Abstract
Mammalian Ste20-like proline/alanine-rich kinase (SPAK) and oxidative stress-responsive 1 (OSR1) kinases phosphorylate and regulate cation-coupled Cl− cotransporter activity in response to cell volume changes. SPAK and OSR1 are activated via phosphorylation by upstream with-no-lysine (WNK) kinases. In Caenorhabditis elegans, the SPAK/OSR1 ortholog germinal center kinase (GCK)-3 binds to and regulates the activity of the cell volume- and meiotic cell cycle-dependent ClC anion channel CLH-3b. We tested the hypothesis that WNK kinases function in the GCK-3/CLH-3b signaling cascade. CLH-3b heterologously expressed in human embryonic kidney (HEK) cells was unaffected by coexpression with the single C. elegans WNK kinase, WNK-1, or kinase-dead WNK-1 dominant-negative mutants. RNA interference (RNAi) knockdown of the single Drosophila WNK kinase had no effect on the activity of CLH-3b expressed in Drosophila S2 cells. Similarly, RNAi silencing of C. elegans WNK-1 had no effect on basal or cell volume-sensitive activity of CLH-3b expressed endogenously in worm oocytes. Previous yeast 2-hybrid studies suggested that ERK kinases may function upstream of GCK-3. Pharmacological inhibition of ERK signaling disrupted CLH-3b activity in HEK cells in a GCK-3-dependent manner. RNAi silencing of the C. elegans ERK kinase MPK-1 or the ERK phosphorylating/activating kinase MEK-2 constitutively activated native CLH-3b. MEK-2 and MPK-1 play important roles in regulating the meiotic cell cycle in C. elegans oocytes. Cell cycle-dependent changes in MPK-1 correlate with the pattern of CLH-3b activation observed during oocyte meiotic maturation. We postulate that MEK-2/MPK-1 functions upstream from GCK-3 to regulate its activity during cell volume and meiotic cell cycle changes.
Keywords: cell volume sensing, meiotic maturation, oxidative stress-responsive 1, cation-chloride cotransporters, germinal center kinase, Caenorhabditis elegans
members of the sterile, or Ste20, superfamily of serine/threonine kinases consist of the structurally distinct p21-activated kinase (PAK) and germinal center kinase (GCK) families. The GCK family is divided into eight subfamilies (10, 11). Members of the GCK VI subfamily have emerged as key regulators of diverse epithelial solute transport processes, cell volume-dependent ion transporters and channels, and systemic salt and water balance (12, 27, 47). For example, the Na-K-2Cl and K-Cl cotransporters NKCC1 and KCC2 play essential roles in cell volume control and numerous other physiological processes. NKCC1 and KCC2 are activated by cell shrinkage and swelling, respectively, and by changes in intracellular Cl− concentration (19). Both transporters interact with mammalian Ste20-like proline/alanine-rich kinase (SPAK) and oxidative stress-responsive 1 (OSR1). SPAK and OSR1 mediate transporter phosphorylation in response to cell volume changes, and phosphorylation in turn regulates transporter activity (12, 27).
CLH-3b, a ClC anion channel expressed in the Caenorhabditis elegans oocyte, is activated by cell swelling and oocyte meiotic cell cycle progression. Activation of the channel plays a role in regulating the timing of the contractions of surrounding smooth muscle-like sheath cells that mediate ovulation (43). The SPAK/OSR1 ortholog GCK-3 binds to the channel COOH terminus and mediates phosphorylation of two nearby serine residues. Phosphorylation in turn inhibits channel activity (14, 16). GCK-3 also plays an essential role in whole animal volume recovery following hypertonicity-induced water loss and shrinkage (6).
Volume regulation was likely one of the earliest homeostatic processes that arose during cellular evolution. While the solute accumulation and loss mechanisms that mediate cell volume regulation are well described, a fundamental problem that remains to be resolved is identification of the mechanisms by which cells detect osmotic perturbations and activate diverse regulatory responses. The discovery of the evolutionarily conserved roles of mammalian SPAK/OSR1 and C. elegans GCK-3 in regulating volume-sensitive channels and transporters represents a key step in this direction. Recent studies in mammals have shown that with-no-lysine, or WNK, serine/threonine kinases function upstream of SPAK and OSR1. Mammals have four WNK kinases: WNK-1, WNK-2, WNK-3, and WNK-4 (27). Multiple studies have shown that WNK kinases function to phosphorylate and activate SPAK and OSR1 (12, 27). In C. elegans, the single WNK homolog WNK-1 binds to and functions, together with GCK-3, to regulate whole animal osmotic balance (6).
The emerging picture from studies in mammals and C. elegans is that WNK and GCK VI kinases are components of evolutionarily conserved signaling cascades that regulate transporters and channels required for cellular and systemic osmotic homeostasis. However, this picture is far from complete. At least two studies suggest that SPAK/OSR1 may be regulated by protein kinase C isoforms (33, 46). Ahlstrom and Yu (1) recently demonstrated that inactivating mutations in WNK-4 do not prevent SPAK and OSR1 phosphorylation. They also showed that an unidentified 40-kDa kinase is capable of phosphorylating both proteins. In addition, the activities of SPAK and OSR1 are regulated by autophosphorylation, and OSR1 autophosphorylation is sensitive to Cl− concentration (18), suggesting that these kinases may be modulated directly by cell volume changes.
The goal of the current study was to determine the role of WNKs in regulating the volume-sensitive activity of the ClC anion channel CLH-3b. Using human embryonic kidney (HEK) cells, Drosophila S2 cells, and C. elegans oocytes, we demonstrate that GCK-3-dependent regulation of CLH-3b does not require the activity of upstream WNKs. Instead, we find that extracellular signal-regulated kinase (ERK) signaling functions, together with GCK-3, to regulate heterologously expressed CLH-3b and CLH-3b expressed endogenously in worm oocytes. ERK signaling plays an essential role in regulating the development and ovulation of oocytes in C. elegans (49). Regulation of CLH-3b by GCK-3 and ERK signaling thus provides a mechanism to tightly couple channel activity to oocyte development, meiotic cell cycle progression, and ovulation in vivo (43).
