Abstract
Recent studies have shown mitochondrial fragmentation during cell stress and have suggested a role for the morphological change in mitochondrial injury and ensuing apoptosis. However, the underlying mechanism remains elusive. Here we demonstrate that mitochondrial fragmentation facilitates Bax insertion and activation in mitochondria, resulting in the release of apoptogenic factors. In HeLa cells, overexpression of mitofusins attenuated mitochondrial fragmentation during cisplatin- and azide-induced cell injury, which was accompanied by less apoptosis and less cytochrome c release from mitochondria. Similar effects were shown by inhibiting the mitochondrial fission protein Drp1 with a dominant negative mutant (dn-Drp1). Mitofusins and dn-Drp1 did not seem to significantly affect Bax translocation/accumulation to mitochondria; however, they blocked Bax insertion and activation in mitochondrial membrane. Consistently, in rat kidney proximal tubular cells, small interfering RNA knockdown of Drp1 prevented mitochondrial fragmentation during azide-induced ATP depletion, which was accompanied by less Bax activation, insertion, and oligomerization in mitochondria. These cells released less cytochrome c and AIF from mitochondria and showed significantly lower apoptosis. Finally, mitofusin-null mouse embryonic fibroblasts (MEF) had fragmented mitochondria. These MEFs were more sensitive to cisplatin-induced Bax activation, release of cytochrome c, and apoptosis. Together, this study provides further support for a role of mitochondrial fragmentation in mitochondrial injury and apoptosis. Mechanistically, mitochondrial fragmentation may sensitize the cells to Bax insertion and activation in mitochondria, facilitating the release of apoptogenic factors and consequent apoptosis.
Keywords: mitochondrial dynamics, mitochondria outer membrane permeabilization, cytochrome c
cellular stress, if severe enough, frequently triggers the intrinsic pathway of apoptosis, which is characterized by mitochondrial outer membrane permeabilization (MOMP) (15, 28, 30). MOMP leads to the release from the intermembrane space of several apoptogenic factors or proteins, including cytochrome c (Cyt c) and apoptosis-inducing factor (AIF). Whereas Cyt c is well documented for its ability to initiate the formation of apoptosome to result in caspase activation, AIF is suggested to induce caspase-independent apoptosis (15, 28, 30).
During apoptosis, MOMP is mainly mediated and regulated by Bcl-2 family proteins, which are characterized by the presence of Bcl-2 homology (BH) domains (1, 9, 11, 33). Depending on their functions in apoptosis, Bcl-2 family proteins are divided into pro- and anti-apoptotic members. Structurally, the anti-apoptotic members, such as Bcl-2 and Bcl-XL, contains four BH domains (BH1-BH4), whereas the pro-apoptotic members either have three BH domains or only one BH domain, the BH-3 (1, 9, 11, 33). It is now broadly appreciated that Bax and Bak, two multi-BH domain pro-apoptotic members, are the molecular gateway or mediators of MOMP, which are inhibited by the anti-apoptotic Bcl-2/Bcl-XL under normal conditions or activated by BH3-only proteins during apoptosis. Whereas both Bax and Bak contribute to the development of MOMP, they are distinctly regulated. Especially, Bax resides in cytosol in normal healthy cells, whereas Bak is in mitochondria. As a result, the regulation of Bax during apoptosis is apparently more complex, involving translocation to mitochondria, insertion into the outer membrane, and activation and formation of oligomers. Despite years of investigation, how each of the Bax activation events is regulated, culminating in mitochondrial permeabilization and the release of apoptogenic factors, remains largely unclear (1, 9, 11, 33).
Recent work has suggested a role for alterations of mitochondrial dynamics in the development of MOMP during apoptosis (3, 6, 7, 25, 26). Mitochondria are a class of dynamic organelles, constantly undergoing fission and fusion (7, 21). Interestingly, mitochondrial fission and fusion are governed by distinct proteins. Whereas fission depends on Fis-1 and Drp1, fusion requires a coordinated action of Mitofusins and OPA-1 (7, 21). Under normal conditions, mitochondrial fusion prevails and as a result, the organelles appear long and filamentous. During cell stress, however, the dynamic balance is shifted to fission, leading to mitochondrial fragmentation (5, 13, 19, 27). Importantly, emerging evidence has suggested that mitochondrial fragmentation contributes MOMP and consequent release of apoptogenic factors during apoptosis (3, 6, 7, 25, 26). Despite these findings, it is unclear as to how mitochondrial fragmentation, a seemingly morphological change, can affect MOMP, the formation of porous defects in mitochondrial outer membrane.
In this study, we have further confirmed that prevention of mitochondrial fragmentation, either by blocking fission or by promoting fusion, can suppress MOMP and apoptosis following cellular stress. In addition, we have shown that cells with fragmented mitochondria are more sensitive to MOMP and apoptosis. Importantly, we have demonstrated that fragmented mitochondria are sensitized to Bax insertion and activation, suggesting a mechanism for the involvement of mitochondrial fragmentation in the development of MOMP during apoptosis.
MATERIALS AND METHODS
Cells and reagents.
