Abstract
The effects of genistein, a protein tyrosine kinase (PTK) inhibitor, on voltage-dependent K+ (Kv) 4.3 channel were examined using the whole cell patch-clamp techniques. Genistein inhibited Kv4.3 in a reversible, concentration-dependent manner with an IC50 of 124.78 μM. Other PTK inhibitors (tyrphostin 23, tyrphostin 25, lavendustin A) had no effect on genistein-induced inhibition of Kv4.3. Orthovanadate, an inhibitor of protein phosphatases, did not reverse the inhibition of Kv4.3 by genistein. We also tested the effects of two inactive structural analogs: genistin and daidzein. Whereas Kv4.3 was unaffected by genistin, daidzein inhibited Kv4.3, albeit with a lower potency. Genistein did not affect the activation and inactivation kinetics of Kv4.3. Genistein-induced inhibition of Kv4.3 was voltage dependent with a steep increase over the channel opening voltage range. In the full-activation voltage range positive to +20 mV, no voltage-dependent inhibition was found. Genistein had no significant effect on steady-state activation, but shifted the voltage dependence of the steady-state inactivation of Kv4.3 in the hyperpolarizing direction in a concentration-dependent manner. The Ki for the interaction between genistein and the inactivated state of Kv4.3, which was estimated from the concentration-dependent shift in the steady-state inactivation curve, was 1.17 μM. Under control conditions, closed-state inactivation was fitted to a single exponential function, and genistein accelerated closed-state inactivation. Genistein induced a weak use-dependent inhibition. These results suggest that genistein directly inhibits Kv4.3 by interacting with the closed-inactivated state of Kv4.3 channels. This effect is not mediated via inhibition of the PTK activity, because other types of PTK inhibitors could not prevent the inhibitory action of genistein.
Keywords: tyrosine kinase inhibitor, closed-state inactivation
the voltage-dependent k+(Kv) 4.3 channel constitutes the principle pore-forming α-subunit underlying the transient outward K+ current, which plays a key role in determining the plateau potential and the duration of the action potential of the heart (8, 30). Kv4.3 is the target for many intracellular signaling pathways, including protein tyrosine kinase (PTK) phosphorylation and dephosphorylation (14). The PTK phosphorylation of Kv4.3 seems to be an important regulatory mechanism in the control of cardiac excitability because Kv4.3 and tyrosine kinases are highly expressed in the heart (10, 14). Kv4.3 is colocalized with c-Src tyrosine kinase at the plasma membrane, and the functional linkage between Kv4.3 and tyrosine phosphorylation has been implicated in some physiological and pathological conditions. For example, increased activity of c-Src kinases can downregulate Kv4.3 channels in the heart, and this process appears to play an important etiological role in both pregnancy-related and pathological cardiac hypertrophy (9, 36). Only a few studies have investigated the role of tyrosine phosphorylation on Kv4.3, although Kv4.3 in rat brain contains five consensus sequences for phosphorylation by PTK (determined using the web-based program Netphos 2.0).
Genistein, an isoflavone compound isolated from the fermentation broth of Pseudomonas, has been characterized as a PTK inhibitor because it potently and selectively inhibits both receptor and nonreceptor tyrosine kinases. For example, genistein was reported to strongly inhibit the tyrosine kinase activity of the epidermal growth factor receptors, pp60v-src and PP110gag-fes, but have a much weaker effect on serine/threonine kinases, protein kinases A and C (3, 4). Since protein tyrosine phosphorylation plays an important role in modulating the activity of a variety of ion channels in excitable cells (19, 23, 33), genistein has been widely used as a pharmacological drug to monitor PTK involvement in the modulation of ion channels from different biological preparations. Using genistein, the PTK-dependent pathway was shown to be involved in the suppression of both the slow delayed-rectifier K+ current in guinea pig ventricular myocytes and the voltage-gated K+ channels expressed in Xenopus oocytes and human embryonic kidney cells (17, 26). Moreover, transient outward K+ currents were inhibited by genistein via PTK-dependent mechanisms in human atrial and rat ventricular myocytes (12, 28, 38). Additional studies show that genistein markedly reduced the amplitude of a slowly inactivating delayed rectifier current and, to a lesser extent, that of a transient K+ current in mouse Schwann cells (31). This action was accompanied by a decrease in tyrosine phosphorylation of the Kv1.4, Kv1.5, and Kv2.1 channel proteins (31). However, ever-increasing data show that genistein has other pharmacological activities, including direct action on ion channels through a PTK-independent mechanism. Via a PTK-independent pathway, genistein directly inhibited several K+ channels: voltage-gated K+ channels in the pulmonary arterial cells of rats and rabbits; a cardiac delayed-rectifier K current in the ventricular cells of the guinea pig; and a cloned human, A-type hKv1.4 in Chinese hamster ovary (CHO) cells (34, 39, 43). Genistein also has been shown to directly inhibit Ca2+ channels in vascular smooth muscle cells isolated from the artery of a rabbit ear (40).
Genistein has been widely used as a valuable pharmacological tool to study the PTK signaling pathway in electrophysiological studies. In the present study, we investigated the effects of genistein on cloned Kv4.3 channels expressed in CHO cells using a patch-clamp technique to determine the direct modulation of Kv4.3 by genistein via a PTK-independent manner.
MATERIALS AND METHODS
Stable transfection and cell culture.
