Abstract
Systemic sclerosis (SSc) is an autoimmune connective tissue disorder characterized by oxidative stress, impaired vascular function, and attenuated angiogenesis. The tight-skin (Tsk−/+) mouse is a model of SSc that displays many of the cellular features of the clinical disease. We tested the hypotheses that abnormal fibrillin-1 expression and chronic phospholipid oxidation occur in Tsk−/+ mice and, furthermore, that these factors precipitate a prooxidant state, collagen-related protein expression, apoptosis, and mesenchymal transition in endothelial cells cultured on Tsk−/+ extracellular matrix. Human umbilical vein endothelial cells were seeded on microfibrils isolated from skin of C57BL/6J (control) and Tsk−/+ mice in the presence or absence of chronic pretreatment with the apolipoprotein Apo A-I mimetic D-4F (1 mg·kg−1·day−1 ip for 6 to 8 wk). Nitric oxide-to-superoxide anion ratio was assessed 12 h after culture, and cell proliferation, apoptosis, and phenotype were studied 72 h after culture. Tsk−/+ mice demonstrated abnormal “big fibrillin” expression (405 kDa) by Western blot analysis compared with control. Endothelial cells cultured on microfibrils prepared from Tsk−/+ mice demonstrated reduced proliferation, a prooxidant state (reduced nitric oxide-to-superoxide anion ratio), increased apoptosis, and collagen-related protein expression associated with mesenchymal transition. Chronic D-4F pretreatment of Tsk−/+ mice attenuated many of these adverse effects. The findings demonstrate that abnormal fibrillin-1 expression and chronic oxidative stress mediate endothelial mesenchymal transition in Tsk−/+ mice. This mesenchymal transition may contribute to the reduction in angiogenesis that is known to occur in this model of SSc.
Keywords: Apo A-I mimetic, apoptosis, collagen-related proteins, proliferation, tight-skin mouse
systemic sclerosis (scleroderma) is an autoimmune, connective tissue disorder that markedly increases fibrosis of the skin and internal organs (3). The tight-skin (Tsk−/+) mouse is an established animal model of systemic sclerosis in which impaired receptor- and flow-mediated vasodilatation, attenuated angiogenic responses to vascular endothelial growth factor (VEGF), and elevated myocardial angiostatin concentrations are notable features. The Apo A-I mimetic D-4F [an amphipathic α-helical peptide that binds with high affinity to oxidized lipids (2)] mitigated these adverse vascular effects in Tsk−/+ mice. We previously demonstrated that chronic D-4F treatment enhances vasoreactivity of pressurized facialis arteries, decreases myocardial inflammation, reduces angiostatin concentrations, and increases angiogenic potential in Tsk−/+ mouse hearts (12) concomitant with a decline in oxidative stress. These data suggested that primary defects in enhanced angiogenic potential of endothelial cell sprouts observed in Tsk−/+ mice may be related to abnormalities in extracellular matrix structure and function (12). The mechanism(s) by which D-4F improved angiogenic potential in Tsk−/+ remains to be defined, but angiogenic tube formation was only present in Tsk−/+ hearts chronically pretreated with D-4F or when the drug was directly added to the Tsk−/+ culture media. Hence, the angiogenic potential of the myocardium may be influenced by not only an abnormal extracellular matrix on which the endothelium proliferates but also chronic oxidative modification of this defective matrix. Thus, we tested the hypothesis that the alterations in the extracellular matrix observed in Tsk−/+ mice produce detrimental changes in nitric oxide-superoxide anion balance, apoptosis, transcription factor activation, and collagen-related protein expression that contribute to endothelial mesenchymal transition in this model of systemic sclerosis.
MATERIALS AND METHODS
Mice.
Female and male C57BL/6J mice (JAX no. 664) and Tsk−/+ breeder pairs (JAX no. 305) were purchased from Jackson Laboratories (Bar Harbor, ME). Female and male Tsk−/+ mice were bred on site. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. All experimental procedures used in this study were approved by the Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, WI) and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Isolation of microfibrils.
