Abstract
Copper is an essential micronutrient in humans and is required for a wide range of physiological processes, including neurotransmitter biosynthesis, oxidative metabolism, protection against reactive oxygen species, and angiogenesis. The first step in the acquisition of dietary copper is absorption from the intestinal lumen. The major human high-affinity copper uptake protein, human copper transporter hCTR1, was recently shown to be at the basolateral or blood side of both intestinal and renal epithelial cell lines and thus does not play a direct role in this initial step. We sought to functionally identify the major transport pathways available for the absorption of dietary copper across the apical intestinal membrane using Caco2 cells, a well-established model for human enterocytes. The initial rate of apical copper uptake into confluent monolayers of Caco2 cells is greatly elevated if amino acids and serum proteins are removed from the growth media. Uptake from buffered saline solutions at neutral pH (but not at lower pH) is inhibited by either d- or l-histidine, unaltered by the removal of sodium ions, and inhibited by ∼90% when chloride ions are replaced by gluconate or sulfate. Chloride-dependent copper uptake occurs with Cu(II) or Cu(I), although Cu(I) uptake is not inhibited by histidine, nor by silver ions. A well-characterized inhibitor of anion exchange systems, DIDS, inhibited apical copper uptake by 60–70%, while the addition of Mn(II) or Fe(II), competitive substrates for the divalent metal transporter DMT1, had no effect on copper uptake. We propose that anion exchangers play an unexpected role in copper absorption, utilizing copper-chloride complexes as pseudo-substrates. This pathway is also observed in mouse embryonic fibroblasts, human embryonic kidney cells, and Cos-7 cells. The special environment of low pH, low concentration of protein, and protonation of amino acids in the early intestinal lumen make this pathway especially important in dietary copper acquisition.
Keywords: anion-dependent transport, chloride-dependent copper transport, copper transport, dietary copper uptake, intestinal copper
copper (Cu) is an essential nutrient for vertebrates and has numerous functions in cellular physiology, including respiration, free-radical defense, angiogenesis, neuronal function, and others (20). In mammals, copper acquisition is initiated by the absorption of dietary copper across the intestinal epithelium, and copper excess is removed by the liver with biliary secretion into the gastrointestinal tract, and excretion with the feces (45, 46). Liver is the major organ to regulate copper homeostasis at the whole body level, by either recycling copper into newly synthesized serum proteins, or clearing copper out of the body (43). Daily dietary copper uptake in the average adult is in the range of 0.6–1.6 mg, and secretion into bile and pancreatic juice is on the order of 4.5 mg/day (47). Most of the copper secreted with the bile into the alimentary canal is reabsorbed by enterocytes, and only a small portion is completely cleared out of the body (29). The biosynthetic requirement for copper in various bodily tissues is satisfied by reabsorbing copper circulating with the blood and complexed to various serum proteins, peptides, and amino acids (9). The mechanism of dietary Cu uptake by the small intestine can be roughly divided into the following steps: 1) transport of copper from the lumen of the gut across the apical surface of intestinal epithelium; 2) intracellular distribution of copper within enterocytes; and 3) efflux of copper from the intestinal epithelium into blood (53). The latter step is mediated by Cu-dependent ATPases such as ATP7A, the Menkes disease protein, and ATP7B, the Wilson disease protein (5, 31). However, the mechanism of the initial stages in copper homeostasis, such as absorption of dietary copper by the gut epithelium, is not well understood.
From yeast to mammals, in eukaryotes the members of the CTR family of copper transporters (SLC31A) mediate high-affinity cellular copper entry, with Km values in the low micromolar range (14, 25, 28). Despite differences in the primary structure, they all are integral membrane proteins that trimerize to form a permeation pathway specific for copper (11) and are inhibited by silver (Ag) ions (28, 53). Mammalian CTR1 proteins are expressed ubiquitously, display high sequence identity, and are targeted primarily to the plasma membrane (25). CTR1 has frequently been assumed to play a direct role in uptake of dietary copper (37), but recent observations call this into question. Mice with intestine-specific CTR1 gene knockout still accumulate high levels of copper in intestinal epithelium (34), suggesting that systems other than CTR1 are involved in intestinal uptake from the gut lumen. Moreover, in cell surface labeling studies it was found that endogenous CTR1 is exclusively localized to the basolateral surface of intestinal and renal epithelial cells, where it mediates copper uptake from the side of blood (53). These studies suggest that intestinal cells acquire their essential Cu from the blood via human CTR1 (hCTR1), and that other mechanisms must mediate apical Cu entry. The localization of hCTR1 in mammalian intestine is controversial as a recent article suggests exclusively apical localization (35), while studies in epithelial cell lines show predominantly basolateral localization and function (53). Although CTR1 is crucial in early embryonic development and its deletion results in an embryonically lethal phenotype (26, 28), fibroblasts derived from the ctr1−/− mouse embryos can transport copper at ∼30% rate of the wild-type cells (28), implying the existence of non-CTR1-mediated cellular copper entry mechanisms.
Candidates for alternative copper uptake pathways include divalent metal transporter DMT1, sodium-dependent amino-acid transporters, and endocytosis. DMT1, also known as Nramp2 or DCT1, is localized at the apical side of intestine and Caco2 monolayers (42) and is involved in the transport of iron and other divalent metals (16). Recently, DMT1 was also proposed to play a relevant role in physiological monovalent Cu(I), rather than divalent Cu(II), entry (3). Copper uptake through endocytosis or macropinocytosis was suggested to occur through membrane ruffling and the formation of vesicles that could engulf the extracellular copper-containing solution, or, alternatively, the endocytosis of CTR1 protein could result in the uptake of copper that is bound to it, in a manner similar to iron uptake via the transferrin cycle (21). In freshwater fish, it has been proposed that transport of dietary copper across the mucosal membrane occurs in a sodium-dependent manner through amino acid transporters, with copper being moved as part of an amino acid complex (17), or, alternatively, by copper leaking through epithelial sodium channels (19).
In the present study we utilized Caco2 monolayers as a model of enterocytes to elucidate the likely functional mechanism of dietary Cu uptake. We show that when amino acids and protein are removed from growth media, Cu enters intestinal cells as a pseudo-substrate for anion exchange systems. The uptake is unaffected by the removal of sodium ions from the media, eliminating a significant role for sodium-dependent uptake pathways, but is greatly inhibited when chloride ions are replaced by sulfate or gluconate. Inhibitors of the anion exchange transporters reduce the uptake rate of copper, and this pathway is operative in other cells in buffered saline media. Our results point to an unexpected role for anion transport systems in metal ion homeostasis and suggest that they play a major role in the initial step of the dietary acquisition of Cu at the apical surface of intestinal cells.
MATERIALS AND METHODS
Cell culture.
All cells were maintained in a humidified incubator at 37°C under a 5% CO2 atmosphere in growth media supplemented with antibiotic-antimycotic mixture (100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml fungizone) from Invitrogen (no. 15240), 5 μg/ml plasmocin (no. ant-mpt, InvivoGen), and 25 mM HEPES, pH 7.4 (no. 15140, GIBCO); cells were routinely subcultured twice a week at 1:5 ratio. More specifically, growth medium of Caco2 cells consisted of DMEM (no. 11995, GIBCO) supplemented with 20% fetal bovine serum (FBS; no. s11550, Atlanta Biologicals), while growth media for human embryonic kidney 293 (HEK293), Cos-7, and mouse embryonic fibroblast (MEF) cells contained DMEM medium supplemented with 10% FBS.
For polarization studies, Caco2 cells (HTB-37 purchased from American Type Culture Collection) were grown on 24-mm Polyester membrane Transwells with 0.4-μm pores (no. 3450, Corning) until confluent, after which cells were grown for additional 2 wk until differentiated, with the growth medium exchanged every 1–2 days. Formation of tight junctions was monitored by the measurement of transepithelial electrical resistance (TEER) using an EVOM meter and STX2 electrodes (World Precision Instruments). Caco2 cells were judged to be differentiated when TEER value was ∼250 Ω·cm2, which usually developed ∼14 days postconfluence.
HEK tet-on cell lines, expressing the COOH-terminally FLAG-tagged hCTR1 (FLAG-hCTR) protein, were generated in a Flip-In system (catalog no. K6010, Invitrogen), as described previously (53). In the absence of tetracycline, a Tet repressor protein represses transcription of the hCTR1 gene, whose expression is induced upon addition of tetracycline to the media. Detection of overexpressed hCTR1 protein in total membranes (see Ref. 27 for the total membrane preparation) was analyzed by Western blot as described previously (53), using antibody to the FLAG-tag epitope at 1:1,000 dilution (no. F-3165, Sigma ) and anti-actin antibody (1:2,000; no. ab20272, Abcam) as a loading control.