The Kv4.3 cDNA was stably transfected into CHO cells (American Type Culture Collection, Manassas, VA) using the lipofectamine reagent (Invitrogen, Grand Island, NY), as described previously (2, 30). CHO cells were cultured in Iscove's modified Dulbecco's medium (Invitrogen), supplemented with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM hypoxanthine, and 0.01 mM thymidine, under a 95% humidified air-5% CO2 environment at 37°C. Transfected cells were exposed to 500 μg/ml geneticin (Invitrogen), and antibiotic-resistant cells were selected and maintained in fresh Iscove's modified Dulbecco's medium containing geneticin. By using a brief trypsin/EDTA (Invitrogen) treatment, transfected CHO cells were passed every 4–5 days and were seeded onto glass coverslips (diameter: 12 mm, Fisher Scientific, Pittsburgh, PA) in a petri dish 24 h before use. For the electrophysiological recordings, a coverslip with adherent cells was transferred to a continually perfused (1 ml/min) recording chamber (RC-13, Warner Instrument, Hamden, CT).
Electrophysiological recordings.
The whole cell current of Kv4.3 was recorded using a patch-clamp technique with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) at room temperature (22–24°C). The data were stored using a Digidata 1200A (Molecular Devices) acquisition board-equipped IBM-compatible computer. Currents were sampled at 5 kHz and filtered at 2 kHz (four-pole Bessel filter). Pulse generation and data acquisition were controlled using pClamp 10.0 software (Molecular Devices). Patch electrodes were fabricated using PG10165–4 glass capillary tubing (World Precision Instruments, Sarasota, FL). Liquid junction potentials between external and pipette solutions were offset. In the whole cell configuration, average series resistances were 3.9 MΩ. The effective series resistances were usually compensated by 80% when the current exceeded 1 nA. Voltage drops, based on the calculated residual series resistance, were <5 mV.
Solutions and drugs.
The pipette solution contained (in mM) 140 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 EGTA, and was adjusted to pH 7.3 with KOH. The bath solution contained (in mM) 140 NaCl, 5 KCl, 1.3 CaCl2, 1 MgCl2, 20 HEPES, and 10 glucose, and was adjusted to pH 7.3 with NaOH. Genistein, tyrphostin 23, tyrphostin 25, lavendustin A, orthovanadate, daidzein, and genistin (Calbiochem, San Diego, CA) were dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO). The concentration of DMSO in the final dilution was <0.1%, and this DMSO concentration had no effect on Kv4.3 currents.
Data analysis.
Analysis of data was performed using pClamp 10.0 software (Molecular Devices) and Origin 8.0 software (Microcal Software, Northampton, MA). The concentration-response curve for Kv4.3 current inhibition by genistein was fitted to the following equation:
| 1 |
in which I is the normalized current inhibition [I = (1 − Idrug/Icontrol)] at test potential, [D] represents various drug concentrations, IC50 is the concentration at half-maximal inhibition, and n is the Hill coefficient. Steady-state activation curves for Kv4.3 channels were fitted with the Boltzmann equation:
| 2 |
where V represents the test potential, and V1/2 and k are the potential at which the conductance was half-activation and the slope, respectively. The steady-state voltage dependence of inactivation for the Kv4.3 channels was investigated using a double-pulse voltage protocol; currents were measured by a 500-ms test potential to +40 mV, while 1-s preconditioning pulses were varied from −110 to 0 mV stepped by 10 mV in the absence and presence of drugs. The experimental points were calculated as shown in the following equation:
| 3 |
in which Imax represents the current measured at the most hyperpolarized preconditioning pulse, and Ic represents the nonzero current that remained activated at the most depolarized 1-s preconditioning pulse. This nonzero residual current was subtracted from the actual value. The resulting steady-state inactivation data were fitted with the Boltzmann equation:
| 4 |
where V is the preconditioning potential.
Results are expressed as means ± SE. Analysis of variance was used for the statistical analyses. A value of P < 0.05 was considered statistically significant.
RESULTS
Concentration-dependent inhibition of Kv4.3 by genistein.
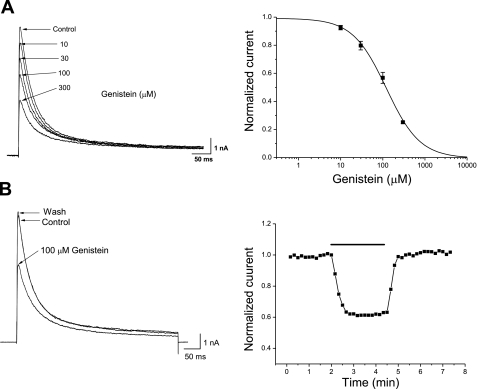
Figure 1A shows the superimposed Kv4.3 currents expressed in CHO cells under control conditions and in the presence of various concentrations of genistein. Under control conditions, Kv4.3 currents were activated to a peak and then were rapidly inactivated during a 500-ms pulse of +40 mV at 10-s intervals, as described previously (2, 22, 30). In the presence of genistein, inhibition of Kv4.3 currents was substantiated by a concentration-dependent reduction in peak current amplitude. To investigate the concentration dependence of genistein action, the pharmacological effect of genistein on the peak amplitude of the Kv4.3 currents was studied. The normalized inhibition was plotted as a function of drug concentration and was fit to the Hill equation, giving an IC50 and a Hill coefficient of 124.78 ± 4.21 μM and 1.22 ± 0.0028 (n = 6), respectively. The Hill coefficient of 1.22 represents a one-to-one drug-receptor interaction. To assess the reversibility of the effect of the drug, the depolarizing pulse of +40 mV was repeated with application of 100 μM genistein (Fig. 1B). When switched to a drug-containing solution, genistein-induced inhibition of Kv4.3 reached a steady state within 1 min, and the currents were restored to 98.2 ± 2.1% of the control value (n = 8) within 1 min, indicating that the effects of genistein were largely reversible on washout.
Fig. 1.