MATERIALS AND METHODS
Transfection and whole cell patch-clamp recording of HEK 293 cells.
HEK 293 cells were cultured in 35-mm-diameter tissue culture plates in MEM (GIBCO, Gaithersburg, MD) containing 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), nonessential amino acids, sodium pyruvate, 50 U/ml penicillin, and 50 μg/ml streptomycin. After reaching 40–50% confluency, cells were transfected using FuGENE 6 (Roche Diagnostics, Indianapolis, IN) with 1 μg of green fluorescent protein (GFP) and 1 μg of CLH-3b ligated into pcDNA3.1 and 2 μg of pBudCE4.1 with GCK-3 ligated into the cytomegalovirus site and WNK-1 ligated into the elongation factor-1α site. Point mutations in WNK-1 were generated using a QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). All mutations were confirmed by DNA sequencing.
After transfection, cells were incubated at 37°C for 24–30 h. At ∼2 h prior to patch-clamp experiments, cells were detached from growth plates by exposure to 0.25% trypsin containing 1 mM EDTA (GIBCO) for 45 s. Detached cells were suspended in MEM, pelleted by centrifugation, resuspended in fresh MEM, and then plated onto poly-l-lysine-coated coverslips. Plated coverslips were placed in a bath chamber mounted onto the stage of an inverted microscope. Cells were visualized by fluorescence and differential interference contrast microscopy.
Transfected HEK 293 cells were identified by GFP fluorescence and patch-clamped using a bath solution containing 90 mM NMDG-Cl, 5 mM MgSO4, 1 mM CaCl2, 12 mM HEPES-free acid titrated to pH 7.0 with CsOH, 8 mM Tris, 5 mM glucose, 80 mM sucrose, and 2 mM glutamine (pH 7.4, 295 mosM) and a pipette solution containing 116 mM NMDG-Cl, 2 mM MgSO4, 20 mM HEPES, 6 mM CsOH, 1 mM EGTA, 2 mM ATP, 0.5 mM GTP, and 10 mM sucrose (pH 7.2, 275 mosM). Cells were swollen by exposure to a hypotonic (225 mosM) bath solution that contained no added sucrose. Cells were shrunken by exposure to a bath solution made hypertonic (400 mosM) by sucrose addition. Swelling-induced currents were measured at −100 mV. Experimental protocols were performed on at least two independently transfected groups of cells.
Patch electrodes were pulled from 1.5-mm-OD silanized borosilicate microhematocrit tubes; electrode resistance ranged from 4 to 8 MΩ. Currents were measured with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA). Electrical connections to the patch-clamp amplifier were made using Ag-AgCl wires and 3 M KCl-agar bridges. Data acquisition and analysis were performed using pClamp 8 software (Axon Instruments).
Western blotting.
Expression of WNK-1 transfected into HEK cells was confirmed using V5 epitope-tagged wild-type kinase and K344M and D481A kinase-dead (KD) mutants. Briefly, equal quantities of control and WNK-1-transfected HEK cells were lysed 24 h after transfection using a modified RIPA buffer containing 0.05% SDS, 1.0% Triton X-100, 150 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, and 1.5 KH2PO4, pH 7.4. Lysates were centrifuged for 20 min at 10,000 g and 4°C to remove cellular debris. Supernatants were precleared with protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated overnight with an anti-V5 antibody (Invitrogen, Carlsbad, CA) and agarose to immunoprecipitate wild-type or WNK-1 mutants. After the supernatant was pelleted by centrifugation, the agarose was washed four times with 1 ml of RIPA buffer and the supernatant was resuspended in 4× lithium dodecyl sulfate sample buffer (Invitrogen) and heated to 70°C for 20 min. Proteins were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. WNK-1 kinases were identified by Western blotting using an anti-V5 antibody (Invitrogen).
RNA interference, transfection, and whole cell patch-clamp of S2 cells.
Drosophila S2 cells were cultured at 25°C in six-well plates and grown in Schneider's Drosophila medium (S2 medium, GIBCO) supplemented with 10% heat-inactivated fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin. Double-stranded RNA (dsRNA) for RNA interference (RNAi) experiments was synthesized using established methods (17a). Briefly, DNA templates were obtained by PCR, and sense and antisense RNA was synthesized by T7 polymerase (MEGAscript, Ambion, Austin, TX). Template DNA was digested with DNase I, and RNA was precipitated with 3 M sodium acetate and ethanol. Precipitated RNA was washed with 70% ethanol, air-dried, and dissolved in water. RNA size, purity, and integrity were assessed on agarose gels. dsRNA was formed by annealing sense and antisense RNA in a 65°C water bath and allowing the bath to slowly return to room temperature.
At 24 h prior to RNAi experiments, ∼106 S2 cells were seeded into 35-mm plates. Cultured cells were gently washed with serum-free S2 medium and then treated with 1 ml of serum-free medium containing 40 μg of dsRNA. The cells were allowed to sit at room temperature for 30 min; then 1 ml of standard S2 medium was added to the dish, and the plates were placed in the incubator overnight. On the following day, cells were transfected with 2 μg of GFP, 2 μg of GCK-3, and 2 μg of CLH-3b ligated into pAC5.1 using a CalPhos Transfection Kit (ClonTech, Mountain View, CA).
