Abstract
Neuropoietic cytokines such as ciliary neurotrophic factor (CNTF) and leukemia inhibitory factor (LIF) stimulate the functional expression of T-type Ca2+ channels in developing sensory neurons. However, the molecular and cellular mechanisms involved in the cytokine-evoked membrane expression of T-type Ca2+ channels are not fully understood. In this study we investigated the role of LIF in promoting the trafficking of T-type Ca2+ channels in a heterologous expression system. Our results demonstrate that transfection of HEK-293 cells with the rat green fluorescent protein (GFP)-tagged T-type Ca2+ channel α1H-subunit resulted in the generation of transient Ca2+ currents. Overnight treatment of α1H-GFP-transfected cells with LIF caused a significant increase in the functional expression of T-type Ca2+ channels as indicated by changes in current density. LIF also evoked a significant increase in membrane fluorescence compared with untreated cells. Disruption of the Golgi apparatus with brefeldin A inhibited the stimulatory effect of LIF, indicating that protein trafficking regulates the functional expression of T-type Ca2+ channels. Trafficking of α1H-GFP was also disrupted by cotransfection of HEK-293 cells with the dominant-negative form of ADP-ribosylation factor (ARF)1 but not ARF6, suggesting that ARF1 regulates the LIF-evoked membrane trafficking of α1H-GFP subunits. Trafficking of T-type Ca2+ channels required transient activation of the JAK and ERK signaling pathways since stimulation of HEK-293 cells with LIF evoked a considerable increase in the phosphorylation of the downstream JAK targets STAT3 and ERK. Pretreatment of HEK-293 cells with the JAK inhibitor P6 or the ERK inhibitor U0126 blocked ERK phosphorylation. Both P6 and U0126 also inhibited the stimulatory effect of LIF on T-type Ca2+ channel expression. These findings demonstrate that cytokines like LIF promote the trafficking of T-type Ca2+ channels.
Keywords: cytokine, expression, signaling
neuropoietic cytokines such as leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) are a large family of trophic factors that play a critical role in cell proliferation, differentiation, and survival during normal development and in response to injury of the nervous system. For example, CNTF and LIF promote long-term cell survival and differentiation of spinal cord and ciliary ganglion neurons (2, 4, 29, 36, 37). Neuropoietic cytokines like LIF stimulate neuronal survival and differentiation through the activation of the Janus-activated kinase (JAK) signaling pathway, which leads to the stimulation of transcription factors such as signal transducer and activator of transcription (STAT) (reviewed in Ref. 22). This signaling pathway is triggered when LIF causes the dimerization of the LIF receptor β (LIFRβ) molecule, and the signaling protein gp130. LIF-induced dimerization of the gp130-LIFRβ complex results in the phosphorylation of JAKs (15, 23, 45; reviewed in Ref. 20). Once activated, JAKs phosphorylate various tyrosine residues on the cytoplasmic tail of gp130, which then becomes a docking site for STAT transcription factors and proteins containing an src homology 2 (SH2) domain. Cytokine-evoked activation of the JAK/STAT signaling pathway followed by dimerization and nuclear translocation of STAT transcription factors results in long-term changes in gene expression (50). Activation of gp130-LIFRβ receptor complex can also lead to stimulation of other signaling molecules including mitogen-activated protein (MAP) and phosphatidylinositol 3-kinase (PI3-kinase) (1, 6).
In addition to the long-term effect on cell survival and differentiation, it is increasingly evident that neuropoietic cytokines like CNTF and LIF can have an acute effect on cell function. For example, we previously demonstrated (39, 52) that CNTF and LIF regulate the functional expression of low-voltage-activated (LVA or T-type) Ca2+ channels in nodose sensory neurons. T-type Ca2+ channel expression reaches a maximum after 12-h exposure to CNTF and persists in the presence of the protein synthesis inhibitor anisomycin (38). The lack of effect of protein synthesis inhibitors combined with our findings that T-type Ca2+ channel transcripts are already present at embryonic day (E)7 suggest that the functional expression of T-type Ca2+ channels is regulated by a posttranslational mechanism (39). The present work was undertaken to explore the possibility that the neuropoietic cytokine LIF evokes T-type Ca2+ channel trafficking.
Voltage-gated Ca2+ channels are a major conduit of Ca2+ influx, which regulates multiple aspects of neuronal physiology including gene expression and neurotransmitter release. Ca2+ influx through T-type Ca2+ channels, in particular, can influence several cellular processes such as neurite outgrowth, electrical excitability, and pain transmission (7, 11, 19, 21, 30, 42, 53). There is considerable evidence demonstrating significant changes in the expression pattern of voltage-gated Ca2+ channels during development (30, 33, 38). Therefore, understanding what factors regulate the functional expression of T-type Ca2+ channels may have important implications in understanding neuronal development and differentiation.
In this study we have examined whether the neuropoietic cytokine LIF promotes the trafficking of the T-type Ca2+ channel α1H-subunit [tagged to green fluorescent protein (GFP)] by stimulating JAK and ERK signaling. Our data indicate that LIF evokes a considerable increase in the functional and membrane expression of T-type Ca2+ channels in HEK-293 cells as determined by whole cell recordings and changes in membrane fluorescence. Furthermore, our data demonstrate that LIF-evoked stimulation of T-type Ca2+ channels requires transient activation of the JAK and ERK signaling pathways.
METHODS
Cell cultures and transfection.
HEK-293 cells (American Type Culture Collection) were maintained in DMEM-F-12 (GIBCO BRL) supplemented with 10% FBS and 1% penicillin-streptomycin under standard tissue culture conditions (5% CO2, 37°C). Cells were grown either in 35-mm petri dishes or on poly-d-lysine-coated glass coverslips (for whole cell recordings). Cells were transiently transfected by the calcium phosphate method with 4 μg of plasmid DNA. After transfection, cells were maintained in a 5% CO2 incubator at 37°C and used for electrophysiological and fluorescence experiments between 36 and 72 h. The rat α1H-GFP subunit was kindly provided by Dr. Kurt Bean (Colorado State University) (54). For cotransfection of HEK-293 cells, the α1H-GFP and dominant-negative (DN) ADP-ribosylation factor (ARF) plasmids were mixed at a 1-to-2 ratio. The DN ARF plasmids were obtained from Dr. Julie Donaldson [National Institutes of Health (NIH)]. For some experiments, cells were treated with brefeldin A, latrunculin, or phalloidin for 1 h before the addition of LIF followed by overnight culture for electrophysiological experiments the following day.
Nodose ganglia were isolated from chicken embryos at E7 as described previously (38, 39). Briefly, nodose ganglia were excised into a HEPES-based, Ca2+- and Mg2+-free solution, mildly trypsinized (0.05% trypsin for 12 min), and dissociated by trituration. For whole cell recordings, nodose neurons were allowed to attach to round glass coverslips (12 mm) for 1.5 h in supplemented culture medium. Culture medium consisted of Eagle's minimum essential medium (MEM, BioWhittaker, Walkersville, MD) supplemented with 10% heat-inactivated horse serum, 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 50 ng/ml brain-derived neurotrophic factor (BDNF). Cell cultures were maintained in a 5% CO2 incubator at 37°C for up to 24 h. Cell cultures were stimulated with LIF overnight in order to promote T-type Ca2+ channel expression.
Electrophysiology.