Mfn1−/−, Mfn2−/−, and control wild-type (wt) mouse embryonic fibroblasts (MEF) were kindly provided by Dr. David Chan at California Institute of Technology (Pasadena, CA). The cells were characterized previously (8). The rat kidney proximal tubular cell line (RPTC) was originally obtained from Dr. Ulrich Hopfer at Case Western Reserve University (Cleveland, OH). The R3 and R24 cell clones were generated by stable transfection of RPTC cells with short hairpin RNAs (shRNAs) targeting Drp1 as described in our recent work (4). HeLa cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Mfn1 and Mfn2 plasmids were obtained from Dr. David Chan, and dn-Drp1 was from Dr. Alexander van der Bliek at the University of California School of Medicine at Los Angeles. pDsRed2-Mito (MitoRed) and pAcGFP1-Mito (MitoGreen) was purchased from BD Clontech (Palo Alto, CA). Antibodies were from the following sources: mouse monoclonal anti-Cyt c from BD Pharmingen (San Diego, CA); mouse monoclonal anti-Bax (1D1, specific for rat Bax) from MeoMarkers (Fremont, CA), rabbit polyclonal anti-Bax (for human, mouse Bax) from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit polyclonal anti-Bax (NT, specific for active Bax) and anti-Bak from Upstate (Lake Placid, NY); and secondary antibodies from Jackson ImmunoResearch (West Grove, PA). Other reagents and chemicals including cisplatin and azide were purchased from Sigma (St. Louis, MO).
Cell injury models.
Cells were treated with cisplatin or azide to induce cellular stress and injury by protocols modified from our previous studies (4, 5, 12, 17, 22, 29). Cisplatin is a chemotherapy drug that induces apoptosis in tumors and normal tissues by multiple mechanisms including DNA damage. In this study, cells were incubated with indicated concentrations of cisplatin in culture medium. Azide blocks cellular respiration at complex IV. In this study, cells were incubated with 10 mM azide in glucose-free medium, a treatment that leads to ATP depletion and consequent mitochondrial leakage of apoptogenic factors such as Cyt c. When the azide-treated cells are returned to glucose-containing medium, the cells develop apoptotic morphology.
Plasmid transfection.
Cells were plated at 40–60% confluence for transfection using Lipofectamine LTX with Plus Reagent (Invitrogen). Usually, 1 μg plasmid DNA was transfected to cells in each 35-mm dish. To reveal mitochondrial morphology, 0.5 μg pDsRed2-mito plasmid or pAcGFP1-Mito was transfected alone or 0.1 μg cotransfected with other plasmids. The transfected cells were subjected to indicated treatments and examined after overnight growth.
Analysis of mitochondrial morphology.
Mitochondrial morphology was examined as described in our recent studies (4, 5, 10). Briefly, cells were transfected with pDsRed2-Mito or pAcGFP1-Mito, which led to the expression of MitoRed or MitoGreen mitochondrial-targeted fluorescent proteins, in mitochondria to label the organelles. The transfected cells were then subjected to control or experimental treatments to evaluate mitochondrial morphology by fluorescence microscopy. Filamentous mitochondria showed a long thread-like tubular structure, whereas fragmented mitochondria were punctate and sometimes rounded. For quantification, the cells with different mitochondrial morphologies were counted to determine the percentage of cells with fragmented mitochondria. Most of the cells had either fragmented or filamentous mitochondria, whereas a small percentage (<10%) of cells contained both fragmented and filamentous mitochondria. If a cell happened to have mitochondria with mixed morphologies, we classified the mitochondrial morphology according to the majority (>70%) of the mitochondria.
Evaluation of apoptosis.
Apoptosis was examined morphologically as described in our previous work (4, 5, 10, 12, 17, 22, 29). Briefly, after various treatments, cells were stained with Hoechst 33342 and examined by phase-contrast and fluorescence microscopy. Apoptotic cells were identified by characteristic morphology including cellular condensation, formation of apoptotic bodies, and condensation and fragmentation of the nucleus. About 200 cells were examined in each dish to determine the percentage of apoptotic cells.
Analysis of the release of Cyt c and AIF.
Cyt c and AIF normally reside in mitochondria in the intermembrane space, and upon apoptotic stimulation, they are released from mitochondria. To determine the release of these proteins during apoptosis, cells were fractionated by using digitonin at low concentrations (4, 12, 24, 29). Briefly, cells were permeabilized with 0.05% (wt/vol) digitonin in an isotonic sucrose buffer for 2–4 min. The cytosol released by digitonin from the same number cells was collected from different treatment groups for immunoblot analysis using specific antibodies to Cyt c or AIF.
Immunofluorescence of Bax.
Indirect immunofluorescence of Bax was conducted as described in our previous studies (4, 12, 24, 29). Briefly, cells grown on glass coverslips were fixed with a modified Zamboni's fixative containing picric acid and 4% paraformaldehyde. The fixed cells were incubated with a blocking buffer containing 2% normal goat serum and then exposed to the primary anti-Bax antibody. Finally, the cells were incubated with FITC-labeled goat anti-mouse secondary antibody. The signals were examined by fluorescence microscopy.
Immunoprecipitation analysis of active Bax.
Bax activation involves the conformational changes to expose the NH2-terminus of the protein. As a result, antibodies specific to the NH2-terminal sequence of Bax only bind and react with active Bax. To detect Bax activation, in this study we used the anti-Bax NT antibody from Upstate Biotechnology that was generated against the Bax NH2-terminal sequence for immunoprecipitation to pull down active Bax. Briefly, cell lysate was collected in the immunoprecipitation buffer containing CHAPS. The lysate of 500 μg protein was subjected to immunoprecipitation by incubation with 1 μg anti-Bax NT and protein A/G agarose beads. After centrifugation and washes, the precipitated proteins were collected and examined by immunoblot analysis.
