Abstract
Background
The World Health Organization recommends isoniazid preventive therapy (IPT) for preventing tuberculosis in HIV-infected adults, although few countries have instituted this policy. Both IPT and highly active antiretroviral therapy (HAART) used separately result in reductions in tuberculosis risk. There is less information on the combined effect of IPT and HAART. We assessed the effect of IPT, HAART or both IPT and HAART on tuberculosis incidence in HIV-infected adults in South Africa.
Methods
Two clinical cohorts of HIV-infected patients were studied. Primary exposures were receipt of IPT and/or HAART and the primary outcome was incident tuberculosis. Crude incident rates and incident rate ratios were calculated and Cox proportional hazards models investigated associations with tuberculosis risk.
Results
Among 2778 HIV-infected patients followed for 4287 person-years, 267 incident tuberculosis cases were diagnosed [incidence rate ratio (IRR) = 6.2/100 person-years; 95% CI 5.5–7.0]. For person-time without IPT or HAART, the IRR was 7.1/100 person-years (95% CI 6.2–8.2); for person-time receiving HAART but without IPT, the IRR was 4.6/100 person-years (95% CI 3.4–6.2); for person-time after IPT but prior to HAART, the IRR was 5.2/100 person-years (95% CI 3.4–7.8); during follow-up in patients treated with HAART after receiving IPT the IRR was 1.1/100 person-years (95% CI 0.02–7.6). Compared to treatment-naive patients, HAART-only patients had a 64% decreased hazard for tuberculosis [adjusted hazard ratio (aHR) = 0.36; 95% CI 0.25–0.51], and patients receiving HAART after IPT had a 89% reduced hazard (aHR = 0.11; 95% CI 0.02–0.78).
Conclusion
Tuberculosis risk is significantly reduced by IPT in HAART-treated adults in a high-incidence operational setting in South Africa. IPT is an inexpensive and cost-effective strategy and our data strengthen calls for the implementation of IPT in conjunction with the roll-out of HAART.
Keywords: HAART, isoniazid, preventive treatment, sub-Saharan Africa, tuberculosis
Introduction
The effectiveness of isoniazid preventive therapy (IPT) to prevent tuberculosis has been well established in HIV-negative individuals and communities [1–3], and in HIV-infected persons [4–9]. The World Health Organization now recommends the use of IPT in HIV-infected patients in countries with high prevalence of HIV infection [10]. Very few countries, however, have instituted this policy [11]. Studies from a variety of settings all show a decreased risk of tuberculosis in HIV-infected adults who start highly active antiretroviral therapy (HAART), although rates of tuberculosis in these populations remains unacceptably high [12–15]. Furthermore, tuberculosis remains the leading opportunistic infection and cause of death in patients on HAART in developing settings [16–18].
A recent study from Rio de Janeiro, Brazil found that whereas IPT and HAART were both effective in reducing tuberculosis risk in HIV-infected adults, the combination of the two therapies was more protective than either alone, resulting in an 80% reduction in tuberculosis rates compared to patients receiving neither therapy [19]. Similar data from sub-Saharan Africa, with far higher incidence of tuberculosis, have not been presented. In this study, we analyzed data from two clinical cohorts of HIV-infected adults in South Africa.
Methods
Clinical cohorts
We studied HIV-infected adults over 18 years of age receiving primary HIV care at two clinics affiliated with the University of the Witwatersrand: the Perinatal HIV Research Unit (PHRU) in Soweto, a large, urban setting, and the Tintswalo Hospital, a remote, rural clinic in Mpumalanga Province. A package of preantiretroviral care is provided – predominantly by nurse practitioners –including CD4 monitoring, tuberculosis screening at every visit with appropriate follow-up and care for those with tuberculosis, co-trimoxazole preventive treatment for those who are eligible, and treatment of ambulatory HIV-related illnesses. Six months of IPT is offered at both sites; at the PHRU it is given to those with a positive tuberculin skin test (TST) of greater than 5 mm induration. At Tintswalo, IPT was given to HIV-infected persons without first ascertaining their TST response. However, a government decision to stop providing isoniazid tablets in an attempt to promote fixed-dose antimycobacterial combination tablets ensured that few patients received IPT. Since 2004 at the PHRU and 2005 at Tinstwalo, patients are offered antiretroviral therapy when they are diagnosed with WHO stage 3 (pulmonary tuberculosis) or 4 (extra-pulmonary tuberculosis) disease, or when their CD4 cell count falls below 200 cells/μl, consistent with South African government guidelines [20]. All patient care is provided free of charge.