Cos-7 cells, stably transfected with the SLC26A6 gene (PAT1) and maintained in geneticin (catalog no. 11811-031, GIBCO), were a generous gift from Dr. M. Soleimani (University of Cincinnati and Veterans Affairs Medical Center, Cincinnati, OH); MEF−/− cells with ctr1 gene deletion were generated in the lab of Dr. Dennis Thiele (Duke University, Durham, NC; 28).
Reagents and buffers.
Choline chloride, sodium-gluconate, potassium-gluconate, magnesium gluconate, bumetanide, glybenclamide, niflumic acid, 4-acetamido-4′-isothiocyanato-2,2′-stilbenedisulfonic acid disodium salt hydrate (SITS), 4,4′-diisothiocyanato-2,2′-stilbenedisulfonic acid disodium salt hydrate (DIDS), l-histidine, and d-histidine, and other amino acids were all purchased from Sigma.
The composition of the transport buffers used was as follows: salt buffer or buffered saline medium (chloride buffer) containing (in mM) 150 NaCl, 5 KCl, 2.5 MgCl2, and 25 HEPES, pH 7.4; gluconate buffer (chloride-free buffer) containing (in mM) 150 sodium gluconate, 5 potassium gluconate, 2.5 magnesium gluconate, and 25 HEPES, pH 7.4; sulfate buffer (chloride-free buffer) containing (in mM) 100 Na2SO4, 3.3 K2SO4, 3.75 MgSO4, and 25 HEPES, pH 7.4; and choline buffer (sodium-free buffer) containing (in mM) 150 choline chloride, 5 KCl, 2.5 MgCl2, and 25 HEPES, pH 7.4. In cases where pH of buffers was lowered from 7.4 to 6.4 or 6.0, 25 mM HEPES was substituted with 25 mM PIPES. The bicarbonate-buffered saline medium (bicarbonate buffer) contained (in mM) 150 NaCl, 5 KCl, 2.5 MgCl2, and 25 NaHCO3, pH 7.4.
Effects of DMEM and serum on copper fluxes were tested using the following transport buffers: 1) DMEM, 10% FBS, and 25 mM HEPES, pH 7.4; 2) salt buffer with 10% FBS (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 10% FBS, and 25 mM HEPES, pH 7.4); and 3) salt buffer supplemented with selected DMEM components (in mg/l: 84 arginine, 62.6 cysteine, 584 glutamine, 30 glycine, 42 histidine, 104.8 isoleucine, 104.8 leucine, 146.2 lysine, 30 methionine, 66 phenylalanine, 42 serine, 95.2 threonine, 16 tryptophan, 103.8 tyrosine, 94 valine, 4 calcium pantothenate, 4 choline chloride, 4 folic acid, 7.2 inositol, 4 nicotinamide, 4 pyridoxine, 0.4 riboflavin, 4 thiamine, 4,500 glucose, and 110 sodium pyruvate, in saline buffer of 150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, and 25 mM HEPES, pH 7.4).
Hypotonic saline medium was prepared by addition of extra 10, 25, or 50 mM sodium gluconate to the standard salt buffer, as follows: 150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, and 25 mM HEPES, pH 7.4, supplemented with either 10, 25, or 50 mM Na-gluconate.
64Cu uptake.
Assays for copper uptake in Transwells employed Caco2 cells plated on 4.67-cm2 permeable membrane supports in Transwells (Corning) and grown as described above, until polarized. Before the assay, the cells were washed twice in transport medium and fresh transport medium was then added to the apical (1.35 ml) and to the basal (1.8 ml) compartments. Cells were equilibrated with the medium for 30 min at 37°C. Cu uptake was initiated by addition of 10× copper solution containing trace levels of 64Cu (Division of Radiological Sciences, Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO) and the desired concentration of CuCl2. Assays were run for 1 h at 37°C, and copper transport was terminated by addition of ice-cold stop buffer (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4, and 10 mM Na2EDTA), after which cells were washed three additional times with ice-cold stop buffer. The wash media were then aspirated, and membrane supports were excised from the Transwells with a scalpel and placed in a container with Eco-Lume scintillation liquid (no. 882470, ICN Biomedicals) for scintillation counting (model LS6500, Beckman-Coulter). Experiments done on impermeable surfaces were carried out as described above, except that after the last wash, cells were resuspended in 0.1 N NaOH and removed for scintillation counting, with a small aliquot being retained for determination of protein concentration. All transport determinations were carried out in triplicate. 64Cu content of the initial tracer-containing buffer was determined for the calculation of specific activity. Cu uptake was then expressed as pmoles of Cu taken up by the cells per milligram protein per hour, following determination of the protein content of the cell monolayer by the Bio-Rad protein assay (model 500-0006, Bio-Rad). Effects of drugs, histidine, ascorbate, or competition by Fe and Mn ions with 64Cu uptake were determined by inclusion of a 50-fold molar excess of FeSO4 or MnNO3, or the indicated concentration of drug or amino acid, in the transport medium for 30 min before the assay, for equilibration. The assay was begun by addition of 10× CuCl2 labeled with trace amounts of 64Cu, was run for 1 h at 37°C, and was then terminated with addition of ice-cold stop buffer, as described previously (48).
Statistical analysis.
Data are shown as means ± SD. Statistical analysis was performed with Student's t-test, and P < 0.05 was considered statistically significant. The data presented are representative of at least three independent experiments, and in each experiment all determinations were carried out in triplicate.
RESULTS
The following investigations were undertaken to functionally identify possible transporters contributing to the initial step in the acquisition of copper in human intestinal cells, because recent studies suggested that systems other than hCTR1 may play such roles.
Copper uptake in FBS-supplemented growth medium (DMEM) or in HEPES-buffered salt medium.
In our earlier work, we characterized the relative ability of polarized epithelial cells to carry out copper uptake across either their apical or basolateral plasma membrane by measuring radioisotopic copper uptake in growth medium supplemented with 10% FBS. The results of these experiments revealed a striking asymmetry in the rates of copper uptake, with significantly higher copper transport taking place across the basolateral side. This correlated well with the subcellular localization of endogenous hCTR1 determined from cell surface biotinylation and by confocal microscopy (53).
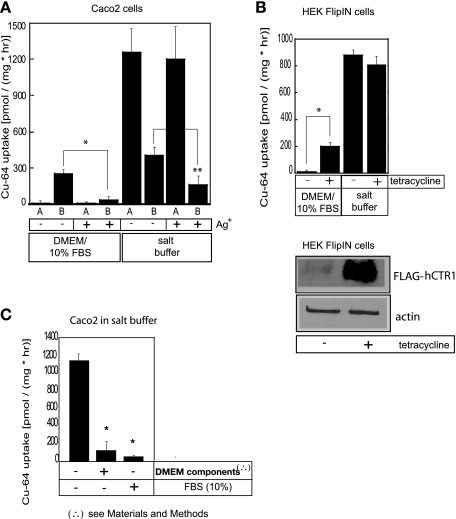
In the present work, when radioisotopic copper uptake rates were measured in HEPES-buffered salt solution, the asymmetry in the rates of copper transport across each side of polarized Caco2 cells was no longer as pronounced, and their relative amplitude changed dramatically (Fig. 1A). The ratio of apical to basolateral copper uptake changed from 1:10 in the growth medium, to about 3:1 in the salt buffer. The absolute values of the apical fluxes were much higher in the salt buffer (Fig. 1A), and the copper transport across the apical membrane was not sensitive to the presence of silver ions known to inhibit CTR1-mediated copper transport (28). In salt solution, however, there is some (∼50%) inhibition of the basolateral Cu uptake by Ag ions reflecting CTR1-mediated uptake, in comparison with ∼90% inhibition in growth media with FBS (see Fig. 1A).
Fig. 1.