Microfibrils were isolated from 6-mo-old C57BL/6J mice and Tsk−/+ mice in the absence or presence of D-4F (1 mg·kg−1·day−1 ip for 6–8 wk). Briefly, after euthanasia, a one-half inch square of skin was harvested and digested in 5 ml of 0.05 M Tris·HCl, pH 7.4, containing 0.4 M NaCl, 0.01 M CaCl2, 2 mM phenylmethanesulfonyl fluoride (PMSF) and 10 mM N-ethyl-maleimide (NEM). Bacterial collagenase Type 1A (Worthington) was added (final concentration 0.2 mg/ml), and digestion continued at 4°C for 48 h with gentle stirring. Digestion was terminated by addition of EDTA (final concentration 10 mM). The digested tissue was centrifuged at 10,000 g for 30 min. Supernatants containing solubilized skin were designated the low-salt extract. The skin pellet was resuspended in 5 ml 0.05 M Tris·HCl, pH 7.4, containing 1 M NaCl, 10 mM EDTA, 2 mM PMSF, and 10 mM NEM, and then extracted for another 48 h at 4°C with gentle stirring. The mixture was centrifuged for 30 min (10,000 g). The supernatant from the centrifugation was labeled the high-salt extract. The samples were desalted using PD-10 desalting columns equilibrated in distilled water (5). The desalted, aqueous samples were stored at −80°C until analysis.
Western blot analysis.
Western blot analysis was performed on the high-salt microfibril preparations isolated from C57BL/6J or Tsk−/+ mice in the presence or absence of D-4F. Immunoblots were performed for fibrillin-1 (GeneTex, Irvine, CA), the major component of the preparation, and 4-hydroxynonenal (4-HNE) (Calbiochem/EMD Chemicals, Gibbstown, NJ) a major breakdown product of oxidized phospholipids and polyunsaturated fatty acids (1, 11). Western analysis was performed on cell lysates of endothelial cells seeded on the different matrices for 48 h, and the membranes were immunoblotted for rabbit anti-VE-cadherin, rabbit anti-heat shock protein (HSP) 47, rabbit anti-Twist (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-discoidin domain receptor 2 (DDR2; Abcam, Cambridge, MA), rabbit anti-fibroblast-specific protein 1 (FSP-1; R&D Systems, Minneapolis, MN), and rabbit anti-collagen-1 (Rockland Immunochemicals, Gilbertsville, PA).
Endothelial cell proliferation.
Twenty-four-well cluster plates were coated with the high-salt extract (20 μg/ml) from C57BL/6J or Tsk−/+ mice in the presence or absence of D-4F pretreatment. Human umbilical vein endothelial cells (HUVECs; Cell Applications, San Diego, CA) were cultured with endothelial base media (EBM; Cell Applications) and seeded on the different matrices at 10,000 cells/well. After 48 h the number of cells per well was determined with a hemocytometer. Proliferation was also determined using an MTS assay (CellTiter AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI) at the same time point, and the absorbance was read on a Bio-Tek microplate reader.
Apoptosis.
Apoptosis was assessed using the TUNEL Assay (Calbiochem/EMD Chemicals) according to manufacturer's instructions using HUVECs cultured on chamber slides coated with microfibril high-salt extract (20 μg/ml) isolated from C57BL/6J or Tsk−/+ mice in the presence or absence of D-4F pretreatment. Apoptosis was also assessed in 24-well plates by FLICA (fluorochrome inhibitor of caspases) using the FAM Caspase Activity Kit (Imgenex, San Diego, CA) and read on a Molecular Devices Spectramax Gemini EM microplate reader, wavelength 450-nm absorbance.
Preparation of chamber slides.
Four-well chamber slides were coated with the high-salt extract (20 μg/ml) from C57BL/6J or Tsk−/+ mice in the presence or absence of D-4F pretreatment. HUVECs were cultured with EBM and seeded on the different matrices at 25,000 cells/well from C57BL/6J or Tsk−/+ mice in the presence or absence of D-4F pretreatment.
Nitric oxide and superoxide anion balance.