Analysis of Bax insertion by alkaline treatment.
Exposure to an alkaline buffer can strip off the loosely attached proteins, but not the inserted proteins, from mitochondria. Alkaline exposure was conducted as described in our previous work (32). Briefly, cells were permeabilized with 0.05% digitonin to release cytosolic fraction. The membrane-bound organellar fraction containing mitochondria was collected, washed with PBS, and then incubated on ice in 0.1 M Na2CO3 at pH 11.5 for 30 min. After 1 h of centrifugation at 100,000 g, the pellet was collected for immunoblot analysis to reveal the inserted Bax that was resistant to alkaline stripping.
Analysis of Bax oligomerization.
Bax oligomerization was analyzed following chemical cross-linking as described in our previous work (16). Briefly, cells were permeabilized with 0.05% digitonin to release cytosolic fraction. After centrifugation, the membrane-bound organellar fraction containing mitochondria was collected and incubated with 1 mM dithiobis[succinimidyl propionate] (DSP from Pierce, Rockford, IL) for 30 min of cross-linking at room temperature. The cross-linked samples were subjected to immunoblot analysis of Bax under nonreducing conditions.
Immunoblot analysis.
Protein concentration of cell lysate was determined by using the bicinchoninic acid reagent (Pierce, Rockford, IL). Immunoblot analysis was performed by a standard protocol. Briefly, equal amount of proteins or protein samples collected from the same number of cells were resolved by reducing SDS-gel electrophoresis and electroblotted onto PVDF membranes. The blots were incubated sequentially with a blocking solution, a specific primary antibody, and a horseradish-peroxidase-conjugated secondary antibody. Finally, antigens on the blots were revealed using the enhanced chemiluminescence kit (Pierce).
Statistics.
Quantitative data were analyzed by Student's test and expressed as means ± SD. Statistical differences between the means were determined using analysis of variance (ANOVA) followed by Tukey's post test. P < 0.05 was considered to reflect significant differences. Qualitative data including cell images and immunoblots were representatives of at least three experiments.
RESULTS
Expression of Mfn1, Mfn2, or dn-Drp1 inhibits mitochondrial fragmentation, Cyt c release, and apoptosis.
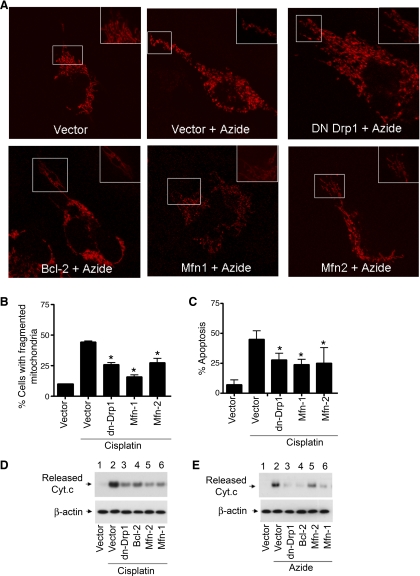
Our previous work showed that HeLa cells had long and filamentous mitochondria, which became fragmented during cell stress. Moreover, blockade of mitochondria fragmentation by expressing a dominant negative mutant of Drp1 could suppress mitochondrial outer membrane leakage, the release of apoptogenic factors (e.g., Cyt c) and attenuate subsequent apoptosis (4, 5). While those results suggest a critical role for mitochondrial fragmentation in apoptosis, it can be argued that Drp1 per se (and not mitochondrial fragmentation) is the key. To address this question, we employed a different approach to prevent mitochondrial fragmentation, i.e., by expressing Mitofusins Mfn1 and Mfn2. Two cell injury models, involving the use of the mitochondrial respiration inhibitor azide and the chemotherapy drug cisplatin, respectively, were examined. As shown in Fig. 1A, control HeLa cells contained long filamentous mitochondria, which became small discrete fragments during azide treatment. Transfection with Mfn1 or 2 prevented azide-induced mitochondrial fragmentation. Consistent with our previous study (5), mitochondrial fragmentation was also attenuated by dn-Drp1 and Bcl-2 (Fig. 1A). In the cisplatin injury model, we quantified the effects by counting the cells with fragmented mitochondria (Fig. 1B). In empty vector-transfected cells, cisplatin induced mitochondrial fragmentation in 45% cells, which was suppressed to 20–25% by Mfn1, Mfn2, and dn-Drp1. Importantly, transfection with Mfn1, Mfn2, or dn-Drp1 significantly suppressed cisplatin-induced apoptosis (Fig. 1C). At the mitochondrial level, we further showed that Mfn1 and Mfn2 as well as dn-Drp1 and Bcl-2 could block the release of Cyt c (Cyt c) during cisplatin and azide treatments (Fig. 1, D and E). Together, these results suggest that maintenance of mitochondrial morphological dynamics during cell stress by either blocking fission (via dn-Drp1) or enhancing fusion (via Mfn1, Mfn2) can suppress mitochondrial injury and consequent apoptosis.
Fig. 1.