A: concentration dependence on the genistein-induced inhibition of voltage-dependent K+ channel (Kv) 4.3. Whole cell Kv4.3 currents were elicited by 500-ms step depolarization to +40 mV from a holding potential of −80 mV at 10-s intervals. The control current and currents following the addition of 10, 30, 100, and 300 μM genistein are indicated. The reduction in the peak amplitude of Kv4.3 current at +40 mV was used as an index of inhibition. B: reversible inhibition of Kv4.3 by genistein. Whole cell currents were elicited by 500-ms depolarizing pulses of +40 mV from a holding potential of −80 mV at 10-s intervals under control conditions and in the presence of genistein. The time course of inhibition in the presence of 100 μM genistein is shown. Maximal inhibition occurred ∼1 min after drug application began. Complete recovery from inhibition was observed after washout of the drug. The steady-state amplitudes of the currents were plotted as a function of time. The bar indicates the time of genistein application. Values are means ± SE.
Phosphorylation-independent inhibition of Kv4.3 by genistein.
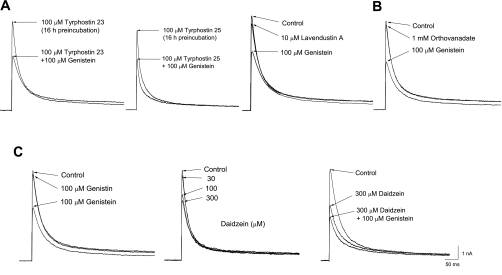
Because Kv4.3 has multiple consensus sites for PTK phosphorylation, we determined whether genistein-induced inhibition of Kv4.3 was mediated through PTK inhibition by using other PTK inhibitors that are structurally and mechanistically different from genistein (tyrphostin 23, tyrphostin 25, and lavendustin A), and orthovanadate, a protein tyrosine phosphatase inhibitor (Fig. 2). Because the maximal inhibitory effect of tyrphostins was observed after 16 h of incubation (25), we tested the effects of genistein after 16 h of incubation with either tyrphostin 23 or tyrphostin 25. Preincubation with 100 μM of either tyrphostin 23 or tyrphostin 25 had no effect on the activation and inactivation kinetics of Kv4.3 currents compared with control currents (Fig. 2A). The addition of 100 μM genistein to a bath solution containing 100 μM of either tyrphostin 23 or tyrphostin 25 decreased the Kv4.3 currents by 48.76 ± 3.19 and 43.74 ± 3.33%, respectively (n = 6). A 10-min treatment with 10 μM lavendustin A did not significantly inhibit Kv4.3 currents. Subsequent application of 100 μM genistein reduced the peak amplitude of Kv4.3 currents by 50.34 ± 3.21% (n = 6). A similar experiment with orthovanadate was carried out, resulting in genistein-induced inhibition of Kv4.3 by 52.57 ± 2.31% (n = 5), while exposure to 1 mM orthovanadate did not significantly inhibit Kv4.3 currents (Fig. 2B). We tested two other inactive structural analogs: genistin and daidzein (Fig. 2C). Although genistin (100 μM) did not affect Kv4.3 currents (n = 6), daidzein decreased the peak amplitude of Kv4.3 in a concentration-dependent manner (n = 6). However, complete inhibition could not be achieved, and the degree of inhibition by daidzein was less than that by genistein at each concentration examined. In addition, daidzein had no effect on the activation or inactivation kinetics of the Kv4.3 currents. Daidzein (300 μM) inhibited Kv4.3 by 41.24 ± 3.27% (n = 6). Coapplication of daidzein (300 μM) and genistein (100 μM) produced an inhibition of Kv4.3 of 53.09 ± 2.94%. This value was not significantly different from that seen in the presence of genistein alone. These results strongly suggest that genistein-induced inhibition of the Kv4.3 currents was independent of phosphorylation and dephosphorylation processes, but might have resulted from a direct interaction between genistein and the Kv4.3 channels.
Fig. 2.
The effects of other types of tyrosine kinase inhibitors (tyrphostin 23, tyrphostin 25, lavendustin A; A), orthovanadate (B), and inactive structural analogs (genistin, daidzein; C) on genistein-induced inhibition of Kv4.3. Representative superimposed currents were produced by applying 500-ms depolarizing pulses from a holding potential of −80 to +40 mV every 10 s. The control current, the currents recorded after preincubation with tyrphostin 23 and 25, and after a 10-min exposure to lavendustin A, orthovanadate, or inactive structural analogs, and the current measured after an additional 3-min treatment with 100 μM genistein are shown.
Voltage-dependent inhibition of Kv4.3 by genistein.
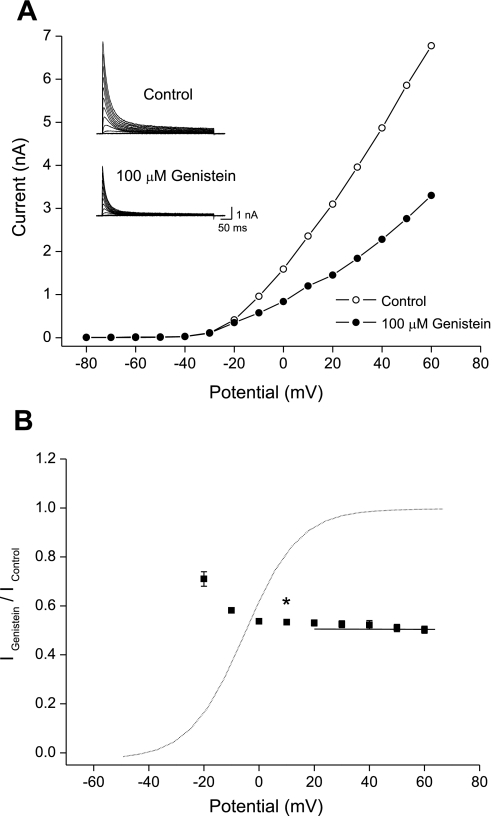
Figure 3A shows the effect of 100 μM of genistein on the current-voltage relationships for Kv4.3. Under control conditions, the Kv4.3 current was activated at pulses greater than −30 mV, and the current-voltage relationship was almost linear for depolarizing pulses between −10 and +60 mV. The inhibition of Kv4.3 currents by genistein was observed in the entire voltage range for which Kv4.3 was activated. By plotting normalized inhibition vs. potential, a high degree of inhibition was observed, with strong voltage dependence between −20 and +20 mV, which included the channel-opening voltage range (Fig. 3B). At a depolarizing potential of −20 mV, 100 μM genistein inhibited the Kv4.3 currents by 28.6 ± 1.4% (n = 6). This inhibition increased continuously to 46.8 ± 2.0% (n = 6) at +20 mV (ANOVA, F5,25 = 24.75, P < 0.05). However, inhibition in the voltage range between +20 and +60 mV, where the channels are fully activated, was not voltage dependent.