On the next experimental day, previously prepared HUVECs were incubated for 30 min with 4-amino-5-methylamino-2′7′diaminofluorescein (DAF; 10 μM; Invitrogen, Carlsbad, CA) and dihydroethidium (10 μM, DHE, Invitrogen) for the determination of nitric oxide (NO) and O2·− levels, respectively. The fluorescent stain was removed and the cells were allowed to stabilize for 30 min. The slides were then sealed with an aqueous mounting media and analyzed immediately by confocal microscopy (Eclipse TE2000-U; Nikon). Images were recorded with EZ-C1 2.10 software (Nikon). Images were captured using 488-nm excitation for DAF and 546 for DHE and >519- and 589-nm emission, respectively (6, 16). Fluorescence intensity was measured by determining pixel intensity of each cell, and only viable cells were counted.
HUVECs were plated in 24-well plates on the different matrices. DAF and DHE were added (10 μM) for 30 min and then plates were read on a fluorometer (Fluorstar Omega Microplate Reader, BMG Labtech).
Immunohistochemistry.
HUVECs were seeded on coated chamber slides (25,000 cells/well). After 48 h, the slides were fixed for 10 min with 1% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.5% Triton X-100 in PBS for 5 min at room temperature. After a single wash with PBS (5 min), primary antibodies were applied. Proteins investigated were rabbit anti-FSP-1 (Neomarker, Fremont, CA) (1:200) and rabbit anti-Twist (Santa Cruz), rabbit anti-HSP47 (Santa Cruz), goat anti-DDR2, rabbit anti-collagen-1 (Millipore, Billerica, MA), and rabbit anti-VE-cadherin (Santa Cruz). The slides were incubated for 30 min at 37°C. The slides were then washed with PBS (2 × 5 min) followed by incubation with the appropriate secondary antibody labeled with Alexa 488 (Invitrogen). The secondary antibodies were incubated for 30 min at 37°C. Next, the slides were washed with PBS (2 × 5 min) followed by a 3-min incubation with To-Pro3 (1 μg/ml, Invitrogen). The slides were sealed with an aqueous mounting media and stored at −20°C for subsequent analysis by confocal microscopy. Fluorescence intensity was measured by determining pixel intensity of each cell.
Statistical analysis.
Statistical differences in fluorescence intensity were determined by two-way ANOVA using SigmaStat 3.5 software by SigmaStat. Differences between test groups were examined either by the Student's t-test or one-way ANOVA with Bonferroni correction for selected pairs of test groups. The null hypothesis was rejected when P < 0.05.
RESULTS
Western blot analysis.
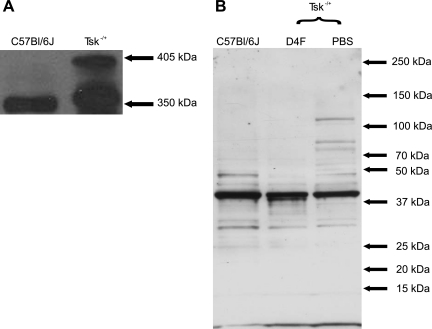
Microfibrils isolated from the skin of Tsk−/+ mice contained abnormal “big” fibrillin-1 (two bands at 350 and 405 kDa) independent of D-4F treatment. In contrast, microfibrils isolated from C57BL/6J mice contained only normal 350-kDa fibrillin-1 (Fig. 1A).
Fig. 1.
Immunoblots of fibrillin-1 and 4-hydroxynonenal (4-HNE) adducts in microfibril preparations. A: microfibrils isolated from tight-skin (Tsk−/+) mice (right lane) contain both 350-kDa and 405-kDa fibrillin molecules compared with microfibrils isolated from C57BL/6J mice (left lane), which contain 350-kDa fibrillin. No “big” fibrillin was present in the microfibrils of C57BL/6J mice. B: immunoblots of 4-HNE show that microfibrils isolated from PBS-treated Tsk−/+ mice (right lane) are oxidatively modified and contain higher levels of 4-HNE than microfibrils isolated from C57BL/6J mice (left lane) and that D-4F treatments decrease 4-HNE adducts in Tsk−/+ mice (center lane). The byproduct of phospholipid oxidation has an affinity for histidine residues.
