Abstract
Matriptase, a type 2 transmembrane serine protease, and its inhibitor hepatocyte growth factor activator inhibitor (HAI)-1 are required for normal epidermal barrier function, and matriptase activity is tightly regulated during this process. We therefore hypothesized that this protease system might be deregulated in skin disease. To test this, we examined the level and activation state of matriptase in examples of 23 human skin disorders. We first examined matriptase and HAI-1 protein distribution in normal epidermis. Matriptase was detected at high levels at cell-cell junctions in the basal layer and spinous layers but was present at minimal levels in the granular layer. HAI-1 was distributed in a similar pattern, except that high-level expression was retained in the granular layer. This pattern of expression was retained in most skin disorders. We next examined the distribution of activated matriptase. Although activated matriptase is not detected in normal epidermis, a dramatic increase is seen in keratinocytes at the site of inflammation in 16 different skin diseases. To gain further evidence that activation is associated with inflammatory stimuli, we challenged HaCaT cells with acidic pH or H2O2 and observed matriptase activation. These findings suggest that inflammation-associated reactive oxygen species and tissue acidity may enhance matriptase activation in some skin diseases.
Keywords: protease, hepatocyte growth factor activator inhibitor-1
epidermal differentiation is a carefully orchestrated process that leads to formation of the critical protective barrier provided by the skin (11, 13). The process involves progressive remodeling of cell morphology and tissue structure, and, not surprisingly, these events involve significant proteolysis that must be activated and inactivated in a controlled manner (32, 43). The importance of proteolysis in epidermis is illustrated by many genetic skin disorders that are caused by defects in proteolysis and by the phenotypes seen in animal models in which protease systems have been manipulated (7, 9, 10, 12, 16, 21, 24, 27, 28, 37, 39, 44, 45). Among the proteases and protease inhibitors in epidermis, matriptase, a type 2 transmembrane serine protease, prostasin, a glycosylphosphatidylinositol (GPI)-anchored serine protease that is a skin-selective downstream substrate of matriptase, and hepatocyte growth factor activator inhibitor (HAI)-1, a membrane-associated Kunitz-type serine protease inhibitor that is an endogenous inhibitor of matriptase and prostasin, form a unique proteolysis network that exhibits a remarkably tight regulatory and functional linkage (8, 14, 29). Activation of prostasin by active matriptase is tightly coupled with matriptase autoactivation, and both active matriptase and active prostasin are rapidly inhibited by HAI-1 (8). Both matriptase and prostasin knockout mice display similar skin defects that result in severe neonatal dehydration and death (21, 24), and HAI-1-deficient mice and matriptase hypomorphic mice display an ichthyosis-like phenotype (28, 38). In addition, matriptase is also essential for epidermal homeostasis in adult mouse skin, since postnatal ablation of matriptase causes a loss of tight junction formation leading to reduced barrier integrity (26). Furthermore, in humans, matriptase mutations are associated with autosomal recessive ichthyosis and hypotrichosis (ARIH) (1–3). Compromised desquamation and impaired profilaggrin processing are observed in the skin of ARIH patients.
The central role of matriptase-mediated proteolysis in epidermal differentiation and barrier formation suggests that deregulation of matriptase proteolysis might be associated with or contribute to development of a variety of skin diseases. Matriptase activity is regulated at three levels, control of matriptase expression, interaction with its inhibitor HAI-1, and control of zymogen activation (23). Matriptase is synthesized as a zymogen (inactive proenzyme) and only acquires proteolytic activity after autoactivation, a process that involves interaction between two matriptase zymogen molecules leading to proteolytic cleavage of both. This activation step converts the single-chain matriptase zymogen to a two-chain active form (4, 31). Matriptase zymogen activation is controlled by exogenous stimuli (5, 18, 20) and dependent upon assembly of the activation complex (20). Matriptase autoactivation is induced by an array of structurally unrelated molecules in a relatively cell type-specific manner. For example, we have shown that matriptase activation is induced by the lysophospholipid sphingosine 1-phosphate in mammary epithelial cells (5) and by androgens in LNCaP prostate cancer cells (18). Although matriptase expression is observed in almost all epithelial tissues in vivo, matriptase activation is limited (42). Robust matriptase activation occurs in epithelial cells of the lactating mammary gland (22, 40) and also in human stomach chief cells (42). Little is known, however, about how matriptase activation is regulated in skin and whether inappropriate matriptase zymogen activation is induced during the development and progression of skin diseases.
In the present study, we examined the matriptase expression and activation state in normal skin and in 23 different skin disorders. Our findings show that compared with normal epidermis, the ratio of matriptase to HAI-1 is not markedly altered in most skin disorders. However, increased matriptase zymogen activation was observed in ∼70% of the specimens of skin diseases examined and that activation was observed in keratinocytes close to areas of inflammation. Moreover, exposure of HaCaT cells to mild acidic buffer or hydrogen peroxide (H2O2) results in increased matriptase activation, suggesting that extracellular acid conditions or production of reactive oxygen species (ROS), both of which are associated with inflammation, may be responsible for the increase in matriptase zymogen activation seen in these skin disorders.
MATERIALS AND METHODS
Cell culture.
