Footnotes
Disclosures
None.
References
- 1.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang K, Kaufman RJ. Unfolding the toxicity of cholesterol. Nat Cell Biol. 2003;5:769–770. doi: 10.1038/ncb0903-769. [DOI] [PubMed] [Google Scholar]
- 3.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 5.Bates SR, Murphy PL, Feng ZC, Kanazawa T, Getz GS. Very low density lipoproteins promote triglyceride accumulation in macrophages. Arteriosclerosis. 1984;4:103–114. doi: 10.1161/01.atv.4.2.103. [DOI] [PubMed] [Google Scholar]
- 6.Huff MW, Evans AJ, Sawyez CG, Wolfe BM, Nestel PJ. Cholesterol accumulation in J774 macrophages induced by triglyceride-rich lipoproteins. Comparison of very low density lipoprotein from subjects with type III, IV, and V hyperlipoproteinemias. Arterioscler Thromb. 1991;11:221–233. doi: 10.1161/01.atv.11.2.221. [DOI] [PubMed] [Google Scholar]
- 7.Jong MC, Hendriks WL, van Vark LC, Dahlmans VE, Groener JE, Havekes LM. Oxidized VLDL induces less triglyceride accumulation in J774 macrophages than native VLDL due to an impaired extracellular lipolysis. Arterioscler Thromb Vasc Biol. 2000;20:144–151. doi: 10.1161/01.atv.20.1.144. [DOI] [PubMed] [Google Scholar]
- 8.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 9.Saraswathi V, Hasty AH. Inhibition of long-chain acyl coenzyme A synthetases during fatty acid loading induces lipotoxicity in macrophages. Arterioscler Thromb Vasc Biol. 2009;29:1937–1943. doi: 10.1161/ATVBAHA.109.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 12.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 13.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 14.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–1705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babaev VR, Patel MB, Semenkovich CF, Fazio S, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J Biol Chem. 2000;275:26293–26299. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- 16.Van Eck M, Zimmermann R, Groot PH, Zechner R, Van Berkel TJ. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:E53–E62. doi: 10.1161/01.atv.20.9.e53. [DOI] [PubMed] [Google Scholar]
- 17.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 18.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 19.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li CL, Johnson GR. Murine hematopoietic stem and progenitor cells: I: enrichment and biologic characterization. Blood. 1995;85:1472–1479. [PubMed] [Google Scholar]
- 21.Chandak PG, Radovic B, Aflaki E, Kolb D, Buchebner M, Frohlich E, Magnes C, Sinner F, Haemmerle G, Zechner R, Tabas I, Levak-Frank S, Kratky D. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J Biol Chem. 2010;285:20192–20201. doi: 10.1074/jbc.M110.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herijgers N, Van Eck M, Groot PH, Hoogerbrugge PM, Van Berkel TJ. Effect of bone marrow transplantation on lipoprotein metabolism and atherosclerosis in LDL receptor-knockout mice. Arterioscler Thromb Vasc Biol. 1997;17:1995–2003. doi: 10.1161/01.atv.17.10.1995. [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 24.Nhan TQ, Liles WC, Schwartz SM. Role of caspases in death and survival of the plaque macrophage. Arterioscler Thromb Vasc Biol. 2005;25:895–903. doi: 10.1161/01.ATV.0000159519.07181.33. [DOI] [PubMed] [Google Scholar]
- 25.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spα/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, Hla T, Claffey K, Smith JD, Wu D. Phospholipase C β3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J Clin Invest. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- 28.Ensrud K, Grimm RH., Jr. The white blood cell count and risk for coronary heart disease. Am Heart J. 1992;124:207–213. doi: 10.1016/0002-8703(92)90942-o. [DOI] [PubMed] [Google Scholar]
- 29.Sweetnam PM, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145:416–421. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 30.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 31.Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25:1287–1292. doi: 10.1016/j.ehj.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Nasir K, Guallar E, Navas-Acien A, Criqui MH, Lima JA. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol. 2005;25:1966–1971. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- 33.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, Lusis AJ, Rajavashisth TB. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol. 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 35.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese RV., Jr. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 38.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.