HEK-293 cells or nodose neurons were visualized with an Olympus X71 inverted microscope (Nashua, NH) equipped with Hoffman optics. Recordings were performed at room temperature (22–24°C). Recording electrodes were made from thin-wall borosilicate glass (3–4 MΩ) and filled with a solution consisting of (in mM) 120 CsCl, 2 MgCl2, 10 HEPES-KOH, 10 EGTA, 1 ATP, and 0.1 GTP, pH 7.4 with CsOH. Normal external saline for measurements of Ca2+ currents contained (in mM) 145 tetraethylammonium chloride (TEACl), 10 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4 with CsOH. Ca2+ currents were generated by applying either a 750-ms voltage ramp from −100 to +80 mV or a 400-ms depolarizing step to various potentials from a holding potential of −100 mV. Voltage commands, data acquisition, and analysis were performed with a MultiClamp 700a amplifier and pCLAMP software (Axon Instruments, Foster City, CA). Pipette offset, whole cell capacitance, and series resistance (usually <10 MΩ) were compensated automatically with the MultiClamp 700B Commander. Sampling rates were between 5 and 10 kHz. For quantitative analyses, we normalized for cell size by dividing current amplitudes by cell capacitance, determined by integration of the transient current evoked by a 10-mV voltage step from a holding potential of −60 mV (38, 39). The current-voltage (I-V) relationship was obtained as previously described by Pachuau and Martin-Caraballo (39) with a 400-ms depolarizing step to various potentials from a holding potential of −100 mV. Steady-state activation curves were obtained from the I-V relationships with the equation G = I/(V − Vr), where I is the current at a given voltage, V is the voltage command, and Vr is the reversal potential of calcium currents obtained by extrapolating the ascending portion of the I-V curve (39). Conductance values were normalized to the maximum conductance at −10 mV and plotted as a function of voltage before being fitted with a Boltzmann equation in the form G/Gmax = [1 + exp(V1/2 − V)k]−1 where G is conductance at membrane voltage V, Gmax is maximal conductance at −10 mV, V1/2 is the half-activation voltage, and k is the slope factor. Analysis of the activation and inactivation kinetics was performed on current traces generated by applying a series of 10-mV voltage steps between −30 and +40 mV from a holding potential of −100 mV. The activation (τactivation) and inactivation (τinactivation) time constants were obtained by fitting the rising and decay phases of the transient T-type Ca2+ currents with one exponential function in the form I(t) = Aexp(−t/τ), where A is peak current and τ is the time constant.
Fluorescence microscopy.
Images were obtained from control (nontreated) or LIF-treated HEK-293 cells transfected with α1H-GFP subunits. Cells were visualized with a DeltaVision reconstruction microscopy system using a ×60 oil-immersion objective (Applied Precision, Issaquah, WA). All images were collected under the same experimental conditions (brightness, resolution, and exposure time). Cell boundaries were established with differential interference contrast (DIC) images of cultured cells. Membrane-bound fluorescence was quantified from background-subtracted fluorescence images. The fluorescence intensity of the membrane was then divided by the number of pixels to obtain the average membrane fluorescence. Image analysis was performed with ImageJ software (NIH).
Western blot analysis.
Immunoblot analysis of ERK and STAT3 phosphorylation was performed as previously described by Trimarchi et al. (52). Briefly, cell cultures were maintained in serum-free EMEM culture medium for 2 h before stimulation. Controls consisted of nonstimulated samples. Immediately after stimulation, cultures were returned to the incubator for varying lengths of time as indicated. Cultures were then washed twice with ice-cold PBS and lysed in 2× Laemmli sample buffer. Samples were resolved by sodium dodecyl sulfate-polyacrylamide (10%) gel electrophoresis (SDS-PAGE). After protein transfer into PVDF membranes, membranes were blocked with Aquablock blocking buffer (EastCoastBio, North Berwick, ME) overnight at 4°C. Membranes were incubated with 1:1,000 dilutions of primary antibodies (dissolved in blocking buffer) for 4 h [rabbit anti-phospho-STAT3 (Cell Signaling Technology, Danvers, MA)/mouse anti-STAT3 (BD Biosciences Pharmingen, San Diego, CA) or mouse anti-phospho-ERK (Sigma, St. Louis, MO)/rabbit anti-ERK (Santa Cruz Biotechnology, Santa Cruz, CA)]. Each membrane was probed with two antibodies simultaneously to allow estimates of the ratio of ERK to phospho-ERK and the ratio of STAT3 to phospho-STAT3. After four washes, membranes were incubated with 1:10,000 fluorescent secondary antibodies in the dark. The following secondary antibodies were used: Alexa Fluor 700 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) and IRDye 800 anti-rabbit IgG (Rockland, Gilbertsville, PA). After the membranes were washed three times, images of Alexa Fluor 700 and IRDye 800 were acquired with the 700- and 800-nm channels of the Odyssey infrared imaging system (LICOR Biosciences, Lincoln, NE). Each experiment was repeated at least three times.
Data analysis.
Averaged data values are presented as means ± SE where indicated. Statistical analyses consisted of Student's unpaired t-test when single comparisons were made or one-way ANOVA followed by post hoc analysis using Tukey's honestly significant difference test for unequal n for comparisons between multiple groups (Statistica Software, Tulsa, OK). Throughout, P ≤ 0.05 was regarded as significant. For electrophysiological experiments, data were collected from a minimum of two platings (i.e., from multiple cultures).
Chemicals and drugs.
LIF was obtained from R&D Systems (Minneapolis, MN). U0126, P6, brefeldin A, phalloidin, and latrunculin were obtained from Calbiochem (Gibbstown, NJ).
RESULTS
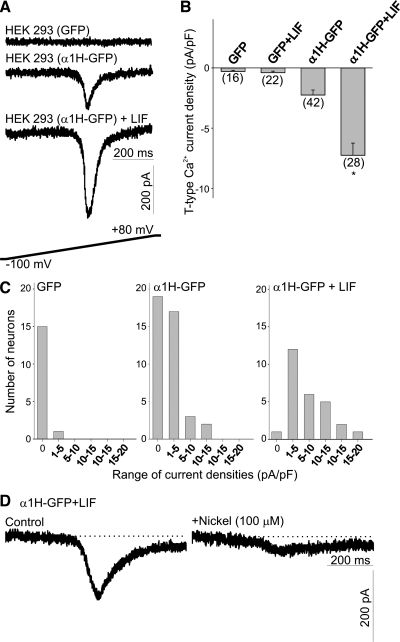
T-type Ca2+ currents were elicited by a 750-ms voltage ramp from −100 mV to +80 mV (0.24 V/s; stimulation protocol is represented in Fig. 1A, bottom). HEK-293 cells transfected with GFP alone had no significant amount of T-type Ca2+ current (Fig. 1A). Transfection of HEK-293 cells with the α1H-GFP subunits generated a transient current (Fig. 1A). The amplitude of T-type Ca2+ currents increased significantly after overnight exposure to LIF (10 ng/ml, Fig. 1, A and B). There were no significant differences in the voltages at which the ramp stimulation induced peak inward currents [α1H-GFP = −14.1 ± 1.6 mV (n = 24), α1H-GFP+LIF = −10.5 ± 1.5 mV (n = 36); P > 0.05]. To quantify changes in channel expression as a function of cell size, we normalized the amplitude of the T-type Ca2+ currents against cell capacitance. Overnight treatment of HEK-293 cells with LIF caused a significant increase in cell capacitance [α1H-GFP = 29.5 ± 3.1 pF (n = 27), α1H-GFP+LIF = 46.9 ± 3.7 pF (n = 41); P ≤ 0.01]. The resulting changes in current density are represented in Fig. 1B. T-type Ca2+ current density was low in GFP-transfected cells. Stimulation of GFP-expressing cells with LIF caused no expression of T-type Ca2+ current (Fig. 1B). Transfection of HEK-293 cells with α1H-GFP subunits evoked a significant increase in the averaged current density. Stimulation of α1H-GFP-transfected HEK-293 cells with LIF overnight evoked an approximately threefold increase in the T-type Ca2+ current density compared with nonstimulated cells (Fig. 1B). Overnight exposure of α1H-GFP-transfected HEK cells to LIF resulted in a rightward shift in the distribution of current densities compared with the current density distribution obtained in cells not treated with LIF (Fig. 1C). Consistent with the high sensitivity of α1H-subunits to low nickel concentrations (27), T-type Ca2+ currents generated by LIF stimulation were inhibited by 100 μM nickel ions (Fig. 1D). Thus these results demonstrate that after transfection of HEK-293 cells with α1H-GFP subunits, a basal level of functional channels can be detected by whole cell recordings. Considering the increase in cell capacitance and current density, LIF evokes a significant increase in the number of functional channels in the membrane (LIF-inducible expression).
Fig. 1.