Transfected S2 cells were patch-clamped 48 h after transfection. Patch-clamp analysis was conducted as described for HEK 293 cells with use of a bath solution containing 85 mM CsCl, 5 mM MgSO4, 4 mM calcium gluconate, 10 mM HEPES, 12 mM glutamine, 10 mM glucose, and 125 mM sucrose (pH 6.8, 325 mosM) and a pipette solution containing 135 mM CsCl, 2 mM MgSO4, 10 mM HEPES, 1 mM EGTA, 6 mM CsOH, 2 mM ATP, 500 nM GTP, and 10 mM sucrose (pH 7.2, 305 mosM). Hypotonic (245 mosM) and hypertonic (400 mosM) bath solutions were made by removal or addition of sucrose.
RNAi and whole cell patch-clamp of C. elegans oocytes.
RNAi was performed by feeding worms a strain of Escherichia coli engineered to transcribe dsRNA homologous to a target gene. The strains were obtained from a commercially available RNAi feeding library (Geneservice, Cambridge, UK) and contained a fragment that corresponds to wnk-1 (GenBank accession number NM_069202.4) nucleotides 3986–4760, mpk-1 (GenBank accession number NM_001027412.2) nucleotides 9328–10674, or mek-2 (GenBank accession number NM_058686.3) nucleotides 151–1315. Basic local alignment research tool searches of C. elegans genomic and expressed sequence tag databases failed to identify coding regions with nucleotide sequence homologous to the WNK-1, MPK-1 or MEK-2 RNAi constructs, indicating that off-target effects are unlikely. A bacterial strain expressing 202 bases of dsRNA that are not homologous to any predicted C. elegans genes was used as a control for nonspecific RNAi effects. dsRNA feeding was carried out for 3 days by transfer of eggs isolated from wild-type N2 worms to agar plates seeded with control or wnk-1, mek-2, or mpk-1 RNAi bacteria.
Oocytes were isolated from gravid young adult worms and patch-clamped, as described previously (43). Bath and pipette solutions contained 116 mM NMDG-Cl, 2 mM CaCl2, 2 mM MgCl2, 25 mM HEPES, and 71 mM sucrose (pH 7.4, 340 mosM) and 116 mM NMDG-Cl, 2 mM MgSO4, 20 mM HEPES, 6 mM CsOH, 1 mM EGTA, 48 mM sucrose, 2 mM ATP, and 0.5 mM GTP (pH 7.2, 315 mosM), respectively.
Cell volume measurements.
Cell volume was measured in all patch-clamp experiments to ensure that volume changes were comparable between protocols. HEK and S2 cells and oocytes were visualized by video-enhanced differential interference contrast microscopy. Relative cell volume change was determined as follows: (experimental CSA/control CSA)3/2, where CSA is the cell cross-sectional area measured at a single focal plane located at the point of maximum cell or oocyte diameter. Rates of cell volume change were similar in all studies and are not reported.
RT-PCR.
RT-PCR was carried out on S2 cells and C. elegans gonads to assess the effectiveness of RNAi knockdown of endogenous WNKs. S2 cells were treated with dsRNA as described above. After treatment, cells were dislodged from their dish as follows: medium was pipetted across the plate, and the cells were spun down, resuspended in fresh medium, and counted. Total RNA was isolated in triplicate from ∼5 × 105 S2 cells using an RNAqueous Micro kit (Ambion). RT-PCR was performed on equal amounts of total RNA from each sample using a Titan One-Step RT-PCR kit (Roche, Indianapolis, IN) and primers to amplify a 532-bp fragment of Drosophila WNK (dWNK). The PCR product was analyzed on a 1% agarose gel alongside molecular weight markers.
C. elegans gonad arms were dissected in egg buffer containing 118 mM NaCl, 48 mM KCl, 2 mM CaCl2, 2 mM MgCl2, and 25 mM HEPES, as described previously (43). Gonads were washed by transfer three times to 35-mm petri dishes containing 2 ml of egg buffer. Single gonads were then transferred to a PCR tube with 5 μl of wash solution and subjected to five, 10-min cycles of freezing at −80°C and thawing for 10 min at room temperature. RT-PCR was performed using a Titan One-Step RT-PCR kit with primers to amplify a 1,100-bp fragment of WNK-1. The PCR product was analyzed on a 1% agarose gel alongside molecular weight markers.
Quantification of CLH-3b current properties.
Coexpression of CLH-3b with GCK-3 causes striking changes in channel voltage sensitivity and the kinetics of hyperpolarization-induced activation (14). Since CLH-3b is inwardly rectifying and active only at hyperpolarized voltages, we used current-to-voltage plots (see Fig. 5B) to estimate channel activation voltage. A line was first drawn by linear regression analysis of currents measured between 0 and 60 mV, where channel activity is low (see Fig. 5B). A second line was drawn by linear regression analysis of currents measured between the first test voltage at which inward current was detected and a second voltage 20 mV more negative. The point at which these two lines intersect is defined as the activation voltage.
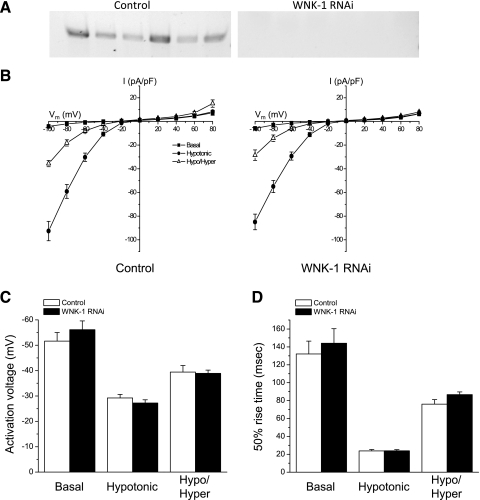
Fig. 5.