Expression of Mfn1, Mfn2, and dn-Drp1 inhibits mitochondrial fragmentation, cytochrome c (Cyt c) release, and apoptosis. A: representative mitochondrial morphology. HeLa cells were cotransfected with Mito-Red and with one of the following plasmids: dn-Drp1, Bcl-2, Mfn1, Mfn2, or empty vector. After overnight transfection, the cells were untreated or treated with 10 mM azide for 3 h. Mitochondrial morphology was examined and recorded by fluorescence microscopy. Insets: boxed area in higher magnification. B: mitochondrial fragmentation during cisplatin treatment. HeLa cells were cotransfected with MitoRed and Mfn1, Mfn2, dn-Drp1, or empty vector. The cells were then treated with 20 μM cisplatin for 16 h to evaluate mitochondrial morphology by fluorescence microscopy. The cells containing fragmented mitochondria and those with filamentous mitochondria were counted to calculate the percentage of cells with mitochondrial fragmentation. C: apoptosis during cisplatin treatment. HeLa cells were transfected as described in A and treated with cisplatin for 24 h to examine apoptosis by morphological criteria. The percentage of apoptosis was determined by counting the cells with typical apoptotic morphology. D: Cyt c release during cisplatin treatment. HeLa cells were transfected as described in A and treated with cisplatin for 24 h. The cells were premeabilized with low concentration digitonin to collect the cytosolic fraction to analyze the released Cyt c by immunoblotting. E: Cyt c release during azide treatment. HeLa cells were transfected as described in A and then subjected to 3 h of ATP depletion with 10 mM azide treatment in glucose-free buffer. The cells were premeabilized with low concentration digitonin to collect the cytosolic fraction to analyze the released Cyt c by immunoblotting. Data in are B and C are expressed as means ± SD (n = 3); *significantly different from the cisplatin-treated empty vector-transfected group.
Mfn1, Mfn2, and dn-Drp1 do not block Bax translocation to mitochondria during cell stress.
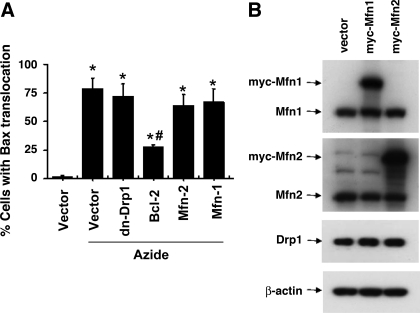
Bax and Bak are considered the “gateway” to mitochondrial outer membrane permeabilization and the release of apoptogenic factors during apoptosis (1, 9, 11, 31, 33). Whereas Bak is constitutively localized in mitochondria, Bax normally resides in the cytosol and translocates to mitochondria upon apoptotic stimulation. At mitochondria, Bax is further activated to insert into the outer membrane and form oligomers, which may directly release the apoptogenic factors or indirectly by destabilizing the membrane lipids (1, 9, 11, 33). Thus to understand how mitochondrial fragmentation affects mitochondrial injury, we examined the effects of Mfn1, Mfn2, and dn-Drp1 expression on Bax accumulation to mitochondria by immunofluorescence staining (Fig. 2A). For this purpose, cells were cotransfected with MitoRed and one indicated plasmid (empty vector, dn-Drp-1, Bcl-2, Mfn1 or Mfn2). After azide treatment, the cells were subjected to Bax immunofluorescence staining (labeled by green FITC) and examined to count the cells with Bax staining in mitochondria. As shown in Fig. 2B, except Bcl-2, none of the transfected genes prevented Bax accumulation to mitochondria during azide treatment. By immunoblot analysis, we confirmed the expression of Myc-Mfn1 and -Mfn2 after transfection, which did not affect the expression of Drp1 (Fig. 2B).
Fig. 2.
DN-Drp1, Mfn1, and Mfn2 do not block Bax translocation to mitochondria during azide treatment. A: Bax translocation analyzed by immunofluorescence staining. HeLa cells were cotransfected with MitoRed and one indicated plasmid (empty vector, dn-Drp-1, Bcl-2, Mfn1, or Mfn2). After azide treatment, the cells were fixed for Bax immunofluorescence staining (labeled by green FITC) and examined to count the cells with Bax translocation to mitochondria. Data are expressed as means ± SD (n = 4); *P < 0.01 vs. control; #P < 0.01 vs. azide treated vector-transfected group. B: Myc-Mfn1 and Myc-Mfn2 expression after transfection. Whole cell lysate was collected for immunoblot analysis using specific antibodies to Mfn1, Mfn2, Drp1, and β-actin.
Mfn1, Mfn2, and dn-Drp1 suppress Bax activation and insertion in mitochondria membrane.