Effect of leukemia inhibitory factor (LIF) on T-type Ca2+ channel expression in α1H-green fluorescent protein (GFP)-transfected HEK-293 cells. A: representative traces of inward Ca2+ currents generated by a voltage ramp in HEK-293 cells transfected with GFP alone or transfected with GFP-tagged α1H-subunits before and after stimulation with LIF (10 ng/ml). T-type Ca2+ currents were generated by a 750-ms depolarizing voltage ramp from −100 mV to +80 mV. Stimulation protocol for T-type Ca2+ currents is shown at bottom. B: mean T-type Ca2+ current densities generated in α1H-GFP-transfected HEK-293 cells after overnight treatment with LIF. Current densities were obtained by dividing current amplitude by cell capacitance. Note little T-type Ca2+ current expression in HEK-293 cells transfected with GFP alone or in GFP-expressing cells treated with LIF. Transfection of HEK-293 cells with GFP-tagged α1H-subunits evoked a significant increase in T-type Ca2+ current densities that was further enhanced by stimulation with LIF. Numbers in parentheses are n cells used. C: histograms of T-type Ca2+ current densities in HEK-293 cells transfected with GFP or cells transfected with α1H-GFP before and after LIF treatment. D: the T-type Ca2+ current generated in α1H-GFP-transfected HEK-293 cells was eliminated after incubation with 100 μM Ni2+. *P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells.
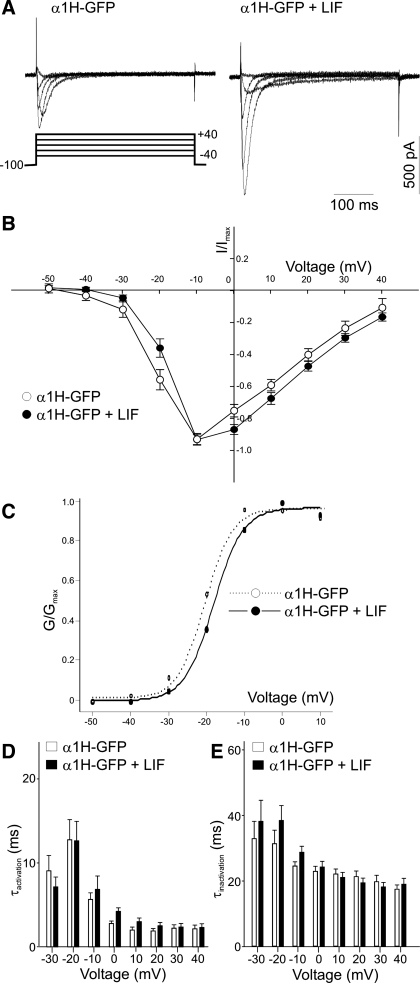
To investigate whether LIF causes a significant change in the channel properties we measured the I-V relationship, steady-state activation, and current kinetics in α1H-GFP-transfected HEK-293 cells before and after LIF treatment. The I-V relationship and steady-state activation were obtained by applying a 400-ms depolarizing step to various potentials from a holding potential of −100 mV (Fig. 2A). As shown in Fig. 2B, there were no significant changes in the voltage dependence of the normalized amplitude of T-type Ca2+ currents generated in LIF-treated and untreated HEK-293 cells. The steady-state activation of T-type Ca2+ currents in control and LIF-treated cells was determined by plotting the relative conductance against depolarizing voltage steps and fitting that relationship with a Boltzmann equation (Fig. 2C). The resulting fitting values V1/2 (step potential resulting in half-maximal activation of normalized conductance) and k (steepness of the curve) were assessed in order to determine possible changes in the steady-state activation in control and LIF-treated cells. There were no significant differences in the V1/2 and k values of nontreated and LIF-treated cells [α1H-GFP: V1/2 = −21.0 ± 1.5 mV, k = 3.2 ± 0.2 mV (n = 9); α1H-GFP+LIF: V1/2 = −17.2 ± 1.3 mV, k = 3.4 ± 0.3 mV (n = 13); P > 0.05]. To determine whether LIF treatment alters the activation and inactivation kinetics of T-type Ca2+ currents, we fitted the rising and decay phases of the transient current with a single-exponential curve. Treatment of α1H-GFP-transfected HEK-293 cells with LIF did not alter τactivation or τinactivation compared with untreated cells (Fig. 2, D and E). These results suggest that LIF does not alter the electrophysiological properties of T-type Ca2+ channels.
Fig. 2.
Characteristics of the current-voltage (I-V) relationship, steady-state activation, and activation (τactivation) and inactivation (τinactivation) time constants in α1H-GFP-transfected HEK-293 cells before and after LIF treatment. A: representative traces of inward Ca2+ currents evoked in α1H-GFP-transfected HEK-293 cells before and after LIF treatment. T-type Ca2+ currents were generated by 400-ms depolarizing pulses from −50 to +40 mV from a holding potential of −100 mV. B: comparison of the voltage dependence of T-type current densities in α1H-GFP-transfected HEK-293 cells in control (nontreated) and after LIF treatment. I/Imax, relative current. C: voltage dependence of steady-state activation. Lines represent the best fit obtained with a Boltzmann equation for the steady-state activation values in α1H-GFP-transfected HEK-293 cells before (α1H-GFP) and after LIF (α1H-GFP+LIF) treatment (−LIF, n = 9; +LIF, n = 13). G/Gmax, relative conductance. D and E: LIF treatment had no significant effect on τactivation (D) and τinactivation (E) of α1H-GFP-transfected HEK-293 cells after LIF stimulation. The activation and inactivation kinetics of the transient currents were determined by applying a series of 10-mV voltage steps between −30 and +40 mV from a holding potential of −100 mV (−LIF, n = 15; +LIF, n = 17; P > 0.05 at all voltages tested).
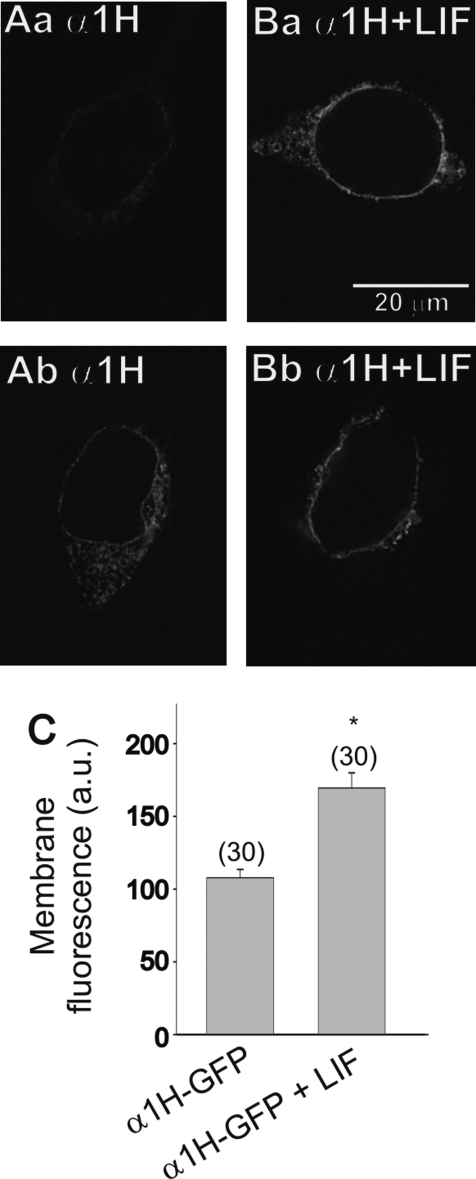
We assessed the membrane localization of GFP-tagged T-type Ca2+ channels by fluorescence microscopy. Images of α1H-GFP-transfected HEK-293 cells before and after stimulation with LIF are represented in Fig. 3, A and B. In agreement with our current density measurement, stimulation of α1H-GFP-transfected HEK cells with LIF caused a significant increase in the intensity of membrane fluorescence compared with nonstimulated cells (Fig. 3C). These data together with our T-type Ca2+ current density results indicate that LIF promotes the trafficking and functional expression of T-type Ca2+ channels in the membrane.
Fig. 3.