Data collection
At baseline, all patients are interviewed using a common questionnaire to obtain demographic, behavioral, socioeconomic and health history information. Similarly, at each subsequent study visit, patients are interviewed using the same instrument at both sites to obtain an interval medical history. Time between data collection visits is scheduled to be 4–7 months but patients may visit the clinic at any time. Follow-up data collection focus on clinical diagnoses, symptoms and longitudinal data including height and weight, smoking status, alcohol consumption and measures of socioeconomic status. Laboratory tests include CD4 and full blood counts at recruitment and every 6 months. Sputum is taken for acid fast bacilli smear and tuberculosis cultures on all participants with symptoms suggestive of tuberculosis; induced sputum is collected if sputum production is problematic. Sputa are sent to a national tuberculosis reference laboratory for smear and culture in which tuberculosis culture is by liquid culture in all instances. Data collection and analysis has been approved by the Human Research Ethics Committee of the University of the Witwatersrand and the institutional review board at the Johns Hopkins School of Medicine.
Statistical analysis
The primary outcome was a diagnosis of tuberculosis; all diagnosed cases of tuberculosis in participants were included, whether they were diagnosed in the cohort clinics or elsewhere. Diagnoses were based upon bacteriological testing, clinical diagnoses and reports of being started on tuberculosis therapy at other institutions. The primary exposures of interest were receipt of IPT and/or HAART. Follow-up time was categorized into four exposure categories: follow-up time in patients when they had received neither IPT nor HAART, follow-up time in those who received IPT but whose follow-up time did not have HAART; follow-up time in those who received HAART without prior IPT and follow-up time on HAART in those who had IPT and then received HAART. All but one patient in the latter group received IPT prior to HAART. Kaplan–Meier curves of incident tuberculosis for each of these four groups were generated and compared using the log-rank test. Incidence rates for the primary exposures were calculated per 100 person-years and exact confidence intervals (CI) were calculated based on the Poisson distribution. Incidence rate ratios (IRR) adjusted and unadjusted Cox proportional hazards regression models were generated.
Follow-up time started at the patient’s baseline cohort visit and ended at first incident tuberculosis diagnosis or at their last recorded clinic visit date if they did not have tuberculosis. Patients who had a past history of tuberculosis disease, and those who enrolled in the clinics with active tuberculosis or were diagnosed within 60 days of their first visit were excluded from the analysis as these patients would not have the same opportunity to enter into the primary exposure categories (specifically IPT) under investigation as those who had no prior tuberculosis history. Baseline CD4 cell counts were included in the analysis to avoid time-dependent confounding. Antiretroviral therapy and isoniazid therapy were treated as time-dependent variables, coded as 0 prior to initiation and 1 following. If a patient with isoniazid exposure began antiretroviral therapy, person-time no longer accumulated in the isoniazid or antiretroviral therapy category, but rather person-time began in the category labeled ‘IPT and HAART.’ This person-time movement also occurred for patients with antiretroviral therapy exposure who then began isoniazid therapy. All analyses were conducted using SAS (Version 9.1; Cary, North Carolina, USA) and Stata (Version 10.0; College Station, Texas, USA).
Results
A total of 3868 HIV-infected adults had at least two data collection visits between 1 June 2003 and 31 December 2007; 252 were excluded from analysis because they did not have any CD4 results available. These patients did not differ in any characteristic of interest in the current analyses (data not shown). Another 838 patients were excluded because they had a history of tuberculosis either at entry into the clinic or prior. Among the remaining 2778 patients, 2210 (80%) were female, 2044 (74%) were from the urban site and 734 (26%) from the rural site (Table 1).
Table 1.