Copper uptake in growth media and transport buffer. A: polarized Caco2 cells. Caco2 cells were grown in Transwells until polarized, as determined by the transepithelial resistance reading of 250 Ω·cm2. Before the experiment, cells were washed and growth medium was replaced with the transport buffer consisting of either DMEM medium supplemented with 10% FBS and buffered at pH 7.4, or saline buffer composed of 150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, and 25 mM HEPES, pH 7.4. Uptake was initiated by addition of 5 μM CuCl2 labeled with trace amounts of 64Cu to either the apical (A) or basolateral (B) compartments of each Transwell at 37°C. The radioactivity retained by the cells after 1 h was determined by scintillation counting. Effect of silver on copper uptake was measured following the addition of 50 μM AgNO3 to the transport buffer on both sides of the Transwells. Additions to the apical side of Transwell are indicated with letter A, and additions to the basolateral side are indicated with letter B. (A, apical; B, basolateral). Data are displayed as means ± SD; *, **P < 0.005. B: human embryonic kidney (HEK) cells. HEK cells, which overexpress human (h)CTR1 in the presence of tetracycline, were grown in the presence (1 μg/ml) (+) or absence (−) of the antibiotic for 48 h. For copper uptake assays, the HEK cells were seeded in 12-well plates and grown for 2 days. Just before the uptake studies, cells were washed and growth medium was replaced with transport buffer consisting of either FBS-supplemented DMEM or salt buffer. 64Cu uptake was assayed as described above for Caco2 cells. Mean ± SD values are shown for each point. *P < 0.005. Below, Western blot analysis of expression levels of FLAG-tagged human copper transporter 1 (hCTR1) protein in uninduced (−) or tetracycline-induced (+) HEK cells. Total membranes (50 μg), prepared from the cells used for the 64Cu uptake experiments, were loaded on 10% SDS-PAGE gel. Proteins were blotted onto nitrocellulose membrane and probed with the antibody to the FLAG-tag epitope of hCTR1 to detect overexpressed protein. Anti-actin antibody was used as a loading control. C: DMEM components and 10% FBS inhibit apical copper uptake. Caco2 cells were grown in Transwells until polarized. Apical copper uptake was assayed by addition of 5 μM radioactively labeled CuCl2. Experiments were done in salt buffer alone, in salt buffer with the added components of DMEM (as listed in materials and methods), or in salt buffer with 10% FBS. Assays were run for 1 h at 37°C, terminated with cold stop buffer, and rinsed 2× more with cold stop buffer. Filters were excised and radioactivity was determined using a scintillation counter. Mean ± SD values obtained from triplicate measurements are shown for each point. *P < 0.005.
To see whether the enhanced rate of Cu uptake, revealed in intestinal cells when salt solutions are used in place of the growth media, is a general cellular phenomenon of Cu transport, we examined Cu uptake in different cell lines. We measured copper fluxes into HEK cells, in which an epitope-tagged form of hCTR1 is overexpressed under the control of a tetracycline-regulated promoter (33). It can be seen that copper uptake rates in HEK cells are quite low when uptake is measured in FBS-supplemented growth medium but are greatly enhanced when hCTR1 levels are increased by overexpression of hCTR1, following growth in tetracycline (Fig. 1B). When these measurements were repeated in HEPES-buffered saline medium, we observed elevated copper transport rates whether or not hCTR1 expression was induced by tetracycline (Fig. 1B). Apparently, the high rates of copper uptake in saline media obscured the lower rates induced by hCTR1 expression. Thus, it seems likely that the mechanism of the rapid copper uptake, observed in salt media in HEK cells, is related to the high rates of copper uptake observed at the apical side of Caco2 cells in saline buffer.
Dependence of copper uptake rates on ionic composition of the medium.
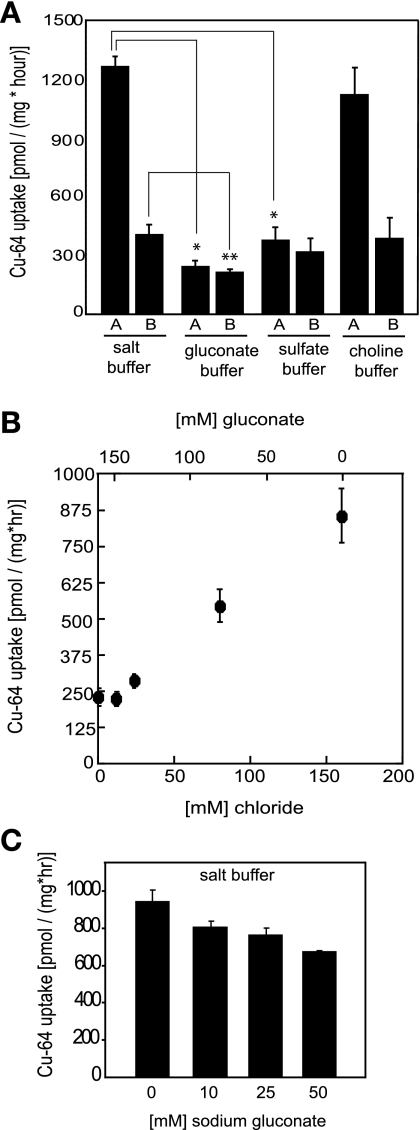
To evaluate the influence of the major ionic components of the media on the rate of copper uptake in the salt buffer, we substituted chloride with either gluconate or sulfate, keeping the relative ionic strength of the buffer constant. Total replacement of chloride in the buffer by gluconate resulted in a dramatic reduction of copper uptake in polarized Caco2 cells (Fig. 2A). The rates were inhibited by about 80% and 30% across apical and basolateral surfaces, respectively. Substitution of chloride ions by sulfate had a similar effect (Fig. 2A). Total replacement of sodium ions (Na), the major extracellular cation by choline, had no effect on the rate of copper entry (Fig. 2A). These results suggested that the major entry mechanism for copper ions from buffered salt solutions did not require Na ions, but depended on the presence of chloride ions in the extracellular media. Variation in the total chloride ion concentration from 0 to 160 mM, at the expense of gluconate in transport buffer, resulted in stimulation in the rate of apical copper entry (Fig. 2B). It appears that the major apical copper entry pathway from saline media shows a strong requirement for chloride ions, with a relatively low apparent affinity. Experiments carried out in HEK cells showed a similar ionic dependence (data not shown). Chloride removal reduced most of the apical uptake of 64Cu, and the residual transport in the absence of Cl ions presumably utilizes a different pathway (see discussion).
Fig. 2.
Copper uptake and ionic composition of transport buffer. A: replacement of chloride and sodium ions in transport buffer. Caco2 cells were grown in Transwells and processed for the assay as described in Fig. 1. Transport buffer consisted of either standard salt buffer (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4), or chloride-free gluconate buffer (150 mM Na-gluconate, 5 mM K-gluconate, 25 mM HEPES, pH 7.4), or chloride-free sulfate buffer (100 mM Na2SO4, 3.3 mM K2SO4, 3.75 mM MgSO4, 25 mM HEPES, pH 7.4). In addition, a sodium-free choline buffer was prepared, consisting of 150 mM choline chloride, 5 mM KCl, 2.5 mM MgCl2, and 25 mM HEPES, pH 7.4. All the assays were begun by addition of 5 μM radioactive CuCl2 and were carried out for 1 h at 37°C. Mean ± SD values are displayed at each point. *P < 0.0005, statistically significant decrease of Cu-transport in gluconate and sulfate buffers compared with Cu-uptake in transport buffer; **P < 0.05, statistically significant basolateral Cu uptake in gluconate buffer compared with that in transport buffer. B: dependence of copper uptake in Caco2 cells on increasing chloride ion in transport buffer. Caco2 cells were grown in Transwells until polarized. Copper uptakes were assayed from apical side of polarized monolayers, as described in materials and methods, using 5 μM radioactively labeled CuSO4 for each measurement. Transport medium consisted of gluconate buffer with increasing concentrations of chloride ion (0–160 mM) at the expense of gluconate (160–0 mM). Data are displayed at each point as means ± SD. C: copper uptake in hypotonic saline medium. Caco2 cells were grown in Transwells until polarized and copper uptakes were assayed from the apical side using 5 μM radioactively labeled CuCl2. Transport buffer consisted of either standard saline medium (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4), or hypotonic saline medium prepared by supplementing salt buffer with additional 10, 25, or 50 mM sodium gluconate (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4 plus 10 mM, 25 mM, or 50 mM sodium gluconate). Assays were run for 1 h at 37°C, terminated with cold stop buffer, rinsed twice with cold stop buffer. Filters were excised, and radioactivity was determined using a scintillation counter. Our experiments were carried out three times, and in each experiment all determinations were measured in triplicate and are shown as means ± SD.
The removal of chloride ions from the transport buffer by replacement with gluconate or sulfate (Fig. 2A) could cause Cl exit from the cells, salt loss, and cell shrinkage. Following such an event, volume-regulatory mechanisms, which have been described for many epithelia, would presumably be activated. It is possible that either the change in cell volume itself or the activation of compensatory pathways may affect the observed rates of copper transport. However, we do not think that the dramatic reduction in copper uptake rates, ∼80% upon Cl removal, reflects inhibition of Cu uptake through a volume-sensitive mechanism. Although we have been unable to find a precise value for internal Cl concentration in Caco-2 cells, it is likely to be low, in the range of 10–40 mM, similar to the concentration reported in other mammalian epithelial cells (44). In that case, cell shrinkage would be small even in the absence of volume-compensatory mechanisms. Furthermore, Fig. 2B shows a fairly monotonic effect on the rate of copper uptake across a wide range of extracellular chloride/gluconate concentrations, encompassing Cl concentration ([Cl]) values ranging from very low (which would cause cellular volume shrinkage) to normal, which would lead back to volume increase. Although we cannot conclude that under these conditions there is no modulating effect of cell volume on copper transport, it is more likely that we are observing a genuine requirement for Cl in the transport mechanism (Fig. 2B).