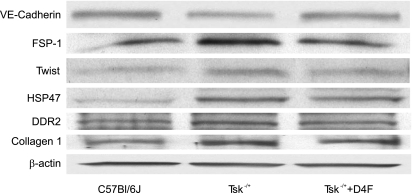
Cell lysates from endothelial cells cultured on Tsk−/+ microfibrils expressed less VE-cadherin compared with those cultured on microfibrils isolated from C57BL/6J mice. Increased expression of FSP-1 and Twist, collagen-1, DDR2, and HSP47 was observed in cell lysates from endothelial cells cultured on Tsk−/+ microfibrils compared with those cultured on microfibrils isolated from C57BL/6J mice (Fig. 2).
Fig. 2.
Western analysis of VE-cadherin, FSP-1, Twist, HSP47, DDR2, and collagen-1 in EC cultured on microfibrils isolated from C57BL/6J, PBS-treated Tsk−/+, and D-4F-treated Tsk−/+ mice. Western analysis from cell lysate confirms immunohistochemical findings. High levels of FSP-1, Twist, collagen-1, DDR2, and HSP47 protein expression were seen in EC seeded on defective Tsk−/+ matrix. D-4F decreases the protein expression of FSP-1 and Twist, collagen-1, DDR2, and HSP47, but not to baseline level. VE-cadherin, a marker of EC expression, is decreased in defective Tsk−/+ matrix.
Microfibrils isolated from Tsk−/+ mice are oxidatively modified.
Increases in 4-HNE were observed in microfibrils isolated from Tsk−/+ mice compared with those from C57BL/6J mice. D-4F pretreatment attenuated this increase in 4-HNE (Fig. 1B). The by-product, 4-HNE, of phospholipid oxidation has an affinity for histidine residues and binds therefore proteins rich in histidine, which explains the multiple bands.
Oxidative stress.
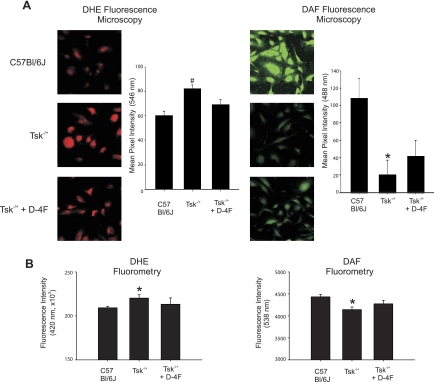
Endothelial cells seeded on microfibrils isolated from Tsk−/+ mice demonstrated decreased DAF and increased DHE fluorescence compared with microfibrils isolated from C57BL/6J mice. Chronic pretreatment of Tsk−/+ mice with D-4F partially reversed these changes in DAF and DHE fluorescence (Fig. 3).
Fig. 3.
Effects of D-4F on·NO and O2·− balance in endothelial cell cultured on microfibrils. A, left: representative superoxide anion (O2·)-dependent hydroethidine (DHE) fluorescence images of endothelial cells (EC) cultured on different microfibril preparations. The levels of O2·− are low in EC cultured on microfibrils isolated from C57BL/6J mice (top). In contrast, O2·− levels in EC cultured on microfibrils isolated from Tsk−/+ mice are increased (center). EC cultured on microfibrils isolated from D-4F-treated Tsk−/+ mice (bottom) generate less O2·− than EC on Tsk−/+ microfibrils (center) but not as low as EC cultured on microfibrils from C57BL/6J mice (top). A, right: representative amino-5-methylamino-2′7′diaminofluorescein (DAF)-nitric oxide (·NO) fluorescence images of EC cultured on different microfibril preparations. EC cultures maintained on microfibrils isolated from C57BL/6J mice generate high levels of intracellular·NO based on intense green fluorescence of DAF-NO (top). EC cultures maintained on microfibrils isolated from Tsk−/+ mice generate low levels of intracellular·NO based on faint DAF-NO fluorescence (center). EC cultured on microfibrils isolated from D-4F-treated Tsk−/+ mice generate levels of DAF-NO that are higher (bottom) than EC cultured on Tsk−/+ microfibrils (center) but not as high as when EC are cultured on microfibrils from C57BL/6J mice (top). Bar graphs depict fluorescence intensity of 60 images for each group and treatment. Tsk−/+ is significantly different (#P < 0.05) from all the other groups. Tsk−/+ is significantly different (*P < 0.05) from C57BL/6J group. B: fluorescence intensity measured with microplate reader shows similar results as in A.
