Abstract
Although focal adhesion kinase (FAK) is typically considered upstream of Akt, extracellular pressure stimulates cancer cell adhesion via Akt-dependent FAK activation. How Akt regulates FAK is unknown. We studied Akt-FAK interaction in colon cancer cells under 15 mmHg increased extracellular pressure. Pressure enhanced Akt-FAK association, blocked by inhibiting FAK or silencing Akt1 but not Akt2, and stimulated FAK serine phosphorylation in Caco-2 and human colon cancer cells from surgical specimens Akt1-dependently. FAK includes three serine (S517/601/695) and one threonine (T600)-containing consensus sequences for Akt phosphorylation. Studying S–>A nonphosphorylatable point mutants suggests that these sites coordinately upregulate FAK Y397 tyrosine phosphorylation, which conventionally initiates FAK activation, and mediate pressure-induced cancer cell adhesion. FAK(T600A) mutation did not prevent pressure-induced FAK(Y397) phosphorylation or adhesion. Akt1 appeared to directly bind FAK, and this binding did not depend on the FAK autophosphorylation site (Y397). In addition, our results demonstrated that Akt phosphorylated FAK at three novel serine phosphorylation sites, which were also not required for FAK-Akt binding. This novel interaction suggests that FAK and Akt may be dual kinase targets to prevent cancer cell adhesion and metastasis.
Keywords: protein kinase B, serine/threonine and tyrosine phosphorylation, cell adhesion, extracellular pressure, cell signaling
cancer cell adhesion is an important step in metastasis (1). We (2, 39, 42, 45) and others (19, 24) have demonstrated that cancer cell adhesion to matrix proteins, endothelial cell monolayers, and murine surgical wounds is increased when tumor cells are exposed to physical forces including increased extracellular pressure. Indeed, brief exposure to increased extracellular pressure significantly promoted cancer cell implantation and impaired tumor-free survival in vivo in a transplantable murine tumor model (10). Malignant colonocytes are subjected to elevated interstitial fluid pressures of similar magnitude in the tumor microenvironment as well as to increased pressure and shear forces during lymphatic or intravascular transit and during surgical manipulation or laparoscopic peritoneal insufflation (6, 23). Therefore, forces such as extracellular pressure may promote cancer metastasis in shed tumor cells by enhancing the adhesiveness of metastasizing tumor cells to distant sites. Delineating the underlying mechanisms by which pressure stimulates cancer cell adhesion will identify targets to inhibit cancer metastasis.
Akt is a serine/threonine (Ser/Thr) kinase with three isoforms (Akt1, Akt2, and Akt3) (7). Akt phosphorylation at serine 473 is stimulated by modest 15 mmHg extracellular pressure increase in colon cancer cells that only express Akt1 and Akt2 (51, 52). Focal adhesion kinase (FAK), a nonreceptor tyrosine kinase, contains an NH2-terminal FERM (band 4.1, ezrin, radixin, moesin homology) domain, followed by an approximately 40 residue linker region, a central kinase domain, two proline-rich regions, and a COOH-terminal focal adhesion targeting domain (Fig. 1A). Like Akt, FAK can also be phosphorylated and activated in response to pressure (39, 41). The well-known upstream regulator of Akt, phosphatidylinositol 3-kinase (PI-3K), is required for pressure-induced human colon cancer cell adhesion (41). Both Akt and FAK are overexpressed with high frequency in many kinds of cancers including colon cancer (5, 34, 46, 55), and increasing evidence suggests that Akt and FAK are promising therapeutic targets for cancer treatment. It therefore becomes important to understand whether and how Akt may regulate FAK.
Fig. 1.
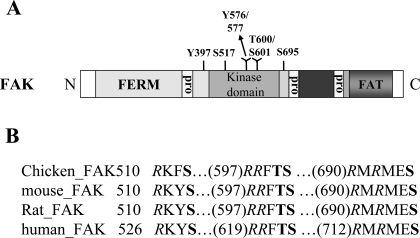
Focal adhesion kinase (FAK) domain structure and phosphorylation sites. A: FAK contains a central kinase domain flanked by an NH2-terminal FERM domain and a COOH-terminal region containing two proline-rich (pro) motifs and a focal adhesion targeting (FAT) domain. The well-established tyrosine phosphorylation sites of FAK at Y397, Y576/577 as well as the four potentially Ser/Thr phosphorylation sites (S517/601/695 and T600) by Akt are highlighted in the diagram. B: these potential Ser/Thr phosphorylation sites (as indicated in boldface) are completely conserved in chicken, mouse, rat, and human FAK. Arginines (R) at positions −5 and/or −3 of the conserved phospho-Akt substrate consensus motif RXRXXS/T are highlighted in italics.
FAK autophosphorylation at tyrosine 397 (Y397) promotes further FAK and downstream signaling that can also regulate cancer cell adhesion. FAK(Y397) phosphorylation can be induced by extracellular stimuli including cell adhesion and mechanical forces. Such FAK(Y397) phosphorylation creates a binding site for the tyrosine kinase Src, which subsequently phosphorylates FAK at other tyrosines, including Tyr576/577, which are important for the maximal activation of FAK and phosphorylation at Y397 (56). In addition to the tyrosine phosphorylation sites of FAK, some serine phosphorylation (S722, 732, 843, 910) sites located at COOH-terminal region of FAK have recently been identified (16, 20–22, 54). More Ser/Thr phosphorylation sites of FAK such as S29, 386, 390, 392, and T13, 388, 407 were identified by mass spectrometry (18). Some of these serine or threonine phosphorylation sites are already known to be functional. For example, S843 phosphorylation of FAK inhibits Y397 phosphorylation, cell spreading, and migration (21), indicating the importance of FAK Ser/Thr phosphorylation and potential cross talk between Ser/Thr and tyrosine phosphorylation sites of FAK. Although upstream regulators of FAK tyrosine phosphorylation sites are known, the upstream mediators that directly phosphorylate these Ser/Thr sites of FAK remain largely unknown with exception of S732, which is phosphorylated by Cdk5 kinase (54).
It is well established now that FAK is an upstream regulator of Akt. However, recent results from our laboratory (41) and from others (44, 48) suggest that FAK may also be regulated by Akt directly or indirectly in some contexts. In the present study, we used bioinformatic, biochemical, molecular biological, and pharmacological approaches in colon cancer cells exposed to extracellular pressure to demonstrate that Akt-1 binds directly to and phosphorylates FAK at S517/601/695. This serine phosphorylation, in turn, seems to facilitate the pressure-dependent tyrosine autophosphorylation of FAK at Y397, the conventional initiator of FAK activation. Although Akt is therefore required for FAK activation in response to pressure, further studies demonstrated that FAK also potentiates Akt activation. Inhibiting FAK pharmacologically or silencing FAK with small interfering RNAs (siRNAs) prevented the increases in phosphorylation of Akt(S473) and FAK(Y397) induced by increased pressure. Thus, FAK and Akt1 bind directly and potentiate each other's activation. This novel mechanism of FAK-Akt interaction suggests that FAK and Akt1 may be important dual therapeutic targets for preventing cancer cell adhesion and, eventually, cancer metastasis.
MATERIALS AND METHODS
Cell culture.
Human colon adenocarcinoma Caco-2 cells were cultured as described previously (2). Single-cell suspensions of primary human colon cancer cells were obtained from surgically resected fresh tumors by mincing and collagenase digestion as previously described (15). The use of human samples was approved by Michigan State University's Institutional Review Board.
Expression vectors and mutagenesis for FAK.
Enhanced green fluorescent protein plasmid (p-EGFP-C3) encoding chicken wild-type FAK (WT-FAK) (25) was used as template to substitute the potential Ser/Thr phosphorylation sites with alanine (A) using QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instruction. Three double mutants (S517/601A, S571/695A, and S601/695A) and one triple mutant (S517, 601, 695/A) and one quadruple mutant (S517, 601, 695A/T600A) of FAK were generated by sequentially performing single point mutations on the WT-FAK expression construct.
Cell transfection and pharmacological treatments.
A nonspecific control siRNA (NT1 siRNA) and other siRNAs (siGENOME SMARTpools) specifically targeting human Akt1, Akt2, and FAK, respectively, were purchased from Dharmacon (Lafayette, CO). We did transfection for siRNAs and plasmids as described previously (52).
