Abstract
Engagement of α5β1-integrin by fibronectin (FN) acutely enhances Cav1.2 channel (CaL) current in rat arteriolar smooth muscle and human embryonic kidney cells (HEK293-T) expressing CaL. Using coimmunoprecipitation strategies, we show that coassociation of CaL with α5- or β1-integrin in HEK293-T cells is specific and depends on cell adhesion to FN. In rat arteriolar smooth muscle, coassociations between CaL and α5β1-integrin and between CaL and phosphorylated c-Src are also revealed and enhanced by FN treatment. Using site-directed mutagenesis of CaL heterologously expressed in HEK293-T cells, we identified two regions of CaL required for these interactions: 1) COOH-terminal residues Ser1901 and Tyr2122, known to be phosphorylated by protein kinase A (PKA) and c-Src, respectively; and 2) two proline-rich domains (PRDs) near the middle of the COOH terminus. Immunofluorescence confocal imaging revealed a moderate degree of wild-type CaL colocalization with β1-integrin on the plasma membrane. Collectively, our results strongly suggest that 1) upon ligation by FN, CaL associates with α5β1-integrin in a macromolecular complex including PKA, c-Src, and potentially other protein kinases; 2) phosphorylation of CaL at Y2122 and/or S1901 is required for association of CaL with α5β1-integrin; and 3) c-Src, via binding to PRDs that reside in the II–III linker region and/or the COOH terminus of CaL, mediates current potentiation following α5β1-integrin engagement. These findings provide new evidence for how interactions between α5β1-integrin and FN can modulate CaL entry and consequently alter the physiological function of multiple types of excitable cells.
Keywords: focal adhesion complex, fibronectin, c-Src, proline-rich domain, protein kinase A
integrins are heterodimeric transmembrane proteins that facilitate adhesion and communication of cells with their surroundings through binding to extracellular matrix proteins (ECM). Extensive studies implicate an essential role for integrins in the transmission of mechanical forces at focal adhesion sites (23, 25, 30). Integrin-mediated mechanotransduction is accomplished by activation of signaling molecules, such as focal adhesion kinase (FAK) and other tyrosine kinases, including c-Src, in conjunction with the recruitment of multiple proteins to focal adhesions and reorganization of the cytoskeleton (36). Integrins thereby translate physical forces into biochemical signals that ultimately alter cellular function.
Ca2+ entry through the L-type voltage-gated Cav1.2 calcium channel (CaL) is required for stretch-induced contraction of vascular smooth muscle (VSM). Intracellular pressurization of isolated arterial myocytes leads to enhancement of CaL current, suggesting that CaL may be modulated by mechanical stress applied to the plasma membrane (22). A link between CaL and integrins in transducing mechanical force has also been demonstrated in arterioles. The application of peptides containing an Arg-Gly-Asp (RGD) sequence, a cryptic binding motif in ECM that becomes exposed during vascular injury (7), inhibits pressure-dependent myogenic tone, through interactions with multiple VSM and endothelial cell integrins (8). Moreover, function-blocking antibodies against α5-, β1-, and β3-integrin significantly reduce the degree of myogenic constriction to pressure elevation (29). The effects of integrin antibodies on myogenic tone are mediated in part by altered Ca2+ entry through CaL because electrophysiological studies reveal selective effects of integrin ligands on CaL current; the engagement and clustering of α5β1-integrin by insoluble fibronectin (FN) or anti-α5-integrin antibody acutely potentiates CaL current, whereas engagement of αvβ3-integrin by vitronectin or soluble ligands inhibits CaL current in isolated VSM cells (41). Using site-directed mutagenesis strategies, two phosphorylation sites within the COOH terminus of the α1C-subunit of CaL (α1C-CaL) were identified as targets of protein kinase A (PKA) and c-Src that become activated downstream from α5β1-integrin ligation (14).
A critical remaining issue is the extent to which α5β1-integrin, PKA, c-Src, and CaL are spatially coupled within a cell. In the present study, we tested the hypothesis that α5β1-integrins spatially associate with CaL and that this association is required to modulate CaL function. Using human embryonic kidney cells (HEK293-T) overexpressing the neuronal isoform of CaL and VSM cells from rat skeletal muscle arterioles as model systems, the interactions between endogenous α5β1-integrin and CaL and between c-Src and CaL were examined using immunoprecipitation (IP), immunoblotting (IB), immunofluorescence confocal microscopy, and patch-clamp methods.
METHODS
Cell culture and transient transfection.
HEK293-T/(TSA201) cells were maintained in 10% FBS (Hyclone)-DMEM (high glucose) supplemented with 2 mM glutamine, 100 units of penicillin, and 100 μg of streptomycin in 5% CO2 at 37°C. When cells reached 70–80% confluence, wild-type (WT) or mutant neuronal Cav1.2 calcium channel (CaL) cDNAs composed of α1C-c (6 μg), β1b (3 μg), and α2δ (2 μg) subunits, in a total volume of 14 μl, were transfected into HEK293-T cells grown in 60-mm tissue culture dishes containing 2.5 ml of DMEM with Lipofectamine 2000 (20 μl) for 18–24 h. Green fluorescent protein (GFP; 0.5–1 μg) was cotransfected with CaL to monitor transfection efficiency. Untransfected cells were used as a control. Cells were incubated for another 24 h posttransfection before passing and plating onto either FN (catalog no. 354403, BD BioCoat, BD Biosciences), BSA (2%), or poly-l-lysine-treated plates for 1 h, followed by protein isolation or immunofluorescence imaging. To account for variation in the expression level of α1C-CaL due to variation in transfection efficiency among the different CaL mutants, the experiments for all CaL mutants were performed in parallel with WT-CaL transfection using the same passage of cells.
For electrophysiological experiments, HEK293-T cells were transfected using calcium phosphate, after which patch-clamp recordings were performed 48–72 h posttransfection as previously described (14). Only single cells expressing GFP were used for electrophysiological protocols.
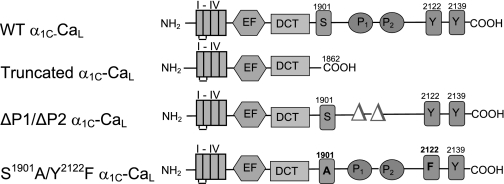
The various mutations in the α1C-COOH terminus were generated using site-directed mutagenesis as described previously (14) and as illustrated in Fig. 1.
Fig. 1.
Schematic depicting the domain structure of wild-type (WT) L-type voltage-gated Cav1.2 calcium channel (CaL) α1C-subunit and mutant α1C-CaL constructs used in this study. I–IV, the four transmembrane repeat of α1C. EF and DCT, EF hand motif and the distal COOH terminus inhibitory domain; P, proline-rich domain; truncated α1C-CaL, WT α1C-CaL missing the distal COOH-terminal 280 amino acids; ΔP1/ΔP2 α1C-CaL, WT α1C-CaL missing the two proline-rich domains (aa 1955–1959; aa 1967–1973); S1901A/Y2122F α1C-CaL, two single-point mutations at serine (S1901A) and tyrosine (Y2122F) in full-length neuronal WT-CaL.
Isolation of skeletal muscle arterioles.
The isolation of first- and second-order arterioles from rat cremaster muscle was performed as described previously (21, 40, 41), except for addition of 1× Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific) into the dissection chamber immediately before dissection. All animal protocols were approved by the University of Missouri Animal Care and Use Committee and conformed to the Public Health Service Policy for the Humane Care and Use of Laboratory Animals (PHS Policy, 1996).
Electrophysiological recording.
Patch-clamp recordings were made using an EPC9 amplifier under the control of Pulse software (HEKA Instruments) in the conventional whole cell mode, as previously described (14). Pipettes were filled with Cs+ pipette solution containing (in mM) 110 CsCl, 20 TEA chloride, 10 EGTA, 2 MgCl2, 10 HEPES, and 1 CaCl2 (pH 7.2 with CsOH). Ba2+ was used as the charge carrier to increase the magnitude of the inward current and to minimize calcium-dependent current inactivation. Cs+ was used to block endogenous K+ current.
Immunoprecipitation and immunoblotting.