As an experimental test of the possible effects of cell shrinkage following chloride replacement, we examined the effect of placing the cells in hyperosmotic media before measuring Cu uptake. Increasing medium osmolarity by the addition of up to 50 mM sodium gluconate (that is 100 mosM) causes only ∼25% reduction in the rate of Cu uptake (Fig. 2C), while smaller increases in tonicity had less effect. The osmolarity of the DMEM growth media is higher (∼360 mosM), not counting the minor contribution from amino acids and vitamins, than the normal osmolarity of blood serum (∼300 mosM). In the experiments shown in Fig. 2C, and in most of our experiments, we used HEPES-buffered saline (salt buffer, 360 mosM) that matched the osmolarity of the growth media. We decided to carry out experiments similar to those shown in Fig. 2C, but with the control salt buffer containing 125 mM instead of 150 mM NaCl, resulting in a final osmolarity of transport buffer being ∼310 mosM, which well approximates the physiological osmolarity of blood. Addition of 10 mM Na gluconate to this transport buffer again caused a decrease in transport rate of ∼30% (from 1,030 ± 33 pmol Cu·mg protein−1·h−1 to 728 ± 133 pmol Cu·mg protein−1·h−1), and the addition of 25 mM Na gluconate caused a similar decrease (to 710 ± 69 pmol Cu·mg protein−1·h−1). Thus, Cu transport in saline buffer with osmolarity similar to that of serum closely matches the rates of copper transport in the elevated osmolarity of the growth media (Fig. 2C), and the effects of increasing tonicity are the same. Therefore, the data in Fig. 2A most likely demonstrate that the Cu uptake is dependent on the presence of chloride and is not due to a volume-dependent regulation of Cu transport.
Inhibition of copper uptake by histidine.
The high uptake rates observed in buffered saline media (this work and Ref. 28) are greatly reduced when transport is measured in DMEM growth medium supplemented with 10% FBS. DMEM is a complex mixture of amino acids, vitamins, and inorganic salts. To examine the effect of DMEM constituents on copper transport (without FBS supplementation), we added the mixture of amino acids and other substrates found in DMEM to our standard salt buffer (see materials and methods). This led to a dramatic inhibition of the high rate of Cu uptake (Fig. 1C). In a similar fashion, FBS on its own (without DMEM) caused a large reduction in the rate of Cu uptake when added to the salt buffer (Fig. 1C).
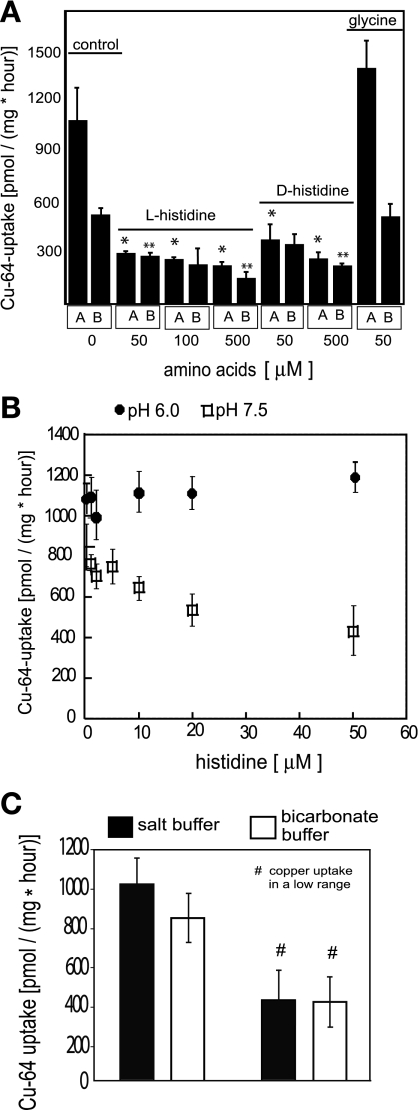
We reasoned that among various amino acids found in DMEM, the ones that are able to bind copper could affect its transport rates (see Fig. 1C). We investigated the effects of histidine, which is known to form stable complexes with copper, on the rates of Cu uptake in Caco2 cells. We found that the addition of histidine to saline solution decreased copper uptake across the apical and the basolateral surfaces of polarized Caco2 cells (see Fig. 3A). At pH 7.4, 100 μM histidine caused ∼80% inhibition of apical copper uptake and 50% inhibition of basolateral uptake. As noted above (Fig. 1C), the addition of 10% FBS to the transport buffer resulted in a 90% inhibition of 64Cu uptake, and albumin has a similar effect (Molloy S and Kaplan JH, unpublished observations). When the naturally occurring l-histidine was substituted with the d-isomer, the inhibitory effect remained unchanged (Fig. 3A), suggesting that the effect did not involve a specific interaction at a protein site. The addition of glycine had no effect on the apical or basolateral rates of copper uptake into Caco2 cells (Fig. 3A). In HEK cells, histidine inhibited ∼70% of the total copper uptake from buffered saline (data not shown). Since albumin and histidine (either d- or l-isomer) are known to bind copper, while glycine does so only weakly, the inhibitory effect of albumin and histidine might be due to the formation of complexes that reduce the level of the transported substrate. This notion is further supported by the observation that lowering the pH from 7.4 to 6.0 results in a loss of the inhibitory effect of histidine on copper uptake (Fig. 3B). Complexation of copper by histidine involves direct coordination with the α-amino residue of histidine in the Cu(His)2 complex (12). The pK of this residue is 9.18, ensuring that, below pH 7.4, <1% of the histidine present would be available for complexation. Therefore, at pH 6.0, inhibition of the copper uptake is no longer apparent (Fig. 3B).
Fig. 3.
Effects of amino acids and carbonate on copper uptake. A: inhibition of copper uptake by histidine. Cells were grown on filters until polarized. Assays were carried out in transport salt buffer consisting of 150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, and 25 mM HEPES, pH 7.4, as described in Fig. 1. Uptake was initiated by addition of 5 μM CuCl2 labeled with trace amounts of 64Cu to either the apical or the basolateral compartments of each Transwell. Where indicated, l-histidine, d-histidine, or glycine was included in either the apical (A) or basolateral (B) compartment of Transwells. Assays were run for 1 h at 37°C, filters with cell monolayers were excised, and the amount of radioactivity in the cells was determined by scintillation counting. All values are displayed as an average of three measurements with the SD shown for each point. *P < 0.005, statistically significant for the apical copper uptake in unsupplemented transport buffer (control), and in buffer supplemented with amino acids; **P < 0.05, statistically significant for the basolateral copper uptake in saline transport buffer (control), and in transport buffer supplemented with amino acids. B: lowered pH reduces inhibition of chloride-dependent apical copper uptake by histidine. l-histidine was added into the transport salt medium, buffered with 25 mM HEPES, pH 7.4, or with 25 mM PIPES buffer, pH 6.0. ●, uptake at pH 6.0; □, uptake at pH 7.4. Values are shown as means ± SD of each point. C: effect of bicarbonate on copper uptake. Caco2 cells were grown in Transwells until polarized and assayed for 64Cu uptake from the apical side as described in materials and methods. Transport buffer consisted of standard HEPES-buffered saline medium (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4) or bicarbonate buffer (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, and 25 mM NaHCO3, pH 7.4). The mean ± SD obtained from triplicate measurements for 10 independent experiments is shown for each point (#three independent experiments that fell into low range of copper uptake).
In the lumen of the proximal intestine, significant quantities of bicarbonate are secreted. To see the extent to which the presence of bicarbonate might affect the rates of Cu uptake, we replaced HEPES with sodium bicarbonate as the buffer. It is apparent that the addition of 25 mM NaHCO3 results in a small reduction (∼20%) in the rapid rate of Cu uptake (see Fig. 3C). During these studies, we observed that, in some preparations, the rate of Cu uptake in salt buffer was ∼50% of its more frequently seen value (∼400 pmol·mg protein−1·h−1 compared with ∼1,000 pmol·mg protein−1·h−1 typically measured). The effect of bicarbonate was less apparent in these preparations (Fig. 3C). We conclude from this that the rapid Cu uptake pathway is operative in the presence of bicarbonate.
The role of the copper oxidation state.