In parallel studies, nontransfected Caco-2 cells were treated with either 20 μM Akt inhibitor IV (Calbiochem, San Diego, CA), the FAK inhibitor 1,2,4,5-benzenetetraamine tetrahydrochloride (Y15) (Sigma-Aldrich, St. Louis, MO), or appropriate vehicles (DMSO and PBS, respectively) for 30 min before use and then throughout the experiment.
Pressure regulation.
Pressure (15 mmHg) was applied as previously described (40).
Adhesion assays.
A calcein-AM-labeling adhesion assay was used for untransfected Caco-2 cells as described previously (52). For Caco-2 cells transfected with the GFP-tagged FAK constructs, the fluorescent adhesion assay could not be used because the plasmid transfection efficiency in Caco-2 cells was relatively low (typically ∼25%). For studies with these cells, we therefore directly counted GFP-fluorescent cells (i.e., successfully transfected cells) in six-well plates by fluorescence microscopy as previously described (51, 52). The two methods yielded similar results.
Pressure-induced signaling.
To avoid the effects of adhesion itself on signaling, for all cell signaling studies, Caco-2 cells in suspension were subjected to ambient pressure or 15 mmHg increased pressure for 30 min in bacteriological plastic plates pretreated with 1% heat-inactivated bovine serum albumin (BSA) in PBS to prevent all adhesion and consequent adhesion-induced signaling. In some studies, cells were pretreated with 20 μM Akt inhibitor IV or Y15 for 30 min.
Western blotting and immunoprecipitation.
Transfected or untransfected cells in suspension were exposed to 15 mmHg pressure for 30 min in plates pretreated with BSA as above to prevent adhesion and consequent adhesion-induced signals. Cells were then lysed on ice and subjected to Western blotting as previously described (39). Membranes were blotted with the following antibodies: phospho-FAK(Y397 or Y576, Invitrogen); total Akt, Akt1, Akt2, phospho-Akt (S473), FAK (polyclone) and phospho-threonine (Cell Signaling Technology, Beverly, MA); phospho-serine (Millipore, Lake Placid, NY), GFP (polyclone, Clontech, Mountain View, CA); and HA.11 (Covance, Berkeley, CA). GAPDH (glyceraldehyde-3-PDH) or actin was used as loading controls.
Immunoprecipitation and coimmunoprecipitation were performed as previously described (9, 40), with modification, except that we used SignalBoost Immunoreaction kit (Calbiochem) while detecting phospho-serine or phospho-threonine to enhance the signal-to-noise ratio. Proteins were immunoprecipitated with mouse monoclonal antibodies targeting FAK (clone 4.47, Millipore), GFP (Clontech), or hemagglutinin (HA; 12CA5, Roche Applied Science, Indianapolis, IN).
Glutathione S-transferase pulldown assay.
Glutathione-Sepharose 4B beads (15 μl) were washed twice in ice-cold PBS and resuspended in 400 μl PBS. Active, glutathione S-transferase (GST)-Akt1 fusion proteins (2 μg; Cell Signaling Technology) or GST proteins (gift of Mr. J. Chen) were then added and incubated for 1 h. Glutathione-Sepharose 4B beads coupled to GST or GST-Akt1 were then washed twice with PBS by centrifuge for 5 min at 500 g and incubated with nontransfected or transfected Caco-2 cell lysates (600–800 μg protein) overnight at 4°C. As for the transfected cells, Caco-2 cells were transfected either with plasmids encoding GFP-WT-FAK, GFP-FAK(3S/3A), and GFP-FAK4A or with HA-tagged WT-FAK and FAK(Y397F) mutants. Nontransfected or transfected Caco-2 cell lysates were prepared in cell lysis buffer lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 50 mM NaF, 10 mM sodium pyrophosphate, 2 mg/ml aprotinin, and 2 mg/ml leupeptin (pH 7.4)]. Following incubation, beads were washed twice with lysis buffer without SDS and protease inhibitors. Proteins were eluted with Laemmli SDS sample dilution buffer, separated by 10% SDS-PAGE, and immunoblotted with GST and FAK antibodies (Cell Signaling Technology) or reprobed with HA monoclonal antibody (Covance) after the membrane was stripped.
Statistical analysis.
Statistical analysis was performed using Student's t-tests or ANOVA as appropriate, seeking 95% confidence. Data are expressed as means ± SE.
RESULTS
FAK is a potential substrate of Akt by phosphorylating FAK at Ser/Thr sites.
Computational analysis of the FAK sequence with Scansite software (32) revealed three serine and a single threonine-containing consensus sequences for Akt phosphorylation within the FAK sequence (Fig. 1A). Three putative phosphorylation sites, S517, 601, and T600 (with numbering based on the amino acid sequence of chicken FAK), are located in the kinase domain of FAK (Fig. 1A) and another site, S695, is between the kinase domain and the proline-rich (Pro) motif. All four potential phosphorylation sites are completely conserved in FAK across different species (from chicken to human; Fig. 1B). Other sites in the Akt-phosphorylated consensus site RXRXX(S/T) of FAK are also almost 100% conserved except for F516 in chicken FAK (Fig. 1B), indicating that these putative Ser/Thr phosphorylation sites in FAK may be important for FAK function. In addition, it is noteworthy that, although RMRMES695 contains the precise consensus sequence predicted for an Akt substrate, i.e., RXRXX(S/T), the three other sites (S517, 601, and T600) are not located within a perfect consensus motif sequence. That is, the arginine (R) at position −5 in the classical consensus sequence is lacking in each case. In fact, several reports regarding identified Ser/Thr phosphorylation sites in other Akt substrates have revealed that the R residue position −5 of the consensus sequence is not absolutely required for phospho-Akt substrate consensus motif RXRXXS/T (3, 14, 47, 49). Therefore, we next sought to experimentally validate these four predicted Akt phosphorylation sites in FAK.
Pressure stimulated serine phosphorylation but inhibited threonine phosphorylation of FAK in Caco-2 colon cancer cells and primary human colon cancer cells.
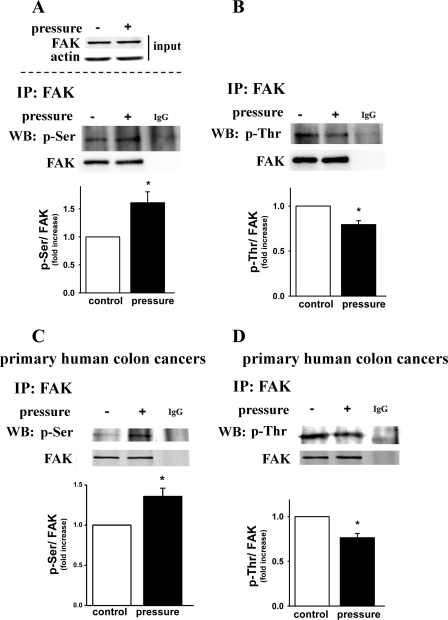
Exposure to 15 mmHg increased extracellular pressure for 30 min significantly promoted serine phosphorylation (p-Ser) of immunoprecipitated FAK (using normal mouse IgG in parallel as negative control) in Caco-2 colon cancer cells (Fig. 2A, n = 4, P < 0.05) as well as in primary cells freshly isolated from human colon cancers (Fig. 2C, n = 4, P < 0.05), whereas pressure reduced threonine phosphorylation of FAK in both Caco-2 cells (Fig. 2B, n = 4, P < 0.05) and human primary colon cancer cells (Fig. 2D, n = 4, P < 0.05). In Fig. 2, A–D, the absence of a significant band in the control sample immunoprecipitated only with untargeted IgG suggests the specificity of the bands we have identified. In addition, consistent with our previous report (41), 15 mmHg increased extracellular pressure for 30 min did not affect the expression of total FAK (Fig. 2A, top).
Fig. 2.
Pressure had opposite effects on the serine and threonine phosphorylation of FAK in Caco-2 colon cancer cells (A and B) and exerted similarly opposite effects in primary cells isolated directly from resected human colon cancers (C and D). A–D: protein samples from the cell lysates were immunoprecipitated (IP) with mouse anti-FAK monoclonal antibody or normal mouse IgG (for pressurized cell lysates) as control and analyzed by Western blot (WB) using phospho-Ser/Thr-specific and FAK antibodies. Endogenous FAK expression levels were also detected for whole Caco-2 cell lysates (input), with or without exposure to pressure (A). Actin was used as loading control. Typical blots are represented, and the graphs summarize densitometric analysis of the ratios of p-Ser (A and C) or p-Thr (B and D) to FAK in each experiment (n = 4, *P < 0.05). All observations were normalized against the respective ambient pressure controls. Pressure significantly promoted Ser phosphorylation (p-Ser) of FAK in Caco-2 cells (A) and human primary colon cancer cells (C) while reducing threonine phosphorylation of FAK in Caco-2 cells (B) and human primary colon cancer cells (D).