Effect of WNK-1 RNAi on CLH-3b activity in C. elegans oocytes. A: RT-PCR of WNK-1 expression in single gonads isolated from control worms fed bacteria expressing a scrambled dsRNA and single gonads isolated from worms fed WNK-1 dsRNA-producing bacteria. Each lane on the gels represents a RT-PCR reaction from a single gonad. B: CLH-3b current-to-voltage relationships in oocytes isolated from control worms and wnk-1(RNAi) worms. Currents were recorded under basal conditions, 5 min after cell swelling in hypotonic (270 mosM) bath solution (hypo), and 5 min after shrinkage of swollen oocytes by exposure to a 435 mosM bath solution (hypo/hyper). C and D: activation voltages and 50% rise times under basal conditions, in swollen oocytes, and in swollen oocytes shrunken by exposure to a 410 mosM bath solution. Values are means ± SE (n = 8–9).
CLH-3b channels are gated open by membrane hyperpolarization. Channel activation kinetics are described by mono- or biexponential fits describing slow or fast and slow time constants, respectively. The nature of the fit is dictated by channel phosphorylation, which appears to inhibit a fast gating process (14). To simplify presentation and interpretation of activation kinetics under different experimental conditions, time constants are not used. Instead, the time required for whole cell current to reach 50% activation when membrane voltage is stepped from 0 to −100 mV for 1 s is quantified. This time is defined as the 50% rise time.
For quantification of rates of current change induced by cell swelling or shrinkage, membrane voltage was stepped from 0 to −100 mV for 500 ms every 1 s. Initial rates of current activation by swelling and inactivation by shrinkage were determined by linear regression analysis performed during the initial 15–25 s of current change.
Pharmacological studies.
U-0126 and PD-098059 (Enzo Life Sciences, Plymouth Meeting, PA) were dissolved as stock solutions in DMSO. The maximum DMSO concentration in all solutions was 0.1%. Control patch-clamp experiments were performed with a bath solution containing 0.1% DMSO.
Statistical analyses.
Values are means ± SE. Statistical significance was determined using Student's two-tailed t-test for paired or unpaired means. For comparison of three or more groups, statistical significance was determined by one-way analysis of variance with Bonferroni's post hoc test. P ≤ 0.05 was taken to indicate statistical significance. All graphs are plotted on common scales to facilitate comparison between experimental groups.
RESULTS
WNK kinases do not function in GCK-3-dependent regulation of CLH-3b.
CLH-3b activity is inhibited via phosphorylation of its COOH terminus by the STK39/SPAK ortholog GCK-3. Phosphorylation reduces whole cell current amplitude, strongly hyperpolarizes channel activation voltage, and slows the rate of hyperpolarization-induced channel activation. These changes are most likely brought about by inhibition of channel fast gating. Cell swelling reverses these changes (14, 16). If WNK kinases regulate GCK-3 during cell volume changes, then changes in WNK activity should alter swelling- and/or shrinkage-induced changes in CLH-3b activity and functional properties. We carried out a series of experiments to determine whether WNK kinases function upstream of GCK-3. As shown by us previously (14, 16), exposure of HEK cells coexpressing CLH-3b and GCK-3 to a hypotonic bath causes rapid current activation. When swollen cells are exposed to a hypertonic bath, CLH-3b inactivates rapidly. Initial rates of current activation and inactivation for cells expressing GCK-3 were ∼0.8 and ∼−0.35 pA·pF−1·s−1, respectively (Fig. 1, A and B). Hypertonic bath inhibited whole cell current ∼85% (Fig. 1C). Coexpression of GCK-3 together with wild-type C. elegans WNK-1 had no significant (P > 0.05) effect on these rates or on the extent of hypertonicity-induced current inhibition (Fig. 1, B and C), indicating that C. elegans WNK-1 is not required for GCK-3 function.
Fig. 1.
Effect of with-no-lysine (WNK) expression on cell swelling (A)- or shrinkage (B and C)-induced changes in CLH-3b current amplitude. Cells were transfected with germinal center kinase (GCK)-3 and CLH-3b and either C. elegans WNK-1 or one of two WNK-1 kinase-dead (KD) mutants, K344M or D481A, predicted to function as dominant-negative mutants. Cell swelling was induced by exposure of cells to a 200 mosM bath solution for 1 min. Swollen cells were shrunken by exposure to a 400 mosM bath solution. Values are means ± SE (n = 4–5). D: Western blot analysis of control human embryonic kidney (HEK) cells and HEK cells transfected with V5-tagged WNK-1, K344M, or D481A. WNK-1 and the KD mutants are detected as a doublet with molecular weights of ∼200,000–220,000.
Mammalian cells express four WNK kinases, WNK-1, WNK-2, WNK-3, and WNK-4 (27). It is conceivable that endogenous WNK kinases regulate GCK-3. To test this possibility, we coexpressed GCK-3 with one of two different WNK-1 mutants, K344M and D481A. K344 and D481 are homologous to K233 and D368 in human WNK-1 and are essential for catalytic activity. The K233A and D368A mutants are kinase dead (KD) (52). Because WNK-1 and GCK-3 physically and functionally interact (6), these mutants are expected to act as dominant-negatives and inhibit potential interactions of GCK-3 with endogenous, mammalian WNKs. As shown in Fig. 1, A–C, coexpression of GCK-3 with either KD mutant had no significant (P > 0.05) effect on initial rates of current activation and inactivation or on the extent of hypertonicity-induced current inhibition.
To ascertain whether WNK-1 and the KD mutants were expressed in HEK cells, we performed Western blot analysis using V5-tagged kinases. As shown in Fig. 1D, the three kinases were detected as doublets with molecular weights of ∼200,000–220,000. The predicted molecular weight of C. elegans WNK-1 is ∼202,000. The doublet may reflect different phosphorylation states of the kinases.
