Abstract
20-hydroxyvitamin D2 [20(OH)D2] inhibits DNA synthesis in epidermal keratinocytes, melanocytes, and melanoma cells in a dose- and time-dependent manner. This inhibition is dependent on cell type, with keratinocytes and melanoma cells being more sensitive than normal melanocytes. The antiproliferative activity of 20(OH)D2 is similar to that of 1,25(OH)2D3 and of newly synthesized 1,20(OH)2D2 but significantly higher than that of 25(OH)D3. 20(OH)D2 also displays tumorostatic effects. In keratinocytes 20(OH)D2 inhibits expression of cyclins and stimulates involucrin expression. It also stimulates CYP24 expression, however, to a significantly lower degree than that by 1,25(OH)2D3 or 25(OH)D3. 20(OH)D2 is a poor substrate for CYP27B1 with overall catalytic efficiency being 24- and 41-fold lower than for 25(OH)D3 with the mouse and human enzymes, respectively. No conversion of 20(OH)D2 to 1,20(OH)2D2 was detected in intact HaCaT keratinocytes. 20(OH)D2 also demonstrates anti-leukemic activity but with lower potency than 1,25(OH)2D3. The phenotypic effects of 20(OH)D2 are mediated through interaction with the vitamin D receptor (VDR) as documented by attenuation of cell proliferation after silencing of VDR, by enhancement of the inhibitory effect through stable overexpression of VDR and by the demonstration that 20(OH)D2 induces time-dependent translocation of VDR from the cytoplasm to the nucleus at a comparable rate to that for 1,25(OH)2D3. In vivo tests show that while 1,25(OH)2D3 at doses as low as 0.8 μg/kg induces calcium deposits in the kidney and heart, 20(OH)D2 is devoid of such activity even at doses as high as 4 μg/kg. Silencing of CY27B1 in human keratinocytes showed that 20(OH)D2 does not require its transformation to 1,20(OH)2D2 for its biological activity. Thus 20(OH)D2 shows cell-type dependent antiproliferative and prodifferentiation activities through activation of VDR, while having no detectable toxic calcemic activity, and is a poor substrate for CYP27B1.
Keywords: melanocytes, melanoma cells, keratinocytes, leukemia
the photochemical isomerization of 7-dehydrocholesterol (7DHC) after absorption of UVB photons to the pre-vitamin D3 intermediate, followed by its slow isomerization to three main products including D3, tachysterol, and lumisterol, represent the most fundamental reactions in the photobiology of the skin (6, 22). Similar photochemical process occur in the case of plant-derived ergosterol leading to generation of vitamin D2 (supplementary Fig. S1). After entering the circulation, vitamins D3 and D2 are successively hydroxylated in the liver and the kidney to 1,25(OH)2D3 and 1,25(OH)2D2, respectively, which in addition to regulation of body calcium metabolism, mediate several systemic and local pleiotropic effects (reviewed in Refs. 6 and 21). Some experts (2, 11), although not all (23), express the opinion that active forms of vitamin D3 are more potent than those of vitamin D2. 1,25(OH)2D3 is also produced in the epidermis from D3 and has significant local actions on formation of the skin barrier, functional differentiation of adnexal structures, and modulation of the skin immune system (reviewed in Refs. 6, 21, and 33).
The above active forms of vitamin D3 and D2 bind to the vitamin D receptor (VDR) and induce conformational changes in the receptor, which then heterodimerizes with the retinoic acid receptor (RXR). The complex is subsequently translocated to the nucleus where it regulates transcription of genes containing the VDRE in their promoter region (13, 43, 71, 73). The final phenotypic effects include tumorostatic and anticarcinogenic activities where proliferation, differentiation, and apoptosis of cells of different lineages are affected and protection of DNA against oxidative damage (15, 22, 28, 43, 54). In addition, 1,25(OH)2D3 and its derivatives also display potent anti-leukemic activities (25, 40, 42, 44, 55, 65, 74). The above activities of 1,25(OH)2D3 and structural analogs of this compound make them of interest for the treatment of cancer and other hyperproliferative disorders (38). Unfortunately, the toxic effect of hypercalcemia caused by pharmacological doses of 1,25(OH)2D3 or 1,25(OH)2D2 impairs their use in pharmacological therapy. This has induced an extensive effort in chemical synthesis of vitamin D analogs that display reduced calcemic activity but retain powerful antiproliferative activity (38, 63, 64).
Cytochrome P450scc (P450scc) is a mitochondrial enzyme that catalyzes the first step of steroidogenesis where the side chain of cholesterol is cleaved producing pregnenolone (68). We have documented that recombinant P450scc, or isolated adrenal mitochondria containing P450scc, can hydroxylate vitamins D2 (46, 56), D3 (19, 58, 70) and their corresponding precursors, ergosterol (57), and 7-DHC (19, 60, 62). The latter reaction was also demonstrated in adrenal glands incubated ex vivo, which produced 7-dehydropregnenolone, its hydroxyderivatives and 7-dehydroprogesterone (62). P450scc converts vitamin D3 to 20-hydroxyvitamin D3 [20(OH)D3] (19, 58), 20,23-dihydroxyvitamin D3 [20,23(OH)2D3], and 17,20,23-trihydroxyvitamin D3 (70) and converts vitamin D2 to 20-hydroxyvitamin D2 [20(OH)D2], 17,20-dihydroxyvitamin D2 [17,20(OH)2D2], and 17,20,24-trihydroxyvitamin D2 [17,20,24(OH)3D2] (46, 56). 20(OH)D3 (58, 70) and 20(OH)D2 (56) are the main products of these reactions indicating that they can readily dissociate from the active side of the enzyme, which indicates their potential to enter the extracellular environment.
Our recent studies documented that 20(OH)D3 and 20,23(OH)2D3 (26, 27, 61, 76), as well as other novel secosteroidal products of P450scc action (62, 77), are biologically active and regulate the behavior of a number of cell types including the inhibition of proliferation and stimulation of differentiation of human keratinocytes and leukemia cells (26, 27, 61, 62, 76, 77). 20(OH)D3 and 20,23(OH)2D3 act through the VDR as partial receptor agonists (26, 27, 76) having antiproliferative activity as potent as 1,25(OH)2D3, but unlike 1,25(OH)2D3, only weakly stimulate the expression of the CYP24 gene (26, 27, 76). Furthermore, 20(OH)D3 shows a lack of calcemic activity at concentrations as high as 3 μg/kg, a dose that is calcemic for 1,25(OH)2D3, 25(OH)D3, and 1,20(OH)2D3 (61).
In the current study we have examined the biological activity of 20(OH)D2, the major product of vitamin D2 hydroxylation by P450scc (56) (Supplemental Fig. S1) and compared it with 1,25(OH)2D3, the classical form of vitamin D3.In our experiments we have used normal and malignant skin epidermal cells as well as K562 and HL60 leukemia cell lines that are well-defined targets of active forms of vitamin D (3, 5, 7, 32, 51). We also compared the calcemic effects of 20(OH)D2 with those of 1,25(OH)2D3 by histologically analyzing tissues for calcium deposition, indicative of calcemic toxicity.
MATERIALS AND METHODS
Preparation and Purification of 20(OH)D2
20(OH)D2 was enzymatically generated through hydroxylation of vitamin D2 (Sigma-Aldrich, St. Louis, MO) using an in vitro reconstituted P450scc system as described previously (46, 56). The product was purified by preparative TLC followed by isocratic RP-HPLC on a C18 column (Brownlee Aquapore, 25 cm × 1.0 cm, particle size 20 μm) using 90% methanol in water as mobile phase at a flow rate of 1.5 ml/min, and the identity was confirmed based on mass and UV spectra, as well as on retention times compared with standards previously characterized by NMR (56). The concentration of 20(OH)D2 was determined using an extinction coefficient of 18,000 M−1·cm−1 at 263 nm (20). The compounds were aliquoted, dried, and stored at −80°C until use.