Extracellular pressure-induced increased FAK serine phosphorylation in Caco-2 cells was prevented by pharmacologic or genetic approaches disrupting Akt.
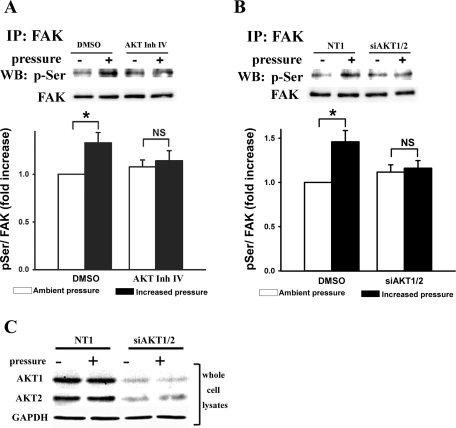
Because three of the four potential phosphorylation sites are serine residues, we next sought to determine whether Akt could regulate the pressure-induced increase in p-Ser of FAK. Either inhibiting Akt activity with Akt inhibitor IV or suppressing Akt expression with combined Akt1 and Akt2 siRNAs (siAKT1/2) prevented the pressure-induced increase in FAK serine phosphorylation (Fig. 3, A and B, n = 4, P < 0.05), suggesting that Akt mediates the serine phosphorylation of FAK that is induced by extracellular pressure. It should be noted that although Akt inhibitor and siAKT1/2 apparently tended to increase basal FAK serine phosphorylation under ambient pressure, neither of these effects achieved statistical significance (n = 4, P = 0.402 and 0.352, respectively). The suppression of Akt1 and Akt2 by siAKT1/2 was confirmed in Caco-2 whole cell lysates (Fig. 3C).
Fig. 3.
Extracellular pressure-induced increase in p-Ser of FAK in Caco-2 cells was prevented by Akt inhibitor IV (A; 20 μM AKT Inh IV) or silencing Akt expression with combined Akt1 and Akt2 small interfering RNAs (siRNAs) (B; 20 nM siAKT1/2). Cell lysates were immunoprecipitated with mouse anti-FAK monoclonal antibody and then analyzed by Western blot using phospho-Ser-specific and FAK antibodies. Typical blots are represented, and the graphs summarize densitometric analysis of the ratios of p-Ser to FAK in each experiment (n = 4, *P < 0.05; NS, not significant). NT1, nontargeting control siRNA. C: The siAKT1/2-transfected whole cell lysates were directly analyzed by Western blot using Akt1 or Akt2-specific antibodies to confirm the knockdown effect of siAKT1/2 in Caco-2 cells. GAPDH was used as a loading control.
Akt1, not Akt2, siRNA prevented pressure-induced increases in p-Ser of FAK, FAK(Y397) phosphorylation, and association between Akt and FAK.
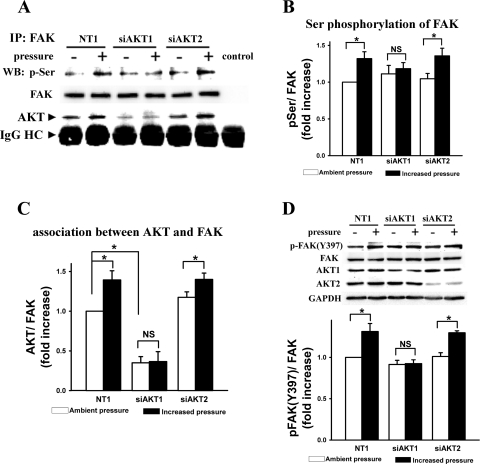
We have previously reported that Akt-mediated colon cancer adhesion induced by pressure requires Akt1 but not Akt2. We therefore sought to determine whether Akt-mediated FAK serine phosphorylation induced by extracellular pressure was also Akt-isoform-specific. Indeed, reducing Akt1, but not Akt2, blocked the pressure-stimulated increase in serine phosphorylation of immunoprecipitated FAK (mouse normal IgG used in parallel as negative control, Fig. 4, A and B, n = 4, P < 0.05). Figure 4A depicts the Akt that was coimmunoprecipitated with FAK, not total cellular Akt. Thus, the lack of change of intensity of the Akt band in Fig. 4A in response to the siRNA silencing of Akt2 suggests not that the siRNA failed to reduce Akt substantially but that reducing Akt2 did not substantially affect the amount of Akt that coprecipitates with FAK. Although both siAKT1 and siAKT2 tended to slightly increase basal FAK serine phosphorylation under ambient pressure (11 ± 12% and 5 ± 7% increase, respectively), neither effect achieved statistical significance (n = 4, P = 0.43 and 0.59, respectively). Furthermore, coimmunoprecipitation demonstrated that reducing Akt1 decreased basal FAK-Akt association 65 ± 8% (Fig. 4, A and C, n = 4, P < 0.05), but reducing Akt2 did not. Furthermore, reducing Akt1 but not Akt2 also prevented the pressure-induced increase in FAK(Y397) phosphorylation (Fig. 4, A and D, n = 4, P < 0.05). Although siAKT2 reduced Akt2 more effectively than siAKT1 reduced Akt1 (Fig. 4D, top, Akt1 and Akt2 bands) in Caco-2 cells, siAKT2 still had no effect on pressure-induced Akt-FAK association or FAK(Y397) phosphorylation (Fig. 4, A, C, and D).
Fig. 4.
Akt1, not Akt2, siRNA prevented pressure-induced increases in p-Ser of FAK (A and B), association between Akt and FAK (A and C) and FAK(Y397) phosphorylation (D). A–C: protein samples from the lysates of Caco-2 cells transfected with 20 nM siAKT1 or siAKT2 or nontargeting control siRNA (NT1) were immunoprecipitated with mouse anti-FAK monoclonal antibody or normal mouse IgG as control and analyzed by Western blot using phospho-Ser-specific and FAK antibodies. Typical blots are represented in A, and the graphs summarize densitometric analysis of the ratios of p-Ser (B) or Akt (C) to FAK in each experiment (n = 4, *P < 0.05). IgG heavy chain (IgG HC) bands underneath Akt bands are also shown. D: the above siRNA-transfected whole lysates were directly analyzed by Western blot using a phospho-specific anti-FAK(Y397) antibody, FAK, Akt1-, or Akt2-specific antibodies. GAPDH was used as a loading control. All observations were normalized against NT1-treated control cells under ambient pressure.
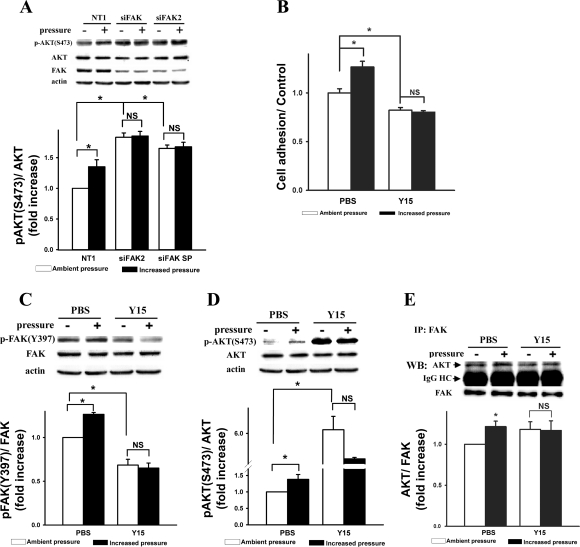
Silencing FAK with FAK-specific siRNA prevented the pressure-induced increases in phosphorylation of Akt at S473 and inhibiting FAK with the FAK-specific inhibitor Y15 prevented pressure-induced increases in Caco-2 cell adhesion, FAK(Y397) phosphorylation, Akt(S473) phosphorylation, and FAK-Akt association.