Fig. 3.
Effects of genistein on the Kv4.3 current-voltage relationship. A: representative whole cell Kv4.3 current traces under control conditions and in the presence of 100 μM genistein. Kv4.3 currents were obtained by applying 500-ms depolarizing pulses from −80 to +60 mV in steps of 10 mV every 10 s from a holding potential of −80 mV. The data were taken from the peak amplitude of Kv4.3 under control conditions and after the addition of 100 μM genistein. B: voltage-dependent inhibition of Kv4.3 currents by genistein was expressed as a relative current (IGenistein/IControl). The peak amplitude of Kv4.3 currents in the presence of genistein was normalized to that at each voltage of the control. In the channel-opening voltage range between −20 and +20 mV, inhibition of Kv4.3 currents by genistein increased and was significantly different from inhibition at +10 mV (n = 6, *P < 0.05). The dotted line represents the steady-state activation curve of Kv4.3 under control conditions. Values are means ± SE.
Effects of genistein on the activation and inactivation kinetics of Kv4.3.
As shown in Fig. 1, genistein decreased the peak amplitude of Kv4.3 currents in a concentration-dependent manner, but did not alter the time course of the Kv4.3 currents during depolarization. Thus we studied the effect of genistein on the activation and inactivation kinetics of Kv4.3. Current traces were fitted to a single exponential function to estimate the rate of current activation. Under control conditions, the time constant of activation was 0.58 ± 0.02 ms (n = 9). In the presence of genistein, the time constants of activation were 0.57 ± 0.04, 0.57 ± 0.03, and 0.58 ± 0.04 ms (n = 9) for 10, 30, and 100 μM, respectively. The kinetics of activation was not significantly affected by genistein. The time course of inactivation of Kv4.3 at 40 mV was fitted to a double-exponential function under control conditions, with a fast time constant of 25.33 ± 1.46 and a slow time constant of 113.46 ± 2.25 ms (n = 9). Genistein did not affect the kinetics of Kv4.3 inactivation (fast time constants: 24.34 ± 1.41, 22.99 ± 1.51, and 23.06 ± 1.64 ms; slow time constants: 115.13 ± 2.87, 116.39 ± 2.72, and 119.13 ± 2.44 ms, n = 9, for 10, 30, and 100 μM, respectively). No significant treatment effect of the different genistein concentrations was found for either the fast or slow time constant.
Voltage dependence of the steady-state activation and inactivation of Kv4.3 by genistein.
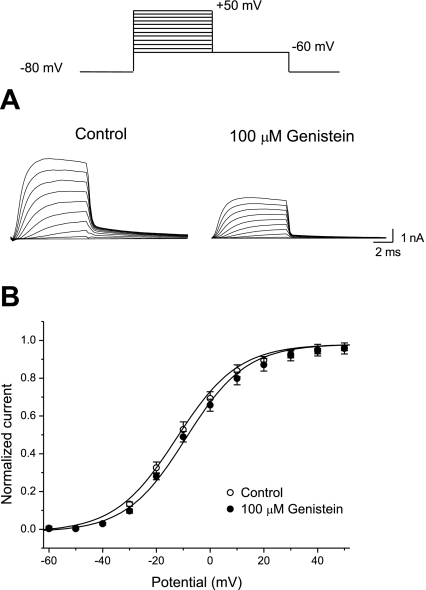
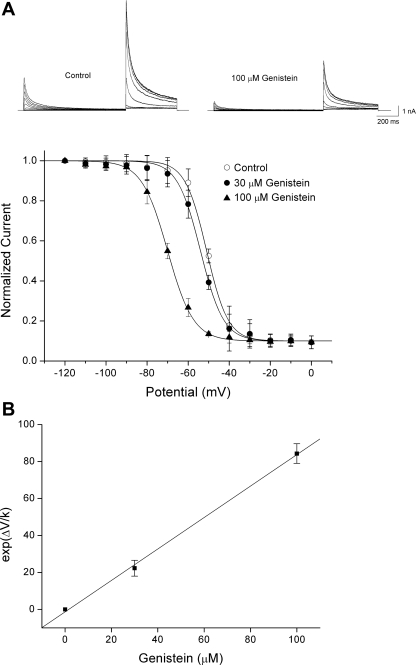
Figure 4A shows the steady-state activation curves of Kv4.3 currents under the control conditions and in the presence of genistein. The potential of the V1/2 and the k of the steady-state activation curves were −9.17 ± 0.03 and 4.33 ± 0.02 mV for the control, and −9.21 ± 0.04 and 4.27 ± 0.05 mV for 100 μM genistein (n = 5), respectively (Fig. 4B). Thus genistein had no effect on the voltage dependence of the steady-state activation. The steady-state inactivation curves for Kv4.3 under the control conditions had a V1/2 of −48.71 ± 1.42 mV and a k of 4.52 ± 0.24 mV (n = 6) (Fig. 5A). Genistein significantly shifted the inactivation curve (V1/2) to a hyperpolarized potential in a concentration-dependent manner (−52.36 ± 2.10 mV at 30 μM and −67.94 ± 2.36 mV at 100 μM; n = 6). However, no significant change in k was observed for the curve in the presence of genistein (4.66 ± 0.32 mV at 30 μM and 4.84 ± 0.31 mV at 100 μM; n = 6). Whereas the apparent dissociation constant (Ki) for genistein-induced inhibition of Kv4.3 in the closed state could be estimated from the reduction in the peak amplitude of Kv4.3, the apparent Ki for genistein-induced inhibition in the inactivated state was estimated from the concentration-dependent shift in the steady-state inactivation curves (6). The theoretical value of Ki was calculated to be 1.17 ± 0.23 μM (n = 6) (Fig. 5B).