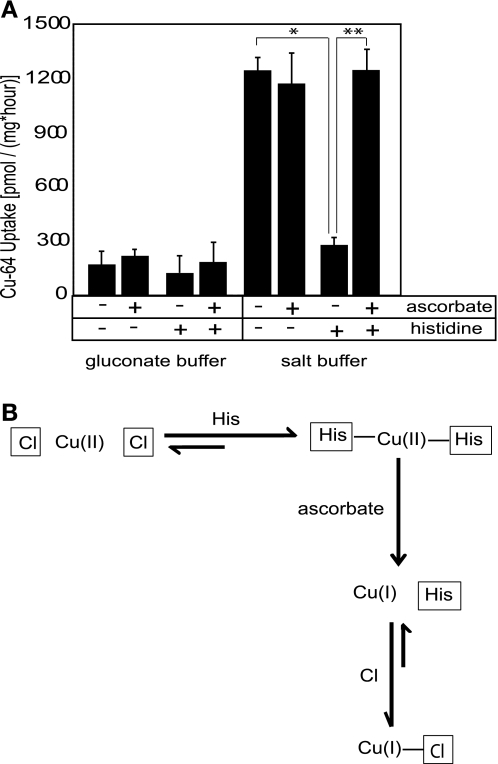
Although previous studies suggest strongly that hCTR1 transports Cu(I), it is not known whether Cu(I) or Cu(II) is involved in the chloride-dependent pathway that we observe. Spectrophotometric measurements utilizing bathocuproine disulfate (10) showed that 1 mM ascorbate reduces all of the Cu(II) chloride used in our transport assays (1–10 μM) to Cu(I), without changing the pH of the transport medium (data not shown). The reducing agent ascorbate had no significant effect on copper uptake in the salt medium at pH 7.4 (Fig. 4A). However, when salt media were supplemented with histidine, and 1 mM ascorbate was added to reduce copper before the uptake measurement, the inhibitory effect of histidine was lost (Fig. 4A). Moreover, the inhibition of copper fluxes in histidine-supplemented medium could be completely reversed by subsequent addition of ascorbate, returning the copper fluxes back to their original value observed in histidine-free buffer (Fig. 4A). These data have two implications: first, that the chloride-dependent pathway is equally effective in transporting Cu(I) or Cu(II) at pH 7.4; and second, that the transport of Cu(I) is not susceptible to inhibition by histidine.
Fig. 4.
The role of the oxidation state of copper. A: ascorbate reverses histidine inhibition of chloride-dependent copper uptake in Caco2 cells. Cu uptakes in Transwells were determined in either salt transport buffer (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4) or chloride-free gluconate buffer (150 mM Na-gluconate, 5 mM K-gluconate, 25 mM HEPES, pH 7.4). Histidine (100 μM) and/or ascorbate (1 mM) was included in the apical compartment of Transwells as indicated in the table. Values are shown as an average ± SD at each point. Two-tailed independent Student's t-test was performed; *P < 0.005 for control vs. histidine-supplemented buffer; ** P < 0.005 for histidine vs. histidine plus ascorbate buffer. B: mechanism of reversal of histidine inhibition by ascorbate. Histidine (pKa 6.0) when placed in solution at pH 7.4 is deprotonated and will compete with chloride ions to form a His2Cu complex with copper (II) via its imidazole nitrogen. Once the copper-histidine complex is formed, it cannot be transported into the cell as a pseudo-substrate of the anion exchanger, which results in an inhibition of copper uptake. However, when copper is reduced by ascorbate, Cu(I)-histidine complexes are less stable than Cu(II)-histidine complexes, and chloride ions, present at ∼150 mM in physiological solution, will compete successfully with histidine for Cu(I). Cu(I)-chloride complexes can enter the cell via the anion exchange mechanism, which results in stimulation of copper uptake (i.e., apparent reversal of histidine inhibition).
To account for these data, we propose (Fig. 4B) that histidine added to the transport buffer will bind Cu(II) to form copper-bis-histidine complexes, which cannot be utilized as a substrate by the relevant anion transporter, hence the inhibition of copper uptake. Addition of ascorbate results in the reduction of Cu(II) in the copper-bis-histidine complexes, and destabilization of these complexes since histidine binds only weakly to Cu(I).
The effects of inhibitors of anion exchange systems.
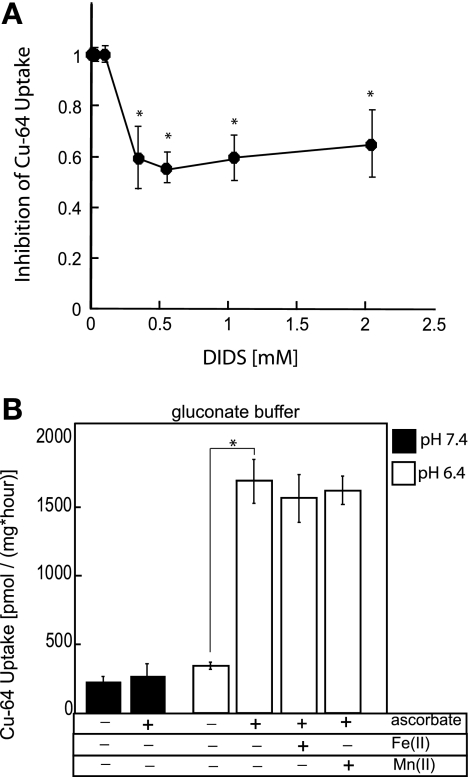
We have suggested that copper ions in saline buffer are complexed with chloride ions and handled as pseudo-substrates of some cellular anion transport system, perhaps an anion exchanger. We tested various inhibitors (Table 1), such as those for chloride channels, 5-nitro-2-β-phenylpropylamino benzoic acid (NPPB); the carbonic anhydrase inhibitor, acetazolamide; NHE sodium-hydrogen exchanger inhibitor, dimethylamiloride; Na+-K+-2Cl− cotransporter (NKCC) inhibitor, bumetanide; organic anion exchanger inhibitor, probenacid, as well as the classic anion-exchanger inhibitor DIDS, and the SLC26A6 anion exchange inhibitors niflumic acid and glybenclamide. Only the stilbene disulfonate DIDS, at relatively elevated concentrations, significantly diminished copper uptake at the apical side of polarized Caco-2 cells (by ∼50%, see Fig. 5A). This is in contrast to chloride-dependent mechanism of copper uptake via anion exchanger AE1 (Band 3) described for human erythrocytes (1), where low micromolar concentrations of SITS or DIDS are reported to inhibit >97% of copper uptake. In contrast to erythrocytes, the increase in the concentration of DIDS to as high as 2 mM did not eliminate more than ∼50% of anion-dependent copper fluxes in Caco2 cells (Fig. 5A). This is reminiscent of the greatly reduced sensitivity of epithelial anion exchangers to the disulfonic stilbenes, where even high concentrations cause only incomplete inhibition (2, 8, 18, 24, 51).
Table 1.
List of inhibitors
| Inhibitor Concentration, μM | Cu Uptake, pmol•mg−1•hour−1 | |
|---|---|---|
| Control (Caco2 cells) | 1,100 ± 120 | |
| Acetazolamide | 100 | 1,375 ± 120 |
| Bumetanide | 20 | 1,430 ± 120 |
| Dimethylamiloride | 500 | 1,485 ± 110 |
| Glybenclamide | 300 | 1,320 ± 110 |
| Niflumic acid | 300 | 880 ± 150 |
| 5-Nitro-2-β-phenylpropylamino benzoic acid | 50 | 1,150 ± 100 |
| Probenacid | 500 | 770 ± 80 |
Effects of inhibitors on the rates of copper uptake were measured at the apical side of polarized Caco2 cells in saline transport buffer (150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, 25 mM HEPES, pH 7.4). Cells were preincubated with indicated concentrations of inhibitors at the apical side for 30 min before the assay, after which the assay was initiated by addition of 5 μM radioactive CuCl2 to the apical compartment of Transwell. Rates were determined as pmoles of radioactive copper per milligram total protein per hour, in triplicate in three independent experiments, and are reported as means ± SD.
Fig. 5.
Role of anion exchangers and DMT1 in copper transport. A: inhibition of apical copper uptake in polarized Caco2 cells by DIDS. Cells were grown on Transwells until polarized. Assays were carried out in transport buffer consisting of 150 mM NaCl, 5 mM KCl, 2.5 mM MgCl2, and 25 mM HEPES, pH 7.4, supplemented with increasing concentration of DIDS. DIDS was added to the upper (apical) compartment of Transwells, and cells were preincubated with the inhibitor for 30 min before the addition of radioactive copper. Assays were carried out for 1 h at 37°C. Mean ± SD values obtained from triplicate measurements are shown at each point. *P < 0.05, Student's t-test on Excel. B: ascorbate stimulates copper uptake in chloride-free medium at low pH. Chloride-free, gluconate transport medium was buffered with 25 mM HEPES, pH 7.4 (solid bars), or 25 mM PIPES, pH 6.4 (open bars). Where indicated, transport buffer was supplemented with 1 mM ascorbate, 50 μM iron(II), or 50 μM manganese(II). Caco2 cells were cultured on Transwells until polarized, and all 64Cu uptakes were measured from the apical side only. Values represent means ± SD displayed at each point. *P < 0.0005, statistically significant.
Role of DMT1 in apical Cu fluxes.