The immortalized, nontumorigenic human keratinocyte line HaCaT was cultured in DMEM (Cellgro, Manassas, VA) supplemented with 10% FBS (Gemini, West Sacramento, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were maintained in a humidified 5% CO2 atmosphere at 37°C.
Antibodies, reagents, and immunostaining.
Human matriptase protein was detected with monoclonal antibodies M32 and M24, both of which recognize the latent and activated forms of the protease (22). Activated matriptase was detected with M69, which recognizes an epitope present only in the activated form of the protease (4, 5). Human HAI-1 was detected with the HAI-1-specific monoclonal antibody M19 (22). These reagents have been adapted for use in both immunoblot and immunohistochemistry analyses. 3,3′-Diaminobenzidine (DAB, K3468) and the secondary antibody (K4063, EnVision+ Dual Link System Peroxidase) were purchased from Dako (Glostrup, Denmark). All other chemical reagents were purchased from Sigma (St. Louis, MO) unless otherwise specified. Immunostaining was performed according to the manufacturer's standard protocol with minor modification (Dako). Frozen and paraffin-embedded human skin tissue sections were obtained from Tri-Service General Hospital, National Defense Medical Center under IRB 099-05-019, approved by the Tri-Service General Hospital Institutional Review Board. The skin specimens appear to be nonacral skin on the basis of the thinness of the cornified layer. Sections were stained with matriptase-specific (M32, M24, and M69) and HAI-1-specific (M19) antibodies at a concentration of 5 μg/ml. Mouse IgG at a concentration of 5 μg/ml was used as a negative control on duplicate sections. Colorimetric reactions for the control slides were developed for the same amount of time as experimental slides.
Immunoblot.
HaCaT cells were lysed in 1% Triton X-100 in phosphate-buffered saline (PBS) containing 1 mM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and protein extracts were resolved by SDS-PAGE under nonboiled and nonreducing conditions and transferred to nitrocellulose membrane (Schleicher and Schuell, Keene, NH) for immunoblot. Binding of the primary antibody was detected with horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and visualized with Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer, Boston, MA).
RESULTS
Matriptase distribution and activity are under tight control in human epidermis.
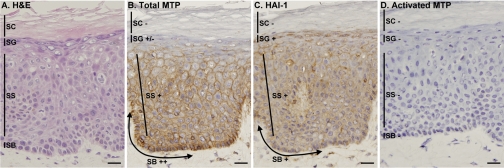
To investigate the role of matriptase and HAI-1 in human skin disease, we examined the distribution of matriptase and HAI-1 and the activation state of matriptase in normal human skin by immunohistochemical staining. In a representative skin section, hematoxylin and eosin (H & E) staining (Fig. 1A) showed the typical epidermal architecture with multiple layers of keratinocytes, including stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. We used monoclonal antibodies that recognize total (latent and active) matriptase and active matriptase and an antibody that recognizes HAI-1. Mouse IgG was also used as negative control. The staining revealed some important and interesting features of the matriptase system in human skin. First, in agreement with in situ hybridization results (30), matriptase protein was detected in the basal and spinous layers (Fig. 1B). This finding is interesting in that it differs from the mouse epidermis, where, as measured by gene trapping assay, matriptase is present only in the upper epidermal layers (29). As expected for a membrane-associated protein, matriptase localized at the cell surface (Fig. 1B), a finding that is consistent with observations in other epithelial cell types (42). However, in contrast to observations in other cell types such as kidney, where matriptase localizes at the basal-lateral surface along the basement membrane, matriptase appears to be absent or present at low levels along the basement membrane in epidermis. It is, however, at the site of cell-cell contact. This difference in subcellular distribution in keratinocytes versus polarized epithelial cells suggests a functional divergence in different tissues. The basal keratinocytes appear to express matriptase at slightly higher levels than spinous cells. Matriptase levels are progressively lower in the uppermost spinous layers, and it is absent in the granular layer and stratum corneum (Fig. 1B). This expression pattern suggests that matriptase may have a role in proliferative and differentiating keratinocytes. HAI-1 was also detected at sites of cell-cell contact in the basal and spinous layers (Fig. 1C). HAI-1 expression levels are similar in the basal and spinous layers. The most striking difference between the patterns of HAI-1 and matriptase expression is that HAI-1 is detected at high levels in the upper granular layer where matriptase is not detected. Although matriptase is abundant in normal human epidermis, it is interesting that it is not activated. As shown in Fig. 1D, cleaved/activated matriptase was not detected in any epidermal layer. The data suggest that normal human skin maintains very low levels of matriptase activation. The lack of detectable matriptase activation in the steady state of normal human skin is consistent with the previous study (1) in which matriptase was detected as the single-chain form by immunoblot.
Fig. 1.
Lack of matriptase activation in normal epidermis. Samples of normal human skin were used to prepare frozen sections that were stained with hematoxylin and eosin (H & E; A) or immunostained with monoclonal antibodies to detect total matriptase M32 (total MTP; B), total hepatocyte growth factor activator inhibitor (HAI)-1 M19 (HAI-1; C), or activated matriptase M69 (activated MTP; D). Mouse IgG was used as the negative control (not shown). SB, stratum basale; SS, stratum spinosum; SG, stratum granulosum; SC, stratum corneum. Staining intensity is expressed as ++ for strong positive, + for positive, +/− for weak positive, and − for negative. Bars = 25 μm.