CLH-3b is strongly inwardly rectifying and is activated by strong hyperpolarization (13, 21) (Fig. 2A). Cell volume-dependent changes in CLH-3b activity are accompanied by changes in the membrane voltage at which the channel activates (i.e., activation voltage) and by the kinetics of hyperpolarization-induced activation, which is quantified as the time required for 50% activation at −100 mV (i.e., 50% rise time) (14, 16). These cell volume- and phosphorylation-dependent changes in biophysical parameters provide an additional, sensitive means to monitor changes in GCK-3 activity that is independent of CLH-3b expression levels. Under isotonic conditions, GCK-3-mediated phosphorylation of CLH-3b hyperpolarizes channel activation voltage by 30–40 mV and slows the kinetics of hyperpolarization-induced current activation seven- to ninefold (14, 16). If WNKs function upstream of GCK-3 and are required for its activity, then inhibition of WNKs should prevent the GCK-3-dependent changes in CLH-3b voltage sensitivity. As shown in Fig. 2, coexpression of GCK-3 with wild-type WNK-1 or the K344M and D481A WNK-1 dominant-negative mutants had no significant (P > 0.05) effect on channel current amplitude, current-to-voltage relationships, activation voltages, or 50% rise time under isotonic conditions.
Fig. 2.
Effect of WNK expression on basal CLH-3b activity and channel biophysical properties. GCK-3 functions normally to hyperpolarize CLH-3b activation voltage, slow hyperpolarization-induced current activation, and decrease whole cell current amplitude at hyperpolarized voltages (14). Coexpression of GCK-3 with WNK-1 or WNK-1 dominant-negative mutants has no effect on CLH-3b amplitude and current (ICLH-3b)-to-voltage (Vm) relationship (A), activation voltage (B), or voltage-dependent activation kinetics (50% rise time; C) under basal conditions. Values are means ± SE (n = 4–5).
We quantified the effects of WNK expression on swelling- and shrinkage-induced changes in channel voltage sensitivity. As shown in Fig. 3, A and B, activation voltages and 50% rise times quantified after 1 min of cell swelling were unaffected (P > 0.3) by coexpression of GCK-3 with WNK-1 or by dominant-negative inhibition of endogenous WNK activity.
Fig. 3.
Effect of WNK expression on cell volume-dependent changes in CLH-3b biophysical properties. Cell swelling for 1 min causes marked depolarization of CLH-3b activation voltage (A) and decrease in 50% rise time (B). Coexpression of GCK-3 with WNK-1 or WNK-1 dominant-negative mutants has no effect on changes in these parameters induced by swelling. Rates of changes induced by cell shrinkage in the CLH-3b activation voltage (C) and 50% rise time (D) are unaffected by coexpression of GCK-3 with WNK-1 or WNK-1 dominant-negative mutants. Cell swelling and shrinkage were induced by exposure to 225 and 400 mosM bath solution, respectively. Values are means ± SE (n = 5–6).
Because we were able to expose cells to hypertonic bath solutions for prolonged periods of time, we monitored the time course of shrinkage-induced increases in channel activation voltage and rise time. The time courses for changes in these biophysical parameters were not significantly (P > 0.3) different in cells expressing GCK-3 alone, GCK-3 and WNK-1, and GCK-3 and WNK dominant-negative mutants (Fig. 3, C and D). Taken together, data in Figs. 1–3 demonstrate that C. elegans WNK-1 and endogenous mammalian WNKs are not required for cell volume-dependent regulation of CLH-3b in HEK cells.
A single WNK homolog, dWNK, is expressed in Drosophila. To further examine the role of WNK kinases in regulating GCK-3, we expressed GCK-3 and CLH-3b in Drosophila S2 cells and assessed the effects of dWNK RNAi on channel activity. S2 cells express a volume-sensitive Cl− channel that is encoded by the bestrophin gene dBest1 (5). This channel activity could conceivably contaminate CLH-3b recordings. However, in our hands, we found that the endogenous S2 cell volume-sensitive anion current had highly variable activity and that current amplitude was ≤3–4 pA/pF after activation by 1 min of cell swelling (data not shown). Expression of CLH-3b, in contrast, generated current amplitudes of 60–225 pA/pF. Because of the low endogenous volume-sensitive current levels and the reduced efficiency of gene silencing when multiple dsRNA species were used, we chose to not silence dBest1 expression.
Results from expression of CLH-3b with wild-type or KD GCK-3 were identical to those observed in HEK cells. In the presence of KD GCK-3, CLH-3b activation voltage and 50% rise time were ∼−35 mV and ∼5 ms, respectively (data not shown). As expected, coexpression of CLH-3b with wild-type GCK-3 reduced whole cell current amplitude (data not shown), hyperpolarized channel activation voltage (Fig. 4B), and slowed the kinetics of hyperpolarization-induced current activation (Fig. 4C).
Fig. 4.
Effect of RNA interference (RNAi) knockdown of Drosophila WNK (dWNK) on CLH-3b biophysical properties. A: RT-PCR of dWNK expression in control (Cntl) Drosophila S2 cells and S2 cells treated with nonspecific (Nus) or dWNK double-stranded RNA (dsRNA). B and C: time-dependent changes in CLH-3b activation voltage and 50% rise time following cell swelling and shrinkage. Activation voltages and 50% rise times reported at −1.5 and 0 min are basal values and values recorded 1 min after cell swelling, respectively. Cell swelling was induced by exposure to a 245 mosM bath solution. At time 0, cells were exposed to a 400 mosM bath solution to induce shrinkage. Values are means ± SE (n = 9).