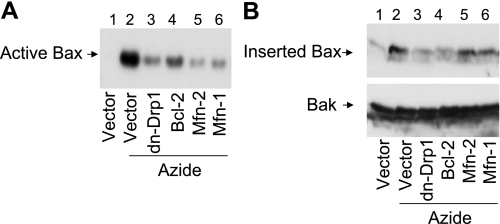
We then examined whether Mfn1, Mfn2, and dn-Drp1 could inhibit Bax activation and insertion into mitochondria. Bax activation was detected by immunoprecipitation using the NT anti-Bax antibody, which was generated by Bax NH2-terminal sequence and specifically recognizes active Bax. As shown in Fig. 3A, active Bax was not detected in untreated cells (lane 1), whereas abundant active Bax was pulled down from azide-treated cell lysate (lane 2). Notably, much less active Bax was precipitated from the cells tranfected with Mfn 1, Mfn2, or dn-Drp1 (lanes 3, 5, and 6). As a positive control, Bax activation was confirmed to be suppressed by Bcl-2 (lane 4). We further analyzed Bax insertion into mitochondrial membrane. To this end, the membrane fraction containing mitochondria was isolated and exposed to an alkaline buffer, which can strip off uninserted proteins (14, 32). As shown in Fig. 3B, after alkaline stripping very little Bax remained in mitochondria in untreated control cells, but still significant amounts of Bax remained in the mitochondria of azide-treated cells (lane 2), indicating Bax insertion into mitochondria membrane. Azide-induced Bax insertion was completely blocked by dn-Drp1 and Bcl-2 and partially attenuated by Mfn1 and Mfn2 expression. Together the results suggest that mitochondrial fragmentation facilitates Bax insertion to and activation in mitochondrial membrane.
Fig. 3.
DN-Drp1, Mfn1, and Mfn2 suppress Bax activation and insertion in mitochondria membrane. HeLa cells were transfected with Mfn1, Mfn2, dn-Drp1, Bcl-2, or control empty vector. The cells were then subjected to 3 h of 10 mM azide treatment in glucose-free buffer. A: active Bax. Cells lysate was collected for immunoprecipitation with an antibody that was specific to active Bax. The precipitate was finally analyzed by immunoblotting of Bax. B: Bax insertion. Membrane fraction containing mitochondria was collected from the cells and incubated for 30 min with an alkaline (pH 11.5) solution. The mitochondrial fraction was then collected by centrifugation for analysis of remaining Bax by immunoblot analysis. Bak was also analyzed to verify that inserted proteins were not stripped off from mitochondrial membrane by the alkali incubation.
Knockdown of Drp1 inhibits Bax activation, insertion, and oligomerization in mitochondria.
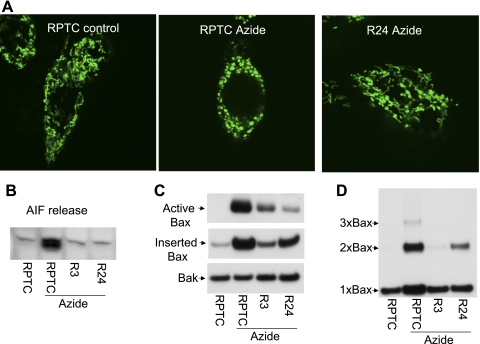
To further confirm the role of mitochondrial fragmentation in Bax regulation in other cell types, we examined RPTC, a kidney epithelial (proximal tubular) cell line. RPTC was used in our recent work to reveal a pathological role for alterations of mitochondrial dynamics in acute kidney injury (4). In that study, we generated two stable cell lines (R3, R24) with Drp1 knockdown via shRNA. We therefore examined azide-induced Bax activation in these cells. We confirmed that the shDrp1 cells were resistant to azide-induced mitochondrial fragmentation [Fig. 4A, quantitative data published previously (4)]. These cells were also markedly resistant to apoptogenic factor release during azide treatment, including Cyt c (Ref. 4) and AIF (Fig. 4B). Importantly, when compared with the parental RPTC, both R3 and R24 cells showed obviously lower Bax activation and mitochondrial insertion during azide treatment (Fig. 4C). We further analyzed the formation of Bax oligomers after chemical cross-linking of the cells. As shown in Fig. 4D, azide treatment induced the formation of Bax dimmer and trimer in RPTC, which was almost completely blocked in R3 cells and partially inhibited in R24 cells. Azide-induced apoptosis was attenuated in R3 and R24 cells (data not shown, published in Ref. 4). The results further indicate that mitochondrial fragmentation contributes to Bax activation and insertion in mitochondria, leading to the release of apoptogenic factors and apoptosis.
Fig. 4.
Knockdown of Drp1 inhibits Bax activation, insertion and oligomerization in mitochondria. R3 and R24 cell clones were generated by stable transfection of Drp-1 short hairpin RNA (shRNA) into rat proximal tubular cells (RPTC). Knockdown of Drp1 in R3 and R24 cells was shown in our recent work. A: representative images of mitochondrial morphology. RPTC and R24 cells were transfected with Mito-Green and then left untreated (control) or treated with 10 mM azide for 3 h to record mitochondrial morphology by fluorescence microscopy. B: apoptosis-inducing factor (AIF) release. Cells were untreated or treated with 10 mM azide for 3 h. The cells were then permeabilized with low concentration digitonin to collect cytosolic fraction for immunoblot analysis of released AIF. C: Bax activation and insertion in mitochondria. RPTC, R3, and R24 cells were untreated or treated with 10 mM azide for 3 h. To analyze Bax activation, cell lysate was collected for immunoprecipitation using an antibody recognizing active Bax, followed by immunoblot analysis of Bax. To analyze Bax insertion, membrane fraction containing mitochondria was isolated and subjected to alkaline (pH 11.5) incubation as described in materials and methods. The fraction was then collected by centrifugation for analysis of remaining Bax by immunoblot analysis. Bak was also analyzed to verify protein loading. D: Bax oligomerization. RPTC, R3, and R24 cells were untreated or treated with 10 mM azide for 3 h. The cells were then cross-linked with 1 mM DSP and further permeabilized with digitonin to collect the membrane fraction containing mitochondria. The membrane fraction was finally subjected to nonreducing gel electrophoresis and immunoblot analysis of Bax.