Proliferation and apoptosis.
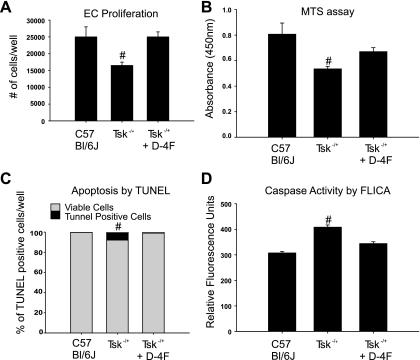
Proliferation of endothelial cells cultured on microfibrils isolated from Tsk−/+ mice (16,500 ± 1,000) was less robust compared with endothelial cells cultured on microfibrils isolated from C57BL/6J (25,000 ± 3,000). Additionally, apoptosis was more pronounced from Tsk−/+ matrix (7.89% ± 2.94%), than C57BL/6J matrix (0.21 ± 0.20%). These differences were abolished by D-4F pretreatment (25,000 ± 1,500 for proliferation and 0.22 ± 0.13% for apoptosis) (Fig. 4). These results were confirmed using MTS assay and caspase-3 activity assay, respectively.
Fig. 4.
Proliferation and apoptosis. EC proliferation is influenced by microfibril preparations. A: cell count. Proliferation is increased in EC cultured on C57BL/6J microfibrils (first bar). EC proliferation is significantly reduced in EC cultured on microfibrils isolated from Tsk−/+ mice (second bar). Proliferation is restored in EC cultured on microfibrils isolated from D-4F-treated Tsk−/+ mice in culture media containing 10 μg/ml D-4F (third bar). Tsk−/+ is significantly different (#P < 0.05) from both groups. B: MTS assay. Proliferation is increased in EC cultured on C57BL/6J microfibrils (first bar). EC proliferation is significantly reduced in EC cultured on microfibrils isolated from Tsk−/+ mice (second bar). Proliferation is restored in EC cultured on microfibrils isolated from D-4F-treated Tsk−/+ mice in culture media containing D-4F (10 μg/ml, third bar). Tsk−/+ is significantly different (#P < 0.05) from C57BL/6J group. C: TUNEL. Percentage of apoptotic cells to viable endothelial cells is increased in cells seeded on Tsk−/+ matrix. (7.8% of 100% viable cells) D: caspase-3 activity by FLICA (fluorochrome inhibitor of caspases) is increased in endothelial cells seeded on defective Tsk−/+ matrix.
Immunohistochemistry.
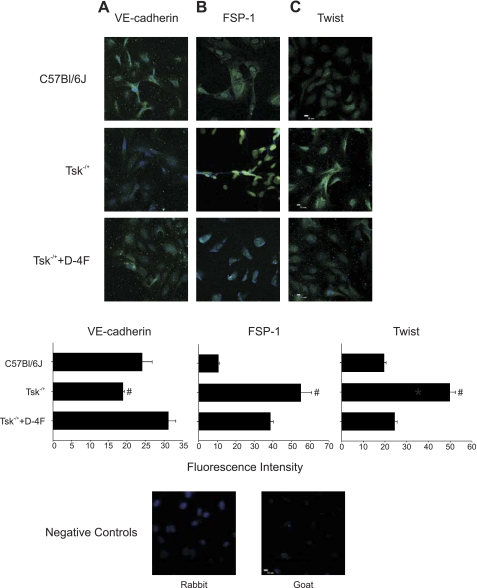
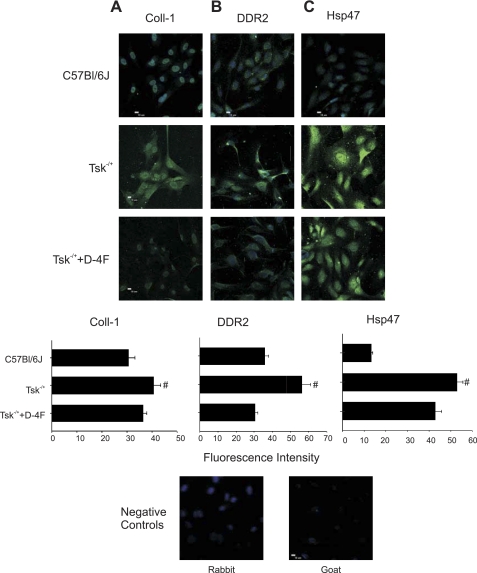
VE-cadherin is normally expressed on cultured endothelial cells and decreases when these cells undergo endothelial mesenchymal transition (15). Endothelial cells cultured on Tsk−/+ microfibrils expressed less VE-cadherin compared with those cultured on microfibrils isolated from C57BL/6J mice. VE-cadherin expression increased in endothelial cells cultured on microfibrils isolated from D-4F-pretreated Tsk−/+ mice (Fig. 5). Endothelial cells in culture normally do not express FSP-1. FSP-1 expression was enhanced in endothelial cells cultured on microfibrils isolated from Tsk−/+ mice compared