Preparation of 1,20(OH)2D2 and Testing of CYP27B1 Activity on 20(OH)D2
Mouse CYP27B1 was expressed in Escherichia coli and purified as described previously (67). Human CYP27B1 was expressed similarly with a COOH-terminal 6-histidine tag. A bacterial membrane fraction containing the human enzyme was prepared and used for catalytic studies since the human enzyme is unstable when extracted from membranes (67). 1α-Hydroxylase activity of purified mouse CYP27B1 was measured in a reconstituted system containing 25(OH)D3 or 20(OH)D2 substrate incorporated into phospholipid vesicles (67). For human CYP27B1, the reconstituted system was similar except that bacterial membranes were used and substrates were added from an ethanol stock. After incubation, secosteroids were extracted with dichloromethane and analyzed by reverse-phase HPLC on a C18 column as before (66). Kinetic parameters were determined by fitting the Michaelis-Menten equation to the experimental data using Kaleidagraph 3.6. The concentration of 25(OH)D3 used was kept below 6 × Km to avoid the substrate inhibition reported to occur at higher concentrations (67).
To produce sufficient product from the action of CYP27B1 on 20(OH)D2 for structural determination by NMR, a 70-ml incubation of 20(OH)D2 in phospholipid vesicles containing 0.025 mol substrate/mol phospholipid was carried out for 1 h with 0.8 μM mouse CYP27B1. The product was extracted with dichloromethane and purified by reverse-phase HPLC (63), which yielded 80 μg.
To test ability of cultured cells to 1α-hydroxylate 20(OH)D2 compared with 25(OH)D3, 10×106 HaCaT keratinocytes were incubated in DMEM without phenol red (Sigma-Aldrich) containing 5 μM 25(OH)D3 or 20(OH)D2 for 24 h. After incubation, the mixtures were extracted twice with 2.5 volumes of dichloromethane. The extract was redissolved in methanol and analyzed using an API-3000 LC-MS/MS (Applied Biosystems, Toronto, Canada) mass spectrometer equipped with an ESI source. HPLC separation was performed with a gradient of methanol in water (65%-100%) at a flow rate of 0.075 ml/min for 20 min using a Zobra Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) (Agilent Technology, Santa Clara, CA).
Defining the Chemical Structure of 1,20(OH)2D2
NMR spectroscopy.
All NMR measurements were performed on a Varian Unity Inova 500 MHz spectrometer equipped with a 3-mm inverse probe (Agilent, Santa Clara, CA). Samples were dissolved in CD3OD and transferred to 3-mm Shigemi NMR tubes (Shigemi, Allison Park, PA). Temperature was regulated at 22°C and was controlled with an accuracy of ±0.1°C. Chemical shifts were referenced to residual solvent peaks for CD3OD (3.31 ppm for proton and 49.15 ppm for carbon). Standard two-dimensional NMR experiments [1H-1H correlation spectroscopy (COSY), 1H-1H total correlation spectroscopy (TOCSY), 1H-13C heteronuclear single correlation spectroscopy (HSQC), and 1H-13C heteronuclear multiple bond correlation spectroscopy (HMBC)] were acquired to fully elucidate the structures of the metabolite. All data were processed using ACD NMR processor (Advanced Chemistry Development, Toronto, ON, Canada), with zero-filling in the direct dimension and linear prediction in the indirect dimension.
Mass spectrometry.
The mass spectrum of 1,20(OH)2D2 was acquired in a Bruker Esquire-LC system (Bruker Daltonics, Billerica, MA) equipped with an electrospray ionization (ESI) source. Data were collected and processed by ACD mass processor (Advanced Chemistry Development).
Cell Culture
Normal human keratinocytes (HEKn) and melanocytes were isolated from neonatal foreskin of African American patients following protocols described previously (26, 27). The protocols were approved by the local IRB. Keratinocytes were grown in serum-free keratinocyte basal medium (KBM) supplemented with keratinocytes growth factors (KGF) (Lonza, Walkersville, MD) on collagen-coated plates (27), whereas normal melanocytes were cultured in MBM-4 medium (Lonza) containing MGM-4 (Lonza). Immortalized human epidermal keratinocytes (HaCaT) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% charcoal-treated fetal bovine serum (CT-FBS) (Hyclone, Logan, UT) and 1% antibiotics [penicillin-streptomycin-amphotericin (Sigma-Aldrich)]. Human SKMEL-188 and hamster AbC1 melanoma cells were grown in Ham's F10 supplemented with 5% CT-FBS and 1% antibiotics (59). All cultures were performed at 37°C in 5% CO2.
DNA Synthesis
Incorporation of [3H]thymidine into DNA was used as a measure of cell proliferation following protocols described previously (26, 76). Briefly, cells were synchronized at G0/G1 phase of the cell cycle by incubation in serum-free media (26, 29, 76), and then 20(OH)D2 was added with fresh media containing growth supplements (as indicated in the figure legends) and incubated for an additional 24–72 h. After a defined period of time, [3H]thymidine (specific activity 88.0 Ci/mmol; Amersham Biosciences, Picataway, NY) was added to a final concentration of 0.5 μCi/ml in medium, and after 4 h of incubation cells were precipitated with 10% TCA, processed as described previously, and subjected to liquid scintillation counting using a beta counter (Direct Beta-Counter Matrix 9600; Packard).
Cell cycle analysis was performed as described previously (26, 29). After synchronization of 40% confluent cultures of HaCaT keratinocytes or melanoma cells at G1/G0 phase, the cultures were maintained in DMEM media containing 5% CT-FBS with or without 10−7 M 20(OH)D2 for 72 h. The cells were then processed for flow cytometry with staining in propidium iodide (Sigma-Aldrich) as described previously. The cell cycle analysis was performed with a FACS Calibur flow cytometer (UTHSC Flow Cytometry and Cell Sorting Laboratory) with 10,000 cells scored.
Cell Viability Assay (MTT Assay)
For determining the number of viable cells in proliferation, we used 5-(dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Promega, Madison, WI). Human melanoma cells (SKMel-188) and neonatal human epidermal kerationocytes (HEKn), isolated from the foreskin from African American patients and in their third passage, were seeded in a 96-well plate and grown until 80% confluent. Cells were then treated with 0.1–100 nM 1,25(OH)2D3, 20(OH)D2, or 25(OH)D3 (or ethanol vehicle), diluted in KGM supplemented with KGF (Lonza) medium and 0.5% BSA for keratinocytes and in F-10 media for melanoma cells, 100 μl/well. After 44 h of incubation, 20 μl of MTT reagent (5 mg/ml) was added to the cells, which were further incubated for 4 h at 37°C. Media were discarded and cells lysed in isopropanol-0.1 N HCl solution for 30 min with shaking at room temperature. The quantity of formazan product is directly proportional to the number of living cells in culture as measured by the amount of 570 nm absorbance. Data were analyzed using GraphPad Prizm and one-way ANOVA.
Colony-Forming Assay
The assay followed standard methodology used in our laboratory as described previously (18, 29, 76). Briefly, cells were plated at a density of 20 cells/cm2 in medium containing 5% CT-FBS. Graded concentrations of 20(OH)D2 or 1,25(OH)2D3 (or ethanol vehicle as a control) were added with media being changed every 3 days. After 7 days of incubation, the colonies were fixed and then stained with 2% crystal violet, and the number and size of the colonies were measured using an ARTEK counter 880 (Dynex Technologies, Chantilly, VA). Colony-forming units were calculated by dividing the number of colonies by the number of cells plated and then multiplying by 100.
Cell Differentiation and Proliferation of Leukemia Cell Lines
HL-60 human promyelocytic and K562 human erythroleukemia cells were cultured in RPMI 1640 medium containing 10% CT-FBS and 1% penicillin-streptomycin-amphotericin antibiotic solution (61).The test compounds were added daily at concentrations of 10−7 M with media being changed every 72 h. After 5 days the cells were stained in 0.4% trypan blue (Sigma) and the viable cells counted with a hemocytometer.
Erythroid differentiation of K562 cells was evaluated after 7 days by benzidine staining. Benzidine-positive cells were scored under light microscopy examination (×20) with a minimum of 200 cells scored, and the induction of differentiation was expressed as the number of benzidine-positive cells per 200 cells. For spectrophotometric analysis, the cells were washed with PBS and lysed in lysis buffer, and the absorbance of supernatants were measured at 600 nm. Data are expressed as fold increases compared with the level of hemoglobin in mock-induced K562 cells.
Differentiation of HL-60 cells toward monocytes-like morphology was assessed by the NBT-reduction test after 5 days as described previously (61). TPA was used as a positive control. The NBT-positive cells were scored under light microscopy examination (×20) with a minimum of 200 cells scored, and the induction of differentiation was expressed as the number of NBT-positive cells per 200 cells. For spectrophotometric analysis, the cells were washed and the insoluble formazan deposits in the resulting pellets were solubilized in 1 ml of 90% DMSO, 0.1% SDS, and 0.01 mM NaOH, the samples were centrifuged 5 min at 1,500 g to remove the cellular debris, and then the absorbance of supernatants was measured at 715 nm. Data are expressed as change in A715/106 cells (16).