As shown in Fig. 4A, dWNK RNAi dramatically reduced expression of the kinase compared with control cells or cells treated with a nonspecific (i.e., Nus) dsRNA. However, loss of kinase activity had no significant (P > 0.4) effect on rates and magnitudes of cell volume-dependent changes in current amplitude. Rates of swelling-induced current activation and shrinkage-induced inhibition were 1.0 ± 0.3 and −0.33 ± 0.08 (SE) pA·pF−1·s−1 (n = 8) in Nus dsRNA-treated cells and 0.91 ± 0.25 and −0.35 ± 0.11 pA·pF−1·s−1 (n = 9) in cells treated with dWNK dsRNA. Shrinkage-induced current inhibition was 74 ± 5% (n = 8) and 74 ± 5% (n = 9) for Nus and dWNK-1 dsRNA-treated cells, respectively. Basal activation voltages and 50% rise times were considerably lower when CLH-3b was expressed in S2 cells (Fig. 4, B and C). However, dWNK silencing also had no significant (P > 0.1) effect on basal values or values observed under hypotonic and hypertonic conditions (Fig. 4, B and C). In addition, the time courses of changes in these parameters during cell shrinkage were unaffected by dWNK knockdown (Fig. 4, B and C).
To determine whether WNK-1 regulates CLH-3b in vivo, we performed experiments in C. elegans oocytes. RNAi knockdown of GCK-3 constitutively activates endogenous CLH-3b (14). If WNK-1 is required for GCK-3 function, knockdown of this kinase should also activate the channel. Oocytes were isolated from worms fed control bacteria producing a scrambled dsRNA or bacteria producing WNK-1 dsRNA. dsRNA feeding knockdown of WNK-1 induces a robust whole animal phenotype (6). To assess the effect of RNAi on wnk-1 expression directly, RT-PCR was performed on single gonads isolated from control and WNK-1 dsRNA-fed worms. As shown in Fig. 5A, WNK-1 RNAi strongly silenced expression of the kinase compared with control dsRNA-fed worms. However, knockdown of WNK-1 had no significant (P > 0.1) effect on basal current amplitude, activation voltage, or 50% rise time (Fig. 5, B–D). It also had no significant (P > 0.2) effect on the changes in these parameters induced by swelling oocytes for 5 min or by shrinking swollen oocytes for 5 min in a 410 mosM hypertonic solution (Fig. 5, B–D).
MAPK signaling regulates CLH-3b activity.
Previous yeast 2-hybrid studies (Nehrke and Strange, unpublished results) suggested that several proteins, in addition to GCK-3, may interact with the CLH-3b COOH terminus. One of these proteins is a predicted scaffolding protein that may form a signaling complex with the C. elegans MAPK MPK-1 and the MAPK kinase MEK-2. Studies from numerous laboratories have shown that Ste20 kinases function in signaling pathways with MAPKs (10).
Given the absence of an apparent role for WNK in regulating CLH-3b activity, we tested the hypothesis that MAPKs function in the GCK-3 signaling pathway. The primary MAPK cascades in mammalian cells are the extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 MAPK signaling pathways (30). C. elegans MPK-1 is an ERK homolog (49). The MAP/ERK kinase (MEK) kinases function to phosphorylate and activate ERK (30, 49). To determine whether MEK/ERK signaling plays a role in regulating CLH-3b, we treated HEK cells with PD-098059 or U-0126. Both inhibitors have been reported to selectively inhibit the ERK-activating kinases (i.e., MEKs) (2, 15, 17).
HEK cells were treated with 10 μM U-0126 or 30 μM PD-098059 for 60 min before patch-clamping. As shown in Fig. 6, neither drug had a significant (P > 0.3) effect on CLH-3b current amplitude, activation voltage, or 50% rise time in cells transfected with KD GCK-3. PD-098059 also had no significant effect on CLH-3b activity in wild-type GCK-3-transfected cells (Fig. 6). In contrast, U-0126 significantly (P < 0.01) increased CLH-3b current amplitude and reduced activation voltage and rise time to values that were similar to those observed in cells lacking GCK-3 activity (Fig. 6). These results suggest that, under basal conditions, U-0126 prevents activation of GCK-3 and, hence, phosphorylation and inhibition of CLH-3b.
Fig. 6.
Effect of mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitors on basal CLH-3b activity and channel biophysical properties. Cells were exposed to 10 μM U-0126 or 30 μM PD-098059 for 60 min before whole cell current measurements were performed. Control cells were exposed to vehicle (0.1% DMSO) only. A–C: CLH-3b current-to-voltage relationships, activation voltages, and 50% rise times in cells expressing KD or wild-type GCK-3. Values are means ± SE (n = 4–6). *P < 0.05; **P < 0.01 compared with control.
We also examined the effect of volume changes on CLH-3b activity in GCK-3-transfected cells treated with U-0126 or PD-098059. Figure 7, A and B, shows the time course of changes in activation voltage and 50% rise time during cell swelling and shrinkage. Cells were swollen for 1 min in 210 mosM medium and then shrunken by exposure to 400 mosM bath solution. As expected, activation voltage and 50% rise time decreased significantly (P < 0.01) following 1 min of swelling and then rose again during shrinkage in control cells treated with vehicle (0.1% DMSO) only. U-0126-treated cells showed no significant (P > 0.3) change in these parameters during cell swelling or shrinkage. This result was also expected, given that U-0126 prevents activation of GCK-3 and, thus, results in constitutively active CLH-3b channels (Fig. 6).
Fig. 7.
Effect of MEK inhibitors on swelling- and shrinkage-induced changes in CLH-3b activity. Cells were swollen by exposure to a 200 mosM bath solution at −1 min. At time 0, cells were exposed to a 400 mosM bath solution to induce shrinkage. A and B: CLH-3b activation voltage and 50% rise time in cells expressing wild-type GCK-3. C: relative whole cell current in cells expressing KD or wild-type GCK-3. Values are means ± SE (n = 4–6). *P < 0.01 compared with control.
The results with PD-098059 were intriguing. Consistent with the apparent lack of effect of PD-098059 on GCK-3 and CLH-3b activity (Fig. 6), swelling caused significant (P < 0.05) drops in CLH-3b activation voltage and 50% rise time in cells treated with the drug (Fig. 7, A and B). The changes observed were not significantly (P > 0.3) different from those observed in control cells (Fig. 7, A and B). However, in contrast to control cells, the effects of swelling on these parameters were not reversed by cell shrinkage (Fig. 7, A and B).