Fig. 4.
Effects of genistein on the steady-state activation of Kv4.3 A: tail currents were recorded at −60 mV after 8-ms depolarizing pulses between −80 and +50 mV in steps of 10 mV, from a holding potential of −80 mV, under control conditions or in the presence of 100 μM genistein. B: the steady-state activation curves were drawn by fitting the normalized tail currents to the Boltzmann equation. Values are means ± SE.
Fig. 5.
Effects of genistein on the steady-state inactivation of Kv4.3. A: the currents were evoked by 1-s prepulses that were varied from −110 to 0 mV stepped by 10 mV and a 500-ms depolarizing pulse to +40 mV in the absence and presence of 30 and 100 μM genistein. Steady-state inactivation curves are shown as a plot of normalized peak currents during the depolarizing pulse as a function of the conditioning potential. The curves represent the best-fit Boltzmann equations. B: plot of exp (ΔV/k) against genistein concentrations. The V1/2 and k values were obtained from the steady-state inactivation curves. The concentration-dependent shift in the midpoint (ΔV) was determined as the difference between V1/2 values in control conditions and at 30 and 100 μM genistein (n = 6). The solid line represents the linear fit of the data: exp (ΔV/k) = 0.64 + 0.85 [genistein], where [genistein] represents the genistein concentration. Ki, the reciprocal of the slope, was calculated from this fit. Values are means ± SE. See materials and methods for definitions of other terms.
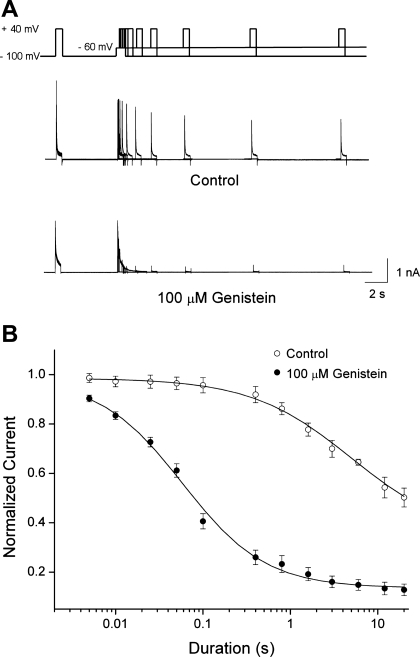
Effect of genistein on closed-state inactivation of Kv4.3.
Previous studies have shown that Kv4.3 channels are predominantly inactivated from the closed state without opening in the subthreshold voltage range (37). We tested the effect of genistein on the kinetics of closed-state inactivation. Under control conditions, a 12-s conditioning pulse to −60 mV inactivated 53.2 ± 4.3% (n = 8) of the Kv4.3 currents (Fig. 6A). The time course for closed-state inactivation was fitted to a single-exponential function with a time constant of 4.33 ± 0.44 s (n = 8). In the presence of 100 μM genistein, the rate of close-stated inactivation was accelerated, and inactivation was almost complete after a 1-s conditioning pulse. Closed-state inactivation was also fitted to a single-exponential function with a time constant of 0.99 ± 0.08 s (n = 8, P < 0.05) (Fig. 6B).
Fig. 6.
Effects of genistein on the kinetics of Kv4.3 closed-state inactivation. A: Kv4.3 currents were recorded at +40 mV using a double-pulse protocol with a conditioning pulse to −60 mV of variable duration. The initial control pulse and conditioning pulse were applied from a membrane potential of −100 mV. B: the current amplitudes evoked by the second pulse, relative to the amplitude resulting from the initial control pulse, were plotted against the duration of the conditioning pulse. The data fit well to a single-exponential function. Values are means ± SE.
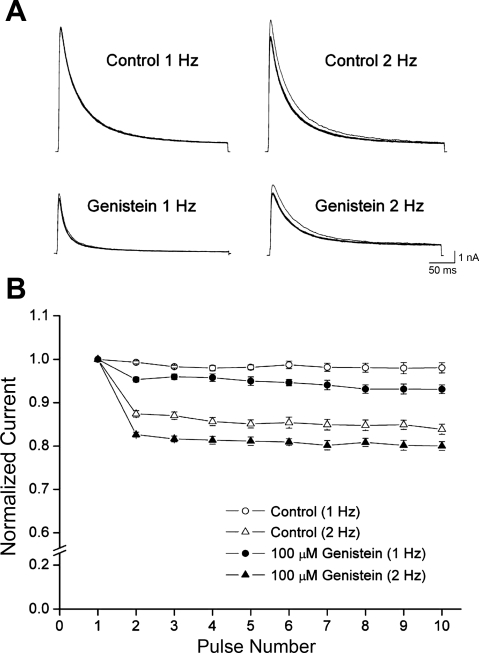
Use-dependent inhibition of Kv4.3 by genistein.