Immunofluorescent Staining
For involucrin immunostaining, normal human epidermal keratinocytes (HeKa) were seeded onto cover slides in six-well plates and treated with 10−7 M 20(OH)D2 or 1,25(OH)2D3 for 24 h. After treatment, cells were washed, fixed in 4% paraformaldehyde, and processed for immunofluorescent analysis as described previously (76, 78). Primary mouse anti-human-involucrin antibody was used at dilution 1:200 in 1% BSA (Sigma-Aldrich), and incubations were for 3 h at room temperature with shaking. After being washed in PBS, cells were incubated in secondary antibody solution of anti-mouse-FITC conjugate (NCL-SAM-FITC) (1:200 in 1% BSA) for 1 h. Nuclei were stained with propidium iodine (Vectashield), and cells were viewed with a fluorescent microscope and photographed under ×40 magnification. Ten to twenty independent images were generated for each condition tested. The percentage of cells expressing involucrin was counted while relative fluorescent intensity and relative fluorescent areas were determined by Image J software downloaded from the NIH web site.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
RNA from cells was extracted using an Absolutely RNA Miniprep kit (Stratagene, La Jolla, CA). A reverse transcription reaction was performed using the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN). The reaction was performed with a LightCycler 480 Probes Master (Roche Applied Science). The primers and probes were designed with the Universal Probe Library (Roche Applied Science), and the sequences are shown in supplemental Table S1. Real-time PCR was performed using TaqMan PCR Master Mix at 95°C for 5 min followed by 45 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 10 s. The data were collected with a Roche Light Cycler 480, and the amount of mRNA was normalized by a comparative Ct method, using cyclophilin B as a housekeeping gene.
Generation of Cell Lines Overexpressing VDR Fused With EGFP
pLenti-CMV-VDR-pgk-puro was constructed by cleavage of the VDR cDNA from the plasmid pRC-CMV-VDR (kind gift from Dr. Daniel Bikle, UCSF) using XbaI and XhoI sites and subcloning it into the same sites of pLenti-CMV-pgk-puro (VVC at UTHSC, Memphis).
pLenti-CMV-VDR-pgk-puro plasmid (Viral Vector Core lab at UTHSC, Memphis, TN) was amplified using primers 5′-ATACATGGATCCCCACCATGGAGGCAATGGCGGCCAGC-3′ (forward); 5′-AGCTTACTCGAGGGAGATCTCATTGCCAAACACT-3′ (reverse), and then digested with BamHI and XhoI and subcloned into the same sites of the pLenti-CMV-p2a-GFP-pgk-puro vector (Viral vector core, UTHSC) (75) to construct pLenti-CMV-VDR-p2a-EGFP-pgk-puro (supplementary Fig. S2). To construct pLenti-CMV-VDR-EGFP-pgk-puro, EGFP cDNA was amplified by PCR from pLenti-CMV-VDR-p2a-EGFP-pgk-puro using primers 5′-GGACCTCTCGAGATGGTGAGCAAGGGCGAGGAG-3′ (forward); 5′- GCGAATTCCTACTTGTACAGCTCGTCCATGCC 3′ (reverse), then digested with XhoI and EcoRI and subcloned into pLenti-CMV-VDR-p2a-EGFP-pgk-puro in which EGFP including p2a gene was removed. The VDR in pLenti-CMV-VDR-p2a-EGFP-pgk-puro was expressed independently of EGFP following 2A peptide self-cleavage, whereas the VDR in pLenti-CMV-VDR-EGFP was expressed with EGFP together as a fusion protein (supplementary Fig. S2). The lentiviral control vector pLenti-CMV-EGFP-pgk-puro was obtained from Viral Vector Core (UTHSC).
Lentivirus was produced in 293FT cells by UTHSC viral vector core using a method described previously (75). Ten multiplicities of infection of lentivirus (construct pLenti-CMV-VDR-p2a-EGFP-pgk-puro) were used to transduce SKMEL-188 melanoma and HaCaT cells in the presence of 6 μg/ml polybrene in the corresponding culture media (see above). The transduction efficiency was determined using a Nikon Eclipse TE300 microscope (Japan) by green fluorescence.
Silencing of VDR in HaCaT Cells Using VDR shRNA Lentivirus Technology
Scrambled and VDR short hairpin RNA (shRNA) lentiviral particles were purchased from Santa Cruz Biotech (Santa Cruz, CA). The viral particles were transduced following the manufacture's protocols. Briefly, lentiviral particles were transduced into HaCaT cells cultured in DMEM media containing 5% FBS in the presence of 5 μg/ml polybrene. The transduced cells were selected by further culturing in media containing 5 μg/ml puromycin. DNA synthesis in transduced cells was tested using protocols described above.
Silencing of CYP27B1 in Epidermal Keratinocytes Using siRNA Technology
Keratinocytes were transfected with 10 nM scrambled or CYP27B1 small interfering RNA (siRNA, Santa Cruz) using Lipofectamine plus (Invitrogen, Carlsbad, CA). After transfection, cells were incubated for 24 h, and medium was replaced with fresh containing the compounds to be tested or vehicle (ethanol) at the concentration of 1 μM and incubated for 24 h. Gene expression levels were determined using RT-PCR as described previously.
VDR Translocation Test
With the use of SKMEL-188 transduced by pLenti-CMV-VDR-EGFP-pgk-puro (VDR and EGFP expressed as fusion protein), VDR translocation was determined by counting cells with a fluorescent nucleus. The transduced cells were incubated with drugs for 30, 90, or 240 min and then fixed with 4% paraformaldehyde (PFA). Fixed cells were mounted with fluorescent mounting media (Dako, Denmark) and analyzed with a fluorescent microscope. At least three pictures were taken from different fields per condition, and the cells containing fluorescent nuclei were counted. Data are presented as a percentage of total cell numbers.
Testing of Calcemic Effect and Histological Markers of Toxicity
The calcemic effects of 20(OH)D2 were compared with those of 1,25(OH)2D3 as described previously for 1,20(OH)2D3 and 20(OH)D3, with some modifications (61). Briefly, 2-mo-old rats were obtained from Jackson Laboratory and fed a normal rat chow for 2 wk (10, 47) before they were divided into seven groups (6 animals per group) and injected with either vehicle (propylene glycol) or three concentrations (0.8, 2.0, and 4.0 μg/kg) of 1,25(OH)2D3 or 20(OH)D2 dissolved in propylene glycol, for 7 consecutive days. A day after the final dosing, selected organs including liver, kidney, heart, skin, and intestine were collected, fixed in 4% formalin and further processed to paraffin blocks. Five-micrometer sections were deparaffinized and processed further for staining with hematoxylin and eosin (H&E). The H&E-stained sections were examined independently by two board-certified anatomic pathologists. Experiments on rats were approved by IRB protocol from Boston University.
Statistical Analysis
Data were analyzed with GraphPad Prizm Version 4.0 (GraphPad Software, San Diego, CA) using the t-test or one-way ANOVA with appropriate post hoc tests. Differences were considered significant when P < 0.05. The data are presented as means ± SD.
RESULTS
Absolute Configuration at C20 in The 20(OH)D2 Metabolite
There are two possible isomers for this 20(OH)D2 metabolite: 20S(OH)D2 and 20R(OH)D2, depending on the absolute configuration at C20. Accumulating evidence strongly suggests that this purified metabolite is 20S(OH)D2 for the following reasons. First, this is a pure compound instead of a mixture of diastereomers as indicated by both HPLC and NMR studies (56). Second, extensive literature indicates that protons of 21-Me have very distinctive chemical shifts for 20S- and 20R-hydroxylation (12, 14, 34, 41, 45). For example, the 1H-NMR chemical shift for 21-Me in 20S-OH-cholesterol is 1.17 to 1.28 ppm (24), and 20R-OH-cholesterol is 1.00 to 1.12 ppm, depending on NMR solvents (24). The value of the proton NMR chemical shift for 21-Me has formed the basis for assigning the absolute configuration at the C20 position for many compounds. The chemical shift of 21-Me in 20(OH)D2 is 1.31 ppm (56), strongly indicating the 20S-configuration in this metabolite. This value is basically the same as that for leucisterol (1.34 ppm), which has very similar structure in the side chain compared with 20(OH)D2 (17). Whereas unambiguous assignment requires challenging stereospecific synthesis of both isomers and X-ray crystallography, a major study currently underway, collectively current evidence strongly suggests the 20S-configuration in this metabolite. It should be noted that the 20S-configuration is consistent with the known mechanism of P450 hydroxylation. The heme-bound oxygen atom abstracts hydrogen from the substrate at C20 forming a hydroxyl group, which is then transferred to the site of hydrogen abstraction on the substrate, all on the same face of the molecule (39).