with controls. D-4F pretreatment of Tsk−/+ mice abolished this effect (Fig. 5). Similarly, endothelial cells seeded on Tsk−/+ microfibrils expressed Twist, whereas those seeded on C57BL/6J microfibrils did not. Endothelial cells cultured on microfibrils of D-4F-pretreated Tsk−/+ mice had reduced Twist expression (Fig. 5). HSP47, DDR2, and collagen-1 expression was enhanced in endothelial cells cultured on Tsk−/+ microfibrils, and these alterations in expression were reduced or abolished by D-4F pretreatment (Fig. 6).
Fig. 5.
Immunohistochemistry of VE-cadherin, fibroblast-specific protein 1 (FSP-1), and Twist in human umbilical vein endothelial cells (HUVECs) cultured on microfibrils isolated from C57BL/6J, PBS-treated Tsk−/+ mice and D-4F-treated Tsk−/+ mice. Shown are representative images of VE-cadherin (A), FSP-1 (B), and Twist (C) expression. A: VE-cadherin expression is decreased in EC cultured on microfibrils isolated from Tsk−/+ mice (center) compared with expression levels in EC cultured on microfibrils isolated from C57BL/6J mice (bright green, top) or from D-4F-treated Tsk−/+ mice (bottom). B: FSP-1 expression is increased (bright green) in EC cultured on microfibrils isolated from Tsk−/+ mice (center) compared with EC cultured on microfibrils isolated from C57BL/6J mice (top) and with EC cultured on microfibrils isolated from D-4F-treated Tsk−/+ mice (bottom). C: Twist expression (bright green) is increased in EC cultured on microfibrils isolated from Tsk−/+ mice (center) compared with microfibrils isolated from C57BL/6J mice (top) or with EC cultured on microfibrils isolated from D-4F-treated Tsk−/+ mice (bottom). Fluorescence intensity is depicted below each panel. Fluorescence intensity was measured by determining pixel intensity of each cell (Tsk−/+ is significantly different from the other groups, #P < 0.05).
Fig. 6.
Immunofluorescence of collagen-1 (Coll-1), discoidin domain receptor 2 (DDR2), and heat shock protein 47 (HSP47) in EC cultured on microfibrils isolated from C57BL/6J, PBS-treated Tsk−/+ mice, and D-4F-treated Tsk−/+ mice. A–C: increased expression (bright green) of collagen-I (A), DDR2 (B), and HSP47 (C) in EC cultured on microfibrils isolated from Tsk−/+ mice compared with expression levels in EC cultured on microfibrils isolated from C57BL/6J and D-4F-treated Tsk−/+ mice. Fluorescence intensity is depicted below each panel. Fluorescence intensity was measured by determining pixel intensity of each cell (Tsk−/+ is significantly different from the other groups, #P < 0.05).