Figure 7A shows the current traces of the Kv4.3 obtained after applying a pulse train at a frequency of 1 or 2 Hz under control conditions or in the presence of 100 μM genistein. Under control conditions, the peak amplitude of the Kv4.3 current decreased by 2.2 ± 0.01 and 17.2 ± 0.02% (n = 5) after the application of a train of depolarizing pulses at 1 and 2 Hz, respectively (Fig. 7B). In the presence of 100 μM genistein, however, a reduction in the peak amplitude was elicited by the first depolarizing pulse, which averaged 7.2 ± 0.5 and 20.4 ± 0.02% (n = 5) inhibition at 1 and 2 Hz, respectively. Thereafter, the current amplitude was slightly affected by the train of pulses. These results suggest that there was a weak use dependence of genistein action.
Fig. 7.
Effect of genistein on use-dependent inhibition of Kv4.3. A: Kv4.3 current traces obtained from applying ten 500-ms depolarizing pulses of +40 mV from a holding potential of −80 mV at 1 or 2 Hz in the absence or presence of genistein. B: plot of normalized current as a function of the number of pulses. The peak amplitudes of the current at every pulse were normalized to the peak amplitude of the current obtained as a result of the first pulse. Values are means ± SE.
DISCUSSION
The results of the present study can be summarized as follows: 1) genistein decreased the peak amplitude of Kv4.3 in a concentration-dependent manner without modifying current kinetics; 2) the inhibition of Kv4.3 by genistein occurred as a result of direct interaction between genistein and the Kv4.3 channels, that is, through a PTK-independent mechanism; and 3) genistein induced a shift in the voltage dependence of the steady-state inactivation curves of Kv4.3 to the hyperpolarizing direction in a concentration-dependent manner and significantly accelerated the closed-state inactivation.
In the present study, several lines of evidence suggest that genistein appears to have direct, PTK-independent effects on the Kv4.3 currents. The time course of genistein inhibition of Kv4.3 was very rapid, and the maximal effect was obtained within 1 min. The reversal of Kv4.3 inhibition occurred just as rapidly, suggesting that both effects might be due to nonspecific inhibition of the channels rather than to inhibition of intracellular PTK activity. Generally, the effects of phosphorylation-mediated events on ion channels occur on a time scale of several minutes (11). If genistein induced the inhibition of Kv4.3 through a PTK-dependent mechanism, then other PTK inhibitors should produce a similar effect. We examined the actions of a variety of PTK inhibitors (tyrphostin 23, tyrphostin 25, and lavendustin A), which are structurally and mechanistically different from genistein. These agents are selective and cell-permeable PTK inhibitors, and the concentrations of PTK inhibitors used in our experiments are sufficient to completely inhibit PTK activity (13, 16, 25). Our data showed that these PTK inhibitors had no effect on the genistein-induced inhibition of Kv4.3. Orthovanadate, which is an inhibitor of protein phosphatases, increases basal tyrosine phosphorylation and is used to antagonize the effects of PTK inhibitor. For example, orthovanadate has been shown to antagonize the inhibitory effect of genistein on L-type Ca2+ currents and delayed-rectifier K+ currents in guinea pig ventricular myocytes (26, 29). In the present study, pretreatment with orthovanadate had no effect on the inhibitory action of genistein on Kv4.3. This result indicates that the basal activity of endogenous tyrosine kinase does not play a significant role in regulating Kv4.3. However, daidzein, an inactive analog of genistein, is structurally related to genistein and showed a similar inhibitory effect on Kv4.3, albeit at a lower potency. Moreover, the kinetics of Kv4.3 currents remained unaffected, and the effect was completely reversed on washout. In addition, genistin, another inactive structural analog, had no effect on either the amplitude or kinetics of Kv4.3 currents. Our results confirmed that the mechanism by which genistein inhibits Kv4.3 is similar to the mechanism by which it is known to inhibit several other ion channels. Genistein inhibited voltage-dependent Na+ channels, whereas daidzein had moderate effects, and genistin had a weak effect (24). Bath application of genistein decreased the L-type Ca2+ current of rat ventricular cells in a concentration-dependent manner, and daidzein had nearly the same inhibitory effect on Ca2+ currents (42). These results suggest that the effect of genistein may be caused by the direct blocking of Ca2+ channels. Genistein inhibited delayed-rectifier K+ currents in guinea pig ventricular myocytes via a tyrosine kinase-independent pathway, and daidzein showed a similar effect but with a lower potency (27, 39). Interestingly, the degree of inhibition is much greater with genistein than that obtained with the same concentration of daidzein. Both genistein and daidzein have two benzene rings (A and B) as a common structural feature, but the structural difference, a hydroxyl group missing from the side chain of the A ring, is thought to be responsible for the lower potency of daidzein during ion channel inhibition. These results suggest that structure-related mechanisms and a phosphorylation-independent signal transduction pathway may contribute to the inhibitory action of genistein and daidzein. Because we only tested the PTK dependence of the inhibitory actions of genistein on Kv4.3, our results cannot rule out the possibility that the inhibition of Kv4.3 by genistein occurs via the nonspecific inhibition of protein kinase A and C.