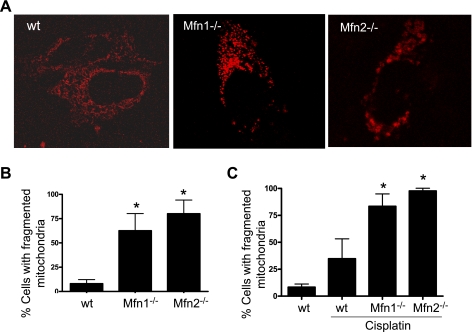
Mfn1 or Mfn2-null cells have fragmented mitochondria.
By promoting fusion (Mfn1, Mfn2) or inhibiting fission (dn-Drp1, siDrp1), the experiments described above showed that keeping mitochondria in a long filamentous shape can suppress Bax activation and insertion in mitochondrial membrane, resulting in suppression of mitochondrial leakage and attenuation of apoptosis. To further validate this conclusion, we went on to examine cells that started with fragmented mitochondria. MEF originated from Mfn1- or Mfn2-null mice have significantly higher mitochondrial fragmentation than wt MEF (8). We confirmed mitochondrial fragmentation in these cells (Fig. 5A). Quantification by cell counting indicated that ∼60% Mfn1−/− and ∼80% Mfn2−/− cells had fragmented mitochondria, whereas less than 10% wt MEF had (Fig. 5B). Interestingly, in both wt and Mfn−/− cells, cisplatin stress could increase mitochondrial fragmentation. After the treatment, mitochondrial fragmentation reached the maximal level of 80–90% in Mfn1 or Mfn2-null cells (Fig. 5C).
Fig. 5.
Mfn1 or MFn2-null cells have fragmented mitochondria. A: representative mitochondrial morphology. Wild-type (wt), Mfn1-null, and Mfn2-null mouse embryonic fibroblast (MEF) cells were transfected with MitoRed to record mitochondrial morphology by fluorescence microscopy. B: quantification of cells with fragmented mitochondria. Cells were transfected as those cells in A and examined by fluorescence microscopy to determine the percentage of cells with fragmented mitochondria. C: mitochondrial fragmentation during cisplatin treatment. wt, Mfn1-null, and Mfn2-null MEF cells were transfected with MitoRed and then treated with 20 μM cisplatin for 16 h. The cells were examined by fluorescence microscopy to determine the percentage of cells with fragmented mitochondria. Data are means ± SD (n = 3); *significantly different wt cells (B) or cisplatin-treated wt group (C).
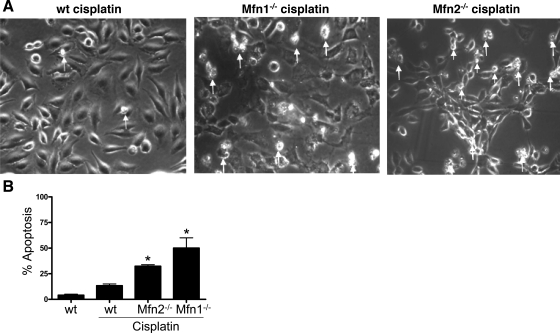
Mfn1 or Mfn2-null cells are more sensitive to apoptosis.
The Mfn-null cells with high background mitochondrial fragmentation provided us a model for further investigation of the effect of mitochondrial fragmentation on cellular sensitivity to mitochondrial injury and apoptosis. Under control conditions, wt and Mfn-null cells did not show obvious apoptosis, despite mitochondrial fragmentation in the latter. Our initial experiments examined cisplatin-induced apoptosis in these cells. The wt MEFs were not very sensitive to cisplatin treatment and, as a result, only induced sparse apoptosis by 20 μM cisplatin (Fig. 6A: left). In sharp contrast, apoptosis was wide spread in Mfn1-null and Mfn2-null MEFs following the same cisplatin incubation (Fig. 6A: middle and right). By counting the cells with apoptotic morphology, it was estimated that cisplatin induced about 30% and 50% apoptosis in Mfn1-null and Mfn2-null cells, whereas only 10% was induced in wt MEFs (Fig. 6B).
Fig. 6.
Mfn1 or MF2-null cells are more sensitive to apoptosis. wt, Mfn1-null, and Mfn2-null MEFs were incubated with 20 μM cisplatin for 24 h to record cell morphology. Apoptotic cells were identified by typical morphology including cellular condensation and fragmentation. A: representative cell morphology. Note: many Mfn1 or Mfn2-null cells had undergone apoptosis and detached form the dish. Arrows: representative apoptotic cells. B: percentage of apoptosis. Data are means ± SD (n = 3); *significantly different from wt cells treated with cisplatin.
