Abstract
During antidiuresis with elevated vasopressin, urea accumulates in the renal medulla to very high concentrations, imposing considerable cellular stress. How local cells cope with urea stress is relevant to the whole kidney because the renal medulla is the major site of residence for the renal stem cells. Previous studies showed that renal cells were incapable of preconditioning in moderate urea concentrations to enhance resistance to urea stress. Instead, preconditioning in moderately high salinity (moderate hypertonicity) has been shown to promote resistance to urea stress due to the induction of the molecular chaperone heat shock protein 70 (Hsp70), which is mediated by the transcription factor tonicity-responsive enhancer binding protein (TonEBP). Here we report that cell lines derived from the kidney and fibroblasts display enhanced resistance to urea stress after pretreatment in moderate, nonstressful concentrations of urea. Using TonEBP knockdown and immunoblot analyses, we demonstrate that TonEBP and Hsp70 are dispensable for the increased resistance to urea stress. These data suggest that cells in the renal medulla are capable of overcoming urea stress by activating distinct cellular pathways.
Keywords: tonicity-responsive enhancer binding protein, heat shock protein 70, hypertonicity, renal medulla
osmolality in the renal medulla is high due to the operation of urinary concentrating mechanism. The cortex is isotonic but the osmolality gradually increases along the cortico-medullary axis starting from the outer medulla down to the tip of the papilla, where tissue osmolality reaches over 2,500 mosmol/kgH2O in rodents during antidiuresis with elevated vasopressin (see Ref. 1 for a review). Urea and salt, two principal solutes in the renal medulla, differ in their osmotic action. Salt distributes mainly in the interstitium, i.e., excluded from the cell, and imposes hypertonic stress to the cell. While urea is an effective osmotic agent driving water reabsorption in the collecting duct, it is generally not excluded from the cell due to high membrane permeability.
Despite the differences in their osmotic action, both hyperosmotic salt (hypertonicity) and urea impose cellular stress as they kill renal cells in culture in a dose-dependent manner (8, 14). Underlying cellular mechanisms involved in the stress appear distinct for the two solutes since urea-induced cell death is not associated with reduced RNA and protein synthesis like hypertonicity-induced cell death (14). On the other hand, cells in the renal medulla in situ cope with the hyperosmolality without signs of distress as cell death is not detectable even during severe antidiuresis (15, 19). Kidney-derived cells in culture can be preconditioned to enhance resistance to hypertonic stress: slow increase in medium tonicity or pretreatment in moderate, nonstressful hypertonicity enhances resistance to hypertonic stress because it allows sufficient accumulation of organic osmolytes (2, 6). The transcription factor tonicity-responsive enhancer binding protein (TonEBP) plays a critical role in the process (see Ref. 5 for a recent review). On the other hand, previous studies have shown that renal cells cannot be preconditioned to urea stress (7, 11). Instead, preconditioning in moderate, tolerable hypertonicity plays a role in overcoming urea stress. Transcriptional induction of heat shock protein 70 (Hsp70), which is mediated by TonEBP in response to hypertonicity (18), is associated with enhanced cellular resistance to urea stress (10, 11).
In this study, we revisited the cellular response to urea stress using renal and nonrenal cell lines. To our surprise, we found that cells were capable of preconditioning to urea stress in a manner independent of Hsp70 and TonEBP.
METHODS
Cell culture.
Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium supplemented with 10% fetal bovine serum, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate (Invitrogen, Carlsbad, CA). Murine inner medullary collecting duct (mIMCD3) cells were maintained in DMEM/F-12 supplemented with 10% FBS, as described previously (13). Mouse embryonic fibroblast (MEF) cells were maintained in DMEM supplemented with 10% FBS as previously described (4). Medium osmolality was raised by adding NaCl or urea to various concentrations as indicated.
Lactate dehydrogenase release assay.
To assess cell death in hyperosmotic conditions, cells were switched to fresh medium containing additional NaCl or urea and cultured for 24 or 48h. Cell culture medium was collected, and adherent cells were lysed in 50 mM Tris, pH 7.5, 150 mM NaCl, and 2% Triton X-100. The amount of lactate dehydrogenase (LDH) in culture medium and cell lysates was quantified using the LDH cytotoxicity detection kit (Clontech, Mountain View, CA). The percentage of LDH (%LDH) release was calculated.
Immunoblot analyses.