Sociodemographic and clinical characteristics of patient population by location of clinic (urban or rural).
| Characteristic | Urban site (N = 2044) | Rural site (N = 734) | Total (N = 2778) |
|---|---|---|---|
| Sex | |||
| Male | 390 (19%) | 175 (24%) | 565 (21%) |
| Female | 1654 (81%) | 556 (76%) | 2210 (80%) |
| Median age (IQR) | 32 (28–37) | 37 (31–44) | 33 (29–39) |
| Education | |||
| None | 28 (2%) | 143 (29%) | 171 (8%) |
| Lower secondary | 710 (45%) | 267 (53%) | 977 (47%) |
| Higher secondary | 545 (34%) | 83 (17%) | 628 (30%) |
| Tertiary | 307 (19%) | 8 (2%) | 315 (15%) |
| Median BMI | 24.6 (21.3–29.3) | 21.0 (18.7–23.9) | 23.6 (20.4–28.1) |
| Median baseline CD4 (IQR) | 307 (177–467) | 169 (86–313) | 266 (139–439) |
| Initiated HAART (%) | 441 (22%) | 379 (52%) | 820 (30%) |
| Median CD4 at HAART initiation (IQR) | 169 (91–286) | 142 (75–203) | 156 (85–252) |
| Initiated INH (%) | 346 (17%) | 9 (1%) | 355 (13%) |
BMI, body mass index; HAART, highly active antiretroviral therapy; IQR, interquartile range.
The 2778 patients had 4287 person-years of follow-up, during which 267 incident tuberculosis cases were diagnosed. The overall tuberculosis incidence was 6.2/100 person-years (95% CI 5.5–7.0) (Table 2). There were 2815 person-years of follow-up in the treatment-naive group (median individual follow-up was 1.0 years); 952 person-years on HAART, but not proceeded by IPT (median individual follow-up 1.9 years; 1.6 following HAART); 427 person-years after IPT only (median follow-up 1.08 years; 1.07 following IPT) and 132 person-years for those who received both IPT and then HAART (median follow-up 2.5 years; 0.7 IPT only and 1.8 with both IPT and HAART).
Table 2.
Incidence rates, incidence rate ratio, hazard ratio and adjusted hazard ratios for risk factors for tuberculosis.
| Tuberculosis cases | Person-years | Incidence rate (95% CI) per 100 person-years | Incidence rate ratio (95% CI) | Hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Total | 267 | 4287 | 6.2 (5.5–7.0) | – | – | – |
| Sex | ||||||
| Female | 198 | 3468 | 5.7 (5.0–6.6) | REF | REF | REF |
| Male | 68 | 814 | 8.3 (6.5–10.6) | 1.46 (1.09–1.94) | 1.46 (1.11–1.92) | 1.22 (0.91–1.62) |
| Clinic location | ||||||
| Urban | 192 | 3403 | 5.6 (4.9–6.5) | REF | REF | REF |
| Rural | 75 | 883 | 8.5 (6.8–10.6) | 1.51 (1.13–1.98) | 1.51 (1.16–1.98) | 1.36 (1.02–1.81) |
| Age at baseline visit (years) | ||||||
| 17–29 | 62 | 1325 | 4.7 (3.6–6.0) | REF | REF | REF |
| 30–39 | 125 | 1992 | 6.3 (5.3–7.5) | 1.34 (0.98–1.85) | 1.34 (0.98–1.82) | 1.35 (0.99–1.83) |
| >39 | 80 | 965 | 8.3 (6.7–10.3) | 1.77 (1.26–2.51) | 1.77 (1.28–2.48) | 1.59 (1.12–2.26) |
| CD4 cell count at baseline visit (cells/μl) | ||||||
| <100 | 64 | 598 | 10.7 (8.4–13.7) | REF | REF | REF |
| 100–199 | 64 | 916 | 7.0 (5.5–8.9) | 0.65 (0.45–0.94) | 0.65 (0.46–0.93) | 0.68 (0.48–0.96) |
| 200–349 | 59 | 1125 | 5.2 (4.1–6.8) | 0.49 (0.34–0.71) | 0.49 (0.34–0.69) | 0.40 (0.27–0.57) |
| >349 | 80 | 1649 | 4.9 (3.9–6.0) | 0.45 (0.32–0.64) | 0.45 (0.33–0.63) | 0.35 (0.24–0.50) |
| IPT and HAART history | ||||||
| Naive | 200 | 2815 | 7.1 (6.2–8.2) | REF | REF | REF |
| HAART only | 44 | 952 | 4.6 (3.4–6.2) | 0.65 (0.46–0.91) | 0.62 (0.44–0.87) | 0.36 (0.25–0.51) |
| IPT only | 22 | 427 | 5.2 (3.4–7.8) | 0.73 (0.44–1.13) | 0.71 (0.45–1.10) | 0.87 (0.55–1.36) |
| IPT and HAART | 1 | 93 | 1.1 (0.2–7.6) | 0.15 (0.004–0.85) | 0.14 (0.02–1.03) | 0.11 (0.02–0.78) |
CI, confidence interval; HAART, highly active antiretroviral therapy; IPT, isoniazid preventive therapy.