DISCUSSION
The primary objective of the current study was to examine potential mechanisms by which abnormal extracellular matrix in Tsk−/+ mice impairs endothelial function. To accomplish this task, we adapted the methodologies of Kielty et al. (6) to isolate microfibrils from the skin of Tsk−/+ mice. After confirming that these microfibril preparations contained “big” fibrillin-1, we then used this technique to determine its relative effects on endothelial cell function and phenotype. Our results indicate that microfibrils isolated from Tsk−/+ mice impair endothelial cell function compared with those obtained from C57BL/6J mice by several mechanisms. Indeed, abnormal big fibrillin expression was associated with oxidative stress (reduced nitric oxide-to-superoxide anion ratio), increased apoptosis, and altered expression of several crucial proteins linked to mesenchymal transition. These findings suggest that the Tsk−/+ mouse model of systemic sclerosis is characterized by endothelial mesenchymal transition resulting from abnormal extracellular matrix. Notably, chronic pretreatment with D-4F largely reversed many of these adverse effects, indicating that the presence of oxidized phospholipids plays a central role in the observed endothelial mesenchymal transition. Western blot analysis demonstrated the presence of big and normal fibrillin-1 based on bands at 405 and 350 kDa, respectively, whereas only normal fibrillin-1 (350 kDa) was expressed in C57BL/6J mice. The use of a microfibril preparation containing big fibrillin-1 from Tsk−/+ mice strongly suggests that endothelial function and phenotypic alterations may have been at least partially related to the presence of this abnormal fibrillin-1 isoform. We previously reported that hearts from Tsk−/+ mice contained greater oxidized phospholipid concentrations compared with their D-4F-pretreated counterparts. The presence of these oxidized phospholipids was correlated with myocardial fibrosis and inversely related to angiogenic potential (12). As a consequence of these findings, we hypothesized that skin microfibrils isolated from Tsk−/+ mice may also be oxidatively modified, and therefore we examined these microfibrils by Western blot analysis for differences in adducts of 4-HNE, a well-known fission product of polyunsaturated fatty acid oxidation (1, 13). We found that microfibrils isolated from Tsk−/+ mice contained greater numbers of 4-HNE-modified protein bands than those obtained from C57BL/6J mice. In addition, 4-HNE-modified bands were reduced in microfibrils derived from D-4F-pretreated Tsk−/+ mice, suggesting that chronic administration of D-4F decreased oxidative stress. This observation is consistent with our previous finding that chronic D-4F treatment reduces proinflammatory high-density lipoprotein concentrations in the same murine model (12).
Our results further demonstrate that microfibrils isolated from Tsk−/+ mice inhibit endothelial proliferation and promote prooxidant shifts in nitric oxide-superoxide anion balance. It appears highly likely that these effects played an important role in enhanced apoptosis (as determined using TUNEL assay) observed in endothelial cells cultured on Tsk−/+ microfibrils. Notably, this prooxidant state and its associated proapoptotic effect were markedly attenuated in endothelial cells cultured on microfibrils isolated from D-4F-pretreated mice. The abnormal microfibrils isolated from Tsk−/+ mice also induced endothelial mesenchymal transition based on classical markers and activation of signaling pathways that are known to mediate this process (15). When endothelial cells undergo endothelial mesenchymal transition, expression of the endothelial cell marker VE-cadherin decreases (14) concomitant with increases in FSP-1 (14, 10) and Twist (a transcription factor implicated the endothelial cell to fibroblast transition) (4). The presence of abnormal or disrupted extracellular matrix also promotes HSP47 and collagen-1 expression in endothelial cells (8), thereby also influencing their phenotype (15). Transcriptional regulation of genes involved in endothelial mesenchymal transition also appears to target the gene encoding for FSP-1. Our results support these previous findings and provide evidence that endothelial cells from Tsk−/+ mice are predisposed to mesenchymal transition, at least in part, by phospholipid oxidation. Indeed, endothelium cultured on Tsk−/+ mouse microfibrils did not express VE-cadherin, whereas FSP-1 and Twist expressions were enhanced. Our findings also support those of other immunofluorescence studies indicating that the phenotype of transitioned endothelial cells is profibrotic (9). Marked increases in collagen-1, HSP47, and DDR2 expression were also observed