Cells were washed with ice-cold PBS and lysed in 50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM dithiothreitol, and protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN) for 30 min at 4°C. Lysates were centrifuged for 5 min at 15,000 g to remove insoluble materials. Supernatants were separated on SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. Nonspecific binding sites were blocked with 5% nonfat milk in TTBS (25 mM Tris, pH 7.4, 137 mM NaCl, 3 mM KCl, 0.05% Tween 20) for 30 min at room temperature. The membrane was incubated with 1:2,000 dilution of anti-Hsp70 antibody (StressGen, Victoria, Alberta, Canada) or anti-TonEBP antibody (9), or with 1:5,000 dilution of anti-heat shock constitutive 70 (Hsc70) antibody (StressGen) in blocking solution for 60 min. The membrane was then incubated with 1:5,000 dilution of anti-mouse IgG, anti-rabbit IgG, or anti-rat IgG conjugated with horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA). Enhanced chemiluminescence assay was performed to visualize horseradish peroxidase using a commercial kit.
RESULTS
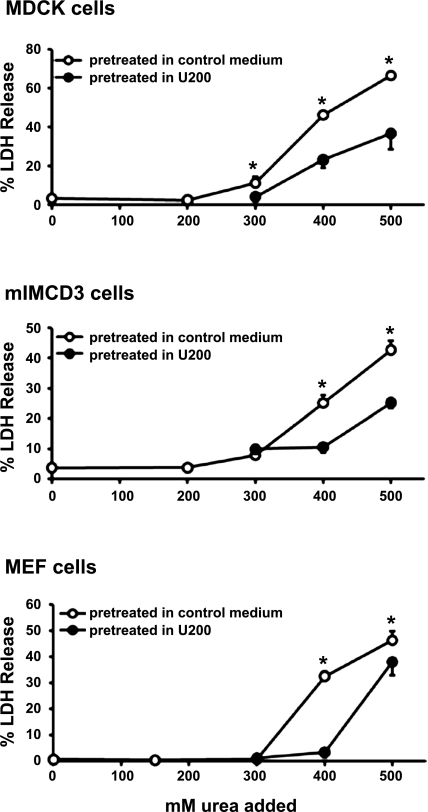
Since cells in the renal medulla displayed few signs of death even when local osmolality is very high (15, 19), we hypothesized that renal cells were capable of overcoming urea stress. To test the hypothesis, we examined the effects of pretreatment with moderate urea concentrations on sensitivity to stressful concentrations of urea as described previously (7, 11). We first examined the sensitivity of cells to urea by measuring %LDH release to assess cell death. In all the experiments presented here, %LDH release was consistent with gross morphological appearance of cell death. As shown in open symbols in Fig. 1, two lines of renal epithelial cells (MDCK and mIMCD3) and a fibroblast cell line (MEF) displayed dose-dependent cell death when urea concentration exceeded 300 mM. After the cells had been pretreated for 6 h in a moderate urea concentration, 200 or 150 mM, in which cells had not displayed measurable cell death, all of the three cell lines became more resistant to urea in that the cell death curve shifted to the right. We also observed improved survival under hypertonicity (hyperosmotic salt) after the cells had been pretreated with moderate hypertonicity (data not shown and see below). The data shown in Fig. 1 demonstrate that both renal and nonrenal cells are inherently capable of preconditioning to promote resistance to urea stress.
Fig. 1.
Effects of preconditioning in moderate urea concentration on resistance to higher urea concentrations. Madin-Darby canine kidney cells (top) and murine inner medullary collecting duct (mIMCD3) cells (middle) were pretreated for 6 h in hyperosmotic medium containing 200 mM urea (U200; filled circles) or kept in control medium (open circles). The cells were then cultured for 48 h in control medium or hyperosmotic medium made by addition of up to 500 mM urea as indicated in the abscissa. Mouse embryonic fibroblast (MEF) cells (bottom) were treated the same way except that 150 mM urea (U150) was used for pretreatment followed by 24 h in control medium or hyperosmotic medium with varying concentrations of urea. Cell death was assessed by percentage of lactate dehydrogenase (LDH) release: %LDH in the medium over total LDH (LDH in the medium plus LDH in adherent cells). Values are means ± SD; n = 4 passages. *P < 0.05 compared with corresponding filled circles.