Identification of 1,20(OH)2D2
Mass spectrometry (Fig. 1A) and NMR analysis (Figs. 1B, 2, and supplementary Fig. S3) of the metabolite produced by the action of CYP27B1 on 20(OH)D2 confirmed its structure to be 1α,20-dihydroxyvitamin D2. The calculated mass for 1,20(OH)2D2 is 428.3, and the observed molecular ion is 451 [M + Na]+ (Fig. 1A).
Fig. 1.
Mass (A) and proton NMR (B) spectra of 1,20(OH)2D2.
Fig. 2.
Representative two-dimensional nuclear magnetic resonance (NMR) spectra of 1,20(OH)2D2. A and B: 1H-13C heteronuclear single quantum correlation spectroscopy (HSQC); C: 1H-1H total correlation spectroscopy (TOCSY); D: 1H-1H correlation spectroscopy (COSY).
The sites of hydroxylation were unambiguously assigned to be at 1 and 20 positions based on the NMR spectra for this metabolite. All five methyl groups (18-, 21-, 26-, 27-, 28-CH3) are intact (Figs. 1B and 2A). The doublet of 21-CH3 in vitamin D2 became a singlet in the metabolite (1H at 1.29 ppm, 13C at 28.8 ppm, Fig. 2A, 1H-13C HSQC, projection), indicating the loss of scalar coupling from 20-CH. Thus the presence of a 20-OH group in the metabolite is unambiguously established. This is consistent with our previously reported results (56). For the other hydroxylation site, 1H-NMR and 1H-13C HSQC revealed a methine peak at 4.35 ppm (13C at 71.2 ppm, Fig. 2B), which is in the same spin system with the methine at C3 (4.13 ppm) based on the 1H-1H total correlation spectroscopy (TOCSY) spectrum (Fig. 2C). Therefore, the hydroxylation must be on the A ring. However, this unknown methine group is not adjacent to the C3 methine because they do not couple with each other as indicated by the 1H-1H COSY NMR spectrum (Fig. 2D). Thus the only possible site for the hydroxylation is the C1 position. The methine proton at the C1 position is a triplet (Fig. 2, B–D), with a coupling constant of 5.0 Hz to the adjacent two protons at C2. This small coupling constant indicates its equatorial (1α-OH) rather than axial (1β-OH) position, which would result in a much larger coupling constant. Thus the above analysis unambiguously confirms the metabolite to be 1α,20(OH)2D2, consistent with the well-established 1α-hydroxylase activity of CYP27B1 on a range of vitamin D analogs (66, 67). In the rest of the text, for simplicity, we refer to this metabolite as 1,20(OH)2D2.
20(OH)D2 Regulates Behavior of Normal and Malignant Skin Cells
We tested the effect of 20(OH)D2 on proliferation of HaCaT keratinocytes by assaying [3H]thymidine incorporation into DNA (Fig. 3, A and B).It inhibited DNA synthesis in time- and dose-dependent manners with more pronounced effects seen at 72 h than at 48 h of culture; the EC50 value was 3.15 × 10−11 M, which was lower than for 1,25(OH)2D3 (Fig. 3B).
Fig. 3.
Vitamin D derivatives inhibit DNA synthesis in normal and malignant skin cells in a time- and dose-dependent manner. HaCaT keratinocytes were an incubated for 48 h (A) or 72 h (B) in the presence of graded concentrations of 20(OH)D2 or 1,25(OH)2D3. Dose-dependent inhibition of the proliferation of immortalized normal epidermal melanocytes (PIG1 line) (C), neonatal epidermal melanocytes (D), SKMEL-188 human (E), and AbC1 hamster (F) melanoma cells by 20(OH)D2 or 1,25(OH)2D3 was measured after 72 h of exposure, with the exception of D, where it was measured after 48 h. Data are shown as means ± SD (n ≥ 3); *P < 0.05; **P < 0.01.
Next, we tested the response of normal epidermal melanocytes and melanoma cells to 20(OH)D2 at concentrations 10−11 to 10−7 M and compared this to HaCaT keratinocytes. The dose-dependent inhibition curves were constructed and the EC50 values were 2.05 × 10−9, 1.21 × 10−10, 3.1 × 10−10, and 1.93 × 10−11 M for immortalized normal epidermal melanocytes (PIG1 line), neonatal epidermal melanocytes, SKMEL-188, and AbC1 melanoma lines, respectively (Fig. 3). Thus the effect was dependent on cell type with HaCaT keratinocytes and melanoma cells being slightly more sensitive than immortalized normal melanocytes. We also compared the antiproliferative activity of 20(OH)D2 to that of 1,25(OH)2D3 for normal melanocytes and melanoma cells (Fig. 3, D–F). Interestingly, 20(OH)D2 was more potent than 1,25(OH)2D3 (EC50 = 3.6 × 10−10 M) in inhibition of normal melanocytes, however, was equipotent with 1,25(OH)2D3 in inhibiting SKMEL-188 melanoma proliferation (EC50 = 3.1 and 2.2 × 10−10 M). We have also used the MTT assay to show that human melanoma growth is inhibited by 20(OH)D2 (P < 0.01).
Morphological evaluation of the cells showed a lack of identifiable cytotoxic effects (not shown). The flow cytometry studies have confirmed the above finding showing that 20(OH)D2 slows cell cycling [to a similar degree as 1,25(OH)2D3] of HaCaT and melanoma cells through an increase in G1/G0 and a decrease in S and G2/M phases, with no effect on subG1 (a marker of apoptosis) (supplementary Fig. S4).
To further define the tumorostatic effect of 20(OH)D2, we examined its effect on the ability to form colonies by human SKMEL-188 melanoma cells compared with 1,25(OH)2D3. 20(OH)D2significantly inhibited colony formation at concentrations as low as 10−11 M. Analysis of all colonies formed (size >0.2 mm) showed that 20(OH)D2 was more potent than 1,25(OH)2D3 (Fig. 4).
Fig. 4.
The comparison of inhibitory effects of 20(OH)D2 (A) and 1,25(OH)2D3 (B) on the ability of human SKMEL-188 melanoma cells to form colonies. After 7 days, colonies were stained with crystal violet, and the numbers over 0.2 mm and over 0.5 mm were counted. Data are shown as means ± SD (n ≥ 3). *P < 0.05; **P < 0.01. ***P < 0.001. Bottom: representative plates of melanoma cells treated with vehicle (control) or 10−7 M 20(OH)D2 or 1,25(OH)2D3.
20(OH)D2 Inhibits Proliferation and Stimulates Differentiation of Normal Human Epidermal Keratinocytes and is More Potent Than 25(OH)D3
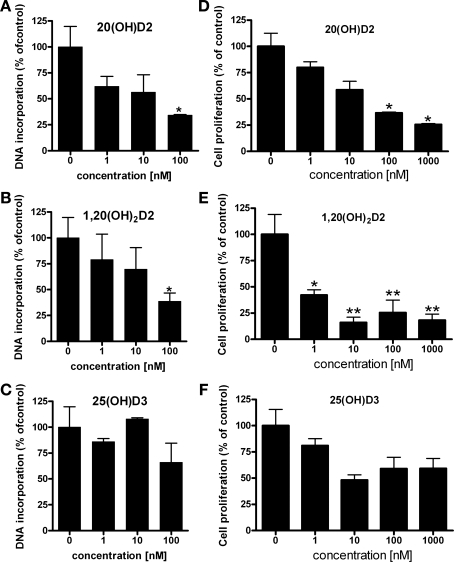
20(OH)D2 inhibited growth of normal epidermal keratinocytes in a similar manner to that by 1,25(OH)2D3, and was significantly more potent than 25(OH)D3 (supplementary Fig. S5A). The inhibition of proliferation by 20(OH)D2 was reflected by the attenuation of the expression of cyclin B1, D1, and E1 mRNA expression (supplementary Fig. S5B). Next, we compared the effects of 20(OH)D2 with those of 25(OH)D3 and 1,20(OH)2D2 on human keratinocytes growth (Fig. 5). 20(OH)D2 and 1,20(OH)2D2 inhibited DNA synthesis similarly, whereas 25(OH)D3 had no significant effect (Fig. 5, A–C). We also measured cell proliferation using the MTT assay and, again, while 20(OH)D2 and 1,20(OH)2D2 shown strong inhibition, 25(OH)D3 was without significant effect (Fig. 5, D–F).