Proteins from HEK293-T cells were isolated as described by Ling et al. (27) with minor modifications. The lysis buffer was composed of 1% Triton X-100 containing the following: 2.5 mM Tris, pH 7.4, 13.8 mM NaCl, 0.3 mM KCl, 2 mM EDTA, 1 mM EGTA, 10 mM NaF, 1 mM benzamidine, 1 mM Na3VO4, 4 mM Pefabloc, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml of calpain I and II, and 10 μg/ml pepstatin A. Isolation of total proteins in arterioles was performed as described above except for using 0.25% Triton X-100, along with 2% n-octyl-β-d-glucopyranoside to lyse arterioles. Arterioles (1st order and 2nd order) were snap-frozen in liquid nitrogen after dissection. Vessels were cut into smaller pieces and pulverized with a plastic pestle in 0.65-ml centrifuge tubes in the presence of an optimal amount of lysis buffer. Tubes containing arterioles were frequently dipped into liquid nitrogen and kept on dry ice during pulverization. Three hundred to four hundred micrograms of total protein (150–250 μg for arterioles) were precleared with protein A/G agarose beads (catalog no. SC-2003; Santa Cruz) in the presence of either rabbit or mouse IgG (catalog no. PP64 and PP54, respectively; Millipore), followed by overnight IP in lysis buffer at 4°C with one of the following antibodies: 1) either rabbit anti-Cav1.2 polyclonal antibody (Ab) (3.2 μg; catalog no. AB5156, Millipore) or pan Calcium Channel Ab (catalog no. Ab6298, Abcam), recognizing the α1C-subunit of CaL (α1C-CaL); 2) mouse anti-human β1-integrin monoclonal Ab (5 μg, clone B3B11, catalog no. MAB2251Z, Millipore); 3) purified mouse anti-human α5-integrin Ab (5 μg, clone 1/CD49e, catalog no. 610634, BD Biosciences); 4) mouse anti-pp60c-Src (4 μg, clone GD11, catalog no. 05-184, Millipore); or 5) anti-phosphorylated-Tyr Ab (5 μg, clone 4G10, catalog no. 05-321, Millipore). The immune complex was captured using either protein A (catalog no. SC-2001, Santa Cruz) or protein G agarose beads (catalog no. 17088601, Amersham) prior to SDS-PAGE (6%), followed by overnight transfer to nitrocellulose membrane (0.45 nm) and IB. To confirm binding specificity, the whole cell lysate was also immunoprecipitated with 1) control IgG for the appropriate host species and captured by protein A or protein G beads, or 2) protein A or protein G beads alone. Equal loading of total protein was confirmed by Ponceau staining (Sigma) before IB. CaL was detected using rabbit anti-CaL α1C-subunit Ab (catalog no. AB5156, 1:6,500 dilution) for HEK293-T cells or pan Calcium Channel Ab (1:2,500 dilution) for rat arterioles. Endogenous β1-integrin was detected using mouse anti-human β1-integrin Ab or rabbit anti-rat β1-integrin Ab (catalog no. MAB2251Z, 1:1,400 dilution and catalog no. AB1952P, 1:1,500 dilution, respectively; Millipore). c-Src or phosphorylated c-Src was detected using anti-pp60c-Src (catalog no. 05-184, 1:1,200 dilution, Millipore) or rabbit anti-rat phospho-Src family (Tyr416) Ab (catalog no. 2101S, 1:1,000 dilution, Cell Signaling Technology), respectively. The immune complexes were incubated with either goat anti-rabbit IgG-horseradish peroxidase (HRP)-conjugated antibody (H&L, 1:120,000 dilution, catalog no. AP132P, Millipore) or donkey anti-mouse-IgG-HRP-conjugated antibodies (H&L, 1:60,000 dilution, catalog no. 715-035-150, Jackson Research Laboratory) followed by detection with Pierce SuperSignal extended Dura or Femto substrate (Thermo Scientific). After probing, the blot was stripped with Restore Western blot stripping buffer (Thermo Scientific) and reprobed with either anti-α1C-CaL, anti-β1-integrin, or anti-c-Src Ab to assess the relative loading. For detection of the immune complex from arterioles, the blot was processed as described above except that SuperSignal Western Blot Enhancer (catalog no. 46641, Thermo Scientific) was used according to the manufacturer's instruction before IB. The results were captured by exposure to Kodak Biomax MR, MS, or Light film (Sigma), and the band intensity for the protein of interest was quantified using Bio-Rad Quantity One. Quantification of co-IP was determined as described by Cherubini et al. (4). The ratio of immunoprecipitated α1C-CaL to β1-integrin in WT-α1C-CaL-expressing cells was set as 1. The ratio of immunoprecipitated α1C-CaL to β1-integrin in mutant α1C-CaL-expressing cells was expressed relative to WT-α1C-CaL.
Immunofluorescence confocal imaging.
To determine the distribution and expression of α1C-CaL and β1-integrin, cells were plated on poly-l-lysine or FN-coated tissue culture dishes with coverglass bottoms (Fluorodish, catalog no. FD35-100, WPI) for 1 h after 24 h of transfection, followed by fixation with 2% paraformaldehyde and staining as described previously (40). α1C-CaL was determined using the same antibody for IP, followed by donkey anti-rabbit IgG conjugated to Alexa 488 (1:1,600 dilution, catalog no. A21206, Invitrogen). β1-Integrin was detected using mouse anti-human β1-integrin Ab (1:75 dilution, clone HUTS 21, catalog no. 556037, BD Biosciences), followed by goat anti-mouse IgG conjugated with Alexa 555 (1:1,400 dilution, catalog no. A21424, Invitrogen). Counterstaining of nuclei was performed using TO-PRO-3 (0.5 μM, catalog no. T3605, Invitrogen). Staining of paxillin or vinculin was determined using rabbit anti-paxillin polyclonal Ab (1:300 dilution, catalog no. AB3794, Millipore) or mouse anti-vinculin antibody (clone V284, 1:80 dilution; catalog no. 05-386, Millipore). Cells were incubated with donkey anti-rabbit IgG conjugated with Alexa 488 alone or goat anti-mouse IgG conjugated to Alexa 555 alone, or the combination of both as a negative control to confirm the specificity of staining. ProLong Gold Antifade Reagent (catalog no. P36934, Invitrogen) was applied to the sample after staining, followed by storage at −30°C until images were captured.
Immunofluorescence images were taken using a laser-scanning confocal system (Leica TCS SP5 Microsystems) attached to an upright microscope (Leica DM 6000 CS) and were collected using a ×63 oil objective (numerical aperture 1.42). Excitation of the fluorophores was achieved using argon 488, He/Ne 543, or He/Ne 633 nm laser combinations with pinhole size set at 1 airy disk. Images were taken at 512 × 512 pixels with a step size of 0.2 μm and a zoom factor of 5. The image size was ∼49.2 μm2.
To prevent bleed-through of different wavelengths that might interfere with colocalization analysis, all the images were taken using a sequential acquisition procedure at wavelengths of 488/519 nm (excitation/emission) for the α1C-CaL-subunit; 543/569 nm (excitation/emission) for β1-integrin, and 633/656 nm (excitation/emission) for nuclear staining.
Quantification of α1C-CaL association with β1-integrin.
The association of α1C-CaL with β1-integrin was analyzed with ImageJ software (version 1.39d, National Institutes of Health, Bethesda, MD). The degree of α1C-CaL association with β1-integrin was quantified using Pearson's coefficient, Mander's coefficient, and intensity correlation analysis (ICA) (24, 33). The advantage of using ICA analysis is that it avoids taking the overlap of randomly distributed proteins into account for colocalization if the intensity of the two target proteins does not vary coincidently (24). ICA values are distributed between −0.5 and +0.5. ICA values close to 0 indicate random staining, values −0.5 ≤ ICA < 0 represent segregated staining, and values 0 < ICA ≤ +0.5 represent interdependent staining. The degree of α1C-CaL association with β1-integrin was normalized to the average ICA value for the coassociation of paxillin with vinculin (0.25), which was shown previously to be very strong (37).
Statistical analysis.
Results are presented as means ± SE from at least three independent experiments for IP or four independent experiments for immunofluorescence confocal imaging (IF). Statistical differences were analyzed using GraphPad InStat or Prism (version 3.06 and version 5.0, respectively; GraphPad, San Diego, CA) with either analysis of variance or unpaired t-tests. P < 0.05 was considered significant.