when endothelial cells were cultured on microfibrils isolated from Tsk−/+ mice. Similar findings were described by Zeisberg et al. (14). Importantly, decreases in these essential biomarkers of fibrosis occurred when the endothelial cells were cultured on microfibrils isolated from D-4F-pretreated Tsk−/+ mice, indicating that a chronic reduction in oxidized phospholipid adducts by D-4F mediates this antifibrotic effect. In addition, the prooxidant phenotype was greatly reduced in endothelial cells cultured on microfibrils isolated from D-4F-pretreated Tsk−/+ mice. Thus, oxidized lipids appear to play a central role in the mechanisms by which genetic defects in fibrillin-1 promote endothelial mesenchymal transition in systemic sclerosis. D-4F does not physically modify “big” fibrillin-1 into normal fibrillin-1 (data not shown). Thus, it is likely that the attenuated endothelial mesenchymal transition response and decrease in collagen-1 deposition occur as a result of the ability of D-4F to bind or scavenge oxidized phospholipids (2). A prooxidant phenotype also did not occur in endothelial cells seeded on intact microfibrils isolated from C57BL/6J mice (the background strain for the Tsk−/+ mouse model), emphasizing the importance of intact extracellular matrix. However, oxidized phospholipid adducts were reduced in Tsk−/+ mice chronically treated with D-4F, and, consequently, the increase in oxidative stress and its resultant endothelial mesenchymal transition did not occur. Whether the oxidized lipids also play a central role in the mechanisms by which other chronic states of oxidative stress promote endothelial mesenchymal transition is clearly an intriguing hypothesis that will require additional investigation to confirm or refute.
In conclusion, our findings indicate that the Tsk−/+ mouse model is characterized by a fundamental microfibril defect that induces cultured endothelial cells to assume a prooxidant phenotype, which contributes to mesenchymal transition. Chronic administration of the oxidized phospholipid scavenger D-4F reduced this baseline oxidative stress in Tsk−/+ mice and decreased endothelial apoptosis and mesenchymal transition. Such a D-4F-induced reduction in endothelial mesenchymal transition may reduce fibrosis, enhance microvascular function, and promote angiogenesis. Our current and previous results with D-4F may have potentially important therapeutic microvascular implications in patients with systemic sclerosis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-089779.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Comporti M. Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic Res 28: 623–635, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res 42: 1096–1104, 2001 [PubMed] [Google Scholar]
- 3.Flavahan NA, Flavahan S, Mitra S, Chotani MA. The vasculopathy of Raynaud's phenomenon and scleroderma. Rheum Dis Clin North Am 29: 275–291, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Hu L, Roth JM, Brooks P, Ibrahim S, Karpatkin S. Twist is required for thrombin-induced tumorangiogenesis and growth. Cancer Res 68: 4296–4302, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Kielty CM, Cummings C, Whittaker SP, Shuttleworth CA, Grant ME. Isolation and ultrastructural analysis of microfibrillar structures from foetal bovine elastic tissues. Relative abundance and supramolecular architecture of type VI collagen assemblies and fibrillin. J Cell Sci 99: 797–807, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Kielty CM, Hanssen E, Shuttleworth CA. Purification of fibrillin-containing microfibrils and collagen VI microfibrils by density gradient centrifugation. Anal Biochem 255: 108–112, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Kojima H, Urano Y, Kikuchi K, Higuchi T, Hirata Y, Nagano T. Fluorescent indicators for imaging nitric oxide production. Angew Chem Int Ed Engl 38: 3209–3212, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG. Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int 58: 587–597, 2000 [DOI] [PubMed] [Google Scholar]
- 9.O'Riordan E, Mendelev N, Patschan S, Patschan D, Eskander J, Cohen-Gould L, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol Heart Circ Physiol 292: H285–H294, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Weihrauch D, Xu H, Shi Y, Wang J, Brien J, Jones DW, Kaul S, Komorowski RA, Csuka ME, Oldham KT, Pritchard KA. Effects of D-4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in tight-skin mice. Am J Physiol Heart Circ Physiol 293: H1432–H1441, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol 50: 319–336, 2003 [PubMed] [Google Scholar]
- 14. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119: 1429–1437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]