Fig. 5.
Comparison of the antiproliferative activities of 20(OH)D2,25(OH)D2 and 1,20(OH)2D2 in human epidermal keratinocytes. A–C: rate of proliferation was determined from the amount of [3H]thymidine incorporated into DNA after 48 h of culture. Data are presented as means ± SD (n = 3). *P < 0.05. D–F: epidermal keratinocytes were treated with graded concentrations of the ligands for 24 h, and the number of viable cells was measured using the MTT assay as described in materials and methods. Data are shown as means ± SD (n = 3); *P < 0.05. **P < 0.01.
20(OH)D2 stimulated involucrin gene expression, which was accompanied by a significant increase in involucrin protein expression (Fig. 6), confirming that 20(OH)D2 induces the keratinocyte differentiation program. In additional experiments we compared the effects of 20(OH)D2 with those of 25(OH)D3, using 1,25(OH)2D3 as a positive control (supplementary Fig. S6A). Again, 20(OH)D2 was markedly more potent than 25(OH)D3 in the induction of involucrin gene expression (11-fold difference). Further testing has also shown that 20(OH)D2 and 1,20(OH)2D2 are significantly more potent (2- and 1.5-fold, respectively) in the induction of VDR gene expression than 25(OH)D3 (supplementary Fig. S6B).
Fig. 6.
20(OH)D2 induces differentiation of normal human epidermal keratinocytes. 20(OH)D2 (10−7 M) induced time-dependent involucrin gene expression (A) that was accompanied by increased expression of involucrin protein (B), which was significant in terms of increased number of cells expressing involucrin (C), increase in total relative fluorescence (D) and increased fluorescent area (E). Data are shown as means ± SD (n = 10–20). **P < 0.01. ***P < 0.001.
The above experiments clearly show that 20(OH)D2 is more potent than 25(OH)D3 in the inhibition of cell proliferation and induction of differentiation.
20(OH)D2 is a Poor Inducer of CYP24 Gene Expression Compared With 1,25(OH)2D3
Induction of CYP24 expression by 20(OH)D2 required a comparatively high concentration of the ligand (10−6 M), and induction was low or absent at a concentration of 10−8 M. This is in contrast to strong stimulation of CYP24 expression by 1,25(OH)2D3 at the same concentrations (Fig. 7). This difference was similar to that described previously for the related 20(OH)D3 versus 1,25(OH)2D3 (34) or 20,23(OH)2D3 versus 1,25(OH)2D3 (26). We have also compared the effect of 10−7 M 20(OH)D3 with that of 1,20(OH)2D3 on normal keratinocytes after a 24 h incubation and found that 20(OH)D3 is about eith times less potent than 1,20(OH)2D3 (P < 0.001) in the stimulation of CYP24 expression (supplementary Fig. S7A). In an additional experiment using siRNA technology, we found that silencing of CYP27B1 completely abrogated the effect of 20(OH)D2 and only partially the effect of 25(OH)D3 on CYP24 expression (supplementary Fig. S7B). The latter is consistent with recent reports indicating that 25(OH)D3 can also act as an agonistic vitamin D receptor ligand (35) or through another mechanism (49).
Fig. 7.
The effect of 20(OH)D2 on CYP24 mRNA expression compared with 1,25(OH)2D3 in normal epidermal keratinocytes. Keratinocytes were treated with graded concentrations of 20(OH)D2 or 1,25(OH)2D3 for the times listed. Total RNA was extracted and subjected to real-time RT-PCR analysis with cyclophilin B used as a housekeeping gene. Data are presented as means ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Phenotypic Effects of 20(OH)D2 Are Mediated Through Interaction With VDR
We have performed several experiments documenting that 20(OH)D2 exerts its phenotypic effects through activation of VDR (Fig. 8).First, stable overexpression of VDR by lentiviral technology significantly increased inhibition of cell proliferation by 20(OH)D2 in keratinocytes and melanoma cells (Fig. 8, A and B). Second, silencing of the VDR gene attenuated 20(OH)D2-mediated inhibition of cell proliferation (Fig. 8C). Finally, 20(OH)D2 induced time-dependent translocation of VDR from the cytoplasm to the nucleus at a comparable rate to that seen for 1,25(OH)2D3 (Fig. 9).
Fig. 8.
The inhibition of skin cell proliferation by 20(OH)D2 is dependent on vitamin D receptor (VDR) expression. Stable overexpression of VDR by lentiviral technology enhanced inhibition of cell proliferation by 20(OH)D2 in SKMEL-188 melanoma (A) and HaCaT keratinocytes (B). Silencing of VDR by short hairpin RNA (shRNA) attenuated 20(OH)D2-mediated inhibition of HaCaT proliferation (C). Insets in A and B represent cells transfected with VDR-GFP, whereas the inset in C shows inhibition on VDR gene expression by VDR shRNA. Data are shown as means ± SD (n ≥ 3). #P < 0.05; ##P < 0.01. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control.
Fig. 9.
20(OH)D2 (10−7 M) induces time-dependent translocation of VDR from the cytoplasm to the nucleus. Data are presented as means ± SD (n ≥ 3). *P < 0.05; **P < 0.01; ***P < 0.001. EtOH: 0.1% ethanol.
Anti-Leukemic Effects of 20(OH)D2
To study the anti-leukemic effect of 20(OH)D2, we used K562 and HL60 lines to evaluate erythroid and monocytic differentiation, respectively. Thus, after 5 days of treatment, both 10−8 M and 10−6 M 20(OH)D2 or 1,25(OH)2D3 induced significant increases in the number of cells synthesizing hemoglobin, with 1,25(OH)2D3 being significantly more potent (supplementary Fig. S8A). The same effect was seen when the level of cell differentiation was determined from the relative concentration of hemoglobin as hemin, measured spectrophotometrically in an equal number of cells (supplementary Fig. S8B).
The induction of differentiation of HL-60 cells toward monocyte-like morphology was evaluated by nitro blue tetrasolium (NBT) reduction and cell morphology, after 5 days of treatment. Both compounds tested induced cell differentiation in a dose-dependent manner as measured by the percentage of cells positive for NBT and by the absorbance at 715 nm of the supernatants from lysates (supplementary Fig. S8, C and D). Again 1,25(OH)2D3 was more potent than 20(OH)D2. Similarly, 1,25(OH)2D3 was more potent in the reduction of cell number than 20(OH)D2 (supplemental Table S2).
20(OH)D2 is Noncalcemic
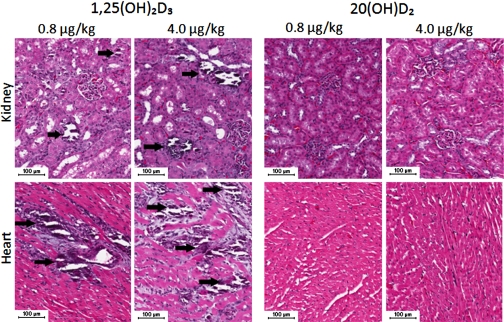
To test the systemic toxicity due to hypercalcemia, rats were treated ip with 0.8, 2, or 4 μg/kg of either 20(OH)D2 or 1,25(OH)2D3 every day for 7 days. Histological examination of the organs harvested at the end of the experiment showed that 1,25(OH)2D3 induced calcium deposits in the heart and kidney at doses as low as 0. 8 μg/kg, while this effect was absent for 20(OH)D2 even at dose as high as 4 μg/kg (Fig. 10).This shows that the cytotoxic effect of 1,25(OH)2D3 is associated with renal or cardiovascular insufficiency due to ectopic calcification. Examination of other organs including liver, lungs, small bowel, and skin showed that there was a lack of calcification or morphologically identifiable toxic effects for both 20(OH)D2 and 1,25(OH)2D3.