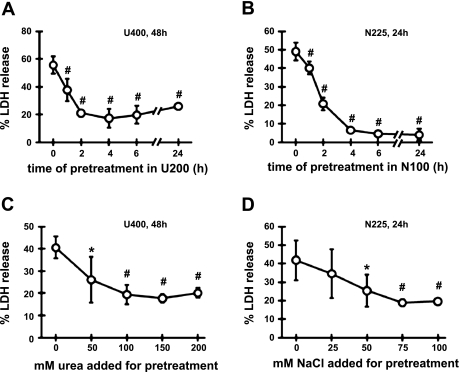
We next compared preconditioning in urea vs. preconditioning in hypertonicity using mIMCD3 cells (Fig. 2). The condition described in Fig. 1 was used for preconditioning in urea: pretreatment in U200 followed by 48 h in U400 (hyperosmotic medium containing additional 400 mM urea). For hypertonic preconditioning, the cells were pretreated in N100 (medium containing additional 100 mM NaCl) followed by 24 h in N225 (medium containing additional 225 mM NaCl). Preconditioning in U200 was completed by 4 h while preconditioning in N100 was complete by 4 to 6 h as shown in Fig. 2, A and B. Likewise, preconditioning reached the maximum at osmolality around 450 mosmol/kgH2O for both urea (150 mM urea added) and hypertonicity (75 mM NaCl added) as shown in Fig. 2, C and D. Thus, characteristics of hyperosmotic preconditioning are similar to both urea and salt in terms of time course and dose response.
Fig. 2.
Preconditioning to urea stress vs. adaptation to hypertonic stress in mIMCD3 cells: time course and dose dependence. A: cells were pretreated with U200 for 0 to 24 h as indicated in the abscissa, followed by 48 h in medium containing 400 mM urea (U400). B: cells were pretreated with hypertonic medium made by addition of 100 mM NaCl (N100) for 0 to 24 h as indicated in the abscissa, followed by 24 h in medium containing additional 225 mM NaCl (N225). C: cells were cultured for 6 h in control medium or hyperosmotic medium containing up to 200 mM urea as indicated in the abscissa, followed by 48 h in U400. D: cells were cultured for 6 h in control medium or hypertonic medium containing up to 100 mM of additional NaCl as indicated in the abscissa, followed by 24 h in N225. %LDH was measured to assess cell death. Values are means ± SD; n = 3 passages. *P < 0.05 and #P < 0.01 compared with 0 h (A and B) or 0 mM (C and D).
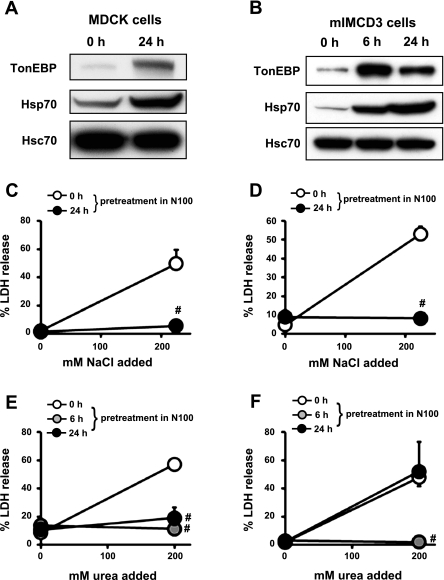
It was reported previously that hypertonic preconditioning promoted cellular resistance to urea stress by virtue of its ability to induce Hsp70 (11). We reexamined this using the two kidney-derived cell lines. We assessed the effects of pretreatment with mild hypertonicity (N100) for 6 h or 24 h. As expected, the pretreatment enhanced Hsp70 expression (Fig. 3, A and B), and resistance to stressful hypertonicity (N225) as indicated by a dramatic decrease in LDH release (Fig. 3, C and D). The pretreatment also led to increased resistance to high urea (U400) in MDCK cells (Fig. 3E), as reported earlier (11). In mIMCD3 cells, however, hypertonic pretreatment for 24 h did not affect resistance to U400 (Fig. 3F), despite prominent induction of Hsp70 (Fig. 3B). Thus, induction of Hsp70 did not necessarily correlate with greater resistance to urea stress, disputing the role of Hsp70 in the process.
Fig. 3.
Effects of hypertonic induction of heat shock protein 70 (Hsp70) on cellular resistance to urea stress. A and B: MDCK (A) and mIMCD3 cells (B) were cultured in N100 for 0 h, 6 h, or 24 h as indicated, and immunoblotted for tonicity-responsive enhancer binding protein (TonEBP), Hsp70, and heat shock constitutive 70 (Hsc70). Representative sets of three independent experiments are shown. C–F: MDCK (C and E) and mIMCD3 cells (D and F) were pretreated in hypertonic medium (N100) for 0 h (open circles), 6 h (shaded circles), or 24 h (filled circles). In C and D, the pretreated cells were cultured for 24 h in control medium or hypertonic medium containing additional 225 mM NaCl. In E and F, the pretreated cells were cultured for 48 h in control medium or hyperosmotic medium containing 400 mM urea. Cell death was assessed by %LDH release. Values are means ± SD; n = 3 passages. #P < 0.01 compared with 0 h pretreatment (open circles).