RESULTS
CaL association with α5β1-integrin depends on adhesion to fibronectin.
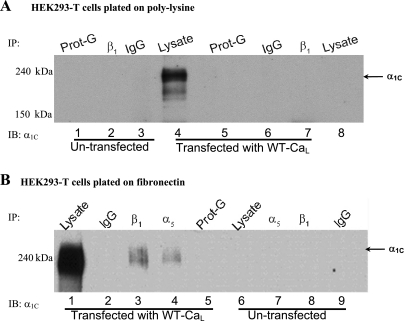
To determine whether α1C-CaL associated with α5β1-integrin, IP of whole cell lysates from HEK293-T cells expressing the neuronal CaL using either anti-β1- or anti-α5-integrin antibody was followed by IB to probe for the presence of α1C-CaL. The neuronal CaL isoform was used as a model system because α1C-CaL mutants had been previously generated by site-directed mutagenesis strategies; neuronal and smooth muscle (SM) cell (SMC) CaL isoform share 90% homology in amino acid sequence, and we previously demonstrated that heterologously expressed rat neuronal and SMC isoforms (Cav1.2c and Cav1.2b, respectively) showed a similar degree of current potentiation following α5β1-integrin ligation (14). We first determined the association between α1C-CaL and β1-integrin in cells plated on poly-l-lysine-treated dishes. As shown in Fig. 2A, no appreciable α1C-CaL was observed after IP using anti-β1-integrin Ab in either control (untransfected cells, lane 2) or cells expressing WT α1C-CaL (lane 7) when plated on poly-l-lysine-treated dishes. However, when cells expressing WT α1C-CaL were plated on FN (Fig. 2B), α1C-CaL was detected after IP of the lysate using either anti-α5- (lane 4) or anti-β1-integrin antibody (lane 3). No specific α1C-CaL band was observed in cells expressing α1C-CaL plated on BSA-coated dishes after IP using anti-β1-integrin or anti-c-Src Ab (Supplemental Fig. S1A, lanes 6 and 7, respectively) or after IP of the lysate using mouse IgG or protein-G (Fig. 2B, lanes 2, 5, and 9). These results indicate that the association of α1C-CaL with α5 or β1-integrin in HEK293-T cells is specific and depends on cell adhesion to FN.
Fig. 2.
CaL association with α5- or β1-integrin depends on adhesion of human embryonic kidney cells (HEK293-T) to fibronectin (FN). A: representative blot showing coimmunoprecipitation (co-IP) protocol on protein lysed from control (untransfected) or neuronal WT-CaL-expressing HEK293-T cells plated onto poly-l-lysine-treated dishes. No α1C-CaL appears to be pulled down by IP with anti-β1-integrin antibody (Ab) in either control (untransfected) or WT CaL-expressing cells (lanes 2 and 7, respectively). Expression of α1C-CaL was confirmed in cells expressing WT-CaL by immunoblotting (IB) with anti-α1C-CaL Ab, showing a specific band at molecular mass 190 to 240 kDa (lane 4). B: representative blot showing pull-down of α1C by anti-α5 or anti-β1-integrin Ab (lanes 4 and 3, respectively) only from cells expressing WT-CaL, not control (untransfected) cells (lanes 7 and 8, respectively), when plated onto FN-coated plates before IP and IB. All blots were repeated at least three times. Prot-G, protein-G.
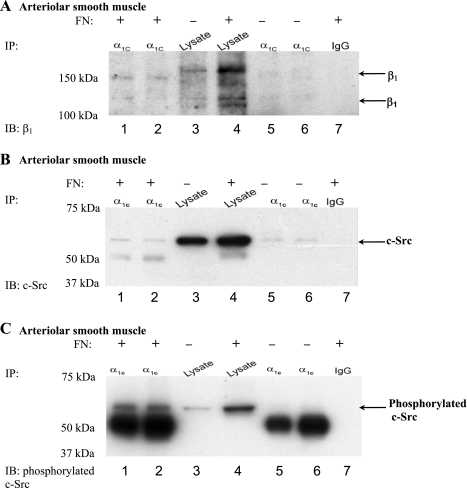
To confirm that the effect of FN on α1C-CaL association with β1-integrin was not an artifact of CaL overexpression in HEK293-T cells, we examined the same interaction in lysates from arteriolar SM. Arterioles were dissected and then incubated with exogenous FN abluminally (300 μg/ml) for 1 h. When arteriolar SM lysates were immunoprecipitated with anti-α1C-CaL, β1-integrin was detected only in arterioles incubated with FN (Fig. 3A, lanes 1 and 2), compared with control (lanes 5 and 6). The same blot was also stripped and reprobed for the expression of α1C-CaL. In addition, incubation with FN did not substantially change the level of α1C-CaL expression in arterioles (Supplemental Fig. S1B). These results indicate that the association of α1C-CaL with β1-integrin in SMC depends on ligation of β1-integrin by FN.
Fig. 3.
α1C-CaL associates with β1-integrin in arteriolar smooth muscle incubated with FN. A: representative blot showing co-IP protocol on protein lysed from arteriolar smooth muscle (pulled down with anti-α1C-CaL). β1-Integrin and α1C-CaL coimmunoprecipitated when arterioles were incubated with FN for 1 h (lanes 1 and 2, respectively). Lanes 1 and 6 were immunoprecipitated with anti-α1C-CaL from Chemicon, whereas lanes 2 and 5 were immunoprecipitated with anti-α1C-CaL from Abcam. Lanes 3 and 4 were lysates alone isolated from control arterioles or arterioles incubated with FN, respectively. The absence of coimmunoprecipitated β1-integrin by rabbit IgG (lane 7) indicates binding specificity. B: representative blot showing co-IP protocol on protein lysed from arteriolar smooth muscle (pulled down with anti-α1C-CaL). c-Src coimmunoprecipitated with α1C-CaL whether or not arterioles were incubated with FN (lanes 1 and 2 vs. lanes 5 and 6, respectively). IP was performed as described in A. Abluminal incubation of arterioles with exogenous FN did not change the degree of association between α1C-CaL and c-Src (lane 2, 90% and lane 5, 86% of arterioles alone, respectively). The absence of coimmunoprecipitated c-Src by rabbit IgG (lane 7) indicates binding specificity. C: representative blot showing co-IP protocol on protein lysed from arteriolar smooth muscle (pulled down with anti-α1C-CaL). Phosphorylated c-Src was only observed in arterioles incubated with exogenous FN (lanes 1 and 2). IP was performed as described in A. The absence of phosphorylated c-Src by rabbit IgG (lane 7) indicates binding specificity.
c-Src, a tyrosine kinase activated upon integrin ligation by ECM ligands (18), has been shown to modulate CaL function in SMC (2, 3, 19, 39). Thus, we also examined whether c-Src associated with α1C-CaL in arteriolar SM and whether FN was important for this association. As shown in Fig. 3B, c-Src was detected in immunoprecipitates using two different anti-α1C-CaL antibodies (Fig. 3B, lanes 1 and 2). However, abluminal incubation with FN did not substantially change the degree of c-Src association with α1C-CaL (Fig. 3B, lanes 1 and 2 vs. lanes 5 and 6). Next, we tested whether incubation with FN altered the phosphorylation status of c-Src in association with α1C-CaL. A basal level of phosphorylated c-Src was observed in untreated control lysate (Fig. 3C, lane 3) and in lysate from arteriolar SM incubated with FN (lane 4). In contrast to Fig. 3B, phosphorylated c-Src coassociated with α1C-CaL only in arteriolar SM incubated with FN, not in control arteriolar SM (Fig. 3C, lanes 1 and 2 vs. lanes 5 and 6, respectively). These results suggest that the association of α1C-CaL with c-Src in arteriolar SM is independent of FN, whereas the association of α1C-CaL with phosphorylated c-Src depends on integrin engagement by FN.
The COOH terminus of α1C-CaL is essential for association with β1-integrin.