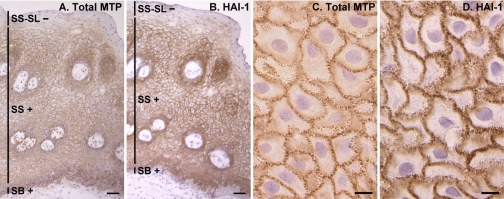
To confirm the distribution of matriptase in the human stratified squamous epithelium, we monitored matriptase and HAI-1 distribution in the oral mucosa. The oral epithelium has a structure similar to epidermis that includes basal and spinous layers. The surface layer of the oral mucosa is a parakeratinized epithelium in which the keratinocytes retain their nuclei, and it lacks the granular and cornified layers seen in the epidermis. Normal human oral mucosa was therefore assessed for matriptase expression and activation and HAI-1 presence (Fig. 2). The expression profiles of matriptase and HAI-1 in the basal and spinous layers mirrored those found in skin, with decreasing levels and eventual loss of matriptase expression in the upper oral mucosal layers (Fig. 2A). In contrast, unlike epidermis where HAI-1 is observed in the granular layer, HAI-1 was not detected in the surface layer of the oral mucosa (Fig. 2B). As in epidermis, activated matriptase was not detected in oral mucosa (not shown). The reduced matriptase level in the granular layer of epidermis and the surface layer of oral mucosa suggests that matriptase may not have a role in the late stages of surface epithelial differentiation, but suggests an important role in the basal and granular layers. In contrast, the increased expression of HAI-1 in the epidermal granular layer but its loss in the surface layer of the oral mucosa suggests that HAI-1 may play an important role in the epidermal terminal differentiation, a skin-specific process.
Fig. 2.
Expression and subcellular localization of matriptase and HAI-1 in human oral mucosa. Sections of paraffin-embedded human oral mucosal tissue were stained by immunohistochemistry for total matriptase with the MAb M24 (A) and for HAI-1 with MAb M19 (B), as indicated. The micrographs are also presented at higher magnification (C and D) for the subcellular localizations. Bars = 50 μm (A and B), 10 μm (C and D). SS-SL, stratum spinosum-surface layer.
An interesting feature of the subcellular staining of matriptase and HAI-1 at cell-cell contacts was noted in both skin and oral mucosa. At higher magnification, it was evident that matriptase (Fig. 2C) and HAI-1 (Fig. 2D) staining is not homogeneously distributed along the cell periphery, particularly in the spinous layer. Instead, staining for both proteins is concentrated in barlike structures studded on the surface of the cells in a discontinuous pattern (Fig. 2, C and D). This staining pattern is reminiscent of the characteristic intercellular bridges found in the stratum spinosum, where cytoplasmic fibrils (tonofibrils) interact with desmosome of adjacent cells (15). This pattern suggests that this protease system is concentrated at the cell surface in specialized membrane subdomains, which connect two keratinocytes. Taken together, these data suggest that this cell surface protease/protease inhibitor pair is targeted to cell-cell junctions at sites of cell-cell contact and their expression is dynamically regulated during epidermal differentiation. Furthermore, matriptase zymogen activation appears to be under very tight control and occurs at very limited levels in normal skin and oral mucosa.
Active matriptase is present in keratinocytes bordering site of inflammation in skin disease.
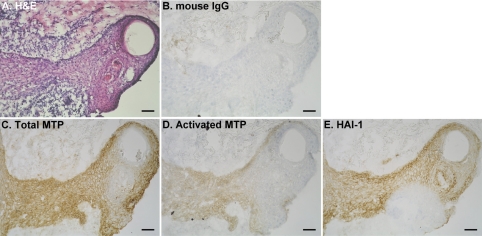
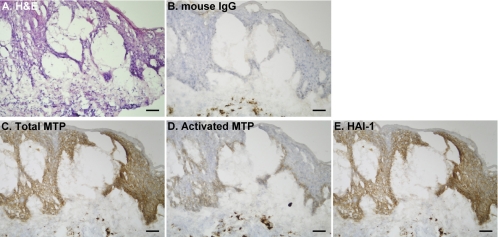
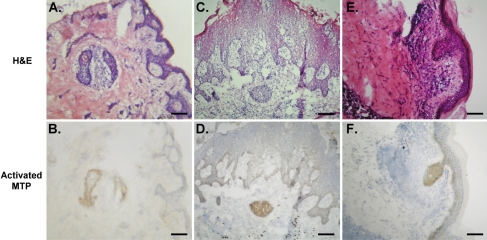
Having determined the patterns of HAI-1 and matriptase expression and the level of matriptase activation in normal skin, we set out to determine how these might be altered in a variety of skin diseases. To do this we screened 45 samples of tissue representing 23 distinct skin disorders (Table 1) for the presence of total matriptase and HAI-1 and activated matriptase. In most of these skin disorders, the levels of matriptase and HAI-1 and the matriptase-to-HAI-1 ratio were similar to those observed in normal skin. In contrast, activated matriptase was detected in 29 of 45 specimens, representing 16 different skin disorders. A representative example of staining observed in samples from eight different patients with ruptured epidermal cysts is shown in Fig. 3. Matriptase (Fig. 3C) and HAI-1 (Fig. 3E) generally colocalize at the cell-cell junction in viable keratinocytes throughout the sections. However, in contrast to normal epidermis, cleaved/activated matriptase zymogen was detected in a subpopulation of these keratinocytes (Fig. 3D), most of which are surrounded and/or infiltrated by inflammatory cells (Fig. 3A). The tissue section was also stained with mouse IgG as a negative control (Fig. 3B). Another example of a skin disorder in which increased matriptase activation is evident is chronic eczema. Matriptase and HAI-1 were detected in all viable keratinocytes (Fig. 4, C and E); however, activated matriptase was detected mainly on basal cells (Fig. 4D) that are surrounded by inflammatory cells (Fig. 4A). The tissue section was also stained with mouse IgG as a negative control (Fig. 4B). Increased matriptase activation was also observed in three other skin disorders, including inflamed intradermal nevus (Fig. 5B), prurigo nodularis (Fig. 5D), and natural killer cell lymphoma with skin metastasis (Fig. 5F). H & E staining of tissue sections of the three skin disorders (Fig. 5, A, C, and E) further showed that the keratinocytes with activated matriptase are surrounded and/or infiltrated with inflammatory cells. Similar to the situation seen with ruptured epidermal cyst and chronic eczema, activated matriptase was evident in the keratinocytes at sites of inflammation and immune cell infiltration (Fig. 5). Taken together, these results suggest that the normal tight control of matriptase activation may be lost in specific skin disorders and may be associated with inflammation.
Table 1.
Expression and activation of matriptase in human skin disorders
| Specimen | Description | No. of Cases | M69 Positive | M32 Intensity | M19 Intensity |
|---|---|---|---|---|---|
| 1 | IDN | 8 | 5/8 | ++++ | ++++ |
| 2 | Blue nevus | 1 | 0/1 | +++ | +++ |
| 3 | Seborrheic keratosis | 5 | 1/5 | ++ | ++ |
| 4 | Skin tag | 4 | 2/4 | ++ | ++ |
| 5 | Wart | 1 | 0/1 | + | + |
| 6 | EC | 8 | 8/8 | +++ | +++ |
| 7 | Pityriasis rosea | 1 | 1/1 | ++++ | ++++ |
| 8 | Actinic keratosis | 1 | 1/1 | ++++ | ++++ |
| 9 | Sebaceous hyperplasia | 1 | 1/1 | ++++ | ++++ |
| 10 | Folliculitis | 1 | 1/1 | ++++ | ++++ |
| 11 | Natural killer cell lymphoma with skin metastasis | 1 | 1/1 | +++ | +++ |
| 12 | Keloid scar | 1 | 0/1 | ++++ | + |
| 13 | Compound nevus | 1 | 0/1 | + | + |
| 14 | IDN and ruptured EC | 2 | 2/2 | +++ | +++ |
| 15 | Prurigo nodularis | 1 | 1/1 | +++ | +++ |
| 16 | Chronic eczema | 1 | 1/1 | ++++ | ++++ |
| 17 | Pilar sheath acanthoma | 1 | 1/1 | ++++ | ++++ |
| 18 | Tophi | 1 | 1/1 | +++ | +++ |
| 19 | Parakeratosis | 1 | 1/1 | ++++ | ++++ |
| 20 | Drug eruption | 1 | 1/1 | +++ | +++ |
| 21 | Melanocyte nevus | 1 | 0/1 | ++ | ++ |
| 22 | ALHE | 1 | 0/1 | ++ | ++ |
| 23 | Granuloma | 1 | 0/1 | ++ | ++ |
| Total | 45 | 29/45 | 45/45 | 45/45 |
IDN, intradermal nevus; EC, epidermal cyst; ALHE, angiolymphoid hyperplasia with eosinophilia.
Fig. 3.
Increased matriptase activation in ruptured epidermal cyst. Tissue sections of frozen human epidermal cyst specimens were analyzed by immunohistochemistry for morphology by H & E staining (A) and for total matriptase (C), activated matriptase (D), HAI-1 (E), and negative control using mouse IgG (B). Bars = 50 μm.
Fig. 4.
Increased matriptase activation in chronic eczema. Tissue sections of frozen human skin specimens from chronic eczema patients were analyzed by immunohistochemistry for morphology by H & E staining (A) and for total matriptase (C), activated matriptase (D), HAI-1 (E), and negative control using mouse IgG (B). Bars = 50 μm.
Fig. 5.
Increased matriptase activation in inflamed intradermal nevus, prurigo nodularis, and natural killer cell lymphoma with skin metastasis. Tissue sections of frozen human skin specimens from 3 skin disorders, including intradermal nevus (A and B), prurigo nodularis (C and D), and natural killer cell lymphoma with skin metastasis (E and F), were analyzed by immunohistochemistry for morphology by H & E staining (A, C, E) and for activated matriptase (B, D, F). Bars = 50 μm.
Extracellular acidity and elevated reactive oxygen species induce matriptase activation.