To determine the contribution of DMT1 to the apical copper transport in Caco2 cells, we conducted competition studies between radioisotopic copper and Fe(II) or Mn(II), two well-characterized and favored substrates of DMT1, using chloride-free medium at low pH, and in the presence of ascorbate. Neither Fe(II) nor Mn(II) had any effect on radioisotopic copper uptake (Fig. 5B), implying a minor (if any) role for DMT1 in copper transport.
Anion-dependent copper uptake in various cell lines.
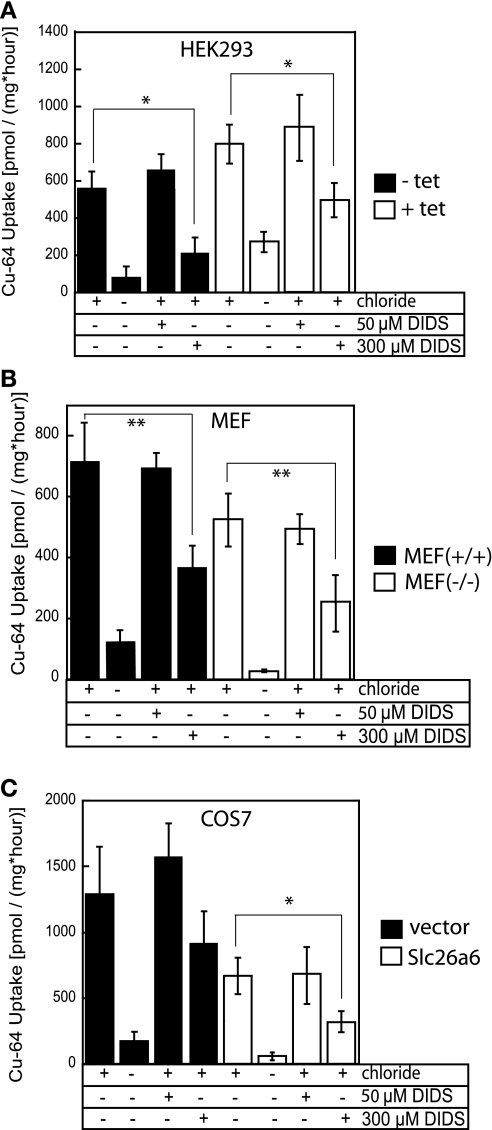
The similar observations of enhanced Cu uptake in saline buffer, and its strong dependence on the presence of chloride ions, were made for epithelial intestinal Caco2 cells and for nonepithelial HEK cells (Fig. 1, A and B), prompting us to examine these phenomena in several other cell lines. Removal of chloride reduced the uptake of copper by at least 50% in HEK cells, MEF, and African green monkey kidney fibroblast-like Cos-7 cells (Fig. 6, A, B, and C, respectively). This demonstrates that the anion-dependent uptake mechanism is conserved in a variety of cell lines. It is also likely to be mediated by a relatively DIDS-insensitive anion exchange mechanism, because 300 μM DIDS inhibited no more than 50–70% of copper fluxes in these cell lines (Fig. 6).
Fig. 6.
Effects of chloride and DIDS on copper uptake in variety of cell lines. Cells were grown on plastic dishes, and 64Cu assays were performed as described in materials and methods. DIDS inhibitor, at either 50 or 300 μM, was added to the transport buffer, and cells were incubated with inhibitors for 30 min before the beginning of the assay, after which 64Cu was added. A: HEK 293 cells. HEK cells were grown in the absence (solid bars) or presence (open bars) of 1 μg/ml tetracycline (tet) for 48 h to cause overexpression of hCTR1. B: mouse embryonic fibroblasts (MEF). Wild-type MEF+/+ (solid bars), or MEF−/− (open bars) derived from CTR1−/− embryos, where the CTR1 gene is deleted (28). C: Cos-7 cells. Wild-type Cos-7 cells (solid bars) and Cos-7 cells stably expressing Slc26a6 (PAT1; open bars) anion exchanger (see materials and methods). All values are shown as an average ± SD of three measurements determined for each point. Two-tailed independent Student's t-test was performed; *P < 0.005, **P < 0.05, statistically significant for control and inhibitor.
DISCUSSION
The precise mechanism by which humans acquire their essential dietary copper has been brought into question by the recent findings that intestinal hCTR1, the major human high-affinity copper transporter, is localized at the basolateral rather than the apical membranes of epithelial cells of the intestine and kidney (25, 53). These results, along with related findings (see below), suggest that hCTR1 is not directly involved, or at least is not the major player, in the uptake of copper from the digested dietary components that have left the stomach and entered the intestinal lumen. The present work was undertaken to functionally identify mechanisms by which copper enters enterocytes at the apical side of epithelium, the first step in the process of intestinal copper absorption.
We have shown that a large fraction of the copper uptake depends on the presence of chloride ions, is not dependent on Na ions, and can be inhibited by histidine at neutral pH. We suggest that copper ions in the form of metallo-anion complexes are handled as surrogate substrates by anion transport systems in the apical membrane. This system is inhibited in normal serum but is responsible for much of the copper uptake from the early intestinal lumen. The high levels of copper uptake from buffered salt solutions experimentally observed in a variety of cells, including MEF−/− cells where hCTR1 gene was deleted (28), all exhibit this chloride-dependent mechanism.
Composition of extracellular medium and copper uptake.
The overexpression of hCTR1 in a variety of mammalian cells results in increased rate of copper uptake (14, 28) when assayed in growth media or similarly supplemented solution. However, it has been observed that the rate of copper uptake is significantly higher when transport buffer is used without added components of histidine, albumin, or serum (28). In the present work we have confirmed this finding and show that the ionic composition of the extracellular medium has a profound effect on the rate of copper uptake. This holds true at the apical side of Caco2 cells (see Fig. 1A), as well as in a variety of other cell lines (Fig. 6). The high copper uptake is unaffected by the replacement of Na ions by choline (Fig. 2), but it is dramatically reduced by the replacement of Cl ions by either gluconate or sulfate. This immediately suggested to us the involvement of a Cl-transporting uptake system. Among the many possible candidates, NKCC, or Na+-K+-Cl−-Cl− family of cotransporters, was eliminated because of the lack of effect of Na (see Fig. 2A) and lack of inhibition by bumetanide (Table 1). Many chloride channels can be inhibited by NPPB, but NPPB had no effect on the copper fluxes we measured. However, the chloride-dependent copper uptake was partially inhibited by the stilbene disulfonic acid DIDS, which is widely employed to inhibit the anion exchangers.
Cl−/HCO3− exchangers constitute a large family of proteins that includes AE exchangers (41), as well as structurally unrelated SLC26 transporters (36). The classical anion exchanger AE1 of red blood cells and renal cells is very sensitive to the addition of DIDS in a low micromolar range, while the wide array of epithelial anion exchangers show significantly less sensitivity, and only partial inhibition (50–80%) by DIDS, in the concentration range of 0.1 to 1 mM (2, 8, 18, 24, 51). The copper uptake that we observe in Caco2 cells shows a similarly low sensitivity to these inhibitors: at the apical surface of intestinal cells we see ∼50% inhibition with 0.3 mM DIDS, and other cell lines tested were also relatively insensitive to stilbene disulfonic acid inhibitors.
It is apparent that the Cl-dependent pathway is operative at both surfaces. It seems likely that anion exchanger systems at either surface can mediate Cu uptake. The extent to which such pathways contribute to Cu uptake under physiological conditions will depend on the extent to which the components in the extracellular media (intestinal lumen or serum) inhibit these pathways. It is well known that multiple anion exchangers reside in the membranes of intestinal cells, and there has been little characterization of their ability to mediate the transport of Cu-anion complexes. However, the ability of the AE1 anion exchanger found in red blood cells to mediate such transport, as well as that of other related heavy metal ion complexes, is well documented (1, 23, 32, 40, 41).
It is significant that the inhibition we observed due to the presence of histidine occurs with either of its isomeric forms (l- or d-). Thus it seems unlikely that the copper enters cells as copper-histidine complex via amino acid transporter. In such a mechanism, the protein mediating the transport pathway (such as an amino acid transporter) would interact only with l-isoform of histidine and would be inhibited by the d-isomer. The most abundant anion in the transport medium is chloride (>150 mM), which is known to form (anionic) complexes with copper ions in solution. Chloride will successfully compete with histidine for Cu(I) binding (see scheme in Fig. 4B), resulting in reversal of the inhibition of copper uptake. We propose that the transported substrate in saline solution is a complex of chloride ions and Cu. It has been previously reported (1) that Cu(II) entry into human erythrocytes is largely mediated by the anion exchanger AE1 and is inhibited by histidine. We propose that an anion transporter may be involved in Cu uptake in intestinal cells, mediating the uptake of Cu as a [CuCln] complex, possibly also containing bicarbonate or hydroxyl ions.
Anion transporters and copper transport.