U-0126 has been reported to inhibit the active (phosphorylated) and inactive forms of MEK, whereas PD-098059 binds to inactive MEK and inhibits its phosphorylation and activation by upstream kinases (2, 17). Our findings are consistent with the postulated modes of MEK inhibition by these drugs. U-0126 prevents the inhibition of CLH-3b by GCK-3 under basal conditions and during cell shrinkage (Figs. 6 and 7, A and B), presumably by inhibiting active and inactive MEK. In contrast, PD-098059 has no effect on the basal activity of CLH-3b in GCK-3-transfected cells (Fig. 6) and, instead, only inhibits changes in channel activity induced by cell shrinkage (Fig. 7, A and B). This suggests that shrinkage-induced activation of upstream signaling events that, in turn, activate GCK-3 is prevented by PD-098059 (see discussion and Fig. 9).
Fig. 9.
Working model of the GCK-3 signaling pathway that functions to regulate CLH-3b activity. GCK-3 binds to CLH-3b via a canonical Ste20 binding motif and mediates phosphorylation of 2 downstream serine residues. Phosphorylation inhibits CLH-3b activity, and GCK-3 must bind to the channel's cytoplasmic COOH terminus in order for phosphorylation to occur (14, 16). We postulate that the ERK kinase MPK-1 functions to directly or indirectly activate GCK-3. MEK-2 is a MAP kinase kinase that phosphorylates and activates MPK-1 (49). Cell shrinkage activates the pathway via unknown sensors and upstream signaling components. U-0126 inhibits active and inactive MEK-2 (2, 17) and, thereby, causes constitutive CLH-3b activation under basal conditions (Fig. 6). PD-098095 has been reported to prevent activation of MEK kinases (2, 17) and has no effect on basal CLH-3b activity but prevents cell shrinkage-induced channel inhibition (Fig. 7). In the C. elegans oocyte, major sperm protein released from sperm induces meiotic maturation via activation of a MEK-2/MPK-1 signaling cascade (37). However, as maturation proceeds, activated MPK-1 levels fall rapidly and dramatically (31). We postulate that falling MPK-1 levels result in GCK-3 inactivation, leading to dephosphorylation of CLH-3b via the type I phosphatases GSP-1 and GSP-2 (44). Subsequent channel activation functions to regulate the timing of sheath cell contractions that mediate ovulation (43).
We also examined the effects of U-0126 and PD-098059 on changes in whole cell current amplitude induced by cell volume changes. In cells transfected with CLH-3b and KD GCK-3, shrinkage caused a significant (35–40%, P < 0.04) reduction in current amplitude in control and drug-treated cells (Fig. 7C). Surprisingly, this reduction was not accompanied by changes in channel biophysical properties. Activation voltages and 50% rise times 5 min after induction of cell shrinkage were −23 ± 5, −23 ± 6, and −23 ± 4.5 mV (n = 4–5) and 11 ± 1, 13 ± 1, and 8.5 ± 0.3 ms (n = 4–5) in control cells, cells treated with U-0126, and cells treated with PD-098059, respectively. These values were not significantly (P > 0.2) different from those observed under basal conditions or 1 min after cell swelling (data not shown) and suggest that shrinkage-induced inhibition of CLH-3b is mediated by GCK-3-dependent and -independent mechanisms. GCK-3 mediates channel phosphorylation, which alters voltage-sensitive gating and renders CLH-3b considerably less sensitive to hyperpolarization than in unphosphorylated channels (14, 16) (Fig. 6). The GCK-3-independent inhibition reduces current amplitude without altering CLH-3b biophysical properties and may thus involve a reduction in the number of functional channels in the cell membrane.
Figure 7D shows volume-dependent changes in current amplitude in cells transfected with GCK-3. In control cells treated with vehicle only, cell shrinkage caused a ∼70% reduction in current amplitude compared with that observed under basal conditions. Mean relative current in cells shrunken for 5 min was ∼0.3. Cell swelling had little effect on current amplitude in U-0126-treated cells, while cell shrinkage inhibited current amplitude ∼30%. These findings are identical to those observed in cells transfected with CLH-3b and KD GCK-3 (Fig. 7C) and are consistent with the observation that U-0126 blocks GCK-3-mediated channel inhibition under basal conditions (Fig. 6).
In cells treated with PD-098059, cell swelling caused channel activation, as expected. However, cell shrinkage reduced current amplitude only ∼40% below that observed in swollen cells (Fig. 7D). Mean relative current following cell swelling was ∼1.6 and was reduced to ∼1.2 following 5 min of cell shrinkage. As shown in Fig. 7, A and B, PD-098059 blocks shrinkage-induced increases in channel activation voltage and 50% rise time. These results are consistent with the postulated inhibition by PD-098059 of upstream signaling events that activate GCK-3. The findings are also consistent with the hypothesis that cell shrinkage inhibits CLH-3b via GCK-3-dependent and -independent processes.
We performed experiments in C. elegans oocytes to determine whether MPK-1 and MEK-2 regulate CLH-3b in vivo. As discussed above, RNAi knockdown of GCK-3 in oocytes constitutively activates CLH-3b (14). If MPK-1 and MEK-2 function to activate GCK-3, knockdown of these kinases should also cause constitutive activation of CLH-3b. Figure 8 shows CLH-3b activity in oocytes isolated from worms fed control bacteria producing a scrambled dsRNA or bacteria producing MPK-1 or MEK-2 dsRNA. RNAi silencing of MPK-1 or MEK-2 caused significant (P < 0.05) increases in whole cell current (Fig. 8A) and significant (P < 0.01) decreases in CLH-3b activation voltage (Fig. 8B) and 50% rise time (Fig. 8C). These results demonstrate that MPK-1 and MEK-2 function together with GCK-3 to inhibit CLH-3b activity.