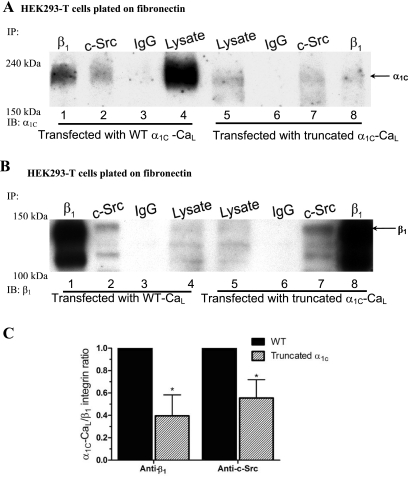
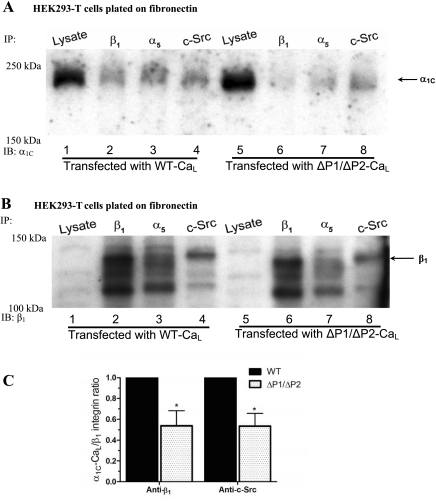
To determine whether the specific regions in the COOH terminus of α1C are required for the association of α1C-CaL with α5β1-integrin, three mutant α1C-CaL constructs were overexpressed in HEK293-T cells (Fig. 1). If any of the specific regions is required for association between the two proteins, we predicted to observe less α1C-CaL pulled down by anti-β1-integrin Ab in cells expressing the mutant α1C-CaL. As shown in Fig. 4A, truncated α1C-CaL exhibited a reduced level of association with β1-integrin (lane 8) and a reduced level of association with c-Src (lane 7), compared with their respective levels of association in WT α1C-CaL (lanes 1 and 2, respectively). Figure 4B shows the same membrane after stripping and reprobing for β1-integrin as a loading control. The expression of β1-integrin, c-Src (Supplemental Fig. S3A), and the degrees of association between β1-integrin and c-Src were not changed in cells expressing truncated α1C-CaL, compared with WT α1C-CaL (quantified data not shown). When normalized to the total amount of WT α1C-CaL, the associations between truncated α1C-CaL and β1-integrin as well as between truncated α1C-CaL and c-Src were reduced to 40 ± 9% and 55 ± 8% of WT α1C-CaL, respectively (Fig. 4C). These results provide evidence for the requirement of the distal COOH terminus in the associations between α1C-CaL and β1-integrin and between α1C-CaL and c-Src.
Fig. 4.
Requirement of the α1C-CaL COOH terminus for association of α1C-CaL with c-Src or β1-integrin. A: representative blot comparing WT- and truncated α1C-CaL immunoprecipitated with either anti-c-Src, anti-β1-integrin Ab, or mouse IgG, then probed for α1C. α1C was only detected in lysates immunoprecipitated with anti-c-Src (lanes 2 and 7) or anti-β1-integrin Ab (lanes 1 and 8) but not with mouse IgG (lanes 3 and 6). Relatively lower amounts of α1C-CaL were pulled down by anti-c-Src or anti-β1-integrin Ab in truncated α1C-CaL (lanes 7 and 8, respectively), compared with WT. B: the same blot in A after stripping and reprobing for β1-integrin as a control for IP and immunoblotting. C: summary graph of the relative levels of immunoprecipitated α1C in cells expressing WT- or truncated α1C-CaL. Values were obtained as described in methods and are based on the average of at least three experiments. *P < 0.05 vs. WT + anti-β1-integrin Ab or WT + anti-c-Src Ab.
To confirm that the reduced associations between α1C-CaL and β1-integrin and between α1C-CaL and c-Src were not due to decreased expression of truncated α1C-CaL construct, we increased the amount of total protein for IP from cells expressing the truncated α1C-CaL construct to twofold that of WT α1C-CaL and reexamined the association of truncated α1C-CaL with β1-integrin or with c-Src. As shown in Supplemental Fig. S2, A and C, reduced degrees of association between α1C-CaL and β1-integrin (Fig. S2A, lane 3), α1C-CaL and phosphorylated tyrosine (Tyr-Pi, Fig. S2A, lane 2), and α1C-CaL and c-Src (Fig. S2A, lane 1) were still observed in cells expressing truncated α1C-CaL, when compared with the degrees of their respective associations in cells expressing WT α1C-CaL (70 ± 7%, 51 ± 4%, and 58 ± 11%; % of WT, respectively). However, the expressions of β1-integrin and c-Src, the degrees of associations between β1-integrin and c-Src, or between β1-integrin and proteins containing phosphorylated tyrosine were not changed in cells expressing truncated α1C-CaL, compared with WT α1C-CaL (Supplemental Figs. S2B and S3B; quantified data not shown). These results suggest a requirement for the distal COOH terminus in α1C-CaL association with the β1-integrin and c-Src.
The collaborative contribution of PKA and c-Src in the association of CaL with β1-integrin.
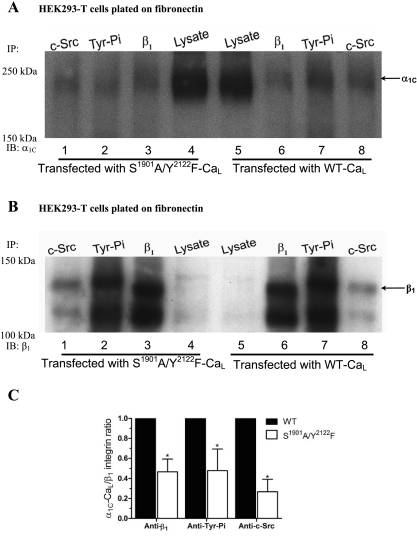
We previously showed that α5β1-integrin engagement leads to potentiation of CaL current via phosphorylation of α1C-CaL serine (S1901) and tyrosine (Y2122) by PKA and c-Src, respectively (14). Thus, a next logical step was to determine whether phosphorylation of α1C-CaL by PKA and c-Src is required for CaL association with β1-integrin or c-Src. As shown in Fig. 5A, reduced associations between α1C-CaL and β1-integrin (lane 3), α1C-CaL and Tyr-Pi (lane 2), and α1C-CaL and c-Src (lane 1) were observed in cells expressing S1901A/Y2122F-CaL, compared with WT α1C-CaL (Fig. 5C, 47 ± 8%, 48 ± 12%, and, 27 ± 7%; % of WT, respectively). However, the expressions of β1-integrin (Fig. 5B) and c-Src, the degrees of associations between β1-integrin and c-Src, or between β1-integrin and proteins containing phosphorylated tyrosine were unchanged in cells expressing S1901A/Y2122F-CaL, compared with WT α1C-CaL (Supplemental Fig. S4A, quantified data not shown). These results suggest that phosphorylation of α1C-CaL by PKA and/or c-Src is required for the associations between α1C-CaL and β1-integrin and between α1C-CaL and c-Src.
Fig. 5.
S1901 and Y2122 in α1C-CaL are required for α1C-CaL association with β1-integrin or c-Src. A: representative blot comparing WT α1C-CaL and S1901A/Y2122F α1C-CaL immunoprecipitated with either anti-c-Src, anti-phosphorylated tyrosine (Tyr-Pi), or anti-β1-integrin Ab, and probed for α1C. Relatively lower amounts of α1C-CaL appear to be pulled down by anti-c-Src, anti-Tyr-Pi, or anti-β1-integrin Ab (lanes 1, 2, and 3, respectively) in S1901A/Y2122F α1C-CaL-expressing cells, compared with WT-α1C-CaL. B: the same blot in A after stripping and reprobing for β1-integrin. C: summary graph showing the relative levels of immunoprecipitated α1C in WT- or S1901A/Y2122F-CaL. Values were obtained as described in methods and are based on the average of at least three experiments. *P < 0.05 vs. WT + anti-β1-integrin Ab, WT + anti-Tyr-Pi Ab, or WT + anti-c-Src Ab.
The proline-rich domains in the COOH terminus are required for the association of α1C-CaL with β1-integrin.