Our previous report in another colon cancer cell line, SW620, suggested that Akt and FAK may be mutually regulated in pressure-stimulated colon cancer cells because silencing FAK prevented pressure induction of Akt(S473) phosphorylation, an indicator of Akt activity, without altering basal Akt(S473) phosphorylation under ambient pressure (41). Therefore, we further investigated how FAK might in turn regulate pressure-induced Akt(S473) phosphorylation and Akt-FAK association in Caco-2 cells. The pressure-induced increase in Akt(S473) phosphorylation was prevented in Caco-2 cells by silencing FAK expression with two different FAK siRNAs, siFAK and siFAK2 (Fig. 5A, n = 4, P < 0.05). Unexpectedly, basal Akt(S473) phosphorylation under ambient pressure was increased by FAK reduction (83 ± 7% and 65 ± 6% for siFAK and siFAK2, respectively; Fig. 5A, top). The increased basal Akt(S473) phosphorylation by FAK-targeted siRNA was confirmed using a third FAK-specific siRNA targeting a third FAK mRNA sequence in Caco-2 cells (not shown) and by the following studies using the FAK-specific inhibitor Y15 (17).
Fig. 5.
Silencing FAK with siRNA prevented the pressure-induced increases in phosphorylation of Akt at S473 (A), and FAK-specific inhibitor Y15 prevented pressure-induced increases in Caco-2 cell adhesion (B), phosphorylation of FAK at Y397 (C), and Akt(S473) phosphorylation (D) and association between Akt and FAK (E). A: suppression of FAK expression by FAK siRNAs blocked pressure-stimulated Akt(S473) phosphorylation in Caco-2 cells and significantly increased basal Akt(S473) phosphorylation under ambient pressure. Protein samples from the lysates of Caco-2 cells transfected with 20 nM siFAK or siFAK2 or nontargeting control siRNA (NT1) were directly analyzed by Western blot using phospho-specific antibody to Akt(S473), as well as antibodies to Akt and FAK. Actin was used as a loading control. Typical blots are represented, and the graph summarizes densitometric analysis of the ratios of Akt(S473) phosphorylation to total Akt (n = 3, *P < 0.05). All observations were normalized against NT1-treated control cells under ambient pressure. B: inactivation of FAK with 20 μM of the FAK-specific inhibitor Y15 blocked pressure-stimulated Caco-2 cell adhesion and statistically significantly reduced the basal cell adhesion. All data were normalized against the PBS-treated control under ambient pressure (n = 4, *P < 0.05). C and D: inhibition of FAK with 20 μM of the FAK-specific inhibitor Y15 blocked the pressure-stimulated FAK(Y397) phosphorylation (C) and Akt(S473) phosphorylation (D) with significantly reduced basal FAK(Y397) phosphorylation and increased basal Akt(S473) phosphorylation. All data were normalized against PBS-treated control cells under ambient pressure (n = 4, *P < 0.05). E: inhibition of FAK with 20 μM of the FAK-specific inhibitor Y15 blocked the pressure-induced increases in association between Akt and FAK. Protein samples from the lysates of Caco-2 cells treated with Y15 or PBS were immunoprecipitated with mouse anti-FAK monoclonal antibody and analyzed by Western blot using Akt and FAK antibodies. Typical blots are represented, and the graph summarizes densitometric analysis of the ratios of Akt to FAK in each experiment (n = 4, *P < 0.05). IgG heavy chain bands underneath Akt bands are also shown.
Pretreatment with 20 μM Y15 for 30 min reduced basal Caco-2 cell adhesion under ambient pressure. More importantly, the pressure-induced increase in Caco-2 adhesion was prevented by treatment with Y15 (Fig. 5B, n = 4, P < 0.05). Inhibiting FAK with Y15 prevented pressure-stimulated FAK(Y397) phosphorylation and Akt(S473) phosphorylation (Fig. 5, C and D, respectively, n = 4, P < 0.05) with reduced basal FAK(Y397) phosphorylation and 614 ± 53% increased Akt(S473) phosphorylation, respectively, under ambient pressure (n = 4, compared with PBS-treated controls). In addition, FAK activation might be required for pressure-induced Akt-FAK association because the FAK inhibitor Y15 prevented increased Akt-FAK association with pressure in coimmunoprecipitation studies (Fig. 5E, n = 4, P < 0.05). Although FAK inhibitor Y15 seemed to modestly increase the baseline of Akt-FAK association under ambient pressure (18 ± 16%), this effect was not statistically significant (n = 4, P = 0.39).
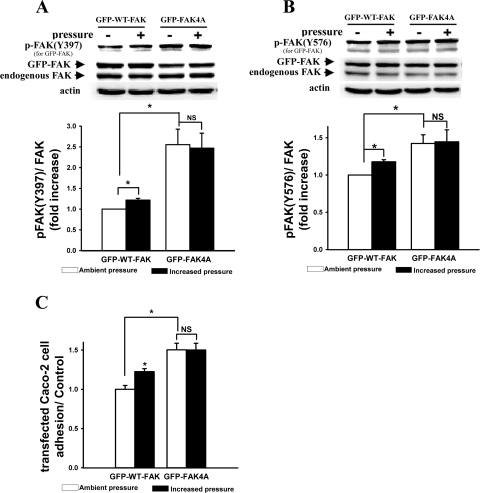
Substitution of all four predicted Akt Ser/Thr phosphorylation sites in FAK with alanines prevented pressure-induced tyrosine phosphorylation of FAK at Y397/Y576 or cancer cell adhesion.
To investigate the functions of these potential Akt Ser/Thr phosphorylation sites in FAK, we generated a quadruple mutant GFP-FAK4A in which all the four potential phosphorylation sites in FAK (S517, 601, 695, and T600A) were substituted with alanines. Overexpressing this quadruple mutant in Caco-2 cells prevented pressure-induced tyrosine phosphorylation of FAK at Y397 and Y576 and cell adhesion with significantly increased basal FAK phosphorylation and cell adhesion under ambient pressure (Fig. 6, A, B, and C, respectively, n = 4, P < 0.05). Since pressure altered threonine phosphorylation of FAK in the opposite direction of serine phosphorylation, we then studied a triple mutant with T600 intact. It is noted that although the expression levels of GFP-FAK (WT or 4A mutant) were similar to those of endogenous FAK, the levels of tyrosine phosphorylation band intensity appear much higher in GFP-FAK (Fig. 6, A and B). Structural differences between the endogenous FAK (human FAK) and the FAK mutants (derived from chicken FAK) may have contributed to different susceptibility to tyrosine phosphorylation between the chicken and human FAK, or the antibodies to FAK and tyrosine phosphorylated FAK that we used may have bound to these antigens from different species with different affinity, resulting in an artifactual difference in band intensity.
Fig. 6.
Effect of transfection with the quadruple mutant (S517, 601, 695A/T600A) of FAK with a green fluorescent protein tag (GFP-FAK4A) on pressure-induced increases in tyrosine phosphorylation of FAK at Y397 (A) and Y576 (B) and cell adhesion (C). All of the four potential phosphorylation sites in FAK (S517, 601, 695, and T600A) were substituted by alanines to create the quadruple mutant (GFP-FAK4A). Protein samples from the lysates of Caco-2 cells transfected with GFP-wild-type (WT)-FAK or GFP-FAK4A expression plasmids, respectively, were directly analyzed by Western blot for FAK(Y397) phosphorylation (A) and FAK(Y576) phosphorylation (B) or total FAK to recognize GFP-tagged FAK and endogenous FAK. Actin served as loading control. A and B: typical blots are represented, and the graphs summarize densitometric analysis of the ratios of p-FAK(Y397, for overexpressed GFP-FAK) or p-FAK(Y576, for overexpressed GFP-FAK) to total FAK (also for overexpressed GFP-FAK) (n = 4, *P < 0.05). All observations were normalized against the GFP-WT-FAK-transfected control under ambient pressure. C: cell adhesion was assessed by counting the GFP fluorescent Caco-2 cells with fluorescence microscopy as described in materials and methods (n = 4, *P < 0.05). All observations were normalized against the GFP-WT-FAK-transfected control under ambient pressure.