Effect of LIF stimulation on membrane fluorescence in α1H-GFP-transfected HEK-293 cells. A, a and b: typical examples of the membrane fluorescence in α1H-GFP-transfected HEK-293 cells. B, a and b: after overnight stimulation with LIF there is a considerable increase in the membrane fluorescence of α1H-GFP-transfected HEK-293 cells. C: comparison of the membrane fluorescence in α1H-GFP-transfected HEK-293 cells before and after LIF treatment. Numbers in parentheses are n cells used. *P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells. a.u., Arbitrary units.
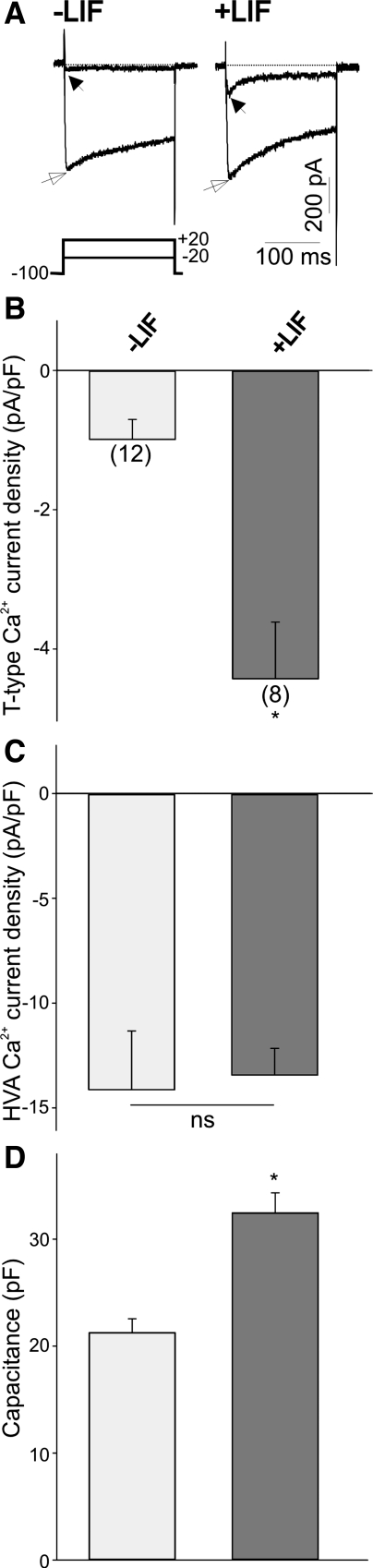
Does LIF also regulate the membrane expression of other voltage-activated Ca2+ channels? To test the specificity of LIF in regulating the functional expression of other ion channels, we used chicken nodose ganglion neurons isolated at E7. We previously demonstrated (38) that nodose ganglion neurons express both T-type and high-voltage-activated (HVA) Ca2+ channels. T-type Ca2+ currents in nodose ganglion neurons are also generated by α1H-subunits (39). Overnight treatment of E7 nodose neurons with LIF (10 ng/ml) caused a significant increase in T-type Ca2+ current amplitude compared with control neurons (Fig. 4A). To compensate for changes in cell size that may occur under different culture conditions, whole cell currents were normalized to cell size by dividing current amplitudes by cell capacitance. As represented in Fig. 4B, treatment of nodose neurons with LIF caused a threefold increase in T-type Ca2+ current densities compared with nontreated cells. In contrast, HVA Ca2+ current densities did not change significantly in the presence of LIF compared with nontreated neurons (Fig. 4C). Similar to the effect of LIF on the cell size of HEK-293 cells, LIF also caused a significant increase in the surface area of the plasma membrane as determined by measurements of cell capacitance in nodose neurons (Fig. 4D). These results suggest that the stimulatory effect of LIF is specific and only affects the functional expression of T-type but not HVA Ca2+ channels.
Fig. 4.
Effect of LIF on T-type and high-voltage-activated (HVA) Ca2+ currents in nodose neurons in vitro. A: representative traces of inward Ca2+ currents in control or LIF-treated nodose neurons. Nodose neurons were isolated at embryonic day (E)7 and treated with LIF overnight, whereas controls represent nontreated cells. T-type Ca2+ currents were generated by a 200-ms depolarizing pulses to −20 mV from a holding potential of −100 mV (filled arrows). HVA Ca2+ currents were generated by a 200-ms depolarizing pulse to +20 mV from a holding potential of −100 mV (open arrows). Stimulation protocol for both T-type and HVA Ca2+ currents is shown at bottom. B: mean T-type Ca2+ current densities after overnight treatment with LIF compared with control. Note that culture of nodose neurons with LIF evokes a significant increase in T-type Ca2+ current densities. Numbers in parentheses are n cells used. C: overnight treatment of nodose neurons with LIF does not alter the mean HVA Ca2+ current densities. ns, Not significant. D: LIF stimulation of nodose neurons evokes a considerable increase in cell capacitance. *P ≤ 0.05 vs. control.
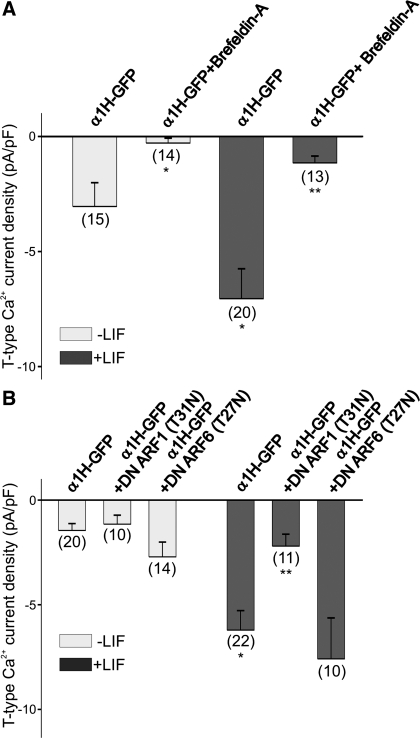
To further investigate the role of LIF in promoting the trafficking of T-type Ca2+ channels to the membrane we used brefeldin A (1 μg/ml), a compound that causes a complete disassembly of the Golgi apparatus and therefore prevents translocation of membrane-bound proteins (9). LIF caused a significant increase in the functional expression of T-type Ca2+ channels as determined by the increased current density (Fig. 5A). Overnight treatment of α1H-GFP-transfected HEK-293 cells with brefeldin A disrupted the basal expression of T-type Ca2+ channels. Brefeldin A also disrupted the LIF-induced expression of T-type Ca2+ channels in α1H-GFP-transfected HEK-293 cells (Fig. 5A). Thus the stimulatory effect of LIF on T-type Ca2+ channel expression was blocked after treatment of α1H-GFP-transfected HEK-293 cells with brefeldin A (Fig. 5A). To test whether brefeldin A has any acute effect on T-type Ca2+ channels already present in the membrane, α1H-GFP-transfected HEK-293 cells were treated with brefeldin A for 1 h before electrophysiological recordings. Acute treatment of α1H-GFP-transfected HEK-293 cells with brefeldin A for 1 h did not alter T-type Ca2+ current density [control = −2.5 ± 0.8 pA/pF (n = 6); +brefeldin A (1 h) = −3.3 ± 1.1 pA/pF (n = 4); P > 0.05 vs. control as determined by Student's unpaired t-test]. Thus these findings demonstrate that brefeldin A does not have any acute effect on T-type Ca2+ currents generated by α1H-subunits already present in the membrane.
Fig. 5.
Disruption of the Golgi apparatus or ADP-ribosylation factor (ARF)1 prevents the stimulatory effect of LIF on T-type Ca2+ channel expression. A: inhibition of the Golgi apparatus with brefeldin A blocked T-type Ca2+ channel expression evoked by LIF (10 ng/ml). Note that brefeldin A inhibited both the basal expression of T-type Ca2+ currents and the LIF-evoked increase in channel expression. *P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells; **P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells treated with LIF. B: cotransfection of HEK-293 cells with α1H-GFP and a dominant-negative (DN) form of ARF1 inhibited the stimulatory effect of LIF on current densities. Note that cotransfection of HEK-293 cells with α1H-GFP and a DN form of ARF6 had no effect on channel expression. There was no effect of ARF1 and ARF6 on the basal level of T-type Ca2+ currents in HEK-293 cells. *P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells; **P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells treated with LIF. Numbers in parentheses are n cells used.