Proline-rich domains (PRDs) are binding motifs for Src homology 3 domain (SH3)-containing proteins, such as c-Src (34). Two PRDs have been identified in the COOH terminus of α1C-CaL and have been shown to mediate the association of CaL with some proteins containing SH3 domains (12, 13). Thus, we investigated the roles of the COOH-terminal PRDs in determining α1C-CaL association with β1-integrin or c-Src by expressing a ΔP1/ΔP2-CaL mutant with selective deletion of both PRDs in the COOH terminus of α1C. As shown in Fig. 6A, significantly less association between α1C-CaL and β1-integrin (lane 6) and between α1C-CaL and c-Src (lane 8), was observed in cells expressing the ΔP1/ΔP2 α1C-CaL mutant, compared with WT α1C-CaL (Fig. 6C, 54 ± 9%, 54 ± 6%; % of WT, respectively). However, the expression of β1-integrin (Fig. 6B), c-Src, and the degree of association between β1-integrin and c-Src was unchanged in cells expressing ΔP1/ΔP2 α1C-CaL (Supplemental Fig. S4B, quantified data not shown). These results suggest that the PRDs in the α1C-CaL COOH terminus are partially required for associations between α1C-CaL and β1-integrin and between α1C-CaL and c-Src.
Fig. 6.
Requirement of proline-rich domains (PRDs) in the α1C-CaL COOH terminus for α1C-CaL association with β1-integrin or c-Src. A: representative blot comparing WT α1C-CaL and ΔP1/ΔP2 α1C-CaL immunoprecipitated with anti-c-Src, anti-α5-integrin, or anti-β1-integrin Ab and probed for α1C. Relatively lower amounts of α1C-CaL appear to be pulled down by anti-c-Src or anti-β1-integrin Ab (lanes 8 and 6, respectively) in cells expressing ΔP1/ΔP2 α1C-CaL, compared with WT α1C-CaL. B: the same blot in A after stripping and reprobing for β1-integrin. C: summary graph showing the relative levels of immunoprecipitated α1C in WT- or ΔP1/ΔP2-CaL. Values were obtained as described in methods and are based on the average of at least three experiments. *P < 0.05 vs. WT + anti-c-Src Ab or WT + anti-β1-integrin Ab.
Functional modulation of CaL current by α5β1-integrin is independent of the PRDs in the COOH terminus.
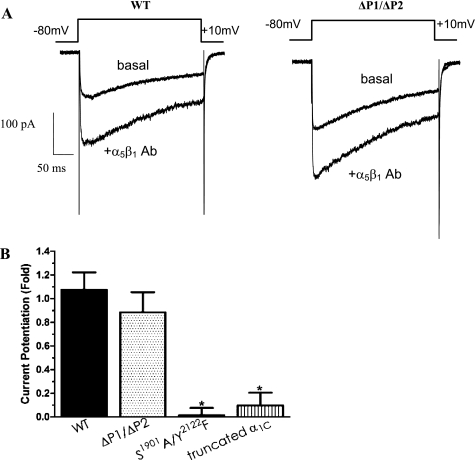
Given the results shown in Figs. 5 and 6, we also investigated the roles of the two PRDs in the modulation of CaL current following α5β1-integrin engagement. As shown in Fig. 7A, the application of α5β1-integrin antibody to the bath solution potentiated whole cell CaL current in HEK293-T cells expressing WT-CaL, consistent with our previous findings (14). To our surprise, no significant electrophysiological difference was observed between WT-CaL and ΔP1/ΔP2-CaL (82% of WT) in the degree of current potentiation by α5β1-integrin engagement, suggesting that the COOH-terminal PRDs are not required for potentiation of CaL current by α5β1-integrin engagement. When the degree of CaL current potentiation following α5β1-integrin ligation in the mutant constructs was normalized to that of WT, both the truncated α1C-CaL and S1901A/Y2122F-CaL mutants displayed significant impairment in the amount of current potentiation by integrin ligation (Fig. 7B, 9% and 1% potentiation of WT-CaL, respectively; see also Ref. 14). The above results confirm that S1901 and Y2122 residues in the COOH terminus of α1C-CaL are required for Ca2+ current potentiation following α5β1-integrin ligation. However, selective deletion of the two PRDs in the COOH terminus of α1C-CaL does not impair the current potentiation by α5β1-integrin engagement.
Fig. 7.
Electrophysiological protocols to determine the effects of COOH terminus-specific regions on modulation of CaL current by α5β1-integrin. A: representative recordings of whole cell CaL current from HEK293-T cells expressing WT-CaL (left) or ΔP1/ΔP2-CaL (right). Top traces in both panels show the amount of basal current in cells expressing WT- or ΔP1/ΔP2-CaL, and bottom traces show the current potentiation by α5β1-integrin Ab (10 μg/ml). No substantial difference in the relative amount of CaL current potentiation was observed in cells expressing ΔP1/ΔP2-CaL, compared with WT-CaL. B: summary graph showing peak current potentiation by α5β1-integrin in WT-CaL, truncated α1C-CaL, or ΔP1/ΔP2-CaL. CaL current potentiated by α5β1-integrin Ab was significantly attenuated in truncated α1C-CaL or in S1901A/Y2122F-CaL, but not in ΔP1/ΔP2-CaL. Number of cells recorded is as follows: WT-CaL, n = 18; n = 8 in cells expressing ΔP1/ΔP2-CaL, S1901A/Y2122F-CaL, or truncated α1C-CaL. *P < 0.05 vs. WT-CaL.
Immunofluorescence imaging analysis demonstrates association of CaL with β1-integrin on the plasma membrane.
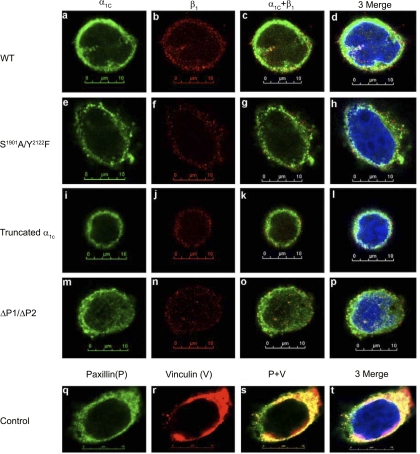
The COOH terminus of α1C-CaL has been shown to mediate membrane targeting of the channel (11), so we further investigated the effect of the abovementioned regions in the COOH terminus on membrane targeting of α1C-CaL and its association with β1-integrin. Immunofluorescence confocal microscopy (IF) was performed to determine the expression, distribution, and relative localization of α1C-CaL and β1-integrin on the plasma membrane. Images of cells without staining or cells stained with either anti-mouse IgG, anti-rabbit IgG, or the combination of both, exhibited only faint, diffuse, and nonspecific staining (Supplemental Fig. S5). As shown in Fig. 8, neither the membrane expression of α1C-CaL nor β1-integrin was detectably altered in S1901A/Y2122F-CaL, truncated α1C-CaL, or ΔP1/ΔP2-CaL, compared with WT-CaL.
Fig. 8.
Confocal immunofluorescence image analysis to determine the distribution and association of α1C-CaL and β1-integrin on the plasma membrane. Representative confocal immunofluorescence images from the cells expressing WT-, truncated α1C-, S1901A/Y2122F-, or ΔP1/ΔP2-CaL. α1C and endogenous β1-integrin expression on the plasma membrane appear as green and red punctate staining in a, e, i, and m and in b, f, j, and n, respectively. The colocalization of α1C-CaL with β1-integrin on the membrane appears as yellow to orange punctate staining (c, g, k, and o). A noticeably higher degree of α1C-CaL colocalization with β1-integrin was seen in cells expressing WT-α1C-CaL, compared with mutant α1C-CaL construct-expressing cells. Merged images of the previous three panels with nuclei counterstaining appear in blue (d, h, l, and p). Endogenous paxillin and vinculin staining in HEK293-T cells appears as green and red in q and r, respectively. The degree of colocalization between paxillin and vinculin (yellow to orange staining; s) appears to be much higher than that between α1C-CaL and β1-integrin in the WT- or mutant α1C-CaL-expressing cells. Merged image with nuclear counterstaining in blue appears in t. Magnification, ×63 oil; scale bar, 10 μm.