It has been previously reported that when human red blood cells are suspended in buffered saline, there is a significant rate of copper uptake that is almost completely inhibited by DIDS (1) or by the addition of histidine to the media. It was suggested that anionic complexes formed between copper and anions (especially chloride) were transported as surrogate substrates by the anion exchange system (1). Such a mechanism has also been reported for the uptake of lead (38, 39), zinc (23), and cadmium (30) in human red blood cells. This suggests that these anion transporters may play a hitherto unexpected role in metal homeostasis in humans (see below).
In the present studies it seems most likely that when cells are suspended in chloride-containing media, anionic copper-chloride complexes are transported into the cells by anion transporters as pseudo-substrates. The formation of copper-histidine complexes decreases the amount of available substrate and results in the inhibition of uptake via the anion transporter. Such inhibition occurs equally readily with l- or d- isoforms of histidine but is greatly reduced at low pH because histidine is protonated under acidic conditions.
Anion exchange: physiological role in apical copper uptake.
On the basis of the present data, it appears that in many physiological situations the presence of histidine, albumin, or other components in the serum would serve to limit the involvement of anionic complexes in cellular copper uptake of copper from blood. Although histidine and histidine-containing small peptides in the early part of the intestinal lumen might otherwise reduce the significance of this pathway, the low luminal pH greatly reduces the capability of these components to form complexes with copper. Under these conditions, the formation of anionic complexes with either chloride or bicarbonate would be favored, and uptake by this pathway enabled.
The energy of the sodium-electrochemical gradient drives the uptake of amino acids at the brush border of intestinal epithelium. Copper readily forms histidine chelates at neutral pH, and therefore some of the dietary copper could be absorbed via amino acid/peptide transporters as has been reported in some fish (17). However, if copper was taken into the cell as copper-amino acid chelate, then removal of sodium from the uptake medium should be inhibitory, and only one isomer of histidine, the naturally occurring l-form, would be recognized. Since sodium had no effect on copper uptake in Caco2 cells, and there was no difference between l- and d-isomers of histidine in their effect on copper uptake and no competition by glycine, the possibility of transport of intact copper-bis-histidine complexes was eliminated as one of the significant mechanisms of intestinal copper transport.
On the basis of our ion-replacement experiments, we found that the high-capacity copper fluxes in HEPES-buffered saline medium are strongly chloride dependent, redox independent, sensitive to histidine inhibition, and independent of the oxidation state of copper. These characteristics correspond closely to the description of copper uptake observed in human erythrocytes mediated by the anion exchange protein Band 3 (AE1), which can be inhibited (>97%) by micromolar concentration of SITS and DIDS (1, 7). In the case of Caco2 cells, it is not likely that the classic anion exchanger of an AE1 family is involved, because DIDS has only partial inhibitory effect on copper uptake even at concentrations as high as 300 μM–1 mM. This suggests the involvement of relatively DIDS-insensitive exchangers that are often observed in epithelial cells, and which may belong to the SLC26A family. Two members of this family are expressed at the mucosal surface of intestinal epithelia, PAT1 (SLC26A6) present in the small intestine (40) and DRA (SLC26A3) found in colon (4), both of which bear <15% homology to an AE family and are not affected by low concentrations of SITS and DIDS. In the present work we carried out an initial examination of copper uptake into Cos-7 cells, which were stably overexpressing PAT1 transporter in the plasma membrane. However, the copper transport by these cells was no different from the original Cos-7 cell line from which they were derived. Thus it seems that PAT1 is unlikely to play a major role in intestinal copper entry. There is a large number and a wide variation among the many anion transporters in cells (13), and any of these may participate in the transport of a surrogate substrate such as chloro-copper complexes. Indeed, in epithelial cells, several members of this family of transporters may contribute to metal ion homeostasis in general, and copper in particular. Of course, other anion transporters might also play this role.
It is generally assumed that dietary Cu is absorbed in the proximal segments of the small intestine and the stomach, and it has been reported that the majority of the Cu absorption in the small intestine of humans takes place in the duodenum (49). Recent studies of the identity and roles of anion transporters in intestinal epithelia revealed a very complex picture. The family of human anion transporters SLC26 is coded by 11 genes. SLC26A3, SLC26A4, and SLC26A6 are expressed in the luminal membranes of many epithelia and carry out Cl−/HCO3− exchange, while SLC26A2 is ubiquitous in epithelia and functions as a SO42− transporter (36). It has also been reported that SLC26A6 (or PAT1) is the predominant apical membrane Cl−/HCO3− transporter in the murine duodenal epithelium (40); furthermore, SLC26A3 (or DRA) is expressed at low levels in the duodenum as is SLC26A4 (or pedrin) (see Ref. 48). In addition, the structurally poorly related, but functionally similar, Cl−/HCO3− exchanger AE4 has also recently been reported to be expressed in the apical membrane of duodenal epithelia of the mouse (52). In this latter work it was reported that at least three anion exchangers are expressed apically in the duodenum, PAT1, DRA ,and AE4, in decreasing levels of expression and that it would be difficult to conclude that one anion exchanger was the major transporter. Thus it is quite possible that several or all of these transporters are able to contribute to Cu uptake in the intestine. Several of these transporters have been reported to be on the basolateral surface of intestinal cells, so that anion transporter-mediated Cu uptake could occur at either cell surface.
Physiological relevance: DMT1 and the chloride pathway.
It has been reported that the divalent metal ion transporter DMT1 is a physiologically relevant transporter of Cu(I), rather than Cu(II), mediating its apical uptake into Caco2 cells (3). To distinguish DMT1-mediated copper transport from anion-dependent copper fluxes, we used chloride-free, histidine-free saline medium, in the presence and absence of ascorbate. The effect of ascorbate was negligible at physiological pH (see filled data in Fig. 5B), but it stimulated copper uptake by approximately three- to fivefold at pH 6.4 (Fig. 5B, histograms 5 and 6). Arredondo et al. (3) made a similar observation that ascorbate stimulates uptake of copper from copper-histidine complexes by approximately sevenfold. However, because histidine was used in those studies, we believe that the effects of ascorbate in that work can be at least partially explained by a relief of histidine-inhibition of Cu(II) uptake and activation of the anion-dependent uptake of Cu(I) complexes.
We investigated the role of DMT1, but detect no inhibition of apical copper uptake by either Fe(II) or Mn(II) ions, the two favored substrates of DMT1 in Caco2 cells. It has been claimed that DMT1 specifically transports copper (I), while the ingested dietary copper is likely to be copper (II), due to the oxidizing function of the environment (47). Therefore, the uptake of copper through DMT1 would have to depend on either the presence of reducing factors derived from food, such as ascorbate, or the action of a curpric reductase at the plasma membrane. No specific mammalian Cu-reductase proteins have been identified so far, although some iron-reducing proteins such as those of STEAP family, or intestinal Dcytb family, have been reported to be capable of copper reduction in vitro (50).
Perhaps the best way to assess a potential physiological role of DMT1 in Cu transport is to examine animal models used to study DMT1, which invariably point to its primary role in iron, rather than copper, metabolism. For example, the phenotype of the Belgrade rat with an inactivating mutation in DMT1 is characterized by iron, rather than copper, deficiency (15). Moreover, human patients with a hereditary mutation in DMT1 suffer from Fe-deficient microcytic anemia but have normal levels of copper (6, 22, 32). Thus, although the high-affinity, redox-dependent uptake through DMT1 might contribute to the dietary acquisition of copper under redox-favorable conditions, it is probably not a major pathway. The residual, chloride-independent apical copper uptake, seen in gluconate or sulfate buffer, may be mediated by DMT1 or some other transporter. The chloride-dependent copper transport is redox independent and is likely to be important in the upper intestine, where the chloride concentration is high due to hydrochloric acid secretion by the stomach, and where peptides and amino acids are protonated due to a low pH, and thus not likely to bind copper. The enhanced levels of anionic complexes with chloride ions (and perhaps bicarbonate or hydroxyl) enable the anion exchange pathways to play a major role.
The total Cu uptake pathway(s) in ctr1−/− MEF cells remain to be elucidated. However, the characteristics of inhibition by histidine and FBS described in this work [and reported earlier (28)] along with the Cl dependence and DIDS inhibition shown in our data (Fig. 6B) make it likely that an anion transporter mediates Cu uptake in these cells. The chloride and anion-dependent pathways, described in the present work, could contribute to Cu uptake by these cells in vivo, depending on the extent to which plasma components completely or incompletely inhibit this uptake. It seems likely that inhibition is less than total, and some contribution to Cu uptake under physiological conditions may be significant. In addition, contributions from other as yet unidentified pathways cannot be excluded.