In eukaryotic cells, membrane trafficking from the Golgi to the membrane is regulated by small GTPases belonging to the ADP-ribosylation factor (ARF) family of proteins (reviewed in Ref. 14). To investigate which ARF proteins may regulate the stimulatory effect of LIF on T-type Ca2+ channel trafficking, HEK-293 cells were cotransfected with α1H-GFP and DN forms of ARF1 and ARF6. The DN-ARF1 and DN-ARF6 forms lack activity as a result of replacing Thr31 or Thr27 (respectively) with asparagines (43). As represented in Fig. 5B, cotransfection of HEK-293 cells with a DN form of ARF1 or ARF6 had no effect on the basal expression of T-type Ca2+ channels. LIF caused a significant increase in the functional expression of T-type Ca2+ channels as determined by the increased current density (Fig. 5B). However, transfection of HEK-293 cells with the DN form of ARF1 caused a significant inhibition in the stimulatory effect of LIF on T-type Ca2+ channel expression (Fig. 5B). In contrast, cotransfection of HEK-293 cells with a DN form of ARF6 had no effect on the stimulatory effect of LIF on T-type Ca2+ channel expression (Fig. 5B). These results suggest that LIF-evoked trafficking of T-type Ca2+ channels is regulated by the activity of ARF1 proteins.
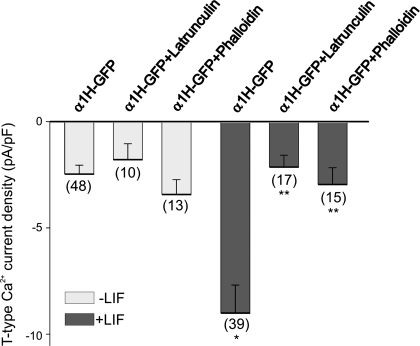
The actin cytoskeleton is an important regulator of T-type Ca2+ channel trafficking (3). To determine whether disruption of the actin cytoskeleton affects the LIF-evoked functional expression of T-type Ca2+ channels, we examined the effect of actin-disrupting drugs on the macroscopic Ca2+ currents generated in α1H-GFP-transfected HEK-293 cells. Pretreatment of α1H-GFP-transfected HEK-293 cells with the actin-depolymerizing agent latrunculin A or with the actin-stabilizing agent phalloidin for 1 h before the onset of LIF exposure was used to assess the role of the actin cytoskeleton on T-type Ca2+ channel expression after LIF treatment. Latrunculin A sequesters actin monomers, preventing actin polymerization and the formation of actin filaments (34), whereas phalloidin is an actin-stabilizing agent (32). In α1H-GFP-transfected HEK-293 cells not treated with LIF, neither latrunculin A (5 μM) nor phalloidin (10 μM) caused any significant change in the basal level of T-type Ca2+ current density (Fig. 6). As reported above, treatment of α1H-GFP-transfected HEK-293 cells with LIF caused a significant increase in the functional expression of T-type Ca2+ channels as determined by changes in current density (Fig. 6). Pretreatment of α1H-GFP-transfected HEK-293 cells with latrunculin A (5 μM) or phalloidin (10 μM) caused a significant inhibition in the LIF-evoked T-type Ca2+ channel expression (Fig. 6). These results demonstrate that disruption of the actin cytoskeleton prevents the stimulatory effect of LIF on T-type Ca2+ channel expression.
Fig. 6.
Effect of actin cytoskeleton-disrupting drugs on LIF-evoked T-type Ca2+ current expression. Pharmacological disruption of actin cytoskeleton with latrunculin A (5 μM) or phalloidin (10 μM) caused a significant inhibition in the LIF-evoked stimulation of T-type Ca2+ channel expression. Cultures of α1H-GFP-transfected HEK-293 cells were pretreated with latrunculin A or phalloidin 1 h before the onset of LIF stimulation. T-type Ca2+ channel expression was determined by whole cell recordings 12 h after the onset of LIF stimulation. *P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells; **P ≤ 0.05 vs. α1H-GFP-transfected HEK-293 cells treated with LIF. Numbers in parentheses are n cells used.
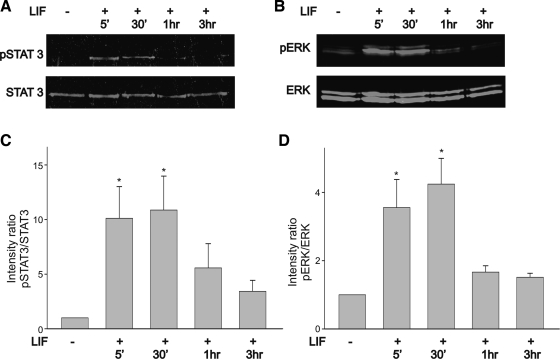
In most cells, LIF-evoked activity is generated by dimerization of the gp130-LIFRβ receptor complex leading to phosphorylation of JAKs and activation of STAT transcription factors (23, 45; reviewed in Ref. 20). In this study we used STAT3 phosphorylation as a reliable biochemical marker of JAK activation. Besides activation of the JAK/STAT signaling cascade, LIF can also stimulate other signaling molecules such as the MAP kinase ERK in a cell-specific manner (15, 23, 55). Initially, we investigated whether stimulation of HEK-293 cells with LIF leads to activation of STAT3 and ERK signaling. Activated STAT3 was probed with an antibody that recognized Tyr705, whereas phosphorylated (p)ERK was assessed with an antibody that recognizes Thr183 and Tyr185. HEK-293 cells were treated with 10 ng/ml LIF for varying lengths of time (5 min, 30 min, 1 h, and 3 h). After incubation with LIF, cells were lysed and assayed for STAT3 and ERK phosphorylation by two-color Western blot detection with the Odyssey infrared imaging system. Treatment of HEK-293 cells with LIF caused increased phosphorylation of STAT3 and ERK (Fig. 7, A and B). Both STAT3 and ERK phosphorylation became evident after 5-min stimulation and reached a peak after 30 min (Fig. 7, A and B). Plots of the intensity ratio of pSTAT3 to total STAT3 indicate that stimulation of HEK-293 cells with LIF evoked a statistically significant increase in STAT3 activation (Fig. 7C). STAT3 and ERK phosphorylation was transient and underwent a considerable reduction after 3-h continuous stimulation with LIF (Fig. 7, C and D). The ratio of pERK as a function of total ERK was used to quantify temporal changes in ERK activation. As represented in Fig. 7D, LIF stimulation of HEK-293 cells caused an approximately threefold increase in the ratio of pERK to total ERK. However, after 3-h stimulation with LIF, the ratio of pERK to total ERK returned to near-normal values.
Fig. 7.
Time course of STAT3 and ERK activation in α1H-GFP-transfected HEK-293 cells following stimulation with LIF (10 ng/ml). A and B: stimulation of cell cultures with LIF generates a considerable increase in STAT3 and ERK phosphorylation (p), respectively. C: phosphorylation pattern of STAT3 as determined by the intensity ratio of pSTAT3 to total STAT3. Stimulation of cell cultures with LIF for 30 min caused a significant increase in the pSTAT3-to-STAT3 ratio. D: phosphorylation pattern of ERK as determined by the intensity ratio of pERK to total ERK. Note that stimulation with LIF for 30 min caused a significant increase in the pERK-to-ERK ratio. The pERK-to-ERK intensity ratio decreased significantly after 3-h stimulation with LIF (n = 4). *P ≤ 0.05 vs. control (no LIF treatment).