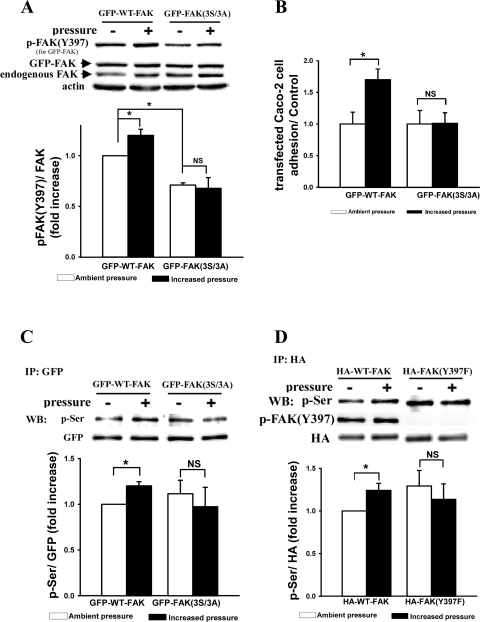
Substituting three predicted Akt serine phosphorylation sites in FAK with alanines prevented extracellular pressure-induced FAK(Y397) phosphorylation, FAK serine phosphorylation, and cell adhesion, and the nonphosphorylatable FAK mutant (Y397F) blocked pressure-induced FAK serine phosphorylation.
Overexpressing the nonphosphorylatable triple mutant, GFP-FAK3A, in Caco-2 cells prevented pressure-induced FAK(Y397) phosphorylation (Fig. 7A, n = 4, P < 0.05). Furthermore, transfection with GFP-FAK3A also blocked pressure-stimulated cell adhesion (Fig. 7B, n = 4, P < 0.05). Interestingly, basal FAK(Y397) phosphorylation under ambient pressure for GFP-FAK3A mutant was significantly reduced as compared with GFP-WT-FAK (Fig. 7A, n = 4, P < 0.05), unlike that of the GFP-FAK4A mutant.
Fig. 7.
Effect of triple mutant (S517, 601, 695/A) of FAK with GFP tag (GFP-FAK3A) on pressure-induced increases in tyrosine phosphorylation of FAK at Y397 (A), cell adhesion (B) and p-Ser of FAK (C) and the effect of nonphosphorylatable FAK mutant (Y397F) on p-Ser of FAK (D). All of the three potentially Ser phosphorylation sites in FAK (S517, 601, 695/A) were substituted by alanines to create the triple mutant (GFP-FAK3A). A: protein samples from lysates of Caco-2 cells transfected with GFP-WT-FAK or GFP-FAK3A expression plasmids, respectively, were directly analyzed by Western blot using FAK(Y397) phosphorylation or total FAK to recognize GFP-tagged FAK and endogenous FAK. Actin served as loading control. Typical blots are represented, and the graph summarizes densitometric analysis of the ratios of p-FAK(Y397, for overexpressed GFP-FAK) to total FAK (also for overexpressed GFP-FAK) (n = 4, *P < 0.05). All observations were normalized against the GFP-WT-FAK-transfected control under ambient pressure. B: cell adhesion of Caco-2 cells transfected with GFP-WT-FAK or GFP-FAK3A expression plasmids, respectively, was assessed by counting the GFP fluorescent Caco-2 cells with fluorescence microscopy as described in materials and methods (n = 4, *P < 0.05). As for cell adhesion, all observations were normalized against the GFP-WT-FAK or GFP-FAK3A-transfected controls, respectively, under ambient pressure. C: cell lysates from Caco-2 cells transfected with GFP-WT-FAK or GFP-FAK3A expression plasmids, respectively, were immunoprecipitated with mouse anti-GFP monoclonal antibody and then analyzed by Western blot using phospho-Ser-specific and GFP polyclonal antibodies. Typical blots are represented, and the graph summarizes densitometric analysis of the ratios of p-Ser to GFP in each experiment (n = 4, *P < 0.05). D: cell lysates from Caco-2 cells transfected with hemagglutinin (HA)-WT-FAK or HA-FAK(Y397A) expression plasmids, respectively, were immunoprecipitated with mouse anti-HA monoclonal antibody and then analyzed by Western blot using phospho-Ser-specific and HA polyclonal antibodies as well as FAK(Y397) phosphorylation antibody to confirm that HA-FAK(Y397A) mutant was not phosphorylated at all at site 397. Typical blots are represented, and the graph summarizes densitometric analysis of the ratios of p-Ser to HA in each experiment (n = 4, *P < 0.05).
More importantly, although pressure promoted serine phosphorylation of overexpressed GFP-tagged WT-FAK (Fig. 7C, n = 4, P < 0.05) as for endogenous FAK (Fig. 2, A and C), increased pressure did not affect the serine phosphorylation of the GFP-FAK3A mutant (Fig. 7C, n = 4, P < 0.05), suggesting that pressure-induced FAK serine phosphorylation occurs at these three Akt-regulated sites. Because the three potential Akt-mediated serine phosphorylation sites affected FAK(Y397) phosphorylation, we then asked whether FAK serine phosphorylation occurred before the FAK autophosphorylation at Y397. Unexpectedly, the FAK serine phosphorylation of immunoprecipitated HA-tagged nonphosphorylatable FAK mutant (Y397F), in contrast to WT-FAK, could not be further induced by pressure (Fig. 7D, n = 4, P < 0.05), suggesting that FAK autophosphorylation at Y397 also affects pressure-induced FAK serine phosphorylation and, therefore, that FAK tyrosine phosphorylation at Y397 and serine phosphorylation at the three sites might be mutually regulated.
Furthermore, the triple mutant GFP-FAK3A and the quadruple mutant GFP-FAK4A, compared with GFP-WT-FAK, have opposite effect on basal level of FAK(Y397) phosphorylation under ambient pressure (increased vs. decreased, compare Fig. 6A to Fig. 7A), again suggesting that T600 in FAK may have opposite effects on FAK(Y397) phosphorylation from that of the three serine phosphorylation sites. To confirm this observation, we constructed a T600A single mutant of FAK as well as double mutants for the three serine sites to narrow down Akt serine phosphorylation sites.
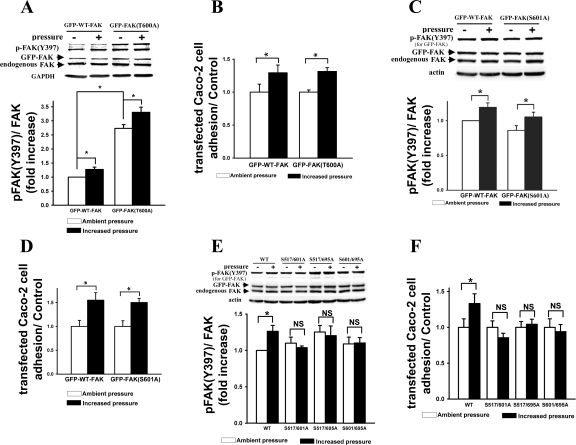
Neither T600/A nor S601A single mutation of FAK prevented pressure-induced FAK(Y397) phosphorylation or cell adhesion, whereas any two combined serine mutation with alanines blocked pressure-induced increased FAK(Y397) phosphorylation and cell adhesion.
Substitution of T600 with alanine inhibited the protein expression of the GFP-FAK(T600A) mutant by 68 ± 2% reduced expression compared with GFP-WT-FAK while transfecting the respective FAK expression plasmids with identical concentrations in Caco-2 cells (not shown). Therefore, to obtain the similar expression levels of WT-FAK and FAK(T600A), we decreased the concentration of GFP-WT-FAK expression plasmid to 0.2 μg/ml while keeping GFP-FAK(T600A) expression plasmid at 2 μg/ml. A single point mutation at T600 with alanine resulted in 2.7 ± 0.1 times increased basal level of FAK(Y397) phosphorylation under ambient pressure compared with WT-FAK, although total GFP-tagged FAK expression was at similar levels (Fig. 8A). However, T600A mutation did not prevent pressure-induced FAK(Y397) phosphorylation (Fig. 8A, n = 4, P < 0.05) or cell adhesion (Fig. 8B, n = 4, P < 0.05).
Fig. 8.
Pressure-induced increase in FAK(Y397) phosphorylation could not be prevented by the single mutants (T600A or S601A) of FAK with GFP tag [GFP-FAK(T600A or S601A)] (A and C, respectively) but was prevented by either of three double mutants (S517/601A, S517/695A, and S601/695A) (E). Similar results were obtained for pressure-induced increase in cancer cell adhesion of Caco-2 cells transfected with either FAK single mutants (B and D) or double mutants (F). As for cell adhesion, all observations were normalized against the GFP-WT-FAK or each FAK mutant transfected controls, respectively, under ambient pressure.