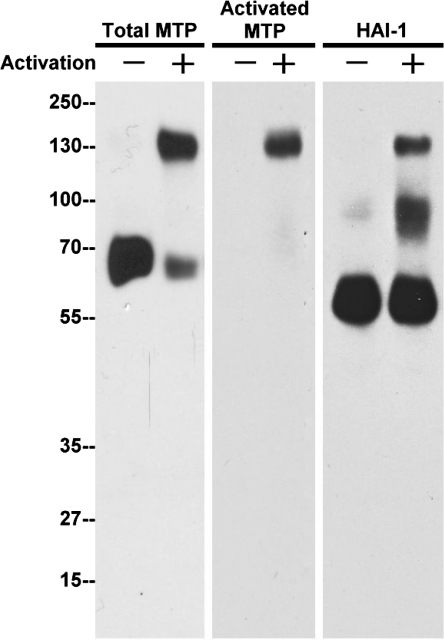
The observation that matriptase activation is increased in keratinocytes close to areas of inflammation in a variety of skin disorders suggests that the activation might be part of the keratinocyte response to inflammation. Inflammation is a regulated response to harmful stimuli such as the presence of pathogens or tissue damage that involves the recruitment of activated inflammatory cells that in turn cause significant changes in stromal microenvironment including making it more acidic and more oxidizing (33–35). We have previously shown (19) that matriptase activation depends on the physical and chemical environment. In terms of the physical environment, the protease has to be anchored on a lipid bilayer for effective and robust zymogen activation. Regarding the chemical environment, extracellular acidity and ionic strength have been shown to regulate matriptase activation. We hypothesize that the increased matriptase activation associated with the inflammation in skin disease might result from inflammation-associated acidification and oxidative stress. To test this, we exposed immortalized human keratinocyte HaCaT cells to mildly acidic conditions or H2O2, treatments that cause oxidative stress. HaCaT cells display some features of normal keratinocyte differentiation. We first examined the impact of mildly acidic conditions (pH 6, 30 min) on matriptase function by running extract on SDS-PAGE under nonboiled and nonreducing conditions. As shown in Fig. 6, untreated HaCaT cells express high levels of total matriptase (70 kDa) and HAI-1 (55 kDa) as shown by immunoblot analysis (total MTP, HAI-1), but no active matriptase (activated MTP) is detected. Upon activation, matriptase forms a complex with HAI-1 that migrates at 120 kDa and is detected by the total matriptase antibody, by anti-HAI-1, and by the anti-active matriptase antibody. As shown in Fig. 6, this band is evident in cells that have been treated with reduced pH conditions.
Fig. 6.
Human keratinocyte HaCaT cells activate matriptase in response to extracellular acidity. Immortalized human keratinocyte HaCaT cells were exposed to a pH 6.0 buffer or basal medium as negative control for 30 min. Cell lysates were prepared and analyzed by immunoblot analysis for total matriptase (total MTP), HAI-1, and activated matriptase (activated MTP). Matriptase and prostasin were robustly activated and rapidly inhibited by HAI-1 to form a 120-kDa complex and an 85-kDa complex, respectively. Both complexes were clearly detected by HAI-1 MAb.
We have previously shown that prostasin, a serine protease, is a specific matriptase substrate in keratinocytes. Matriptase cleavage of prostasin converts it to an active form; this active form of prostasin interacts with HAI-1 to form an 85-kDa prostasin-HAI-1 complex (8). In the present study, the prostasin-HAI-1 complex was detected in extracts prepared from mild acid-treated HaCaT cells with anti-HAI-1 (Fig. 6). These data demonstrate that human keratinocytes display robust matriptase activation in response to extracellular acidosis. This has also been observed in other cell types (41).
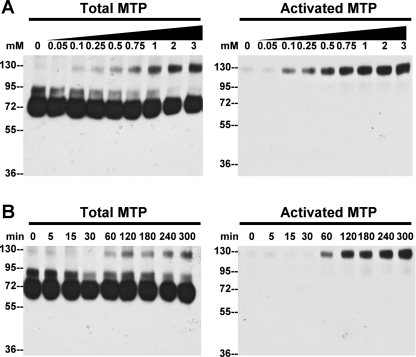
To test the role of ROS in activating matriptase, HaCaT cells were exposed to H2O2. As shown in Fig. 7, treatment of HaCaT cells with increasing concentrations of H2O2 results in increased levels of activated matriptase, which migrates as a complex with HAI-1 at 120 kDa that is detected by anti-total matriptase and anti-activated matriptase (Fig. 7A). ROS-induced matriptase activation appears to occur quite rapidly, since active matriptase is detectable within 60 min after exposure to H2O2 exposure and the level of activated matriptase continues to accumulate thereafter (Fig. 7B). These data demonstrate that H2O2 induces matriptase activation in a dose- and time-dependent manner. Taken together, these data suggest that the increased acidity and accumulation of ROS can lead to matriptase activation in keratinocytes.
Fig. 7.
H2O2 exposure induces matriptase activation in HaCaT cells. HaCaT cells were exposed to increasing concentrations of H2O2 for 6 h (A) or to 500 μM H2O2 for the indicated times (B). Cell lysates were prepared and analyzed by immunoblot analyses for total matriptase (total MTP), activated matriptase (activated MTP), and HAI-1.