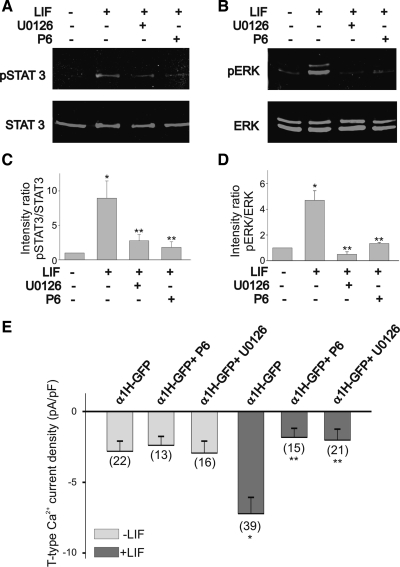
LIF-evoked activation of STAT3 and ERK was prevented by pretreatment of HEK-293 cells with the JAK inhibitor P6 (10 μM). This compound selectively targets kinases of the JAK family and has been widely used to study cytokine-evoked signaling (28, 41). As described above, stimulation of HEK-293 cells with LIF for 30 min caused a significant increase in STAT3 and ERK activation (Fig. 8, A and B, respectively). Pretreatment of HEK-293 cells with P6 for 1 h before stimulation with LIF prevented STAT3 activation, as indicated by changes in the ratio of pSTAT3 to total STAT3 (Fig. 8C). As represented in Fig. 8D, the ratio of pERK to total ERK was significantly reduced after P6 treatment of HEK-293 cells. Interestingly, pretreatment of HEK-293 cells with the selective blocker of ERK phosphorylation, U0126 (10, 47), for 1 h before stimulation with LIF prevented the activation of both STAT3 and ERK (Fig. 8, C and D). Thus LIF-evoked ERK activation was inhibited by pretreatment of HEK-293 cells with 50 μM U0126 (Fig. 8D). Pretreatment of HEK-293 cells with the ERK inhibitor U0126 (10 μM) also decreased STAT3 phosphorylation (Fig. 8C). Thus U0126 not only caused a significant reduction in the phosphorylation ratio of pERK to total ERK but also generated a significant reduction in the ratio of pSTAT3 to total STAT3 activation.
Fig. 8.
Effect of the JAK inhibitor P6 and the ERK inhibitor U0126 on STAT3 and ERK phosphorylation and current densities in α1H-GFP-transfected HEK-293 cells. A and B: LIF-evoked activation of STAT3 and ERK is blocked by the inhibitor of JAK kinases P6 (10 μM). The ERK inhibitor U0126 (50 μM) also blocked STAT3 and ERK activation. C and D: effect of P6 and U0126 on pSTAT3-to-total STAT3 and pERK-to-total ERK intensity ratios. Stimulation of α1H-GFP-transfected HEK-293 cells with LIF caused a significant increase in the pSTAT3-to-total STAT3 ratio. Note that P6 and U0126 caused a significant reduction in the pSTAT3-to-STAT3 intensity ratio. Stimulation of α1H-GFP-transfected HEK-293 cells with LIF also caused a 3-fold increase in the pERK-to-total ERK ratio. Treatment with either P6 or U0126 inhibited the stimulatory effect of LIF on ERK phosphorylation. E: JAK inhibitor P6 blocked the stimulatory effect of LIF on T-type Ca2+ channel expression. Inhibition of ERK activation with U0126 also blocked T-type Ca2+ channel expression evoked by LIF. In these experiments, cultures were pretreated with P6 or U0126 for 1 h before stimulation with LIF. *P ≤ 0.05 vs. control (nontreated cultures); **P ≤ 0.05 vs. LIF-treated cultures. Numbers in parentheses are n cells used.
To determine whether JAK-dependent ERK activation is required for the stimulatory effect of LIF on T-type Ca2+ channel expression, we tested the effect of the JAK inhibitor P6 and the ERK inhibitor U0126 on whole cell Ca2+ currents. HEK-293 cells transfected with α1H-GFP were cultured overnight in the presence of LIF, with or without P6 (10 μM). As stated above, stimulation of HEK-293 cells with LIF evoked a threefold increase in T-type Ca2+ current density compared with nonstimulated cells (Fig. 8E). In cells not stimulated with LIF, P6 did not have any significant effect on the basal T-type Ca2+ current density (Fig. 8E). Similarly, inhibition of ERK activation with U0126 also prevented the stimulatory effect of LIF on T-type Ca2+ current density (Fig. 8E). Treatment of HEK-293 cells with U0126 alone did not have any significant effect on current expression compared with nontreated cultures (Fig. 8E). These results demonstrate that JAK-dependent ERK activation is required for the LIF-evoked expression of T-type Ca2+ channels.
DISCUSSION
We demonstrated previously (39, 52) that in developing nodose neurons stimulation with neuropoietic cytokines such as CNTF or LIF causes a significant increase in the functional expression of T-type Ca2+ channels. In this study we have examined whether LIF stimulates trafficking of T-type Ca2+ channels. The stimulatory effect of LIF on T-type Ca2+ channel trafficking was assessed in HEK-293 cells transfected with the rat α1H channel subunit tagged to GFP. Three main conclusions can be drawn from these experiments. First, LIF evokes a significant increase in the membrane insertion of T-type Ca2+ channels as determined by whole cell currents and fluorescence microscopy. Second, disruption of the Golgi apparatus or overexpression of a DN form of ARF1 prevents the stimulatory effect of LIF on channel trafficking and expression. Third, the functional expression of T-type Ca2+ channels evoked by LIF requires a JAK-dependent, transient activation of ERK signaling.
Our results demonstrate that in HEK cells transfected with α1H-GFP, LIF stimulation causes a significant increase in the functional membrane expression of T-type Ca2+ channels. We should point out that HEK-293 cells normally do not express T-type Ca2+ channels, as demonstrated by our whole cell recordings of GFP-transfected cells. Cells transfected with the α1H-GFP construct generate currents with the typical characteristics of T-type Ca2+ channels, including transient activation and high sensitivity to low nickel concentrations. However, some of the biophysical properties of the T-type Ca2+ currents generated with the α1H-GFP construct including the voltage dependence of the peak currents and the steady-state activation parameters were more positive than previously reported, likely because of the experimental conditions used in the present work (42).
Stimulation with LIF causes a significant increase in whole cell T-type Ca2+ currents over the basal levels of expression. The ability of LIF to promote T-type Ca2+ channel trafficking is significant considering that LIF also evokes a dramatic increase in cell size as demonstrated by our measurements of cell capacitance in both HEK-293 cells and nodose neurons. The increase in cell capacitance is likely due to a stimulatory effect on cell growth. The combined effect of LIF on cell capacitance and current density suggests that LIF promotes the insertion of more T-type Ca2+ channel-forming α1H-subunits into the membrane. Our present results also demonstrate that in chicken nodose ganglion neurons LIF's ability to stimulate the membrane expression of T-type Ca2+ channels is specific. Thus LIF does not appear to alter the functional expression of HVA Ca2+ channels despite a significant increase in membrane surface area. Although the ability of LIF to promote the trafficking of T-type Ca2+ channels is a reasonable conclusion based on our electrophysiological and imaging data, we cannot disregard the possibility that increased synthesis of more channel proteins or changes in unitary conductance could have also contributed to the LIF-evoked changes in current density and membrane expression. Testing of these possibilities required confirmation by Western blot analysis and detailed analysis of single-channel conductance.
The ability of LIF to promote the trafficking of T-type Ca2+ channels to the membrane was confirmed by our fluorescence microscopy results showing a significant increase in the membrane fluorescence of α1H-GFP-transfected HEK-293 cells after LIF stimulation. We should point out that LIF stimulation does not alter the biophysical and pharmacological properties of T-type Ca2+ currents generated by α1H-subunits. This conclusion is consistent with our findings that LIF stimulation does not alter the I-V relationship, steady-state activation, τactivation, or τinactivation. Previous studies have demonstrated that channel phosphorylation by various signaling molecules can modify the gating properties of T-type Ca2+ currents. For example, stimulation of Ca2+/calmodulin-dependent protein kinase (CaMK)II causes a negative shift in the I-V relationship of T-type Ca2+ currents generated by α1H-subunits (56). Similarly, protein kinase C (PKC) activation stimulates T-type Ca2+ currents generated by α1H-subunits by altering the gating properties of the channels, including a shift in the I-V relationship toward more negative potentials (40).