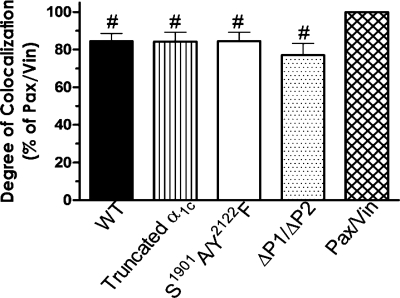
When we examined the association between WT α1C-CaL and endogenous β1-integrin on the plasma membrane, a moderate degree of colocalization was observed. To quantify the degree of colocalization between α1C-CaL and β1-integrin, we used the overlap of paxillin and vinculin staining as a normalization standard for the following analyses, since these two proteins highly associate at the focal adhesion site (ICA value 0.25) (37). The relatively high degree of colocalization between paxillin and vinculin was set as 100% for subsequent analysis of colocalization between α1C-CaL and β1-integrin. We used Pearson's coefficient (R), Mander's coefficient (Rr), and ICA to determine the degree of α1C-CaL-β1-integrin colocalization, as listed in Table 1. The degrees of α1C-CaL colocalization with β1-integrin in WT-CaL, truncated α1C-CaL, S1901A/Y2122F-CaL, or ΔP1/ΔP2 α1C-CaL were significantly less than the degree of colocalization between paxillin and vinculin (60–88% of paxillin-vinculin colocalization).
Table 1.
Semiquantitative analysis of CaL association with β1-integrin
| Rr, % of Pax + Vin | R, % of Pax + Vin | ICA, % | ICA, % of Pax + Vin | |
|---|---|---|---|---|
| Paxillin-vinculin | 100 | 100 | 0.251 ± 0.021 | 100 |
| WT | 60.6 ± 2.7 | 88.5 ± 1.5 | 0.22 ± 0.009 | 85.1 ± 4.2 |
| Truncated-α1C | 59.2 ± 4.8 | 87.9 ± 2.6 | 0.211 ± 0.01 | 84.1 ± 5.0 |
| S1901A/Y2122F | 56.5 ± 4.5 | 84.5 ± 2.5 | 0.21 ± 0.011 | 84.8 ± 5.3 |
| ΔP1/ΔP2 | 55.4 ± 5.1 | 84.1 ± 1.7 | 0.185 ± 0.013 | 75.2 ± 7.4 |
Values are means ± SE, taken from at least eight cells in either wild-type (WT) or mutant L-type voltage-gated Cav1.2 calcium channel (CaL) per experiment. The degree of CaL association with β1-integrin was normalized using the association of paxillin (Pax) and vinculin (Vin) as a reference and set as 100% for Pearson's, Mander's, or intensity correlation analysis (ICA). The value of CaL association with β1-integrin in WT, truncated α1C-, S1901A/Y2122F, or ΔP1/ΔP2-CaL is presented as the percentage ± SE of paxillin-vinculin association. Rr, Pearson's coefficient; R, Mander's coefficient. Statistical analysis was performed on the basis of the average from at least four individual experiments.
Using ICA analysis (Fig. 9), modest, but not significant, decreases in the degrees of α1C-CaL association with β1-integrin were observed in the truncated α1C-CaL, S1901A/Y2122F α1C-CaL, and ΔP1/ΔP2 α1C-CaL mutants, compared with WT-CaL (99 ± 5.9%, 99 ± 6.2%, and 88 ± 5.1% of WT α1C-CaL, respectively). Deconvolution analysis was also used to eliminate the signals collected from out-of-focus emission. However, no substantial differences were noted in the same comparisons when deconvolution analysis was applied (data not shown). Collectively, confocal image analysis demonstrated that the association between α1C-CaL and β1-integrin on the cell membrane was moderate in cells expressing WT α1C-CaL. No significant differences were observed in the degree of α1C-CaL colocalization with β1-integrin between the three mutant α1C-CaL constructs, compared with WT α1C-CaL. Taken together, the IF results suggest that α1C-CaL and β1-integrin colocalize to a moderate degree on the plasma membrane and that the modifications we made in the COOH terminus of α1C-CaL did not significantly alter the membrane targeting of α1C-CaL or β1-integrin on the plasma membrane. Thus, the reduced associations between the mutant forms of α1C-CaL and β1-integrin observed in IP protocol are not due to deficient membrane targeting.
Fig. 9.
Bar graph showing the quantified degree of α1C-CaL association with β1-integrin normalized to the degree of paxillin (Pax)-vinculin (Vin) association using intensity correlation analysis (ICA). The association of paxillin with vinculin was set as 100%. The ICA value of α1C-CaL-β1-integrin association in WT α1C-CaL, truncated α1C-CaL, S1901A/Y2122F-α1C-CaL, or ΔP1/ΔP2 α1C-CaL is presented as the percentage of paxillin-vinculin association. Values were taken from at least 8 cells in either WT- or mutant CaL per experiment, and the values in the graph were calculated on the basis of the average of at least four experiments. Statistical analysis was performed on the basis of the average from at least four individual experiments. #P < 0.01 vs. the degree of colocalization between paxillin and vinculin.
DISCUSSION
We present evidence that the pore-forming α1C-subunit of the L-type calcium channel (α1C-CaL) associates with β1-integrin and c-Src in arteriolar SM as well as when the channel is heterologously expressed in HEK293-T cells. These associations depend on interactions of the cells with the ECM protein FN, which is known from previous studies to potentiate CaL current following α5β1-integrin engagement (40). Furthermore, our results show that the associations between α1C-CaL and α5β1-integrin and between α1C-CaL and c-Src require specific regions in the COOH terminus of α1C-CaL. Two specific domains that are critical for these interactions are the PKA and c-Src phosphorylation sites that we previously identified as essential for modulation of CaL function following α5β1-integrin engagement (14). In addition, we found that CaL preferentially associates with the phosphorylated, active, form of c-Src in arteriolar SM and that this association depends on FN. The latter result provides evidence for a unique local functional regulation of CaL by α5β1-integrin through phosphorylation of c-Src. Collectively, our data support the concept of a macromolecular complex composed of multiple focal adhesion molecules including PKA, c-Src, and some fraction of the CaL population, which is assembled upon integrin ligation and required for potentiation of CaL current following α5β1-integrin engagement.
In this study, three different approaches, IP, IF, and patch-clamp recording of CaL current, were used to assess structural and functional interactions between α1C-CaL and α5β1-integrin. In some respects, the data obtained using these methods support each other, while in other respects the data conflict. How can we reconcile the conflicting results? What do the collective data tell us about the direct or indirect association of the channel with α5β1-integrin? What signaling components are required to mediate α1C-CaL association with α5β1-integrin upon adhesion to FN? How does the spatial interaction between α1C-CaL and α5β1-integrin contribute to the functional regulation of CaL? What is the physiological significance of α1C-CaL association with α5β1-integrin? These issues will be addressed in the following sections.
Direct or indirect association of CaL with α5β1-integrin.
After demonstrating an association between α1C-CaL and α5β1-integrin, using IP and IB methods, we sought to determine the nature of the interaction and the regions in α1C-CaL that were required. Because the crystal structure of α1C-CaL has not been resolved, interactions between these proteins are difficult to predict. If α1C-CaL directly interacts with α5β1-integrin, a potential binding region would be the canonical Arg-Gly-Asp (RGD)-integrin-binding motif. In fact, a previous study by McPhee et al. (31) identified the functional significance of an RGD sequence located in the extracellular loop, between the first membrane-spanning region and the pore, of the G protein-activated inward rectifier K+ channel (GIRK). This RGD sequence mediates the direct interaction between GIRK and the β1-integrin. The functional significance of the interaction appears to involve targeting of GIRK to the plasma membrane and/or regulation of its stability rather than acute regulation of channel function (31). However, in Cav1.2, the only conserved RGD sequence (aa 448–450) is found in a conserved transmembrane region of all three CaL isoforms, so that direct binding of α1C-CaL to RGD-binding integrins is unlikely due to potential steric hindrance. Thus, an indirect association between α1C-CaL and α5β1-integrin through cytoplasmic or cytoskeletal proteins seems more probable.