DISCUSSION
In the present study, we have studied the mode of matriptase activation in disease states and in cultured keratinocytes. Our findings are consistent with the novel idea that matriptase is activated in response to inflammatory stimuli, which leads to reduced pH and increased production of ROS. Given the critical role that matriptase plays in skin barrier formation and the extremely tight regulation of matriptase activity, inappropriate elevation and prolonged zymogen activation may have an adverse impact on epidermal differentiation and enhance the disease phenotype or prolong the recovery from skin diseases.
The distribution profile of matriptase expression in human skin is interesting and may provide important clues regarding its role in skin disease. Epidermal basal keratinocytes express the highest levels of matriptase, suggesting a potential role for the enzyme in regulating epidermal proliferation. Matriptase may also be important for epidermal differentiation because of its high expression in the spinous layer and early granular layer. The significant decrease in matriptase expression in the late granular layer, however, suggests a diminishing role as cells terminally differentiate. The lack of matriptase in the upper epidermal layers was also observed in the noncornified epidermis of oral mucosa (Fig. 2), suggesting a conservation of distribution and perhaps function in different stratified epithelia. In contrast to matriptase, high-level expression of HAI-1 in the late granular layers suggests that the protease inhibitor may have a role in epidermal terminal differentiation during terminal cornification. It is possible that HAI-1 inhibits a protease other than matriptase in these layers and that the specific protease target of HAI-1 may vary during differentiation.
In addition to its differential expression at various stages of epidermal differentiation, the detailed subcellular localization (Fig. 2) and biochemical studies of matriptase activation also provide insights. Matriptase is clearly present in the cell-cell junctions that connect keratinocytes. Given the extremely short half-life of free, active matriptase and the location of the serine protease domain of the enzyme on the extracellular sides of cell-cell junctions, the immediate downstream events resulting from matriptase activation are likely extracellular rather than intracellular (8, 29). Thus in epidermis matriptase may act via “outside in” signaling to regulate epidermal proliferation and differentiation and profilaggrin processing. Impaired profilaggrin processing is observed in matriptase-deficient mice and in patients with matriptase mutations (1, 27). The case for an indirect role of matriptase in profilaggrin processing is also supported by the differential subcellular localization of the two proteins. Profilaggrin processing takes place mainly within the keratohyalin granules (17, 36), organelles that are abundantly seen in the late granular epidermis; however, matriptase is expressed at very low levels at these cells. Although terminal differentiation in the transitional layer plays a critical role in the epidermal barrier formation, the proliferation program in the basal cells and the differentiation in the suprabasal cells can affect subsequent terminal differentiation, and, therefore, defects in proliferation and differentiation can also influence terminal differentiation.
In contrast to our finding of matriptase presence in all living epidermal layers as shown by immunostaining in the present study and by in situ hybridization in our previous study (30), matriptase is reported to be expressed only in the uppermost epidermal layers in mouse epidermis as measured by gene trap (29). Moreover, matriptase is detected in the chief cells of the human stomach by immunostaining (42) but not seen in mouse stomach chief cells as measured by gene trap (25). The difference in matriptase distribution in mouse versus human epidermis and stomach may result from the differences in detection method or may be due to genuine physiological differences between rodents and primates. The less severe clinical phenotype observed with matriptase mutation in humans compared with mice (1) may reflect a genuine difference in physiological role in the two species.
The lack of detectable activated matriptase by immunohistochemistry in normal skin is consistent with a previous study (1), in which only the single-chain matriptase was detected by immunoblot. Activated matriptase is a two-chain form and constantly in complexes with HAI-1. Activated matriptase can be detected by immunoblot either as a 120-kDa complex with HAI-1 under nonreducing and nonboiled conditions or as the dissociated fragments at 45 kDa or 25 kDa under reducing and boiled conditions. The 70-kDa matriptase band is therefore most likely to be the latent matriptase. The latent 70-kDa matriptase was also detected in normal human skin by immunoblot under reducing and boiled conditions in another previous study (6). Interestingly, although a 130-kDa protein band was also detected in this study, this 130-kDa protein band should not be the matriptase-HAI-1 complex that should have been dissociated under boiling treatment. Therefore, the detection of the 130-kDa protein should not be considered as valid evidence for the presence of activated matriptase in normal human skin. The lack of detectable activated matriptase may result from the low level of matriptase activation in homeostatic skin. Furthermore, matriptase-HAI-1 complex is rapidly removed from the cell surface (41, 42). The rapid removal of matriptase-HAI-1 complex could also contribute to the extreme low levels of activated matriptase in normal skin. Nevertheless, the increased activated matriptase detected in skin diseases suggests that matriptase activation is elevated during the disease process.
The functional linkage between matriptase activation and inflammation associated with some skin diseases provides interesting speculation on whether aging and solar damage affect matriptase activation. After scrutinizing the H & E slides available for all cases, except one case of actinic keratosis, we did not see significant signs of solar damage, such as skin atrophy or solar elastosis of papillary dermis. Furthermore, since patient information for the specimens examined is not available, the relationship between age and matriptase activation cannot be established and needs to be further investigated. Although ROS is likely to be the inflammation-associated factor that induces matriptase activation in keratinocytes, the levels of ROS in these skin specimens were not determined in the present study.