One of the main findings of the present study is that genistein inhibited the Kv4.3 currents in a concentration-dependent manner without a significant effect on current kinetics on depolarization. One possible explanation for this result is that genistein inhibits Kv4.3 by preferentially interacting with the closed state of the channel before channel activation. However, there are discrepancies in the reported effects of genistein on voltage-gated K+ channel inhibition, and other mechanisms of action have been suggested for genistein. Genistein slowed the activation kinetics of Kv1.3 and of vascular smooth muscle cell K+ currents during depolarization, but did not change the steady-state activation of Kv1.3 (34, 35). These effects were not mediated by inhibition of tyrosine kinase activity, but might have been due to direct inhibition. The activation kinetics of Kv1.4 were profoundly slowed, and the steady-state activation curve was shifted in a depolarizing direction (43). The inactivation kinetics of Kv1.4 were slowed in the subthreshold range of depolarization, but remained unchanged at depolarizing potentials (43). Genistein produced no significant voltage-dependent changes of transient outward K+ current activation or inactivation (12, 38). In the present study, genistein affected neither the activation nor the inactivation kinetics of Kv4.3, but it shifted the steady-state inactivation curves in a hyperpolarizing direction. This finding can be explained by preferential binding to the channel in the inactivated state with channel inhibition. Our results indicate that the affinity of Kv4.3 for genistein is 100 times greater for the inactivated state than for the closed state. Kv4.3 channels can be inactivated from either the open or closed states, but inactivation most frequently occurs from the closed state without activation (37). Accordingly, genistein caused a marked acceleration of the closed-state inactivation of Kv4.3. A possible mechanism of action is that genistein, which is highly lipophilic, alters the mechanical properties of membranes, thereby modulating the inactivation kinetics of Kv4.3 via an allosteric mechanism. Consistent with our results, genistein modulated gramicidin A channels in planar phospholipid bilayers by altering bilayer mechanical properties via a phosphorylation-independent mechanism, whereas daidzein had only modest effects, and genistin had no effect (18).
Genistein exists in both the monoanionic and neutral forms under physiological conditions (44). Thus genistein inhibited Kv4.3 by at least two different mechanisms: binding to the closed state and accelerating the closed-state inactivation of Kv4.3 channels. Closed states, which are predominant at hyperpolarized membrane potentials, supposedly bind genistein with low affinity, whereas closed-inactivated states bind at subthreshold levels of depolarization with high affinity. Whereas interaction of genistein with Kv4.3 channels was voltage independent in the full activation voltage range, genistein resulted in voltage-dependent inhibition of Kv4.3 over the voltage range that corresponds to channel activation. This result was attributed to the voltage dependence of Kv4.3 inactivation in the subthreshold voltage range and the preferential interaction of genistein with inactivated Kv4.3 channels. This may explain the low degree of use-dependent inhibition of Kv4.3 by genistein.
Genistein is a major bioactive isoflavone, abundant in some vegetables, that has proven to have beneficial cardiovascular effects in both animal and human studies (5). Kv4.3 is responsible for the primary transient outward K+ current in the heart (30). This channel is an important repolarizing current that determines the amplitude and duration of action potential in the heart. Inhibition of transient outward K+ currents, which are mainly mediated by atrial and ventricular Kv4.3 in humans, may contribute to prolongation of action potential duration (8). Consequently, Kv4.3 channels are a potential therapeutic target for antiarrhythmic drugs (41). The inhibitory effect of genistein on Kv4.3 may, therefore, have antiarrhythmic activity that could explain, at least in part, its cardiovascular effects. In failing hearts, however, downregulation of Kv4.3 was reported, and an excessive prolongation of action potential duration may produce proarrhythmic effects (20, 21). Plasma concentrations of genistein were relatively low and generally <40 nM in humans consuming diets devoid of soy, but were considerably higher in vegetarians (32). However, the average plasma concentration of genistein in Japanese subjects consuming a traditional diet was 0.1–0.27 μM, and plasma genistein concentrations as high as 2.4 μM were measured (1, 15). In the present study, we estimated the Ki for the binding to inactivated Kv4.3 to be 1.17 μM: therefore, the effect of genistein on Kv4.3 could be clinically relevant. Thus our observation that genistein inhibits Kv4.3 provides promising information about the molecular action by which genistein exerts cardiac effect. Before extrapolating our results to clinical application, however, it should be noted that the pore-forming α-subunit of the Kv4.3 channel protein itself can form voltage-gated K+ channels in heterologous expression systems (28). Kv4.3 coassembles with a large variety of ancillary subunits in native tissues, and β-subunits are not coexpressed in our expression system. Thus it is yet to be determined whether genistein-induced inhibition of Kv4.3 occurs in native cardiomyocytes, because the interaction between these proteins can alter the drug sensitivity of Kv4.3 channels (7).
In conclusion, the results of the present study suggest that genistein directly inhibits Kv4.3 currents by binding to the closed-inactivated state of the channel. Furthermore, the inhibitory action of genistein is not mediated via an inhibition of the PTK activity. When genistein is used to study the effects of PTK phosphorylation on ion channels, therefore, the nonspecific effects of this drug should be taken into consideration, and the results must be interpreted with great caution. In addition, the present study provides a possible explanation for mechanisms that underlie some of the cardiac effects of genistein.
GRANTS
This work was supported by a grant from the Medical Research Center, Korea Science and Engineering Foundation, Republic of Korea (R13-2002-005-01002-0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Imaizumi (Department of Molecular and Cellular Pharmacology, Nagoya City University, Japan) for the Kv4.3 cDNA.