Substitution of S601, the adjacent site of T600, with alanine failed to prevent pressure-induced FAK(Y397) phosphorylation (Fig. 8C, n = 4, P < 0.05) and cell adhesion (Fig. 8D, n = 4, P < 0.05) with slightly reduced, but not significant, basal level of FAK(Y397) phosphorylation under ambient pressure, as compared with WT-FAK. In contrast to the above two single mutants, any two combined serine mutation with alanines (S517/601A, S517/695A, and S601/695A) blocked pressure-induced increases in FAK(Y397) phosphorylation (Fig. 8E, n = 4, P < 0.05) or cell adhesion (Fig. 8F, n = 4, P < 0.05). All observations were normalized against their respective ambient pressure controls for all adhesion studies in Fig. 8 because of the variable transfection efficiencies in Caco-2 cells between WT-FAK and FAK mutants. Therefore, the apparently unchanged basal cell numbers under ambient pressure conditions (Fig. 8, B, D, and F) did not reflect a lack of effect of transfection on basal adhesion per se, which could not be addressed by this technique.
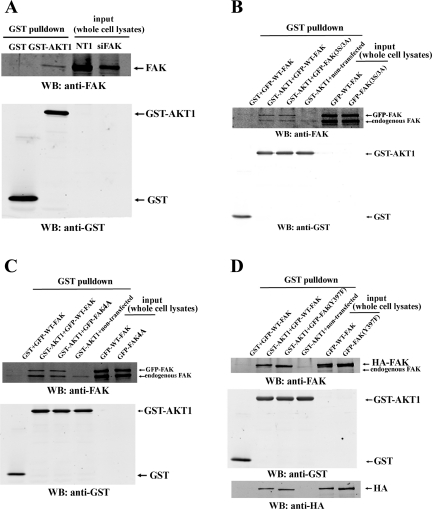
The association between FAK and Akt did not depend on the FAK autophosphorylation site (Y397) or on serine or threonine phosphorylation sites of interest in FAK.
We used a GST pulldown assay to determine whether FAK-Akt association requires the FAK Y397 site or the serine or threonine phosphorylation sites that we had identified as of interest. Consistent with the results of our coimmunoprecipitation studies above, endogenous FAK in pressurized and suspended Caco-2 cells associated with active GST-tagged human Akt1, a purified fusion protein (Fig. 9A). The specificity of this binding between FAK and Akt was confirmed by the absence of the FAK band in the GST control lane. In addition, the identity of this band was further confirmed by Western blotting for FAK in diluted whole Caco-2 cell lysates (independently of GST pulldown) to show that the FAK band in the whole cell lysates resolved at the same apparent molecular weight as the FAK-immunoreactive band from the Akt pulldown, Finally, a lighter exposure of the same two lanes (Fig. 9A, second blot) demonstrated that this FAK band was reduced in the whole cell lysates of Caco-2 cells transfected with siRNA to FAK, as compared with nontargeting siRNA (NT1) control. Like endogenous FAK, the GFP or HA-tagged WT-FAK also bound to GST-Akt1 (Fig. 9, C and D). However, neither the FAK triple [GFP-FAK(3S/3A)], quadruple (GFP-FAK4A) mutation, nor the nonphosphorylatable mutation [FAK(Y397F)] blocked the binding of FAK to Akt (Fig. 9, C and D). The specificities of GFP-FAK or HA-FAK bands were confirmed by the absence of these bands in GST control and another control in which nontransfected cell lysates were incubated with GST-Akt1-coupled beads.
Fig. 9.
The association between FAK and Akt did not depend on FAK autophosphorylation site (Y397) or serine or threonine phosphorylation sites in FAK. A: suspended and pressurized Caco-2 cell lysates were subjected to glutathione S-transferase (GST) pulldown assay as detailed in materials and methods. NT1 or siFAK-transfected Caco-2 whole cell lysates were used as controls. FAK and GST were detected by Western blot. The second blot from top in A represents a lighter exposure of the identical blot shown at top of image to better demonstrate the reduction in the FAK band from the lysate from cells treated with siRNA to reduce FAK. B–D: suspended and pressurized cells transfected with indicated FAK mutants (tagged with GFP or HA as indicated) were lysed and subjected to GST pulldown assay. Nontransfected cell lysates were used as controls. FAK and GST were detected by Western blot. In D, HA was also detected after membranes were stripped. All results (A–D) are representative of at least two independent experiments with similar results.
DISCUSSION
FAK conventionally functions as an upstream regulator of Akt in many physiological and pathological cellular responses induced by various extracellular stimuli (27, 33, 35, 53). Our recent results demonstrate that Akt may also regulate FAK, directly or indirectly, in response to extracellular pressure because inhibiting or silencing Akt blocks pressure-induced increases in autophosphorylation of FAK at Y397 (41). Our present data suggest for the first time that Akt directly regulates FAK by binding to FAK and inducing phosphorylation at three serine sites (S517, 601, 695), which in turn upregulates FAK(Y397) phosphorylation and cancer cell adhesion in response to modest extracellular pressure increases. Although Akt is therefore required for FAK activation in response to pressure, our further studies demonstrate that pressure-induced Akt and FAK activation may be interdependent. Inhibiting or silencing FAK prevented the increases in phosphorylation of Akt(S473) induced by increased pressure. Thus, FAK and Akt bind directly and may potentiate each other's activation. In addition, their binding did not depend on the FAK autophosphorylation site or the Akt Ser/Thr phosphorylation sites we had identified in FAK.
Although the overall effect of pressure on cancer cell adhesion in vitro and phosphorylation of various proteins in our study might seem relatively modest, such seemingly modest in vitro effects cause substantial physiological and/or pathological effects in vivo. A previous report demonstrated that preexposure to increased pressure enhances murine tumor cell implantation to surgical wounds by 43% to 52% (45), while another study demonstrated that preexposure to 15 mmHg-elevated pressure increases CT26 transplantable colon cancer cell adhesion to murine wounds by 66 ± 14% (8). Furthermore, pressure increases peritoneal metastasis by 49 ± 19% and significantly impairs tumor-free survival in murine transplantable tumor models, reducing tumor-free survival rates by ∼50% in various studies (8, 10). Consistent with our observations, Haier and colleagues reported that modest hydrodynamic shear force modestly stimulates tyrosine phosphorylation of FAK in HT-29 colon cancer cells in vitro, and this effect is also important in vivo (50). Finally, other authors have previously investigated the contribution of intracellular signals of similar magnitude to the regulation of anchorage-independent growth (12), cell migration (11), and cyclic strain-induced endothelial cell reorientation (43).
It should be noted that in our previous studies of the effects of similar extracellular pressure increases in vivo in the murine transplantable tumor models, the effects of pressure were transiently applied to the cancer cells themselves ex vivo before adhesion and not to the host organism, thus demonstrating that the effects of pressure that we observed on tumor cell adhesion to surgical wounds and to the peritoneum and the pressure impairment of subsequent tumor-free survival reflected a direct effect of pressure on the tumor cells themselves, not on the host organism. Since patients with cancer commonly have circulating tumor cells (4), but the development of a metastatic tumor from a circulating tumor cell is itself an uncommon event (36), even small changes in the probability of adhesion may have important implications for metastatic tumorigenesis in vivo.
The conserved phospho-Akt substrate consensus motif has commonly been defined as RXRXXS/T (30, 31), where X is any amino acid and S/T is the serine or threonine phosphorylation site. Our bioinformatic analysis revealed one threonine-containing and three serine-containing consensus sequences for Akt phosphorylation within the FAK sequence. These four potential Ser/Thr phosphorylation sites are completely conserved in FAK molecules across various species, suggesting their important roles in maintaining the function of FAK. Previously identified serine phosphorylation (S722, 732, 843, 910) sites are all in the COOH-terminal region of FAK (16, 20–22, 54). In contrast, these four predicted Ser/Thr phosphorylation sites (S517, 601, 695, and T600) are in or near the FAK kinase domain.
Before investigating the functions of these potential Ser/Thr phosphorylation sites, we first sought to determine whether extracellular pressure affected the overall serine or threonine phosphorylation of FAK. Interestingly, our present data demonstrated that pressure had opposite effects on FAK serine and threonine phosphorylation. Pressure promoted FAK serine phosphorylation but inhibited FAK threonine phosphorylation. These findings were confirmed in primary cells from surgically resected human tumors, suggesting that they are not artifacts of the adaptation to cell culture and confirming their clinical relevance.
In addition, nonphosphorylatable mutation at the three serine sites [GFP-FAK(3S/3A)] or the single threonine site [GFP-FAK(T600A)] had opposite effects on basal levels of FAK(Y397) phosphorylation under ambient pressure. Although basal FAK(Y397) phosphorylation for GFP-FAK(T600A) under ambient pressure was dramatically increased compared with that of WT-FAK, the GFP-FAK(T600A) mutation could not prevent the further increase in FAK(Y397) phosphorylation in exposure to pressure.