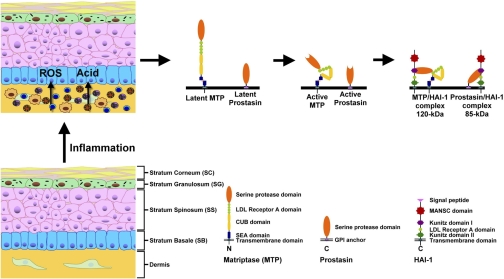
The presence of matriptase accumulation on the surface of basal layer keratinocytes may confer an ability of these cells to respond to changes in the dermis. The increased matriptase activation in keratinocytes observed near areas of inflammation is consistent with this hypothesis. Many skin diseases are accompanied by inflammation associated with recruitment of inflammatory leukocytes leading to cytokine release. Activation of matriptase by H2O2 and acidity may represent a response to accumulation of ROS in the environment. Although the increased matriptase activation in keratinocytes may in turn contribute to the inflammation process, whether matriptase activation affects and/or regulates inflammation remains unclear. Figure 8 presents our model of matriptase regulation in normal and diseased epidermis. In normal epidermis, matriptase and prostasin are activated only under highly restricted conditions to facilitate normal differentiation. In Fig. 8 each protein is shown in an inactive state. In skin diseases that produce inflammation, acid/low pH conditions and ROS production may lead to activation of matriptase and prostasin. It is known that matriptase is required for normal keratinocyte differentiation and barrier assembly, but the role of the abnormally high level of activity associated with inflammation in skin disease will require additional study.
Fig. 8.
Schematic illustration of the regulation of matriptase-driven serine protease network by inflammation in skin diseases. A matriptase-driven serine protease network, comprised of matriptase as the initiator protease, prostasin, a downstream protease substrate of matriptase, and HAI-1, a Kunitz-type serine protease inhibitor, is expressed at high levels at the cell surface of living keratinocytes in normal skin, where the activation of the system is tightly regulated. In some skin disease with inflammation, proinflammatory cells are recruited that release reactive oxygen species (ROS) and increase tissue acidity. Both the ROS and acidity stimulate nearby keratinocytes to activate matriptase. The active matriptase rapidly activates prostasin before the inhibitor HAI-1 forms complexes with the active matriptase and active prostasin.
GRANTS
This study was supported by Taiwan Department of Defense Grant DOD98-14-02 (J.-K. Wang), DOD98-14-05 (C.-J. Chen), and Taiwan Tri-Service General Hospital Grant TSGH-C99-036 (to B.-Y. Wu). This work was also supported by National Institutes of Health Grants R01-CA-104944 (C.-Y. Lin), R01-CA-123223 (M. D. Johnson and C.-Y. Lin), and R01-AR-046494 and R01-AR-094713 (R. L. Eckert).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alef T, Torres S, Hausser I, Metze D, Tursen U, Lestringant GG, Hennies HC. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol 129: 862–869, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, Smitt J, van Marle J, Shohat M, Basel-Vanagaite L. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet 74: 47–53, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben AD, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet 80: 467–477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem 268: 1439–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem 277: 10539–10546, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bocheva G, Rattenholl A, Kempkes C, Goerge T, Lin CY, D'Andrea MR, Stander S, Steinhoff M. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol 129: 1816–1823, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Chavanas S, Bodemer C, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, Bonafe JL, Wilkinson J, Taieb A, Barrandon Y, Harper JI, de Prost Y, Hovnanian A. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet 25: 141–142, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chen YW, Wang JK, Chou FP, Chen CY, Rorke EA, Chen LM, Chai KX, Eckert RL, Johnson MD, Lin CY. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 (HAI-1) during epidermal differentiation. J Biol Chem 285: 31755–31762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, Mauro T, Hupe M, Crumrine D, Roelandt T, Houben E, Elias PM, Feingold KR. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol 172: 86–97, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, Van den Broecke C, Van Damme P, D'Herde K, Hachem JP, Borgonie G, Presland RB, Schoonjans L, Libert C, Vandekerckhove J, Gevaert K, Vandenabeele P, Declercq W. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol 9: 666–674, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Eckert RL. Structure, function, and differentiation of the keratinocyte. Physiol Rev 69: 1316–1346, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Egberts F, Heinrich M, Jensen JM, Winoto-Morbach S, Pfeiffer S, Wickel M, Schunck M, Steude J, Saftig P, Proksch E, Schutze S. Cathepsin D is involved in the regulation of transglutaminase 1 and epidermal differentiation. J Cell Sci 117: 2295–2307, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Elias PM, Friend DS, McNutt NS. Epidermal permeability barrier: transformation of lamellar granule-disks into intercellular sheets by a membrane fusion process. J Invest Dermatol 88: 459–460, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Fan B, Wu TD, Li W, Kirchhofer D. Identification of hepatocyte growth factor activator inhibitor-1B as a potential physiological inhibitor of prostasin. J Biol Chem 280: 34513–34520, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fawcett DW. Intercellular bridges. Exp Cell Res Suppl 8: 174–187, 1961 [DOI] [PubMed] [Google Scholar]