The need for channel trafficking was further confirmed by the effect of brefeldin A and the DN form of ARF1. The effect of brefeldin A is often associated with disruption of the Golgi apparatus and membrane-directed trafficking (26). Brefeldin A inhibits the GTP-dependent interaction of ARF1 with the Golgi membrane required for the assembly of trafficking vesicles (12). Consistent with the idea that membrane trafficking is required for the functional expression of T-type Ca2+ currents, our data show that both basal expression and LIF-evoked stimulation of T-type Ca2+ currents can be eliminated by brefeldin A treatment of HEK-293 cells. Our results demonstrate that ARF1, a likely target of brefeldin A, is critical in regulating the LIF-evoked stimulation of T-type Ca2+ currents. Thus transfection of HEK-293 cells with a DN form of ARF1 caused a significant inhibition of the LIF-evoked stimulation of T-type Ca2+ currents. However, the DN form of ARF1 did not affect basal expression of T-type Ca2+ currents like brefeldin A. This could be explained by other side effects of brefeldin A in cell function (12). Interestingly, our data demonstrate that cotransfection of HEK-293 cells with α1H-GFP subunits and DN-ARF6 has no effect on the stimulatory effect of LIF in promoting channel trafficking. Previous studies have revealed that ARF6 is mainly involved in the recycling of membrane proteins, whereas ARF1 plays a critical role in endoplasmic reticulum-Golgi transport and in protein trafficking in the trans-Golgi network (reviewed in Ref. 13). Thus it appears that recycling of α1H-subunits does not have a significant effect on the LIF-evoked stimulation of T-type Ca2+ currents.
Our present results suggest that LIF-evoked trafficking of T-type Ca2+ channels requires a functional actin cytoskeleton. Thus actin depolymerization with latrunculin A inhibits the stimulatory effect of LIF on T-type Ca2+ currents, whereas the actin-stabilizing agent phalloidin prevents the stimulatory effect of LIF. Latrunculin A acts by sequestering actin monomers, which will ultimately cause depolymerization of actin filaments. Phalloidin blocks actin depolymerization by capping the dissociating end of actin filaments, which prevents their normal disassembly. Thus our results indicate that latrunculin A and phalloidin, which have opposite effects on the actin cytoskeleton, disrupt LIF-evoked stimulation of T-type Ca2+ channel expression. These findings suggest that LIF-evoked stimulation of T-type Ca2+ channel expression requires a dynamic cytoskeleton. This conclusion is supported by recent findings suggesting that the actin cytoskeleton plays a critical role in regulating the membrane trafficking and insertion of T-type Ca2+ channels (3). It is interesting to point out that increased actin depolymerization or stimulation of actin polymerization results in a significant inhibition of T-type Ca2+ channel trafficking. The actin cytoskeleton can play various roles in promoting the trafficking and functional expression of channel proteins in the membrane. First, the actin cytoskeleton can act as a barrier that prevents the trafficking of membrane proteins. This mechanism regulates the vasopressin-mediated trafficking of aquaporin-2 channels in renal epithelial cells (35) and the trafficking of Ca2+-dependent K+ channels induced by protein kinase activation in parasympathetic neurons (9, 35). Second, the actin cytoskeleton can anchor channel proteins to the membrane and promote the stabilization of protein complexes. Such a mechanism regulates the membrane expression of Kv1.2 channels and 5-HT receptors in neurons (16, 31). Under both conditions, latrunculin A and phalloidin have opposite effects on channel trafficking. For example, disruption of the actin cytoskeleton with latrunculin A promotes the vasopressin-mediated trafficking of aquaporin-2 channels in renal epithelial cells, whereas stabilization of the actin cytoskeleton with phalloidin prevents it (35). Since LIF-evoked trafficking of T-type Ca2+ channels is inhibited by both latrunculin A and phalloidin, it is unlikely that in our model the actin cytoskeleton acts as a barrier that prevents trafficking of T-type Ca2+ channels. A third possibility is that a dynamic actin cytoskeleton (with both actin polymerization and depolymerization happening simultaneously) may be necessary for the trafficking of transport vesicles to the membrane. This mechanism facilitates insulin-mediated trafficking of the glucose transporter GLUT4 in myocytes (8, 24) and the dopamine-evoked trafficking of GABAA receptors in cortical neurons (18). In this case, it has been shown that both latrunculin A and phalloidin inhibit the trafficking of channel proteins to the membrane (18, 24). This resembles our observation that LIF-evoked trafficking of T-type Ca2+ channels is disrupted equally by actin-stabilizing and -disrupting agents. Thus it appears that dynamic arrangements of the actin cytoskeleton by actin depolymerization and polymerization rather than the presence of a static cytoskeleton is required for the cytokine-induced T-type Ca2+ channel trafficking in HEK-293 cells.
Previous studies have demonstrated that LIF activation of the tricomponent gp130 receptor complex results in activation of the JAK/STAT and MAP kinase signaling cascades in various cell systems (reviewed in Ref. 22). Cytokine-evoked activation of the JAK/STAT signaling cascade is often associated with long-term changes in gene expression through dimerization of STAT transcription factors followed by nuclear translocation (50). By using STAT activation as a biochemical marker for LIF-evoked signaling, we have demonstrated that STAT3 activation takes place after stimulation of HEK-293 cells with LIF. However, it is not obvious by which mechanism JAK/STAT activation could regulate T-type Ca2+ channel trafficking. Our results demonstrate that LIF stimulation also leads to activation of the ERK signaling pathway. Indeed, our results indicate that JAK activation also leads to transient activation of the ERK signaling pathway. Thus, similar to our previous results in chicken nodose neurons, cytokine-evoked stimulation of T-type Ca2+ channel expression requires transient activation of the JAK and ERK signaling cascade (52). Inhibition of ERK signaling with U0126 not only prevented ERK phosphorylation but also blocked the trafficking and functional expression of T-type Ca2+ channels in HEK-293 cells transfected with α1H-subunits. Interestingly, our data show that U0126 not only blocked ERK activation but also leads to inhibition of STAT3 phosphorylation. This result should not be expected if LIF-evoked activation of JAK leads to the activation of STAT and ERK separately through two parallel pathways (52). Thus, different from our previous results in nodose sensory neurons (52), there appears to be significant cross talk between ERK and STAT3 activation in HEK-293 cells. We should mention that a significant cross talk between ERK and STAT activation has also been demonstrated after interleukin stimulation of T cells and cardiomyocytes (5, 48). Future experiments are required to elucidate how cytokine-induced ERK activation promotes T-type Ca2+ channel trafficking in HEK-293 cells and nodose sensory neurons. However, activated ERK can initiate a variety of intracellular signaling processes through phosphorylation of cytosolic proteins involved in protein trafficking. For example, ERK activation promotes trafficking of inward-rectifier K+ channels in a heterologous expression system (17) and the membrane insertion of 3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in cortical neurons (25, 44).
Cytokine-dependent regulation of T-type Ca2+ channel trafficking can be a fundamental mechanism for regulating cellular excitability. Because of their activation close to resting membrane potential, T-type Ca2+ channels are designed to enhance cellular excitability generated by small membrane depolarizations. This can lead to the generation of burst firing and amplification of synaptic signals in T-type Ca2+ channel-expressing neurons (reviewed in Ref. 42). The present results demonstrate that cytokines like LIF can upregulate T-type Ca2+ channel trafficking and functional expression in a cell system that normally does not express these channels. Cytokine-evoked expression of T-type Ca2+ channels can potentially regulate neuronal excitability in developing neurons and in certain pathological states. For example, we have proposed (38) that interaction with cardiac tissue upregulates the functional expression of T-type Ca2+ channels in developing nodose neurons through the release of a cytokine-like factor. Release of proinflammatory cytokines like LIF after tissue and/or nerve injury (46, 49, 51) could potentially increase cellular excitability and nociception in sensory neurons by promoting T-type Ca2+ channel trafficking and functional expression.
GRANTS
This work was supported by NIH Grant P20-RR-016435 from the Centers of Biomedical Research Excellence Program of the NCRR and the Vermont Genetics Network through NIH Grant P20-RR-16462 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the NCRR.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources (NCRR) or the National Institutes of Health (NIH).