This increased baseline of FAK phosphorylation was also observed in the quadruple mutant (4A). It is noteworthy that this comparison was performed with WT chicken FAK, instead of endogenous human FAK. Therefore, the quadruple or T600A mutation may change the protein conformation of FAK, resulting in the activation of FAK. The active FAK further increased basal cell adhesion in cells transfected with the quadruple mutant just as the specific FAK inhibitor Y15 reduced basal cell adhesion, consistent with a model in which FAK activity influences for cell adhesion. Because three of the four predicted Akt phosphorylation sites in FAK are serine residues and because GFP-FAK(T600A) failed to prevent pressure-induced FAK(Y397) phosphorylation, we focused on the serine phosphorylation of FAK in our further work. Pressure-induced serine phosphorylation (p-Ser) of FAK appeared to depend on Akt, because FAK serine phosphorylation was prevented by inhibiting Akt or silencing Akt expression. Furthermore, isoform-specific siRNA studies suggested that only Akt1 seems involved in this pathway. Indeed, pressure-promoted Akt-FAK association and FAK(Y397) phosphorylation were also sensitive only to Akt1 silencing. The substantially reduced basal level of Akt associating with FAK (Akt band in Fig. 4A) under ambient pressure at presence of siAKT1 highlighted the specificity of the Akt band and suggested a direct binding between Akt1 and FAK. It is unlikely that this apparent isoform specificity might reflect the preponderance of Akt1 in Caco-2 cells because siAKT2 was more effective in reducing Akt2 expression than siAKT1 to Akt1 expression; and because Akt2 is the predominant isoform in Caco-2 cells (41). Indeed, we have previously reported that because Akt1 is less abundant than Akt2 in Caco-2 cells, siAKT2 is even more effective than siAKT1 in knocking down total Akt expression (41).
This pressure-induced Akt dependent regulation of FAK is not straightforwardly one-way. Pressure-stimulated Akt(S473) phosphorylation was in turn prevented by suppression of FAK expression with either a pool of four individual siRNA duplexes (siFAK) or a different single duplex (siFAK2) specifically targeting to FAK. It is noteworthy that basal levels of Akt(S473) phosphorylation under ambient pressure were increased by FAK reduction or FAK pharmacologic blockade. This apparent induction of basal Akt(S473) phosphorylation in response to FAK reduction or blockade may reflect a compensatory negative feedback effect, consistent with the apparent complexity of FAK-Akt interaction. This result also highlights the potential importance of targeting both FAK and Akt together rather than FAK alone for therapeutic effect in the treatment of cancers. Pressure-stimulated increases in Akt(S473) phosphorylation and cancer cell adhesion are also prevented by FAK reduction in our previous study (41), or pharmacological blockade in the present study, suggesting that pressure-induced Akt(S473) phosphorylation is regulated by FAK.
In contrast to the serine point mutations, FAK(T600A) dramatically stimulated FAK(Y397) phosphorylation under ambient pressure. FAK exhibits autoinhibition. The N-FERM domain of FAK binds the FAK kinase domain and then masks the phosphorylation site at Y397, resulting in inactive FAK. Recently described three-dimensional structures of autoinhibited and active FAK reveal a direct contact between the F2 lobe and the kinase C-lobe (26). Substitution of Phe596 at kinase domain with Asp dramatically increases FAK kinase activity by disrupting contact with the kinase C-lobe. Substitution of T600, which is adjacent to F596 in kinase domain, with alanine might have a similar effect on the contact between the F2 lobe and the kinase C-lobe, releasing the FERM domain from the kinase domain, and thus potentiating FAK Y397 phosphorylation. In contrast, the T600A point substitution did not prevent the stimulation of FAK(Y397) phosphorylation or cell adhesion by increased pressure, each of which is mediated by Akt. It therefore seems unlikely that Akt phosphorylates T600 directly in response to pressure, although we cannot exclude the possibility that T600 residue might work collaboratively with the three serine sites in regulating FAK(Y397) phosphorylation and cell adhesion.
Although other serine phosphorylation sites in FAK have been identified, including S843 and S910 (16, 18, 20–22, 54), these reported serine phosphorylation sites are unlikely to be influenced by Akt because they are not at Akt consensus sequences. Among these serine phosphorylation sites is S843, which is the only previously identified serine phosphorylation site affecting FAK(Y397) phosphorylation. In contrast to S843, which inhibits FAK(Y397) phosphorylation, the three serine phosphorylation sites we identify here appear to promote FAK(Y397) phosphorylation. These three novel serine phosphorylation sites in FAK were not previously identified by mass spectrometry (18), perhaps because of the lower abundance of these serine phosphorylation events in activated FAK. However, our present findings suggest that they are functionally important in regulating pressure-induced cancer cell adhesion.
It is noteworthy that all the signaling results at the present study were performed in suspended cells. The regulation of FAK by Akt may be limited in the so-called inside-out signaling, which is from the cytoplasm out to the extracellular response. Like endogenous FAK, GFP-tagged FAK fusion proteins (wild-type or the nonphosphorylatable triple mutant) were also predominantly localized in cytoplasm of adherent transfected Caco-2 cells (data not shown). Time-lapse imaging with a confocal microscope demonstrated that the nonphosphorylatable triple mutant [GFP-FAK(3S/3A)], compared with GFP-WT-FAK, did not noticeably alter the dynamic change of GFP fluorescence during 20 min after transfected Caco-2 cells were plated on collagen I-precoated dishes (not shown). These observations, at least, suggested that serine phosphorylation of FAK by Akt was not required for the intracellular localization of FAK.
Interestingly, in addition to our findings of FAK-Akt interaction under increased pressure, FAK and Akt activities, based on our present results, also seem to be reciprocally interdependent under ambient pressure, in that Akt phosphorylation is stimulated by FAK inhibition or reduction. This may be explained by the above mentioned negative feedback mechanism. Indeed, Akt phosphorylation at Ser473 is not necessarily FAK dependent in all settings. For example, Akt phosphorylation at Ser473 induced by β1-integrins can occur independently of FAK (48). Although one could attempt to explain this by postulating a role for increased PI-3K or decreased phosphatase and tensin homolog (PTEN) activity, these seem less likely candidates because both PTEN and PI-3K can bind to FAK directly and are down- and upregulated by FAK (37, 38, 53). Therefore, it is expected that PTEN and PI-3K, at the presence of FAK inhibition, would be activated and inhibited, respectively, resulting the inhibition/dephosphorylation of Akt at Ser473. Increased TORC2 kinase activity or decreased PHLPP phosphatase could, however, also be candidates for the intermediate in this negative feedback, or there could be some other intermediary signaling mechanism not yet identified. The detailed mechanism of this interesting relationship between FAK and Akt activities under baseline conditions awaits further study beyond the scope of the current article. Several authors have suggested that Akt activity may to some extent act to protect suspended tumor cells against anoikis (13, 28). Since FAK is activated by conventional outside-in signaling in cells after integrin-mediated adhesion (39, 56), then the relative reduction in FAK activation in suspended cells could by this reciprocal relationship result in Akt activation that might help protect cancer cells against anoikis. This interesting hypothesis awaits further exploration beyond the scope of the current article.
FAK-Akt interaction did not depend on the FAK autophosphorylation site or on the Akt Ser/Thr phosphorylation sites in FAK. This unexpected result is not necessarily surprising, however, because the Akt Ser/Thr phosphorylation sites in FAK are not necessarily the binding sites. Autophosphorylation at Y397 is a prerequisite for FAK activation. Therefore, failure of FAK(Y397F) mutant in blocking association between FAK and Akt suggests that FAK kinase activity is not required for FAK binding to Akt. Indeed, increasing recent evidence supports a model in which FAK can also function as a scaffold in diverse signaling events (29, 57).
In summary, Akt1 directly binds to and phosphorylates FAK at three novel serine phosphorylation sites in response to extracellular pressure. This association was not dependent on the FAK autophosphorylation site or the Akt serine or threonine phosphorylation sites identified in FAK. These three serine phosphorylation sites coordinately function together to positively regulate FAK(Y397) phosphorylation and thus to further mediate pressure-induced cancer cell adhesion, probably through regulating the integrin binding affinity to matrix proteins. Pressure-induced phosphorylation/activation of FAK and Akt is interdependent in that FAK and Akt appear to potentiate each other's activation. This novel FAK-Akt interaction suggests that FAK and Akt1 may be dual kinase targets for agents to prevent cancer cell adhesion, and eventually inhibit cancer metastasis.