Fig. 10.
1,25(OH)2D3 induces calcium deposition in kidneys and heart (arrows), whereas 20(OH)D2 is noncalcemic, similar to the control (untreated animals). Bar: 100 μm.
20(OH)D2 is a Relatively Poor Substrate For CYP27B1
To test the likelihood that 20(OH)D2 rapidly undergoes 1α-hydroxylation in target cells to 1α,20-dihydroxyvitamin D2, which could then mediate its biological effects, we tested the ability of vitamin D 1α-hydroxylase (CYP27B1) to metabolize 20(OH)D2. This was done with mouse CYP27B1, where purified bacterially expressed enzyme was available (66, 67), and with human CYP27B1 in bacterial membranes (Fig. 11A).Mouse CYP27B1 converted only 6% of the 20(OH)D2 to 1α,20-dihydroxyvitamin D2, during a 2-min incubation, whereas 88% of 25(OH)D3 was converted to 1,25(OH)2D3 under identical conditions (Fig. 11A, bottom). Detailed kinetic analysis revealed that both sources of enzyme displayed a higher Km with 20(OH)D2 as substrate than with 25(OH)D3, and a lower maximum velocity (kcat or Vmax). The overall catalytic efficiency (kcat/Km for mouse CYP27B1, Vmax/Km for human CYP27B1) was 24-fold lower for 20(OH)D2 than for 25(OH)D3 with the mouse enzyme, and 41-fold lower for the human enzyme.
Fig. 11.
Comparison of CYP27B1 activity toward 25(OH)D3 and 20(OH)D2 in vitro (A) and in vivo (B). A: activity of CYP27B1 was measured using phospholipid (PL) vesicles for the mouse enzyme and with a bacterial membrane fraction for the human enzyme. Kinetic parameters for mouse CYP27B1 were obtained using 12.5 nM enzyme for 25(OH)D3 and 300 nM enzyme for 20(OH)D2. For human CYP27B1 in bacterial membranes, the protein concentration was 0.24 mg/ml for 25(OH)D3 and 2.4 mg/ml for 20(OH)D2. Incubations were for 2 min at 37°C. The chromatograms (bottom) illustrate the metabolism of 25(OH)D3 to 1,25(OH)2D3 (bottom trace) and 20(OH)D2 to a product, identified as 1α,20-dihydroxyvitamin D2 (top trace). Substrate was present in phospholipid vesicles at a ratio to phospholipid of 0.025 mol/mol, and incubations were for 2 min at 37°C with 0.2 μM mouse CYP27B1. No products were present in controls where adrenodoxin was omitted (not shown). B: transformation of 25(OH)D3 or 20(OH)D2 to corresponding dihydroxyvitamin D products by HaCaT keratinocytes. Insets: representative chromatogram of 1,25(OH)2D3 (m/z = 439 [M+Na]+) and 1,20(OH)2D2 (m/z = 451 [M+Na]+) with selective ion monitoring (SIM). Left, shaded line shows detection of 1,25(OH)2D3 (arrow) from HaCaT cells incubated with 25(OH)D3 , whereas solid line shows analysis of control HaCaT cells incubated with the ethanol vehicle only. Right, SIM for 1,20(OH)2D3 with shaded line corresponding to extract from HaCaT cells incubated with 20(OH)D2, and solid line representing extract from control cells incubated with the ethanol vehicle.
The enzymatic analysis showing that 20(OH)D2 is a relatively poor substrate for CYP27B1 was confirmed using cultured cells (Fig. 11B). Thus, whereas incubation of HaCaT keratinocytes with 25(OH)D3 led to production of 1,25(OH)2D3, no 1,20(OH)2D2 was detected in simultaneous incubations with 20(OH)D2 (Fig. 11B).
Biological Activity of 20(OH)D2 on Keratinocytes Does Not Require its Transformation to 1,20(OH)2D2
To test whether 20(OH)D2 activity requires its transformation into 1,20(OH)2D2, we silenced CYP27B1 gene expression using siRNA technology (Fig. 12A).The silencing of the CYP27B1 gene only slightly (by 8%) decreased the stimulation of involucrin expression by 20(OH)D2, with the stimulation still being highly significant compared with the control (vehicle treatment) (Fig. 12B). In addition, CYP27B1 silencing had no effect on 20(OH)D2-induced VDR gene expression (Fig. 12C). Furthermore, silencing of CYP27B1 did not significantly attenuate inhibition of DNA synthesis by 20(OH)D2 (Fig. 12D).
Fig. 12.
Effect of CYP27B1 siRNA on CYP27B1 gene expression (A) and stimulation of involucrin (B) and VDR (C) gene expression and cell proliferation (D). Keratinocytes were transfected with 10 nM scrambled or CYP27B1 small interfering RNA (siRNA) using Lipofectamine plus. After transfection, cells were treated with the compounds to be tested or vehicle (ethanol) at the concentration of 1,000 nM and incubated for 24 h. Gene expression levels were determined using RT-PCR. Total RNA was extracted and subjected to real-time RT-PCR analysis with cyclophilin B used as a housekeeping gene. Data are presented as means ± SD (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. Cells were also treated with the compounds and incubated with thymidine. DNA incorporation is measured using B-counter.
DISCUSSION
In this paper we have extensively investigated the biological activities of 20(OH)D2, a novel and major product of the P450scc-mediated metabolism of vitamin D2 (46, 56). We have also defined the chemical structure of the product generated in vitro by the action of CYP27B1 on 20(OH)D2 as 1,20(OH)2D2 and shown that it is also biologically active. 20(OH)D2 shows potent, cell-type selective, antiproliferative and prodifferentiation effects, having similar (normal and malignant skin cells) or lower potency (leukemia cells) to calcitriol [1,25(OH)2D3] and significantly higher potency than 25(OH)D3. This antiproliferative activity requires the VDR with a ligand-induced receptor translocation to the nucleus. In vivo experiments have shown that 20(OH)D2 at concentrations as high as 4 μg/kg has no identifiable toxic effects, whereas 1,25(OH)2D3 induced calcium deposition in the heart and kidney at the same or even lower (0.8 μg/kg) concentrations. 20(OH)D2 is a poor substrate for CYP27B1, and its antiproliferative and prodifferentiation activities did not require transformation to 1,20(OH)2D2.
Experiments performed on skin cells have demonstrated that 20(OH)D2 inhibits proliferation of epidermal keratinocytes and melanocytes and melanoma cells in dose- (EC50 values were between 10−11 and 10−9 M) and time-dependent manners, with keratinocytes and melanoma cells being slightly more sensitive than normal melanocytes. The antiproliferative activity of 20(OH)D2 toward skin cells was similar to that of 1,25(OH)2D3 and of enzymatically synthesized 1,20(OH)2D2 (the structure of this new compound was confirmed by NMR) but significantly higher than that of 25(OH)D3. Flow cytometry studies demonstrated that 20(OH)D2 slows cell cycling through an increase in G1/G0 and a decrease in S and G2/M phases rather than having a proapoptotic or toxic effect. In keratinocytes this activity includes stimulation of cell differentiation as documented by induction of involucrin expression by 20(OH)D2 at both mRNA and protein levels. In melanoma cells, it includes inhibition of colony formation suggesting that this antiproliferative activity may reflect its antitumorigenic activity. Thus 20(OH)D2, a product of P450scc action on plant-derived vitamin D2 (46, 56), has very similar antiproliferative/prodifferentiation activity to the related product of vitamin D3 metabolism 20(OH)D3 (27, 76) as well as to the classical active form of vitamin D3 1,25(OH)2D3 (8, 22), while being significantly more potent than 25(OH)D3.