- 16. Hart TC, Hart PS, Bowden DW, Michalec MD, Callison SA, Walker SJ, Zhang Y, Firatli E. Mutations of the cathepsin C gene are responsible for Papillon-Lefevre syndrome. J Med Genet 36: 881–887, 1999 [PMC free article] [PubMed] [Google Scholar]
- 17. Kamata Y, Taniguchi A, Yamamoto M, Nomura J, Ishihara K, Takahara H, Hibino T, Takeda A. Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J Biol Chem 284: 12829–12836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiyomiya KI, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and subsequent shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. Am J Physiol Cell Physiol 291: C40–C49, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol 293: C95–C105, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Lee MS, Kiyomiya K, Benaud C, Dickson RB, Lin CY. Simultaneous activation and HAI-1-mediated inhibition of matriptase induced at activation foci in immortal human mammary epithelial cells. Am J Physiol Cell Physiol 288: C932–C941, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, Sandhoff K, Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol 170: 487–496, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem 274: 18237–18242, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, Dickson RB. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci 13: 621–635, 2008 [DOI] [PubMed] [Google Scholar]
- 24. List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene 21: 3765–3779, 2002 [DOI] [PubMed] [Google Scholar]
- 25. List K, Hobson JP, Molinolo A, Bugge TH. Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol 213: 237–245, 2007 [DOI] [PubMed] [Google Scholar]
- 26. List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol 175: 1453–1463, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, Bugge TH. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol 163: 901–910, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagaike K, Kawaguchi M, Takeda N, Fukushima T, Sawaguchi A, Kohama K, Setoyama M, Kataoka H. Defect of hepatocyte growth factor activator inhibitor type 1/serine protease inhibitor, Kunitz Type 1 (Hai-1/Spint1) leads to ichthyosis-like condition and abnormal hair development in mice. Am J Pathol 173: 1464–1475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem 281: 32941–32945, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Oberst MD, Singh B, Ossandon M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51: 1017–1025, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem 278: 26773–26779, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Ovaere P, Lippens S, Vandenabeele P, Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci 34: 453–463, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Press AG, Hauptmann IA, Hauptmann L, Fuchs B, Fuchs M, Ewe K, Ramadori G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther 12: 673–678, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Rahman I, Macnee W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax 51: 348–350, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rahman I, Morrison D, Donaldson K, Macnee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Resing KA, Thulin C, Whiting K, al-Alawi N, Mostad S. Characterization of profilaggrin endoproteinase 1. A regulated cytoplasmic endoproteinase of epidermis. J Biol Chem 270: 28193–28198, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, Schmidt P, Schmahl W, Scherer J, Anton-Lamprecht I, Von Figura K, Paus R, Peters C. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and perturbation of hair follicle cycling. FASEB J 14: 2075–2086, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Szabo R, Kosa P, List K, Bugge TH. Loss of matriptase suppression underlies spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol 174: 2015–2022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toomes C, James J, Wood AJ, Wu CL, McCormick D, Lench N, Hewitt C, Moynihan L, Roberts E, Woods CG, Markham A, Wong M, Widmer R, Ghaffar KA, Pemberton M, Hussein IR, Temtamy SA, Davies R, Read AP, Sloan P, Dixon MJ, Thakker NS. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet 23: 421–424, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Tseng IC, Chou FP, Su SF, Oberst M, Madayiputhiya N, Lee MS, Wang JK, Sloane DE, Johnson M, Lin CY. Purification from human milk of matriptase complexes with secreted serpins: mechanism for inhibition of matriptase other than HAI-1. Am J Physiol Cell Physiol 295: C423–C431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tseng IC, Xu H, Chou FP, Li G, Vazzano AP, Kao JP, Johnson MD, Lin CY. Matriptase activation, an early cellular response to acidosis. J Biol Chem 285: 3261–3270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang JK, Lee MS, Tseng IC, Chou FP, Chen YW, Fulton A, Lee HS, Chen CJ, Johnson MD, Lin CY. Polarized epithelial cells secrete matriptase as a consequence of zymogen activation and HAI-1-mediated inhibition. Am J Physiol Cell Physiol 297: C459–C470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeeuwen PL. Epidermal differentiation: the role of proteases and their inhibitors. Eur J Cell Biol 83: 761–773, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Zeeuwen PL, van Vlijmen-Willems IM, Hendriks W, Merkx GF, Schalkwijk J. A null mutation in the cystatin M/E gene of ichq mice causes juvenile lethality and defects in epidermal cornification. Hum Mol Genet 11: 2867–2875, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Zeeuwen PL, van Vlijmen-Willems IM, Olthuis D, Johansen HT, Hitomi K, Hara-Nishimura I, Powers JC, James KE, op den Camp HJ, Lemmens R, Schalkwijk J. Evidence that unrestricted legumain activity is involved in disturbed epidermal cornification in cystatin M/E deficient mice. Hum Mol Genet 13: 1069–1079, 2004 [DOI] [PubMed] [Google Scholar]