At time of entry into the four time periods, there were no significant differences by age, sex or education. Median CD4 cell count for patients not receiving therapy was 317 cells/μl at the beginning of the treatment-naive time period. At time of IPT, median CD4 was 399 cells/μl, compared to 145 cells/μl at time of HAART initiation for those only receiving HAART and 176 cells/μl for those starting HAART after previously receiving IPT.
Among the 355 patients who received IPT, 282 (79%) had a TST result of which 245 (87%) had >9 mm induration, 27 (9%) had 5–9 mm and 10 (4%) had 0 mm. Of 355 patients who received IPT, 209 (59%) completed at least 6 months of IPT.
The median CD4 cell count at HAART initiation was 156 cells/μl [interquartile range (IQR) 85–252]. Of all the 820 patients who received HAART, the time to tuberculosis diagnosis following HAART initiation among the 44 patients receiving HAART without prior IPT was less than 30 days in five patients (11%) and within 90 days in eight (18%) of diagnosed cases.
Almost all (61 of 62) patients who received both IPT and HAART received IPT prior to starting HAART. The median time between start of IPT and initiation of HAART was 1.0 years (IQR 0.5–1.9). One patient developed tuberculosis after starting both IPT and HAART, with 2.3 years of follow-up after IPT initiation and an additional 0.7 years after HAART initiation.
Men were more likely to develop tuberculosis than women (IRR = 1.46; 95% CI 1.09–1.94) and compared to patients <30 years of age, those who were 30–39 years (IRR = 1.34; IQR 0.98–1.85) or were 40–49 years (IRR = 1.77; 95% CI 1.26–2.51) were at greater risk (Table 2). The rural site had an increased tuberculosis risk (IRR = 1.51; 95% CI 1.13–1.98) compared to the urban site. Patients with initial CD4 cell counts <100 cells/μl had a tuberculosis incidence rate of 10.7/100 person-years (95% CI 8.4–13.7), whereas the risk was lower for patients with higher initial CD4 cell counts (Table 2).
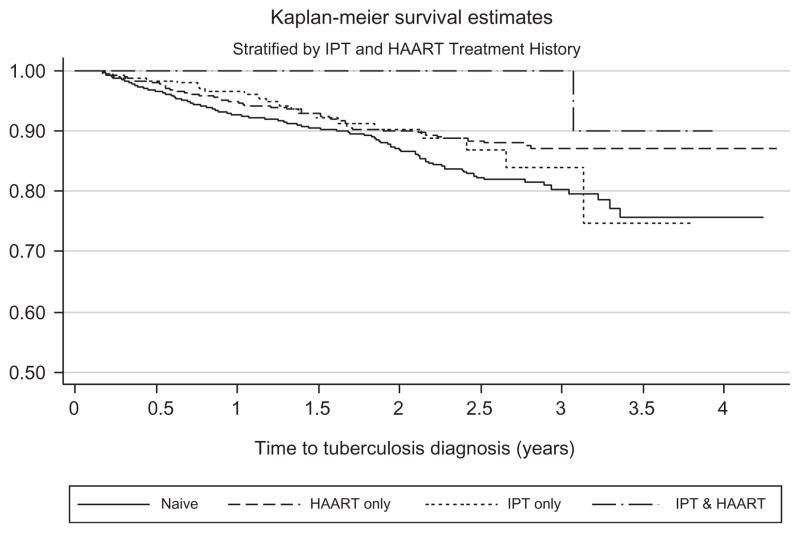
During follow-up time among patients receiving neither IPT nor HAART, tuberculosis incidence was 7.1/100 person-years (95% CI 6.2–8.2) (Table 2). Incidence decreased 27% after receipt of IPT only (IR = 5.2/100 person-years; IRR = 0.73; 95% CI 0.44–1.13) compared to treatment-naive patients, whereas patients receiving HAART only had a reduced incidence of 35% compared to patients whose follow-up included neither HAART nor IPT (IR = 4.6/100 person-years; IRR = 0.65; 95% CI 0.46–0.91). During follow-up time while on HAART among those who had previously received IPT, incidence was reduced by 85% (IR = 1.1/100 person-years; IRR = 0.15; 95% CI 0.004–0.85). The log-rank test for equality of the Kaplan–Meier curves (Fig. 1) supported a significant difference between the curves (P < 0.01).