The similarity of the effects of 20(OH)D2, 1,25(OH)2D3, and of novel 1,20(OH)2D2 raises the question of whether 20(OH)D2 requires further hydroxylation at position 1α, similar to 25(OH)D3, to reach full biological activity. Therefore, we performed additional experiments to examine this possibility. We have found that 20(OH)D2 is a relatively poor substrate for CYP27B1 with the overall catalytic efficiency for CYP27B1 being 24-fold lower for 20(OH)D2 than for 25(OH)D3 with the mouse enzyme and 41-fold lower for the human enzyme. Furthermore, transformation of 20(OH)D2 to 1,20(OH)2D2 in intact keratinocytes could not be detected, which was in contrast to production of 1,25(OH)2D3 when 25(OH)D3 was added to cells. We have also shown that 20(OH)D2 causes significantly higher inhibition of proliferation of human keratinocytes and induction of involucrin and VDR gene expression compared with 25(OH)D3. This is in agreement with our additional work with the analogous vitamin D3 metabolite [20(OH)D3], which is significantly more active than 25(OH)D3 in the inhibition of keratinocyte proliferation and differentiation (76). In addition, silencing of the CYP27B1 gene using siRNA technology neither prevented stimulation of VDR and involucrin gene expression by 20(OH)D2 nor inhibition of keratinocyte proliferation. Therefore, we can safely conclude that the antiproliferative and prodifferentiation activities of 20(OH)D2 do not require its transformation to 1,20(OH)2D2.
We have enzymatically synthesized 1,20(OH)2D2 from 20(OH)D2, and after confirming its structure by NMR, we have tested its biological activity. It had similar or more potent (in some assays) antiproliferative activity against human keratinocytes compared with the 20(OH)D2 precursor. This pattern is very similar to the previously described antiproliferative and prodifferentiation effects of the analogous 1,20(OH)2D3 and 20(OH)D3 in leukemia cells (61) and in human keratinocytes (69). However, the addition of the 1α-hydroxyl group can explain remarkable differences in the stimulation of the expression of CYP24 [the enzyme that inactivates the active forms of vitamin D (50, 52, 53)] between 20(OH)D2 and 25(OH)D3 or 1,25(OH)2D3, with 20(OH)D2 being a rather poor inducer. Importantly, silencing of the CYP27B1 gene completely abrogated the 20(OH)D2-mediated induction of CYP24 expression, indicating that metabolic hydroxylation at the 1α-position is required for this activity. In the related model for vitamin D3, 1,20(OH)2D3 is also more potent than 20(OH)D3 in induction of CYP24 expression, and it does exhibit some calcemic activity, although less than that for 1,25(OH)2D3, whereas 20(OH)D3 is noncalcemic (61). Again, the 20(OH)D2, investigated in this study is nontoxic and noncalcemic at comparatively high concentrations. Therefore, in agreement with the previous data, the current study demonstrates a requirement for the 1α-hydroxyl group for stimulation of CYP24 expression by 20(OH)D2 and 20(OH)D3, and, perhaps its calcemic activity (61), which is in contrast to the regulation of other cellular functions in human keratinocytes such as proliferation and differentiation.
Since the prodifferentiation and antiproliferative activities of 20(OH)D2 toward epidermal cells are very similar to the endogenously produced 1,25(OH)2D3 (5, 9, 33), we have performed detailed experimental studies to define the involvement of the VDR in this process. The evidence for involvement of VDR was provided using genetically modified keratinocytes and melanoma cells. Specifically, silencing of the VDR in skin cells attenuated the inhibitory effect of 20(OH)D2 on cell proliferation, whereas overexpression of VDR enhanced the antiproliferative effect. These data, in conjunction with the 20(OH)D2-induced time-dependent translocation of VDR from the cytoplasm to the nucleus comparable to that for 1,25(OH)2D3, clearly demonstrate the involvement of the VDR in mediation of the above phenotypic actions, which is again similar to what has been observed for 20(OH)D3 (27, 76) and 20,23(OH)2D3 (26). The ability of 20(OH)D2 to bind to the VDR is illustrated by molecular modeling based on the crystal structure of the VDR (supplementary Fig. S9), which shows that both 20(OH)D2 and 1,20(OH)2D2 metabolites overlap with the native ligand well and bind to the VDR with high affinity. The presence of the 20-OH moiety contributes to the binding with increased van der Waals interaction and provides a unique ability to reduce the hypercalcemic effects for this type of analog, which is consistent with literature reports that certain modifications at C20 are very beneficial for reducing hypercalcemia (4, 30, 31, 36, 64), as well as with our present (Fig. 10) and previous studies (61). In addition, the absence of hydrogen bonding interactions from the 1α-OH to the VDR is likely to contribute to the reduced calcemic activity, since addition of 1α-OH to 20(OH)D3, partially restores the calcemic effect (61).
In contrast to normal and malignant skin cells, 20(OH)D2 is less potent than 1,25(OH)2D3 in its anti-leukemic effects, suggesting that the local cellular environment and/or cell lineage affect the receptor activity. Such VDR activity could depend on RXR availability and/or on the presence and activity of coactivators and corepressors (8, 37, 48). The involvement of these factors in 20(OH)D2 and 20(OH)D3 (and their derivatives) mediated-VDR-dependent signaling represents an exciting new challenge in this field, which could also explain the remarkably different activities on the induction of CYP24 expression.
The above properties make 20(OH)D2 an excellent candidate for treatment of cutaneous hyperproliferative disorders or for use as an adjuvant in the therapy of malignant melanoma. Specifically, it has strong antiproliferative and tumorostatic activities similar to 1,25(OH)2D3 without being toxic. 20(OH)D2 shows no calcemic activity at concentrations (0.8–4 μg/kg) that for 1,25(OH)2D3 induce calcium deposits in the kidney and heart. Furthermore, 20(OH)D2 is produced by enzymatic action of P450scc either in a reconstituted system or in isolated mitochondria (56). This makes it a good candidate for a natural product, although confirmation of this awaits its direct detection in animals or plants. Finally, efficient methods of its production via enzymatic or chemical routes are available (supplementary Fig. S1).
In conclusion, evidence is presented that the newly discovered vitamin D2 derivative 20(OH)D2 shows potent and cell-type-dependent antiproliferative and prodifferentiation activity toward normal and malignant cells through activation of VDR, while having low or no toxic/calcemic activity.
GRANTS
This study was supported by National Institutes of Health Grant R01A052190 to A. Slominiski and 1R15CA125623–01A2 from NIH/NCI.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Daniel Bikle (UCSF) for his construct pRC-CMV-VDR containing human VDR.