GRANTS
This work was supported by in part by National Institutes of Health Grant 2R01DK-060771 (to M. D. Basson) and a VA Merit Research Award (to M. D. Basson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. J. L. Guan (University of Michigan) and Dr. D. Schlaepfer (Scripps Research Institute) for generously providing p-EGFP-C3-WT-FAK and HA-FAK, HA-FAK(Y397F) vectors, respectively. We also thank Mr. J. Chen (Michigan State University) for technical assistance in GST pulldown assay and providing GST protein.
REFERENCES
- 1. Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol 3: 221–247, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Basson MD, Yu CF, Herden-Kirchoff O, Ellermeier M, Sanders MA, Merrell RC, Sumpio BE. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem 78: 47–61, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem 277: 33895–33900, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Budd GT. Let me do more than count the ways: what circulating tumor cells can tell us about the biology of cancer. Mol Pharm 6: 1307–1310, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 6: 2417–2423, 2000 [PubMed] [Google Scholar]
- 6. Champault G, Almagro Ruiz M, Panchana G, Barrat C, Catheline JM. [Port-site metastases. A prospective study of 131 cases]. J Chir (Paris) 134: 423–428, 1997 [PubMed] [Google Scholar]
- 7. Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 24: 7482–7492, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Craig DH, Downey C, Basson MD. SiRNA-mediated reduction of alpha-actinin-1 inhibits pressure-induced murine tumor cell wound implantation and enhances tumor-free survival. Neoplasia 10: 217–222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Craig DH, Haimovich B, Basson MD. α-Actinin-1 phosphorylation modulates pressure-induced colon cancer cell adhesion through regulation of focal adhesion kinase-Src interaction. Am J Physiol Cell Physiol 293: C1862–C1874, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Craig DH, Owen CR, Conway WC, Walsh MF, Downey C, Basson MD. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest 118: 3170–3180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai LP, White SR, Waters CM. Cyclic mechanical stretch decreases cell migration by inhibiting phosphatidylinositol 3-kinase- and focal adhesion kinase-mediated JNK1 activation. J Biol Chem 285: 4511–4519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med 15: 1163–1169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz-Montero CM, Wygant JN, McIntyre BW. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur J Cancer 42: 1491–1500, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273: 32377–32379, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Emenaker NJ, Basson MD. Short chain fatty acids inhibit human (SW1116) colon cancer cell invasion by reducing urokinase plasminogen activator activity and stimulating TIMP-1 and TIMP-2 activities, rather than via MMP modulation. J Surg Res 76: 41–46, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Fan RS, Jacamo RO, Jiang X, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor activation rapidly stimulates focal adhesion kinase phosphorylation at Ser-843. Mediation by Ca2+, calmodulin, and Ca2+/calmodulin-dependent kinase II. J Biol Chem 280: 24212–24220, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, Cance WG. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the Y397 site of focal adhesion kinase decreases tumor growth. J Med Chem 51: 7405–7516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grigera PR, Jeffery ED, Martin KH, Shabanowitz J, Hunt DF, Parsons JT. FAK phosphorylation sites mapped by mass spectrometry. J Cell Sci 118: 4931–4935, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Haier J, Nicolson GL. PTEN regulates tumor cell adhesion of colon carcinoma cells under dynamic conditions of fluid flow. Oncogene 21: 1450–1460, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Hunger-Glaser I, Fan RS, Perez-Salazar E, Rozengurt E. PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. J Cell Physiol 200: 213–222, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Jacamo R, Jiang X, Lunn JA, Rozengurt E. FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration. J Cell Physiol 210: 436–444, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Jiang XH, Sinnett-Smith J, Rozengurt E. Differential FAK phosphorylation at Ser-910, Ser-843 and Tyr-397 induced by angiotensin II, LPA and EGF in intestinal epithelial cells. Cell Signal 19: 1000–1010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim ZG, Mehl C, Lorenz M, Gutt CN. Impact of laparoscopic CO2-insufflation on tumor-associated molecules in cultured colorectal cancer cells. Surg Endosc 16: 1182–1186, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Lawler K, Meade G, O'Sullivan G, Kenny D. Shear stress modulates the interaction of platelet-secreted matrix proteins with tumor cells through the integrin αvβ3. Am J Physiol Cell Physiol 287: C1320–C1327, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA 99: 3546–3551, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lietha D, Cai X, Ceccarelli DFJ, Li Y, Schaller MD, Eck MJ. structural basis for the autoinhibition of focal adhesion kinase. Cell 129: 1177–1187, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Z, Zhang HM, Yuan J, Lim T, Sall A, Taylor GA, Yang D. Focal adhesion kinase mediates the IGTP-induced PI3K/Akt survival pathway and further initiates a positive feedback loop of NF-kappaB activation. Cell Microbiol 10: 1787–1800, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mandal M, Younes M, Swan EA, Jasser SA, Doan D, Yigitbasi O, McMurphey A, Ludwick J, El-Naggar AK, Bucana C, Mills GB, Myers JN. The Akt inhibitor KP372–1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol 42: 430–439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18: 516–523, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley L. Determination of the specific substrate sequence motifs of protein kinase c isozymes. J Biol Chem 272: 952–960, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem 275: 36108–36115, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, Brenner DA, Rippe RA. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem 278: 8083–8090, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Siesser PMF, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res 12: 3233–3237, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T. FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J Biol Chem 274: 10566–10570, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9: 541–573, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Tamura M, Gu J, Danen EHJ, Takino T, Miyamoto S, Yamada KM. PTEN Interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem 274: 20693–20703, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280: 1614–1617, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology 126: 8–18, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Thamilselvan V, Basson MD. The role of the cytoskeleton in differentially regulating pressure-mediated effects on malignant colonocyte focal adhesion signaling and cell adhesion. Carcinogenesis 26: 1687–1697, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J 21: 1730–1741, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Thamilselvan V, Patel A, van der Voort van Zyp J, Basson MD. Colon cancer cell adhesion in response to Src kinase activation and actin-cytoskeleton by non-laminar shear stress. J Cell Biochem 92: 361–371, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tureckova J, Vojtechova M, Krausova M, Sloncova E, Korinek V. Focal adhesion kinase functions as an akt downstream target in migration of colorectal cancer cells. Transl Oncol 2: 281–290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van der Voort van Zyp J, Thamilselvan V, Walsh M, Polin L, Basson MD. Extracellular pressure stimulates colon cancer cell adhesion in vitro and to surgical wounds by Src (sarcoma protein) activation. Am J Surg 188: 467–473, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Van Nimwegen MJ, van de Water B. Focal adhesion kinase: A potential target in cancer therapy. Biochem Pharmacol 73: 597–609, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Vandermoere F, Yazidi-Belkoura IE, Demont Y, Slomianny C, Antol J, Lemoine J, Hondermarck H. Proteomics exploration reveals that actin is a signaling target of the kinase Akt. Mol Cell Proteomics 6: 114–124, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Velling T, Nilsson S, Stefansson A, Johansson S. beta1-Integrins induce phosphorylation of Akt on serine 473 independently of focal adhesion kinase and Src family kinases. EMBO Rep 5: 901–905, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Villafuerte BC, Phillips LS, Rane MJ, Zhao W. Insulin-response element-binding protein 1: a novel Akt substrate involved in transcriptional action of insulin. J Biol Chem 279: 36650–36659, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Von Sengbusch A, Gassmann P, Fisch KM, Enns A, Nicolson GL, Haier J. Focal adhesion kinase regulates metastatic adhesion of carcinoma cells within liver sinusoids. Am J Pathol 166: 585–596, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang S, Basson MD. Identification of functional domains in AKT responsible for distinct roles of AKT isoforms in pressure-stimulated cancer cell adhesion. Exp Cell Res 314: 286–296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang S, Basson MD. Integrin-linked kinase: a multi-functional regulator modulating extracellular pressure-stimulated cancer cell adhesion through focal adhesion kinase and AKT. Cell Oncol 31: 273–289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem 279: 33024–33034, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114: 469–482, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Yoeli-Lerner M, Toker A. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle 5: 603–605, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 28: 35–49, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. In press [DOI] [PMC free article] [PubMed] [Google Scholar]