Fig. 1.
Kaplan–Meier estimates of tuberculosis-free survival by treatment category.
Adjusted and unadjusted Cox proportional hazards models suggest similar trends to the IRRs. Compared to treatment-naïve patients, the effect of IPT alone was not statistically significant after adjustment for CD4 cell count and other variables [adjusted hazard ratio (aHR) = 0.87; 95% CI 0.55–1.36]; HAART alone was strongly associated with a decreased risk of incident tuberculosis (aHR = 0.36; 95% CI 0.25–0.51); and patients who received both IPT and HAART showed a strong reduction in tuberculosis risk (aHR = 0.11; 95% CI 0.02–0.78). Rural location and CD4 cell count <100 cells/μl were each strongly associated with an increased risk of tuberculosis in the final adjusted model (Table 2).
Discussion
Although HAART has been demonstrated to reduce incident tuberculosis by 50–80% in HIV-infected patients, active tuberculosis remains the leading serious opportunistic infection and cause of death in HAART-treated adults [16–18]. In this study of HIV-infected South African adults, we found that whereas HAART alone decreased the adjusted risk of tuberculosis by 64%, the incidence of the disease remained high, at 4.6 cases per 100 person-years. This reflects a rate 10 times higher than the overall incidence of tuberculosis in the South African general population and 1000-fold that in the United States. We showed a strong interaction effect: the combined effect of both IPT and HAART, which was associated with an 89% decrease in the adjusted risk for tuberculosis, with a crude incidence of 1.1 cases per 100 person-years. These results are similar to recently reported findings in HIV-infected individuals in Rio de Janeiro, Brazil [19]. Taken together, these studies suggest that in conjunction with HAART initiation, the more widespread use of IPT has the potential to lead to further reductions in tuberculosis incidence among HIV-infected adults in high tuberculosis burden settings.
Multiple studies have shown that IPT reduces tuberculosis incidence in HIV-infected patients [4–6,9,21,22] and a systematic review summarized this risk to be 32% in all HIV-infected patients regardless of TST results [8]. Despite WHO’s recommendation that IPT be offered to all HIV-infected patients in areas with a high tuberculosis burden [10,23], very few countries or HIV care programs in high-burden countries utilize IPT [11]. Concerns about implementing IPT programmatically include difficulties in detecting active tuberculosis, selection for isoniazid resistance and toxicity. Nonetheless, in the thousands of HIV-infected patients who have received IPT as part of clinical trials or clinical programs, none of these issues have been documented as a problem [24].
Achieving high adherence to IPT is a historical operational problem [25,26]. In our study, only 59% of patients received 6 months of IPT. We included in our analysis all participants who started IPT, an intent-to-treat analysis, and still demonstrated considerable benefit. We suggest that expanding the scope of current efforts to strengthen care delivery systems for HAART in resource-poor settings to include better access and adherence to IPT has substantial potential to increase the overall effectiveness of this intervention at a population level.
Tuberculosis is the most common opportunistic disease reported among the >400 000 people who have started HAART in Africa, with incidence rates as high as 13.3/100 person-years within 1 year following HAART initiation in Cape Town [17] and 5.0 in Uganda [27], consistent with the rate found in our patients who received HAART but not IPT. Eighteen percent of the tuberculosis cases that developed post-HAART initiation in our study were diagnosed within 90 days of initiation suggesting either immune reconstitution disease or unmasking of existing infection. Giving IPT before HAART is initiated when CD4 cell counts are >300 cells/μl, as was the case for most of the patients in this study who received both therapies, could substantially reduce this risk.
Our study has several limitations, primarily the small number of patients who had a history of both IPT and HAART. Although this number is small, the dramatic reduction in tuberculosis risk is compelling, particularly since the follow-up for all groups was at least 1 year. The study was observational and is subject to the limitations and potential biases of such analyses; the reduced risk of tuberculosis in those who received IPT then HAART could be because the more robust patients survived long enough to firstly receive IPT and then HAART. However, adjustment for potential confounders did not alter our estimate of the effectiveness of both IPT and HAART. Finally, many of the patients in this study received IPT because of a positive TST, and this may have increased the effectiveness of IPT more than might be seen in clinical settings in which skin testing is not performed.