To determine regions in α1C-CaL that provide binding motifs for other focal adhesion proteins that are known to associate with α5β1-integrin, we used a site-directed mutagenesis strategy. On the basis of differences in the amount of coimmunoprecipitated α1C-CaL with β1-integrin in WT versus mutated α1C-CaL, we identified several regions in the α1C-CaL COOH terminus that are involved in the interaction of α1C-CaL with α5β1-integrin: two phosphorylation sites for PKA and c-Src, along with the PRDs. These findings therefore implicate PKA, c-Src, and potentially other SH3 domain-containing proteins in mediating the interaction of α1C-CaL with α5β1-integrin.
The roles of PKA in the functional regulation of CaL and its association with α5β1-integrin.
PKA is known to regulate all three CaL isoforms. In neurons, PKA colocalizes with adenylate cyclase, β2-adrenergic receptor (β2-AR), A-kinase anchoring protein (AKAP), and protein phosphatase (PP2A) in a macromolecular signaling complex; PKA mediates the increase in neuronal CaL current following stimulation of β2-AR (6). Our results suggest that PKA is also a component of another macromolecular complex containing various focal adhesion proteins recruited following ligation of α5β1-integrin and that signaling between proteins in this complex also can exert a significant degree of control over CaL function. Evidence to support this conclusion derives from two aspects of our results. First, reduced association between α1C-CaL and β1-integrin was observed in an α1C-CaL mutant with altered PKA and c-Src phosphorylation sites. Second, our previous electrophysiological studies demonstrated a substantial reduction in the amount of CaL current that was potentiated following ligation of α5β1-integrin when α1C-CaL mutants with altered PKA and/or c-Src phosphorylation were used (14). When S1901 in α1C is mutated, it not only leads to reduction in the association between α1C-CaL and β1-integrin, but also to reduction in the phosphorylation of CaL by PKA (14). Findings from other laboratories also support the notion that S1901 in the COOH terminus of α1C-CaL is a focal assembly point for PKA, AKAP, PP2A, and WAVE/WASP, which collectively modulate CaL function (5). In support of this idea, the macromolecular complex composed of PKA, AKAP, PP2A, and WAVE/WASP has been shown to provide a spatial link to the integrin-cytoskeleton network in modulation of other cellular functions (15) and engagement of β1-integrin is known to facilitate PKA targeting to specific cellular locations (26, 32). On the basis of all the above, faulty trafficking/targeting of PKA in the cytoplasm could contribute to reduced interaction of PKA with other focal adhesion components in the macromolecular complex, thereby disrupting the spatial link to the integrin-cytoskeleton network and reducing the extent to which CaL function is influenced by α5β1-integrin signaling.
The roles of PRDs and c-Src in functional regulation of CaL and its association with α5β1-integrin.
c-Src is another kinase that is implicated in the signaling pathway between α5β1-integrin and CaL. The involvement of c-Src is supported by several findings in the present study: 1) co-IP of α1C-CaL with c-Src in arteriolar SM appears to be independent of FN; 2) co-IP of α1C-CaL and phosphorylated c-Src is only observed in arteriolar SM incubated with FN; 3) co-IP of α1C-CaL and c-Src is only observed upon adhesion of HEK293-T cells to FN; and 4) mutation of the phosphorylation site for c-Src in the COOH terminus in α1C-CaL (Y2122) leads to reduced co-IP of α1C-CaL with β1-integrin and of α1C-CaL with c-Src. These results are consistent with previous findings that purified c-Src associates with the COOH terminus of α1C-CaL (19, 20) and that a basal level of CaL current depends on c-Src activity in colonic smooth muscle (16). Our co-IP findings are also consistent with a previous functional study from our laboratory showing that the potentiation of CaL current following α5β1-integrin ligation is significantly reduced in Y2122F α1C-CaL constructs (14). Thus, preventing this tyrosine residue from being phosphorylated not only reduces the overall phosphorylation of CaL by exogenous c-Src, but also reduces the spatial interaction between α1C-CaL and β1-integrin and between α1C-CaL and endogenous c-Src, indicating that the interactions between α1C-CaL and β1-integrin and between α1C-CaL and c-Src are required for current potentiation following α5β1-integrin ligation.
Our IP studies also identified a region in the α1C-CaL COOH terminus containing two PRDs (P1/P2) as another potential binding domain for c-Src. Reduced associations between α1C-CaL and β1-integrin and between α1C-CaL and c-Src were observed in the ΔP1/ΔP2 α1C-CaL mutant. Our results agree with a previous study demonstrating that fusion proteins containing α1C PRDs mediate interactions with c-Src in SY5Y cells expressing the neuronal CaL isoform upon IGF stimulation (1). Taken together, the results suggest multiple roles for PRDs in association with the macromolecular complex containing β1-integrin, c-Src, and α1C-CaL.
FAK is another proline-rich tyrosine kinase known to provide a binding site for SH2 and SH3 domain-containing proteins such as paxillin, vinculin, talin, p130Cas, and Crk (42). Although we did not specifically examine whether α1C-CaL associates with FAK, our previous studies in vascular SMC revealed that a significant amount of the CaL current potentiation following α5β1-integrin engagement was attenuated by dialysis of the cells with an antibody against FAK (40). A precedent for an association between FAK and another ion channel has been established by studies of Cherubini et al. (4), who demonstrated the association between FAK and the human ether-a-go-go-related gene (hERG) channel. In addition, PYK2, a family member of the FAK tyrosine kinases, was demonstrated to associate with cardiac α1C-CaL via binding to PRDs (9). On the basis of the above, the PRDs in the α1C-CaL appear to play significant roles in the assembly of a macromolecular complex containing CaL, α5β1-integrin, c-Src, and potentially other protein tyrosine kinases.
It should be noted that the roles of PRDs in the physical and functional association of α1C-CaL with α5β1-integrin may be more complicated than at first suggested by the preceding discussion. When the ΔP1/ΔP2 α1C-CaL mutant is expressed, the IP results (Fig. 6, A and C) reveal a reduced association between α1C-CaL and β1-integrin and between α1C-CaL and c-Src, compared with WT α1C-CaL, whereas the electrophysiological data (Fig. 7, A and B) reveal no substantial impairment in the degree of CaL current potentiation following α5β1-integrin ligation. The discrepancy may arise from two issues. First, the PRD regions that we deleted from α1C-CaL lie within the distal COOH terminus inhibitory region (DCT). Two studies have shown that deletion of the homologous PRDs (aa 1966–2004) in the cardiac CaL isoform leads to enhancement of basal CaL current (13, 38). Deletion of the PRDs removes part of the DCT, which may partially relieve constitutive channel inhibition by the DCT segment, though this has not been specifically tested. However, the major differences between the neuronal, cardiac, and smooth muscle isoforms of the CaL channel reside in the more distal regions of the respective COOH termini, and whether or not the DCT segment exerts strong inhibitory control of the neuronal CaL has not been determined.
A second explanation for the discrepancy between the structural and functional PRD results is the possible contribution of another PRD that has been identified in the II–III linker region of α1C-CaL (aa 857–861). A study by Dubuis et al. (9) suggests that the II-III linker region appears to play a role in extending the activation range of cardiac CaL, which consequently alters CaL function. Our IP results revealed that deletion of the COOH-terminal PRDs only reduced c-Src coassociation with α1C-CaL by 42%, even when the amount of truncated COOH-terminal α1C-CaL mutants for IP was increased by twofold (Supplemental Fig. S2, A and C). Thus, deletion of only the PRDs in the COOH terminus of α1C-CaL may not be sufficient to fully abolish the potentiation in CaL current following α5β1-integrin engagement. Overall, our electrophysiological and IP results agree with the findings of Dubuis et al. and suggest the possibility of another important c-Src binding region in α1C-CaL.
Reduced association between α1C-CaL and β1-integrin is not due to faulty membrane targeting of CaL.