It should be pointed out that although we favor the transport of Cu-anion complexes to account for the Cl-dependent uptake, this has not yet been rigorously established. It is possible that there is an indirect coupling, perhaps via charge neutrality requirements, of anion transport and Cu transport through another transporter. We believe that this is less likely for a number of reasons: the red blood cell data provide compelling evidence that AE1 can mediate Cu transport directly (1); it is not clear that anion transport is required for maintenance of charge neutrality, and if that were the case, then the presence of sulfate-transporting systems would also support a charge requirement, and yet sulfate does not promote Cu transport. Finally, in the case of copper, the rates of uptake are quite low compared with that of other substrates, and it would be anticipated that a variety of anion or cation leak pathways could easily compensate for any imbalance of charges.
The special characteristics of intestinal absorption.
Uptake across the apical surface of intestinal cells is the first step in intestinal absorption, and in general, it has been thought that copper entering intestinal cells is handled in the same fashion, as copper that enters other cells of the body. However, this appears not to be the case, based on recent unexpected observations in studies of copper uptake in mice with an intestine-specific ablation of hCTR1 (34). First, copper was elevated in the intestinal cells of knockout animals, rather than reduced, and second, the intracellular copper in enterocytes was not biologically available for delivery to copper-dependent cellular enzymes. These data suggested that 1) hCTR1 was probably not directly involved in apical Cu entry and 2) hCTR1 was involved in making copper that had entered cells bioavailable. Since hCTR1 mediates the basolateral uptake of copper, enterocytes most likely acquire their copper for biological processes as do other cells of the body, by uptake from the blood via hCTR1, acquisition by intracellular chaperones such as Atox1 or CCS, and delivery to ATP7A and ATP7B for excretion or utilization by the secretory pathway (53).
A portion of copper absorption from the apical side of intestine occurs through a different mechanism, such as transport of copper-chloride complexes via anion transporters reported in this work. The role for these transporters in copper, and potentially other metal ions, homeostasis needs to further explored. The next step in the dietary acquisition process after copper entry across the apical surface, as an anionic complex, needs to be elucidated. It is not clear how the apical pool of copper, entering cells from the gut, is made available to cellular proteins, or how it is transferred to the blood. Additionally, the precise molecular identity of the anion transporter(s) mediating this process in enterocytes needs to be identified, as our work suggests a new and potentially important role for them in metal homeostasis.
GRANTS
This work was supported by National Institutes of Health Grant P01 GM-067166 (to J. H. Kaplan) and by the Training Grant DK-007739 (to A. Zimnicka).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
MEF−/− cells were a generous gift from Dr. Dennis J. Thiele (Duke University, Durham, NC).
Present address of A. M. Zimnicka: Dept. of Medicine, Institute for Personalized Respiratory Medicine, Univ. of Illinois at Chicago, COMRB 3128, Chicago, IL 60612-7227.
REFERENCES
- 1.Alda JO, Garay R. Chloride (or bicarbonate)-dependent copper uptake through the anion exchanger in human red blood cells. Am J Physiol Cell Physiol 259: C570–C576, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Alper SL, Chernova MN, Stewart AK. Regulation of Na+-independent Cl−/HCO3− exchangers by pH. JOP 2: 171–175, 2001 [PubMed] [Google Scholar]
- 3.Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 284: C1525–C1530, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Barmeyer C, Ye JH, Sidani S, Geibel J, Binder HJ, Rajendran VM. Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflügers Arch 454: 441–450, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Barry AN, Shinde U, Lutsenko S. Structural organization of human Cu-transporting ATPases: learning from building blocks. J Biol Inorg Chem 15: 47–59, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Beaumont C, Delaunay J, Hetet G, Grandchamp B, de Montalembert M, Tchernia G. Two new human DMT1 gene mutations in a patient with microcytic anemia, low ferritinemia, and liver iron overload. Blood 107: 4168–4170, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bogdanova AY, Gassmann M, Nikinmaa M. Copper ion redox state is critical for its effects on ion transport pathways and methaemoglobin formation in trout erythrocytes. Chem Biol Interact 139: 43–59, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Brown CD, Dunk CR, Turnberg LA. Cl−/HCO3− exchange and anion conductance in rat duodenal apical membrane vesicles. Am J Physiol Gastrointest Liver Physiol 257: G661–G667, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Cabrera A, Alonzo E, Sauble E, Chu YL, Nguyen D, Linder MC, Sato DS, Mason AZ. Copper binding components of blood plasma and organs, and their responses to influx of large doses of (65)Cu, in the mouse. Biometals 21: 525–543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos C, Guzman R, Lopez-Fernandez E, Casado A. Evaluation of the copper(II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: the CUPRAC-BCS assay. Anal Biochem 392: 37–44, 2009 [DOI] [PubMed] [Google Scholar]
- 11.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA 106: 4237–4242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deschamps P, Kulkarni PP, Sarkar B. X-ray structure of physiological copper(II)-bis(l-histidinato) complex. Inorg Chem 43: 3338–3340, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiology (Bethesda) 23: 104–114, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem 277: 29162–29171, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95: 1148–1153, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals 16: 41–54, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Glover CN, Wood CM. Histidine absorption across apical surfaces of freshwater rainbow trout intestine: mechanistic characterization and the influence of copper. J Membr Biol 221: 87–95, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Halligan RD, Shelat H, Kahn AM. Na+-independent Cl−/HCO3− exchange in sarcolemmal vesicles from vascular smooth muscle. Am J Physiol Cell Physiol 260: C347–C354, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Handy RD, Eddy FB, Baines H. Sodium-dependent copper uptake across epithelia: a review of rationale with experimental evidence from gill and intestine. Biochim Biophys Acta 1566: 104–115, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr 20: 291–310, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 66: 10944–10952, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Iolascon A, d'Apolito M, Servedio V, Cimmino F, Piga A, Camaschella C. Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2). Blood 107: 349–354, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kalfakakou V, Simons TJ. Anionic mechanisms of zinc uptake across the human red cell membrane. J Physiol 421: 485–497, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karniski LP, Aronson PS. Anion exchange pathways for Cl− transport in rabbit renal microvillus membranes. Am J Physiol Renal Fluid Electrolyte Physiol 253: F513–F521, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Klomp AE, Tops BB, Van Denberg IE, Berger R, Klomp LW. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1). Biochem J 364: 497–505, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo YM, Zhou B, Cosco D, Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98: 6836–6841, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laughery MD, Clifford RJ, Chi Y, Kaplan JH. Selective basolateral localization of overexpressed Na-K-ATPase β1- and β2-subunits is disrupted by butryate treatment of MDCK cells. Am J Physiol Renal Physiol 292: F1718–F1725, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277: 40253–40259, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr 63: 797S–811S, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Lou M, Garay R, Alda JO. Cadmium uptake through the anion exchanger in human red blood cells. J Physiol 443: 123–136, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutsenko S, Gupta A, Burkhead JL, Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys 476: 22–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, Divoky V, Prchal JT. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood 105: 1337–1342, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 284: 29704–29713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4: 235–244, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, Thiele DJ. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem 285: 32385–32392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol 587: 2179–2185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp PA. Ctr1 and its role in body copper homeostasis. Int J Biochem Cell Biol 35: 288–291, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Simons TJ. Passive transport and binding of lead by human red blood cells. J Physiol 378: 267–286, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simons TJ. The role of anion transport in the passive movement of lead across the human red cell membrane. J Physiol 378: 287–312, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sterling D, Casey JR. Bicarbonate transport proteins. Biochem Cell Biol 80: 483–497, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Tandy S, Williams M, Leggett A, Lopez-Jimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem 275: 1023–1029, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Tao TY, Gitlin JD. Hepatic copper metabolism: insights from genetic disease. Hepatology 37: 1241–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Treharne KJ, Crawford RM, Mehta A. CFTR, chloride concentration and cell volume: could mammalian protein histidine phosphorylation play a latent role? Exp Physiol 91: 131–139, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Turnlund JR. Human whole-body copper metabolism. Am J Clin Nutr 67: 960S–964S, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Turnlund JR, Keyes WR, Peiffer GL, Scott KC. Copper absorption, excretion, and retention by young men consuming low dietary copper determined by using the stable isotope 65Cu. Am J Clin Nutr 67: 1219–1225, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Van den Berghe PV, Klomp LW. New developments in the regulation of intestinal copper absorption. Nutr Rev 67: 658–672, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Weber PM, O'Reilly S, Pollycove M, Shipley L. Gastrointestinal absorption of copper: studies with 64Cu, 95Zr, a whole-body counter and the scintillation camera. J Nucl Med 10: 591–596, 1969 [PubMed] [Google Scholar]
- 50.Wyman S, Simpson RJ, McKie AT, Sharp PA. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett 582: 1901–1906, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Barone S, Petrovic S, Wang Z, Seidler U, Riederer B, Ramaswamy K, Dudeja PK, Shull GE, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in gastric surface mucous and duodenal villus cells. Am J Physiol Gastrointest Liver Physiol 285: G1225–G1234, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Zimnicka AM, Maryon EB, Kaplan JH. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J Biol Chem 282: 26471–26480, 2007 [DOI] [PubMed] [Google Scholar]