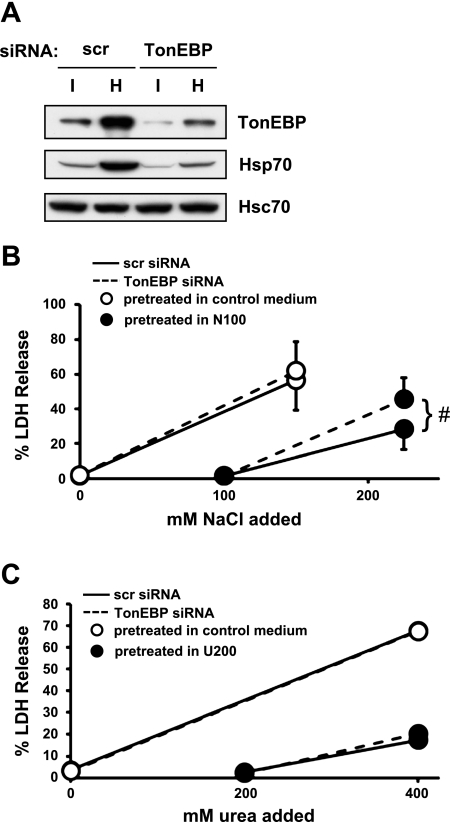
To further define the relationship between urea vs. hypertonic preconditioning, we examined the effects of TonEBP knockdown. TonEBP is a central regulator involved in resistance to hypertonic stress and induction of Hsp70 in response to hypertonicity (see Introduction). We were able to knock down >70% of TonEBP in mIMCD3 cells using targeted small interfering RNA (Fig. 4A). As expected, the knockdown of TonEBP was associated with reduced expression of Hsp70. The process of transfection made cells more sensitive to hypertonicity in that few cells survived N225 with %LDH release close to 100. To keep %LDH release comparable to untransfected cells, i.e., near 60%, N150 was used for cells pretreated with control medium (Fig. 4B). While LDH release in N150 was not affected by the TonEBP knockdown in those cells pretreated in control medium, LDH release increased significantly in N225 in those cells pretreated in N100, demonstrating that TonEBP knockdown compromised cellular preconditioning to hypertonic stress. On the other hand, LDH release in U400 was not affected by the TonEBP knockdown regardless of pretreatment with U200, indicating that cellular resistance to urea stress was not affected. These data demonstrate that TonEBP and Hsp70 are dispensable for preconditioning to urea stress.
Fig. 4.
Effects of TonEBP knockdown on preconditioning to hypertonic stress and urea stress in mIMCD3 cells. Cells were transfected with scrambled (scr) or TonEBP-targeted small interfering RNA (siRNA). A: the transfected cells were immunoblotted for TonEBP, Hsp70, and Hsc70 after they had been cultured for 24 in control medium (I) or N100 (H). B: cells transfected with scr siRNA (solid lines) or TonEBP siRNA (dashed lines) were pretreated for 24 h in N100 (filled circles) or kept in control medium (open circles). The cells were then cultured for 24 h in control medium or hypertonic medium containing additional 100, 150, or 200 mM NaCl as indicated in the abscissa. C: cells transfected with scr siRNA (solid lines) or TonEBP siRNA (dashed lines) were pretreated for 24 h in U200 (filled circles) or kept in control medium (open circles). The cells were then cultured for 48 h in control medium or hyperosmotic medium containing 200 or 400 mM urea as indicated in the abscissa. %LDH was measured to assess cell death. Values are means ± SD; n = 3 passages. #P < 0.01.
DISCUSSION
This study shows that multiple lines of cultured cells are capable of enhancing resistance to urea stress after they are pretreated with moderate and tolerable concentrations of urea. The preconditioning to urea stress shares some features with the preconditioning to hypertonic stress: comparable time course and dose response. On the other hand, they are clearly distinct in that the transcription factor TonEBP is required for the hypertonic preconditioning but not for the urea preconditioning. Although cell lines were used in this study, the observations made here are likely to be relevant to the cells in the hyperosmotic renal medulla: the cells in the renal papilla should be able to survive the high concentration of urea on their own because it takes many hours for the urea concentration to build up (1), allowing sufficient time for the preconditioning to occur. Indeed, cell death is not detectable in the renal papilla even under maximum antidiuresis with full vasopressin action (15, 19). The resistance to urea stress is important not only for the integrity and function of the renal medulla but also for recovery from injuries in the cortex because renal stem cells that respond to such injuries reside in the medulla (12).