REFERENCES
- 1.Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Müller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 18: 270–282, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci 10: 3507–3515, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aromolaran KA, Benzow KA, Cribbs LL, Koob MD, Piedras-Rentería ES. Kelch-like 1 protein upregulates T-type currents by an actin-F dependent increase in alpha1H channels via the recycling endosome. Channels (Austin) 3: 1–11, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Barbin G, Manthorpe M, Varon S. Purification of the chick eye ciliary neuronotrophic factor. J Neurochem 43: 1468–78, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Booz GW, Day JN, Speth R, Baker KM. Cytokine G-protein signaling crosstalk in cardiomyocytes: attenuation of Jak-STAT activation by endothelin-1. Mol Cell Biochem 240: 39–46, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Boulton TG, Stahl N, Yancopoulos GD. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem 269: 11648–11655, 1994 [PubMed] [Google Scholar]
- 7.Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J 24: 315–324, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brozinick JT, Jr, Hawkins ED, Strawbridge AB, Elmendorf JS. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J Biol Chem 279: 40699–40706, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chae KS, Oh KS, Dryer SE. Growth factors mobilize multiple pools of KCa channels in developing parasympathetic neurons: role of ADP-ribosylation factors and related proteins. J Neurophysiol 94: 1597–1605, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chae KS, Dryer SE. The p38 mitogen-activated protein kinase pathway negatively regulates Ca2+-activated K+ channel trafficking in developing parasympathetic neurons. J Neurochem 94: 367–379, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chemin J, Nargeot J, Lory P. Neuronal T-type alpha1H calcium channels induce neuritogenesis and expression of high-voltage-activated calcium channels in the NG108-15 cell line. J Neurosci 22: 6856–6862, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360: 350–352, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Donaldson JG, Honda A. Localization and function of Arf family GTPases. Biochem Soc Trans 33: 639–642, 2005 [DOI] [PubMed] [Google Scholar]
- 14.D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7: 347–358, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dziennis S, Habecker BA. Cytokine suppression of dopamine-beta-hydroxylase by extracellular signal-regulated kinase-dependent and -independent pathways. J Biol Chem 278: 15897–15904, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Emerit MB, Doucet E, Darmon M, Hamon M. Native and cloned 5-HT3A(S) receptors are anchored to F-actin in clonal cells and neurons. Mol Cell Neurosci 20: 110–124, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Giovannardi S, Forlani G, Balestrini M, Bossi E, Tonini R, Sturani E, Peres A, Zippel R. Modulation of the inward rectifier potassium channel IRK1 by the Ras signaling pathway. J Biol Chem 277: 12158–12163, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Graziane NM, Yuen EY, Yan Z. Dopamine D4 receptors regulate GABAA receptor trafficking via an actin/cofilin/myosin-dependent mechanism. J Biol Chem 284: 8329–8336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature 375: 784–787, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1–20, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holliday J, Spitzer NC. Spontaneous calcium influx and its roles in differentiation of spinal neurons in culture. Dev Biol 141: 13–23, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Inoue M, Nakayama C, Noguchi H. Activating mechanism of CNTF and related cytokines. Mol Neurobiol 12: 195–209, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Jiao J, Kaur N, Lu B, Reeves SA, Halvorsen SW. Initiation and maintenance of CNTF-Jak/STAT signaling in neurons is blocked by protein tyrosine phosphatase inhibitors. Brain Res Mol Brain Res 116: 135–146, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kanzaki M, Pessin JE. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem 276: 42436–42444, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Keifer J, Zheng Z, Zhu D. MAPK signaling pathways mediate AMPA receptor trafficking in an in vitro model of classical conditioning. J Neurophysiol 97: 2067–2074, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116: 1071–1080, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J 77: 3034–3042, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucet IS, Fantino E, Styles M, Bamert R, Patel O, Broughton SE, Walter M, Burns CJ, Treutlein H, Wilks AF, Rossjohn J. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood 107: 176–183, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ludlam WH, Kessler JA. Leukemia inhibitory factor and ciliary neurotrophic factor regulate expression of muscarinic receptors in cultured sympathetic neurons. Dev Biol 155: 497–506, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Martin-Caraballo M, Greer JJ. Voltage-sensitive calcium currents and their role in regulating phrenic motoneuron electrical excitability during the perinatal period. J Neurobiol 46: 231–248, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Mason HS, Latten MJ, Godoy LD, Horowitz B, Kenyon JL. Modulation of Kv1.5 currents by protein kinase A, tyrosine kinase, and protein tyrosine phosphatase requires an intact cytoskeleton. Mol Pharmacol 61: 285–293, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Matthews JB, Smith JA, Hrnjez BJ. Effects of F-actin stabilization or disassembly on epithelial Cl- secretion and Na-K-2Cl cotransport. Am J Physiol Cell Physiol 272: C254–C262, 1997 [DOI] [PubMed] [Google Scholar]
- 33.McCobb DP, Best PM, Beam KG. Development alters the expression of calcium currents in chick limb motoneurons. Neuron 2: 1633–1643, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin-A alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol 2: 376–378, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Noda Y, Sasaki S. Trafficking mechanism of water channel aquaporin-2. Biol Cell 97: 885–892, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Oppenheim RW, Prevette D, Yin QW, Collins F, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science 251: 1616–1618, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Oyesiku NM, Wigston DJ. Ciliary neurotrophic factor stimulates neurite outgrowth from spinal cord neurons. J Comp Neurol 364: 68–77, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Pachuau J, Martin-Caraballo M. Expression pattern of T-type Ca2+ channels in embryonic chick nodose ganglion neurons. Dev Neurobiol 67: 1901–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Pachuau J, Martin-Caraballo M. Extrinsic regulation of T-type Ca2+ channel expression in chick nodose ganglion neurons. Dev Neurobiol 67: 1915–1931, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Park JY, Jeong SW, Perez-Reyes E, Lee JH. Modulation of Cav3.2 T-type Ca2+ channels by protein kinase C. FEBS Lett 547: 37–42, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res 66: 9714–9721, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83: 117–161, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Peters PJ, Hsu VW, Ooi CE, Finazzi D, Teal SB, Oorschot V, Donaldson JG, Klausner RD. Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J Cell Biol 128: 1003–1017, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev 19: 2000–2015, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhee KD, Goureau O, Chen S, Yang XJ. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J Neurosci 24: 9779–9788, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutkowski MD, DeLeo JA. The role of cytokines in the initiation and maintenance of chronic pain. Drug News Perspect 15: 626–632, 2002 [PubMed] [Google Scholar]
- 47.Schonhoff CM, Bulseco DA, Brancho DM, Parada LF, Ross AH. The Ras-ERK pathway is required for the induction of neuronal nitric oxide synthase in differentiating PC12 cells. J Neurochem 78: 631–639, 2001 [DOI] [PubMed] [Google Scholar]
- 48.So EY, Oh J, Jang JY, Kim JH, Lee CE. Ras/Erk pathway positively regulates Jak1/STAT6 activity and IL-4 gene expression in Jurkat T cells. Mol Immunol 44: 3416–3426, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci 12: 457–466, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Symes A, Gearan T, Eby J, Fink JS. Integration of Jak-Stat and AP-1 signaling pathways at the vasoactive intestinal peptide cytokine response element regulates ciliary neurotrophic factor-dependent transcription. J Biol Chem 272: 9648–9654, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Thompson SW, Dray A, Urban L. Leukemia inhibitory factor induces mechanical allodynia but not thermal hyperalgesia in the juvenile rat. Neuroscience 71: 1091–1094, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Trimarchi T, Pachuau J, Shepherd A, Dey D, Martin-Caraballo M. CNTF-evoked activation of JAK and ERK mediates the functional expression of T-type Ca2+ channels in chicken nodose neurons. J Neurochem 108: 246–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Umemiya M, Berger AJ. Single-channel properties of four calcium channel types in rat motoneurons. J Neurosci 15: 2218–2224, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkens CM, Beam KG. Insertion of alpha1S II-III loop and C terminal sequences into alpha1H fails to restore excitation-contraction coupling in dysgenic myotubes. J Muscle Res Cell Motil 24:99–109, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Winston LA, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem 270: 30837–30840, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Wolfe JT, Wang H, Perez-Reyes E, Barrett PQ. Stimulation of recombinant Cav3.2, T-type, Ca2+ channel currents by CaMKIIgamma(C). J Physiol 538: 343–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]