Our data show that IPT given prior to HAART significantly reduces tuberculosis risk in HIV-infected patients in South Africa whose tuberculosis risk is extremely high. Whereas HAART alone reduced tuberculosis risk in this population, the combination of both interventions was considerably more effective. Our data support widespread implementation that this inexpensive, simple and clearly effective intervention should be provided to patients in high-burden countries to reduce the extraordinary toll that tuberculosis exacts from people living with HIV.
Acknowledgments
The authors are grateful to the participants in these two wellness programs, Ms. Thembi Dlamini, Ms. Joyce Mthimkhulu who consulted patients at PHRU, and Jonathan Adams and Frans van der Linde for data management.
Patient care is funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) through USAID under the terms of Award no. 674-A-00-05-00003-00. The opinions expressed herein are those of the authors and do not necessarily reflect the views of USAID. National Institutes of Health grants AI066994 and AI001637 and a Fogarty International Center grant (TW007370) supported the analysis.
Authors’ roles: N.M. set up the cohorts with help from P.M.P., J.M.C., G.G., H.S. and R.E.C. J.E.G. conducted the analysis and drafted the manuscript with assistance from R.E.C. and N.M. N.T., M.M. and L.M. were responsible for day-to-day management of cohorts and data. All authors reviewed the submitted draft of the paper.
References
- 1.Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967;95:935–943. doi: 10.1164/arrd.1967.95.6.935. [DOI] [PubMed] [Google Scholar]
- 2.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 3.Ferebee SH. Long-term effects of isoniazid prophylaxis. Bull Int Union Tuberc. 1968;41:161–166. [PubMed] [Google Scholar]
- 4.Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD., Jr Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 5.Mwinga A, Hosp M, Godfrey-Faussett P, Quigley M, Mwaba P, Mugala BN, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS. 1998;12:2447–2457. doi: 10.1097/00002030-199818000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda-Case Western Reserve University Research Collaboration. N Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 7.Churchyard GJ, Fielding K, Charalambous S, Day JH, Corbett EL, Hayes RJ, et al. Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy? AIDS. 2003;17:2063–2070. doi: 10.1097/00002030-200309260-00007. [DOI] [PubMed] [Google Scholar]
- 8.Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2004;1:CD000171. doi: 10.1002/14651858.CD000171.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13:501–507. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 10.Report of a WHO Joint HIV and TB Department Meeting. Report from WHO’s Three I’s Meeting: intensified case finding (ICF), isoniazid preventive therapy (IPT) and TB infection control (IC) for people living with HIV, 2–4 April 2008.
- 11.WHO. Global tuberculosis control: surveillance, planning, financing: WHO report. Geneva: WHO; 2008. [Google Scholar]
- 12.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 13.Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 14.Girardi E, Antonucci G, Vanacore P, Libanore M, Errante I, Matteelli A, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- 15.Jones JL, Hanson DL, Dworkin MS, DeCock KM. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–1031. [PubMed] [Google Scholar]
- 16.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 17.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 18.Saraceni V, King BS, Cavalcante SC, Golub JE, Lauria LM, Moulton LH, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2008;12:769–772. [PMC free article] [PubMed] [Google Scholar]
- 19.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Department of Health SA. National Antiretroviral Treatment Guidelines. 1. 2004. [Google Scholar]
- 21.Halsey NA, Coberly JS, Desormeaux J, Losikoff P, Atkinson J, Moulton LH, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351:786–792. doi: 10.1016/S0140-6736(97)06532-X. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson D, Squire SB, Garner P. Effect of preventive treatment for tuberculosis in adults infected with HIV: systematic review of randomised placebo controlled trials. BMJ. 1998;317:625–629. doi: 10.1136/bmj.317.7159.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO/HTM/TB2004.330. Geneva: WHO; 2004. Interim policy on collaborative TB/HIV activities. [Google Scholar]
- 24.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12:744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowe KA, Makhubele B, Hargreaves JR, Porter JD, Hausler HP, Pronyk PM. Adherence to TB preventive therapy for HIV-positive patients in rural South Africa: implications for anti-retroviral delivery in resource-poor settings? Int J Tuberc Lung Dis. 2005;9:263–269. [PubMed] [Google Scholar]
- 26.World Health Organization. The ProTEST pilot projects: review of achievements, gaps and constraints 1999–2002. Geneva: World Health Organization; 2002. [Google Scholar]
- 27.Moore D, Liechty C, Ekwaru P, Were W, Mwima G, Solberg P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]