Two opposing actions of urea have been recognized. Urea can be a stressor that imposes oxidative stress (20), cell cycle arrest, and apoptosis (8, 14). Conversely, urea can stimulate DNA synthesis (3) and cell proliferation via multiple signaling pathways (reviewed in Ref. 16). At moderate concentrations that do not induce cell death, urea activates transcription of a set of genes including many transcription factors in a manner analogous to epidermal growth factor (17). Urea has also been shown to alleviate the hypertonic stress: addition of urea protects from hypertonicity-induced cell death in both acute phases (14) and chronic phases (6) despite further elevation of osmolality. Data presented here—cellular preconditioning that allows greater resistance to urea stress—add to the complexity of urea actions.
Previous studies did not observe the preconditioning to urea stress (7, 11), in direct contradiction with the observations presented here, although the same cell lines and comparable pretreatment schemes were used. We are unable to provide an explanation for the differences. Since we find the preconditioning not only in kidney-derived epithelial cell lines but also in a fibroblast cell line, we suggest that the process is autonomous and widespread to many cell types like the preconditioning to hypertonic stress (5). Collectively, the data presented here provide new insight into the cellular response to urea stress.
GRANTS
This study was supported by National Institutes of Health Grant DK61677.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Beck FX, Burger-Kentischer A, Müller E. Cellular response to osmotic stress in the renal medulla. Pflügers Arch 436: 814–827, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Cai Q, Ferraris JD, Burg MB. Greater tolerance of renal medullary cells for a slow increase in osmolality is associated with enhanced expression of Hsp70 and other osmoprotective genes. Am J Physiol Renal Physiol 286: F58–F67, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Cohen DM, Gullans SR. Urea selectively induces DNA synthesis in renal epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 264: F601–F607, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101: 10673–10678, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwon MS, Lim SW, Kwon HM. Hypertonic stress in the kidney: a necessary evil. Physiology 24: 186–191, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Kwon MS, Na KY, Moeckel G, Lee SD, Kwon HM. Urea promotes TonEBP expression and cellular adaptation to extreme hypertonicity. Pflügers Arch 459: 183–189, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Leroy C, Colmont C, Pisam M, Rousselet G. Different responses due to acute or progressive osmolality increases in the mIMCD3 cell line. Eur J Cell Biol 79: 936–942, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Michea L, Ferguson DR, Peters EM, Andrews PM, Kirby MR, Burg MB. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am J Physiol Renal Physiol 278: F209–F218, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 96: 2538–2542, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neuhofer W, Lugmayr K, Fraek ML, Beck FX. Regulated overexpression of heat shock protein 72 protects Madin-Darby canine kidney cells from the detrimental effects of high urea concentrations. J Am Soc Nephrol 12: 2565–2572, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Neuhofer W, Müller E, Burger-Kentischer A, Fraek ML, Thurau K, Beck FX. Pretreatment with hypertonic NaCl protects MDCK cells against high urea concentrations. Pflügers Arch 435: 407–414, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awgati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114: 795–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Fluid Electrolyte Physiol 265: F416–F424, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Santos BC, Chevaile A, Hebert MJ, Zagajeski J, Gullans SR. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. Am J Physiol Renal Physiol 274: F1167–F1173, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Terada Y, Inoshita S, Hanada S, Shimamura H, Kuwahara M, Ogawa W, Kasuga M, Sasaki S, Marumo F. Hyperosmolality activates Akt and regulates apoptosis in renal tubular cells. Kidney Int 60: 553–567, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Tian W, Cohen DM. Signaling and gene regulation by urea in cells of the mammalian kidney medulla. Comp Biochem Physiol A 130: 429–436, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Tian W, Cohen DM. Urea stress is more akin to EGF exposure than to hypertonic stress in renal medullary cells. Am J Physiol Renal Physiol 283: F388–F398, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of Hsp70 in response to hypertonicity. Mol Cell Biol 22: 5753–5760, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Z, Cai Q, Michea L, Dmitrieva NI, Andrews P, Burg MB. Proliferation and osmotic tolerance of renal inner medullary epithelial cells in vivo and in cell culture. Am J Physiol Renal Physiol 283: F302–F308, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Z, Yang XY, Cohen D. Urea-associated oxidative stress and Gadd153/CHOP induction. Am J Physiol Renal Physiol 276: F786–F793, 1999 [DOI] [PubMed] [Google Scholar]