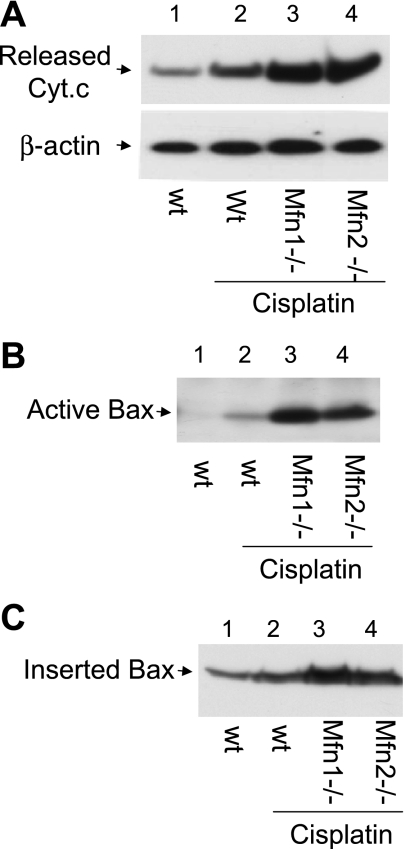
Mfn1 or Mfn2-null cells are more sensitive to Cyt c release, Bax activation, and insertion.
The higher sensitivity of Mfn-null cells to cisplatin-induced apoptosis (shown above in Fig. 6) promoted us to analyze the mitochondrial events of apoptosis in these cells. Under control conditions, Mfn-null cells as wild-type cells did not show Bax activation or Cyt c release (not shown). During cisplatin treatment both Mfn1-null and Mfn2-null cells released significantly more Cyt c from mitochondria than wild-type cells (Fig. 7A: lanes 3, 4 vs. lane 2). Notably, cisplatin induced remarkably higher Bax activation in Mfn-null cells than wild-type cells (Fig. 7B: lanes 3, 4 vs. lane 2). In addition, more Bax was inserted into mitochondria in Mfn-null cells (Fig. 7C: lanes 3, 4 vs. lane 2). The results suggest that cells with fragmented mitochondria are sensitized to Bax activation and insertion, resulting in mitochondrial leakage of apoptogenic factor and activation of the apoptotic cascade.
Fig. 7.
Mfn1 or Mfn2-null cells are more sensitive to Cyt c release, Bax activation, and insertion. wt, Mfn1-null, and Mfn2-null MEFs were incubated with 20 μM cisplatin for 24 h. A: Cyt c release. Cells were permeabilized with low concentration digitonin to collect cytosolic fraction for immunoblot analysis to detect Cyt c that had been released into cytosol during cisplatin treatment. B: Bax activation. Cells were lysed with the CHAPS buffer. The lysate was subjected to immunoprecipitation using the antibody specific for active Bax. The resultant immunoprecipitates were analyzed for Bax by immunoblot analysis. C: Bax insertion. Cells were permeabilized with digitonin to release cytosol and collect the membrane fraction with mitochondria, which was subjected to alkaline incubation as described in materials and methods. After alkaline treatment, Bax remaining in the membrane fraction was analyzed by immunoblot analysis.
DISCUSSION
Changes of mitochondrial dynamics, resulting in fragmentation of the organelles, have been documented during cell stress in a variety of apoptotic models. Emerging evidence has further suggested an important role for mitochondrial fragmentation in mitochondrial injury during apoptosis (3, 7, 25, 26). Importantly, the pathological role of mitochondrial fragmentation has been demonstrated not only by in vitro cell culture studies but also by in vivo animal model examination. Our recent work (4) has revealed fragmented mitochondria in renal tubular cells during renal ischemia-reperfusion injury and cisplatin-induced nephrotoxicity in mice. Notably, pharmacological inhibition of mitochondrial fragmentation can partially, but significantly, protect the kidneys under the injury conditions. Despite these observations, whether and to what extent mitochondrial fragmentation contributes to apoptosis has been questioned by a few other studies (2, 23). Especially, it has been questioned whether mitochondrial fragmentation is a cause or just a consequence of MOMP or mitochondrial outer membrane permeabilization. In addition, as blocking mitochondrial fragmentation in previous studies was mainly achieved by expressing dominant negative Drp1, it has been speculated that maybe it is Drp1 (and not mitochondrial fragmentation) that somehow contributes to MOMP and apoptosis. Finally, it remains largely unknown how changes in mitochondrial dynamics affect mitochondrial injury or MOMP during apoptosis.