REFERENCES
- 1.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342: 1209–1210, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Ahn HS, Kim SE, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. Interaction of riluzole with the closed inactivated state of Kv4.3 channels. J Pharmacol Exp Ther 319: 323–331, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592–5595, 1987 [PubMed] [Google Scholar]
- 4.Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol 201: 362–370, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Altavilla D, Crisafulli A, Marini H, Esposito M, D'Anna R, Corrado F, Bitto A, Squadrito F. Cardiovascular effects of the phytoestrogen genistein. Curr Med Chem Cardiovasc Hematol Agents 2: 179–186, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol 81: 613–642, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bett GC, Rasmusson RL. Modification of K+ channel-drug interactions by ancillary subunits. J Physiol 586: 929–950, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res 79: 659–668, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res 96: 1208–1216, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Eghbali M, Wang Y, Toro L, Stefani E. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med 16: 285–291, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Fadool DA, Levitan IB. Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J Neurosci 18: 6126–6137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Z, Lau CP, Wong TM, Li GR. Protein tyrosine kinase-dependent modulation of voltage-dependent potassium channels by genistein in rat cardiac ventricular myocytes. Cell Signal 16: 333–341, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gazit A, Yaish P, Gilon C, Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem 32: 2344–2352, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Gomes P, Saito T, DelCorsso C, Alioua A, Eghbali M, Toro L, Stefani E. Identification of a functional interaction between Kv4.3 channels and c-Src tyrosine kinase. Biochim Biophys Acta 1783: 1884–1892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooderham MH, Adlercreutz H, Ojala ST, Wahala K, Holub BJ. A soy protein isolate rich in genistein and daidzein and its effects on plasma isoflavone concentrations, platelet aggregation, blood lipids and fatty acid composition of plasma phospholipid in normal men. J Nutr 126: 2000–2006, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, Persons PE, Spada AP, Bednar RA, Levitzki A, Zilberstein A. Kinetic analysis of the inhibition of the epidermal growth factor receptor tyrosine kinase by Lavendustin-A and its analogue. J Biol Chem 266: 21105–21112, 1991 [PubMed] [Google Scholar]
- 17.Huang XY, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell 75: 1145–1156, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Hwang TC, Koeppe RE, 2nd, Andersen OS. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry 42: 13646–13658, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Jonas EA, Kaczmarek LK. Regulation of potassium channels by protein kinases. Curr Opin Neurobiol 6: 318–323, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98: 1383–1393, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kass RS, Cabo C. Channel structure and drug-induced cardiac arrhythmias. Proc Natl Acad Sci U S A 97: 11683–11684, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SE, Ahn HS, Choi BH, Jang HJ, Kim MJ, Rhie DJ, Yoon SH, Jo YH, Kim MS, Sung KW, Hahn SJ. Open channel block of A-type, Kv4.3, and delayed rectifier K+ channels, Kv13 and Kv31, by sibutramine. J Pharmacol Exp Ther 321: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol 56: 193–212, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Yang T, Simon SA. The protein tyrosine kinase inhibitor, genistein, decreases excitability of nociceptive neurons. Pain 112: 131–141, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lyall RM, Zilberstein A, Gazit A, Gilon C, Levitzki A, Schlessinger J. Tyrphostins inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem 264: 14503–14509, 1989 [PubMed] [Google Scholar]
- 26.Missan S, Linsdell P, McDonald TF. Tyrosine kinase and phosphatase regulation of slow delayed-rectifier K+ current in guinea-pig ventricular myocytes. J Physiol 573: 469–482, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Missan S, Zhabyeyev P, Linsdell P, McDonald TF. Insensitivity of cardiac delayed-rectifier IKr to tyrosine phosphorylation inhibitors and stimulators. Br J Pharmacol 148: 724–731, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J Mol Cell Cardiol 48: 12–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogura T, Shuba LM, McDonald TF. L-type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 276: H1724–H1733, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel α-subunit, Kv4.3 in the rat. FEBS Lett 420: 47–53, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Peretz A, Sobko A, Attali B. Tyrosine kinases modulate K+ channel gating in mouse Schwann cells. J Physiol 519: 373–384, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68: 1333S–1346S, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Siegelbaum SA. Channel regulation. Ion channel control by tyrosine phosphorylation. Curr Biol 4: 242–245, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Smirnov SV, Aaronson PI. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ Res 76: 310–316, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Teisseyre A, Michalak K. Genistein inhibits the activity of Kv1.3 potassium channels in human T lymphocytes. J Membr Biol 205: 71–79, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Thorburn J, Thorburn A. The tyrosine kinase inhibitor, genistein, prevents α-adrenergic-induced cardiac muscle cell hypertrophy by inhibiting activation of the Ras-MAP kinase signaling pathway. Biochem Biophys Res Commun 202: 1586–1591, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Bondarenko VE, Qu YJ, Bett GC, Morales MJ, Rasmusson RL, Strauss HC. Time- and voltage-dependent components of Kv4.3 inactivation. Biophys J 89: 3026–3041, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Kumar R, Wagner MB, Cheng J, Mishra M, Joyner RW. Regulation of transient outward current in human atrial myocytes by protein tyrosine kinase pathway. J Cardiovasc Electrophysiol 13: 927–935, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Washizuka T, Horie M, Obayashi K, Sasayama S. Genistein inhibits slow component delayed-rectifier K currents via a tyrosine kinase-independent pathway. J Mol Cell Cardiol 30: 2577–2590, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Wijetunge S, Aalkjaer C, Schachter M, Hughes AD. Tyrosine kinase inhibitors block calcium channel currents in vascular smooth muscle cells. Biochem Biophys Res Commun 189: 1620–1623, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov 8: 982–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoshiki H, Sumii K, Sperelakis N. Inhibition of L-type calcium current in rat ventricular cells by the tyrosine kinase inhibitor, genistein and its inactive analog, daidzein. J Mol Cell Cardiol 28: 807–814, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Zhang ZH, Wang Q. Modulation of a cloned human A-type voltage-gated potassium channel (hKv1.4) by the protein tyrosine kinase inhibitor genistein. Pflügers Arch 440: 784–792, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Zielonka J, Gebicki J, Grynkiewicz G. Radical scavenging properties of genistein. Free Radic Biol Med 35: 958–965, 2003 [DOI] [PubMed] [Google Scholar]