The amino acid residues 1623–1733 in the COOH terminus of cardiac α1C-CaL have been shown to mediate the membrane targeting of the channel (11). Using IF, we detected no significant difference in membrane targeting of α1C-CaL or β1-integrin in S1901A/Y2122F-CaL, truncated α1C-CaL at amino acid residue 1862, or ΔP1/ΔP2-CaL, compared with WT-CaL. Our results suggest that the reduced association between α1C-CaL and β1-integrin observed in the above mentioned α1C-CaL mutants is not due to unsuccessful membrane targeting of α1C-CaL. Our findings agree with those of Gao et al. (11) that the regions in the COOH terminus required for successful membrane targeting of cardiac CaL are amino acid residues 1623–1733. Because those specific regions are not altered in our mutant α1C-CaL constructs, the reduced association between α1C-CaL and β1-integrin in our mutant constructs are unlikely attributable to incorrect membrane targeting of CaL. In addition, our IF results also suggest that the association between α1C-CaL and β1-integrin on the plasma membrane is independent of the PKA/c-Src phosphorylation sites, the PRD domains, or the distal COOH terminus after amino acid residue 1862 in α1C-CaL. These results suggest that α5β1-integrin modulates CaL function independent of the interactions of the two proteins on the plasma membrane. The IF results agree with the IP and patch-clamp findings in that the formation of a macromolecular complex including PKA, c-Src, α5β1-integrin, and CaL in the cytoplasm following α5β1-integrin engagement appears to play an important role in CaL current potentiation by α5β1-integrin.
Physiological significance of CaL association with integrin.
Our findings of an association between CaL and β1-integrin and between CaL and c-Src provide evidence for a macromolecular signaling complex composed of α5β1-integrin, CaL, c-Src, PKA, and possibly other focal adhesion components. Formation of this complex appears to be important for regulation of CaL function following integrin-ECM interaction. Our IP results are consistent with previous findings that c-Src is involved in the increase of CaL current potentiation in response to PDGF (17) or IGF (1) and with the finding that a c-Src-FAK complex forms in colonic smooth muscle in response to PDGF stimulation (16). Interestingly, cross talk between integrins and growth factors has been demonstrated in several studies, with the interaction of FAK and c-Src as a point of convergence (10, 28, 35). Although it is not known whether these two signaling pathways converge to regulate an ion channel, an intriguing possibility is that PDGF or IGF act in conjunction with α5β1-integrin to produce additive or synergistic potentiation of CaL function by integrin.
In summary, this is the first study to provide evidence for the association of α1C-CaL with α5β1-integrin or α1C-CaL with c-Src in arteriolar smooth muscle. This association depends on engagement of α5β1-integrin by FN and specific regions in the α1C-CaL COOH terminus, including two phosphorylation sites for PKA and c-Src and proline-rich domains that provide a binding motif for SH3 domain-containing proteins, such as c-Src. Identification of these regions, along with our electrophysiological findings, suggests that a macromolecular complex is assembled upon engagement of α5β1-integrin and that the interactions of proteins within this complex contribute to modulation of CaL function. Our study provides new insights into a CaL macromolecular regulatory complex that is required for functional regulation of the channel following α5β1-integrin engagement.
GRANTS
This project was funded by National Institutes of Health Grants HL-071796, HL-072989, and P01 HL-095486 (to M. J. Davis). G. W. Zamponi holds funding from the Heart and Stroke Foundation and is a Canada Research Chair and Alberta Heritage Foundation for Medical Research Scientist.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of Judy Davidson, Cara Froyd, Steve Dorman, and Shan-Yu Ho in preparing cell cultures and performing transfections. The technical assistance of Zhaohui Li, Dr. Luke Sun, and Dr. Luis Martinez-Lemus with the initial use of the confocal microscope is greatly appreciated. Rat neuronal WT α1C, β1b, and α2δ DNA, subcloned into pcDNA3.1 vectors, were gifts from Dr. T. Snutch.
REFERENCES
- 1. Bence-Hanulec KK, Marshall J, Blair LA. Potentiation of neuronal L calcium channels by IGF-1 requires phosphorylation of the alpha1 subunit on a specific tyrosine residue. Neuron 27: 121–131, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Callaghan B, Koh SD, Keef KD. Muscarinic M2 receptor stimulation of Cav1.2b requires phosphatidylinositol 3-kinase, protein kinase C, and c-Src. Circ Res 94: 626–633, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Callaghan B, Zhong J, Keef KD. Signaling pathway underlying stimulation of L-type Ca2+ channels in rabbit portal vein myocytes by recombinant Gβγ-subunits. Am J Physiol Heart Circ Physiol 291: H2541–H2546, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E, Di Stefano P, Degani S, Balzi M, Olivotto M, Wanke E, Becchetti A, Defilippi P, Wymore R, Arcangeli A. Human ether-a-go-go-related gene 1 channels are physically linked to beta1 integrins and modulate adhesion-dependent signaling. Mol Biol Cell 16: 2972–2983, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev 89: 411–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293: 98–101, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol 156: 1489–1498, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Dubuis E, Rockliffe N, Hussain M, Boyett M, Wray D, Gawler D. Evidence for multiple Src binding sites on the alpha1c L-type Ca2+ channel and their roles in activity regulation. Cardiovasc Res 69: 391–401, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ Res 89: 1104–1110, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Gao T, Bunemann M, Gerhardstein BL, Ma H, Hosey MM. Role of the C terminus of the alpha 1C (CaV1.2) subunit in membrane targeting of cardiac L-type calcium channels. J Biol Chem 275: 25436–25444, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM. C-terminal fragments of the alpha 1C (CaV1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated alpha 1C subunits. J Biol Chem 276: 21089–21097, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the alpha(1C) subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem 275: 8556–8563, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem 281: 14015–14025, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta 1692: 159–174, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hu XQ, Singh N, Mukhopadhyay D, Akbarali HI. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J Biol Chem 273: 5337–5342, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Hughes AD. Increase in tone and intracellular Ca2+ in rabbit isolated ear artery by platelet-derived growth factor. Br J Pharmacol 114: 138–142, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huveneers S, Danen EH. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci 122: 1059–1069, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Jin X, Morsy N, Shoeb F, Zavzavadjian J, Akbarali HI. Coupling of M2 muscarinic receptor to L-type Ca channel via c-src kinase in rabbit colonic circular smooth muscle. Gastroenterology 123: 827–834, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kang M, Ross GR, Akbarali HI. COOH-terminal association of human smooth muscle calcium channel Cav1.2b with Src kinase protein binding domains: effect of nitrotyrosylation. Am J Physiol Cell Physiol 293: C1983–C1990, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol 261: H1706–H1715, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Langton PD. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J Physiol 471: 1–11, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension 32: 338–345, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci 24: 4070–4081, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Lim CJ, Kain KH, Tkachenko E, Goldfinger LE, Gutierrez E, Allen MD, Groisman A, Zhang J, Ginsberg MH. Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol Biol Cell 19: 4930–4941, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ling S, Sheng JZ, Braun JE, Braun AP. Syntaxin 1A co-associates with native rat brain and cloned large conductance, calcium-activated potassium channels in situ. J Physiol 553: 65–81, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maile LA, Badley-Clarke J, Clemmons DR. The association between integrin-associated protein and SHPS-1 regulates insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Mol Biol Cell 14: 3519–3528, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol 289: H322–H329, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 119: 508–518, 2006 [DOI] [PubMed] [Google Scholar]
- 31. McPhee JC, Dang YL, Davidson N, Lester HA. Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. J Biol Chem 273: 34696–34702, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol 2: 666–668, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Mies F, Spriet C, Heliot L, Sariban-Sohraby S. Epithelial Na+ channel stimulation by n-3 fatty acids requires proximity to a membrane-bound A-kinase-anchoring protein complexed with protein kinase A and phosphodiesterase. J Biol Chem 282: 18339–18347, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen JT, Lim WA. How Src exercises self-restraint. Nat Struct Biol 4: 256–260, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell 6: 1349–1365, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci 122: 179–186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turner CE, Glenney JR, Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol 111: 1059–1068, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac alpha 1 subunit. J Biol Chem 269: 1635–1640, 1994 [PubMed] [Google Scholar]
- 39. Wijetunge S, Hughes AD. Src family tyrosine kinases mediate contraction of rat isolated tail arteries in response to a hyposmotic stimulus. J Hypertens 25: 1871–1878, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem 276: 30285–30292, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J Cell Biol 143: 241–252, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol 9: 858–867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.