The results from the current study have provided information to address the critical questions listed above. First, we have further demonstrated compelling evidence to support a role for mitochondrial fragmentation in the development of MOMP and apoptosis. We show that prevention of mitochondrial fragmentation, either by blocking fission or by enhancing fusion, can suppress MOMP as indicated by lowered release of Cyt c and AIF (Figs. 1, C and D, and 4B). Moreover, upon apoptotic induction, MOMP is exacerbated in cells with fragmented mitochondria (Fig. 7A). Of note, these experiments were conducted in several apoptotic models involving different types of apoptotic treatment (azide, cisplatin) and cells (HeLa, RPTC, MEF). Second, a main approach of this study is manipulating Mfn1 and Mfn2, the key regulators of mitochondrial fusion. The results show that overexpression of Mfn1 and Mfn2, as dn-Drp1, can maintain mitochondrial morphology during cell stress and prevent MOMP and apoptosis. Apparently, the cytoprotective effects of these proteins are unlikely due to the specific involvement of an individual molecule (e.g., Drp1) in MOMP or apoptosis; rather, mitochondrial fragmentation governed by these molecules plays an important role. Third and finally, our results suggest that one mechanism for the involvement of mitochondrial fragmentation in MOMP is by facilitating the insertion and activation of Bax in mitochondrial membrane. Bax activation in apoptosis involves the translocation of the molecule to mitochondria, insertion into the outer membrane, and then oligomerization into homo-oligomers (18). By expressing dn-Drp1, previous work (13) suggested that Bax translocation or accumulation to mitochondria during apoptosis does not depend on mitochondrial fragmentation. This notion is further supported by our results showing that Bax translocation to mitochondria is not blocked when mitochondrial fragmentation is prevented by Mfn1, Mfn2, or dn-Drp1 (Fig. 2). Importantly, our results further demonstrate that Bax insertion to and activation in mitochondrial membrane are significantly suppressed if mitochondrial fragmentation is prevented by expressing these genes (Fig. 3). Similarly, Bax insertion and activation are suppressed in Drp1-knockdown cells, which can maintain filamentous mitochondria during stress (Fig. 4). In addition, Mfn-null cells containing fragmented mitochondria are highly sensitive to Bax insertion (Fig. 7). Collectively, these results suggest that one mechanism for the involvement of mitochondrial fragmentation in apoptosis is by facilitating Bax insertion and activation in mitochondrial membrane.
It is still controversial as to whether mitochondrial fragmentation is upstream of Bax/Bak in apoptosis. We believe this may depend on experimental models. In our models, mitochondrial fragmentation seems to be upstream. In addition, we would entertain the idea of a positive feedback loop in the regulation. In other words, Bax/Bak may contribute to mitochondrial fragmentation and conversely, fragmented mitochondria sensitize Bax/Bak activation. In support of this possibility, our previous work (4) showed that Bak participates in mitochondrial fragmentation by interacting with mitofusins and our present results suggest that fragmented mitochondria are sensitized to Bax/Bak activation.
Our results do not show a linear correlation between mitochondrial fragmentation, Bax activation, Cyt c release, and apoptosis. For example, MFN2 was as effective in blocking mitochondrial fragmentation but less effective than dn-Drp1 in reducing Cyt c release (Fig. 1). This may be related to additional effects of dn-Drp1. In this regard, recent work suggests that Drp1 may directly participate in Bax oligomerization (20). Alternatively, some of the nonlinear correlations could be caused by experimental variations. For example, in Fig. 3 there seems to be more residual active Bax after Bcl-2 transfection than mitofusin or Drp1 transfection. In addition, there are discrepancies between Bax activation (Fig. 3A) and insertion (Fig. 3B) results. The discrepancies might be caused by experimental variations. These experiments, involving immunoprecipitation, mitochondrial fraction isolation, and alkali stripping, are technique demanding and usually associated with bigger variations.
It is currently unclear why fragmented mitochondria are sensitized to Bax insertion and activation. We speculate there are at least two possibilities. First, the biophysical property of the membrane of filamentous mitochondria may be different from that of fragmented mitochondria. In this regard, apparently there are differences in the membrane curvatures in filamentous and fragmented mitochondria. It would be interesting to determine whether membranes with different curvatures show different sensitivity to Bax insertion. Second, when mitochondria change their fission-fusion dynamics and undergo fragmentation, there may be significant changes in the biochemical property of the membrane. In this regard, both proteins and lipids may have changes that affect the docking and insertion of Bax into the membrane. Future research should investigate these possibilities to advance the understanding of mitochondrial dynamics and injury during cell stress.
GRANTS
Z. Dong is a Research Career Scientist of Department of Veterans Affairs (VA). The study was supported in part by grants from the National Institutes of Health and VA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. David Chan at California Institute of Technology for kindly providing the Mfn1 and Mfn2 plasmids and the Mfn1−/−, Mfn2−/−, and wt MEFs. We also thank Dr. Alexander van der Bliek at the University of California School of Medicine at Los Angeles for the dominant negative Drp1 plasmid.
Present address of C. Brooks: Renal Division, Dept. of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115.
REFERENCES
- 1. Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Current Opin Immunol 19: 488–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnoult D, Rismanchi N, Grodet A, Roberts RG, Seeburg DP, Estaquier J, Sheng M, Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol 15: 2112–2118, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle 6: 3043–3047, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Brooks C, Wei Q, Cho S, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA 104: 11649–11654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene 25: 4717–4724, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL2 family reunion. Mol Cell 37: 299–310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho SG, Du Q, Huang S, Dong Z. Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol 299: F199–F206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 116: 205–219, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Dong Z, Wang JZ, Yu F, Venkatachalam MA. Apoptosis-resistance of hypoxic cells: multiple factors involved and a role for IAP-2. Am J Pathol 163: 663–671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93: 97–102, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Jiang M, Pabla N, Murphy RF, Yang T, Yin XM, Degenhardt K, White E, Dong Z. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem 282: 2636–2645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287: F1140–F1147, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36: 487–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142: 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39: 503–536, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283: 6572–6583, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol 26: 7397–7408, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17: 3401–3415, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 1793: 154–170, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev 22: 1577–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279: 52726–52734, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet 43: 95–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Wang X. The expanding role of mitochondria in apoptosis. Genes Dev 15: 2922–2933, 2001 [PubMed] [Google Scholar]
- 31. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yi X, Yin XM, Dong Z. Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 278: 16992–16999, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]