REFERENCES
- 1.Antony P, Sigueiro R, Huet T, Sato Y, Ramalanjaona N, Rodrigues LC, Mourino A, Moras D, Rochel N. Structure-function relationships and crystal structures of the vitamin D receptor bound 2 alpha-methyl-(20S,23S)- and 2 alpha-methyl-(20S,23R)-epoxymethano-1 alpha,25-dihydroxyvitamin D3. J Med Chem 53: 1159–1171, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89: 5387–5391, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bar M, Domaschke D, Meye A, Lehmann B, Meurer M. Wavelength-dependent induction of CYP24A1-mRNA after UVB-triggered calcitriol synthesis in cultured human keratinocytes. J Invest Dermatol 127: 206–213, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Barycki R, Sicinski RR, Plum LA, Grzywacz P, Clagett-Dame M, Deluca HF. Removal of the 20-methyl group from 2-methylene-19-nor-(20S)-1alpha,25-dihydroxyvitamin D(3) (2MD) selectively eliminates bone calcium mobilization activity. Bioorg Med Chem 17: 7658–7669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D and skin cancer. J Nutr 134: 3472S–3478S, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bikle DD. Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism. J Invest Dermatol 128: 2357–2361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem 92: 436–444, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab 21: 375–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest 78: 557–566, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown AJ, Ritter CR, Finch JL, Morrissey J, Martin KJ, Murayama E, Nishii Y, Slatopolsky E. The noncalcemic analogue of vitamin D, 22-oxacalcitriol, suppresses parathyroid hormone synthesis and secretion. J Clin Invest 84: 728–732, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AJ, Ritter CS, Holliday LS, Knutson JC, Strugnell SA. Tissue distribution and activity studies of 1,24-dihydroxyvitamin D2, a metabolite of vitamin D2 with low calcemic activity in vivo. Biochem Pharmacol 68: 1289–1296, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Burstein SH, Peron FG, Williamson E. Reactions of 20-hydroxylated steroids with bovine adrenal tissue preparations. Steroids 13: 399–412, 1969 [DOI] [PubMed] [Google Scholar]
- 13.Carlberg C. Molecular basis of the selective activity of vitamin D analogues. J Cell Biochem 88: 274–281, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Corey EJ, Virgil SC, Sarshar S. New mechanistic and stereochemical insights on the biosynthesis of sterols from 2,3-oxidosqualene. J Am Chem Soc 113: 8171–8172, 1991 [Google Scholar]
- 15.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80: 1689S–1696S, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Fabiani R, De Bartolomeo A, Rosignoli P, Servili M, Selvaggini R, Montedoro GF, Di Saverio C, Morozzi G. Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J Nutr 136: 614–619, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fatima I, Ahmad I, Anis I, Malik A, Afza N, Iqbal L, Latif M. New butyrylcholinesterase inhibitory steroid and peroxy acid from Leucas urticifolia. Arch Pharm Res 31: 999–1003, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Fischer TW, Zmijewski MA, Zbytek B, Sweatman TW, Slominski RM, Wortsman J, Slominski A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol 29: 665–672, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA 100: 14754–14759, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiwatashi A, Nishii Y, Ichikawa Y. Purification of cytochrome P-450D1 alpha (25-hydroxyvitamin D3–1 alpha-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun 105: 320–327, 1982 [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D: A millenium perspective. J Cell Biochem 88: 296–307, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93: 677–681, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda M, Komori T. Structures of thornasterols A and B (biologically active glycosides from asteroidia, XI). Tetrahedron Lett 27: 3369–3372 1986 [Google Scholar]
- 25.James SY, Williams MA, Newland AC, Colston KW. Leukemia cell differentiation: cellular and molecular interactions of retinoids and vitamin D. Gen Pharmacol 32: 143–154, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol 223: 36–48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PloS one 4: e5988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamradt J, Rafi L, Mitschele T, Meineke V, Gartner BC, Wolfgang T, Holick MF, Reichrath J. Analysis of the vitamin D system in cutaneous malignancies. Recent results in cancer research. Fortschritte der Krebsforschung 164: 259–269, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kim TK, Chen J, Li W, Zjawiony J, Miller D, Janjetovic Z, Tuckey RC, Slominski A. A new steroidal 5,7-diene derivative, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity. Steroids 75: 230–239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol 72: 332–343, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Paul S, Atalla N, Thomas PE, Lin X, Yang I, Buckley B, Lu G, Zheng X, Lou YR, Conney AH, Maehr H, Adorini L, Uskokovic M, Suh N. Gemini vitamin D analogues inhibit estrogen receptor-positive and estrogen receptor-negative mammary tumorigenesis without hypercalcemic toxicity. Cancer Prev Res (Phila Pa) 1: 476–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann B. HaCaT cell line as a model system for vitamin D3 metabolism in human skin. J Invest Dermatol 108: 78–82, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Lehmann B. Role of the vitamin D3 pathway in healthy and diseased skin–facts, contradictions and hypotheses. Exp Dermatol 18: 97–108, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 75: 926–935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou YR, Molnar F, Perakyla M, Qiao S, Kalueff AV, St-Arnaud R, Carlberg C, Tuohimaa P. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol 118: 162–170, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Maehr H, Uskokovic M, Adorini L, Penna G, Mariani R, Panina P, Passini N, Bono E, Perego S, Biffi M, Holick M, Spina C, Suh N. Calcitriol derivatives with two different side chains at C-20 III. An epimeric pair of the gemini family with unprecedented antiproliferative effects on tumor cells and renin mRNA expression inhibition. J Steroid Biochem Mol Biol 103: 277–281, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Malloy PJ, Wang J, Jensen K, Feldman D. Modulation of vitamin d receptor activity by the corepressor hairless: differential effects of hairless isoforms. Endocrinology 150: 4950–4957, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther 5: 797–808, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes. Chem Rev 104: 3947–3980, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Meyskens FL, Jr, Kopecky KJ, Appelbaum FR, Balcerzak SP, Samlowski W, Hynes H. Effects of vitamin A on survival in patients with chronic myelogenous leukemia: a SWOG randomized trial. Leuk Res 19: 605–612, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Mijares A, Cargill DI, Glasel JA, Lieberman S. Studies on the C-20 epimers of 20-hydroxycholesterol. J Org Chem 32: 810–812, 1967 [DOI] [PubMed] [Google Scholar]
- 42.Munker R, Kobayashi T, Elstner E, Norman AW, Uskokovic M, Zhang W, Andreeff M, Koeffler HP. A new series of vitamin D analogs is highly active for clonal inhibition, differentiation, and induction of WAF1 in myeloid leukemia. Blood 88: 2201–2209, 1996 [PubMed] [Google Scholar]
- 43.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrinol Rev 26: 662–687, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa K, Sasaki Y, Kato S, Kubodera N, Okano T. 22-Oxa-1alpha,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis 26: 1044–1054, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Nes WR, Varkey TE. Conformational analysis of the 17(20) bond of 20-keto steroids. J Org Chem 41: 1652–1653, 1976 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome p450scc (CYP11A1). Drug Metab Dispos 37: 761–767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishii Y, Abe J, Mori T, Brown AJ, Dusso AS, Finch J, Lopez-Hilker S, Morrissey J, Slatopolsky E. The noncalcemic analogue of vitamin D, 22-oxacalcitriol, suppresses parathyroid hormone synthesis and secretion. Contrib Nephrol 91: 123–128, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol 129: 1367–1378, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng X, Vaishnav A, Murillo G, Alimirah F, Torres KE, Mehta RG. Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J Cell Biochem 110: 1324–1333, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29: 664–673, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Ren Q, Kari C, Quadros MR, Burd R, McCue P, Dicker AP, Rodeck U. Malignant transformation of immortalized HaCaT keratinocytes through deregulated nuclear factor kappaB signaling. Cancer Res 66: 5209–5215, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci 10: 119–134, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Sawada N, Kusudo T, Sakaki T, Hatakeyama S, Hanada M, Abe D, Kamao M, Okano T, Ohta M, Inouye K. Novel metabolism of 1 alpha,25-dihydroxyvitamin D3 with C24–C25 bond cleavage catalyzed by human CYP24A1. Biochemistry 43: 4530–4537, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Schwartz GG, Blot WJ. Vitamin D status and cancer incidence and mortality: something new under the sun. J Natl Cancer Inst 98: 428–430, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Sicinski RR, Prahl JM, Smith CM, DeLuca HF. New 1alpha,25-dihydroxy-19-norvitamin D3 compounds of high biological activity: synthesis and biological evaluation of 2-hydroxymethyl, 2-methyl, and 2-methylene analogues. J Med Chem 41: 4662–4674, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J 273: 2891–2901, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, Li W, Tuckey RC. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol 12: 931–939, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J 272: 4080–4090, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol 206: 780–791, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem 271: 4178–4188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PloS one 5: e9907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PloS one 4: e4309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, Holick MF. Vitamin D and cancer. Anticancer Res 26: 2515–2524, 2006 [PubMed] [Google Scholar]
- 64.Spina CS, Ton L, Yao M, Maehr H, Wolfe MM, Uskokovic M, Adorini L, Holick MF. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol 103: 757–762, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Srivastava MD, Ambrus JL. Effect of 1,25(OH)2 Vitamin D3 analogs on differentiation induction and cytokine modulation in blasts from acute myeloid leukemia patients. Leuk Lymphoma 45: 2119–2126, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Tang EKY, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, Tuckey RC. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3 producing 1α,20,23-trihydroxyvitamin D3 which has altered biological activity. Drug Metab Dispos 38: 1553–1559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang EKY, Voo KJQ, Nguyen MN, Tuckey RC. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1[alpha]-hydroxylase (CYP27B1). J Steroid Biochem Mol Biol 119: 171–179, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Tuckey RC. Progesterone synthesis by the human placenta. Placenta 26: 273–281, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 112: 213–219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J 275: 2585–2596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-dihydroxyvitamin D3. J Mol Biol 350: 65–77, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Vanhooke JL, Benning MM, Bauer CB, Pike JW, DeLuca HF. Molecular structure of the rat vitamin D receptor ligand binding domain complexed with 2-carbon-substituted vitamin D3 hormone analogues and a LXXLL-containing coactivator peptide. Biochemistry 43: 4101–4110, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 19: 2685–2695, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Wu-Wong JR, Nakane M, Ma J, Dixon D, Gagne G. Vitamin D receptor (VDR) localization in human promyelocytic leukemia cells. Leuk Lymphoma 47: 727–732, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Yue J, Sheng Y, Ren A, Penmatsa S. A miR-21 hairpin structure-based gene knockdown vector. Biochem Biophys Res Commun 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol 128: 2271–2280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids 74: 218–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol 218: 593–602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.