Fig. 8.
Effect of MPK-1 or MEK-2 RNAi on CLH-3b activity in C. elegans oocytes. A–C: CLH-3b current-to-voltage relationships, activation voltages, and 50% rise times in oocytes isolated from control, mpk-1(RNAi), and mek-2(RNAi) worms. Values are means ± SE (n = 4–5). *P < 0.01; **P < 0.02; ‡P < 0.03 compared with control.
DISCUSSION
Numerous studies have shown that mammalian WNK kinases function to phosphorylate and activate SPAK and OSR1, which in turn phosphorylate and regulate the activity of the volume-sensitive K-Cl and Na-K-2Cl cotransporters (12, 27). C. elegans WNK-1 physically interacts with GCK-3, and the kinases function together in a common signaling pathway to regulate whole animal osmotic balance (6). The current studies, however, failed to identify a role for WNK kinases in the GCK-3-dependent phosphorylation and inhibition of CLH-3b heterologously expressed in mammalian or Drosophila S2 cells or endogenously expressed in C. elegans oocytes (Figs. 1–5).
Results of previous yeast 2-hybrid studies (Nehrke and Strange, unpublished observations) led us to postulate that the C. elegans MEK/ERK orthologs MEK-2 and MPK-1 play a role in CLH-3b regulation. Pharmacological studies in HEK cells support this hypothesis. U-0126 inhibits the active (phosphorylated) and inactive forms of MEK (2, 17). Consistent with this mode of action, we found that U-0126 completely blocks the inhibitory effects of GCK-3 on CLH-3b current amplitude and voltage dependence under basal conditions and during cell volume changes (Figs. 6 and 7).
PD-098059 is thought to bind inactive MEK and inhibit its phosphorylation and activation by upstream kinases (2, 17). The drug has no effect on inhibition of CLH-3b by GCK-3 under basal conditions (Fig. 6), nor does it impact the effect of cell swelling on channel activity (Fig. 7), but it does inhibit GCK-3-dependent cell shrinkage-induced reductions in current amplitude and channel voltage sensitivity (Fig. 7). The simplest interpretation of these data taking into account previous findings (2, 17) is that PD-098059 inhibits activation of MEK-2 by cell shrinkage.
As we showed previously, knockdown of GCK-3 in the C. elegans oocyte activates CLH-3b (14). Similarly, we found that RNAi silencing of MEK-2 or MPK-1 in vivo constitutively activates the channel (Fig. 8). These results indicate that GCK-3, MEK-2, and MPK-1 function normally to inhibit CLH-3b, which is expressed endogenously in the worm oocyte.
MEK-2 and MPK-1 have been studied extensively in C. elegans. These kinases function together and play central roles in diverse tissue/organ developmental pathways (38, 49), control of stress response mechanisms and longevity (40, 41), whole animal fluid homeostasis (23), and regulation of germline proliferation, development, and apoptosis (3, 7, 31).
Extensive studies of the role of ERK signaling in C. elegans oocyte cell cycle progression and ovulation have been carried out by several laboratories. Briefly, adult C. elegans hermaphrodites possess two U-shaped gonad arms connected via spermatheca to a common uterus. Oocytes form in the proximal gonad and accumulate in a single-file row of graded developmental stages. Developing oocytes remain in diakinesis of prophase I until they reach the most proximal position in the gonad arm, where they undergo meiotic maturation and are then ovulated into the spermatheca for fertilization (reviewed in Refs. 20 and 24). Meiotic maturation is triggered by release of major sperm protein from sperm stored in the spermatheca (37). A signal released from the maturing oocyte induces ovulation by modulating the contractile activity of neighboring sheath and spermatheca cells (25, 36).
The levels of activated MPK-1 fluctuate throughout oocyte development. Major sperm protein is thought to activate MPK-1, which in turn induces meiotic maturation (37, 55). However, as maturation proceeds, activated MPK-1 levels fall rapidly and dramatically (31). We showed previously that endogenous CLH-3b is activated in the absence of cell swelling during oocyte meiotic maturation and that channel activation modulates the timing of sheath cell ovulatory contractions (43), which are tightly coupled to the maturation state of the oocyte (20, 24). CLH-3b activation requires channel dephosphorylation (16) mediated by the type I phosphatases GSP-1 and GSP-2 (originally termed GLC-7α/β) (44). Net CLH-3b dephosphorylation must occur via inhibition of kinase activity and/or activation of phosphatases.
Figure 9 is a working model of the putative CLH-3b regulatory signaling cascade. GCK-3 binds to a regulatory domain on the channel COOH terminus via a canonical Ste20 binding motif. The kinase mediates phosphorylation of two serine residues located 70 and 75 amino acids downstream from the binding site (16), and binding in turn is essential for phosphorylation and channel inhibition (14). While we have not yet demonstrated that GCK-3 directly phosphorylates CLH-3b, this is the most parsimonious explanation of our data (14, 16) and is consistent with studies of SPAK/OSR1 regulation of cation-coupled cotransporters (12, 27). We suggest that MPK-1 functions upstream from GCK-3 and that it directly or indirectly activates the kinase, allowing it to phosphorylate and inhibit CLH-3b. Furthermore, we postulate that the dramatic drop of activated MPK-1 levels after initiation of meiotic maturation (31) results in inactivation of GCK-3 and allows net CLH-3b dephosphorylation via GSP-1/2.
Our model is a departure from many Ste20 signaling paradigms. Ste20 kinases have largely been described as activators of MAPK signaling cascades (10). However, there are exceptions to this generalization. For example, some mammalian members of the GCK II and GCK III subfamilies phosphorylate diverse substrates and appear to function independently of MAPK signaling (35, 42). Certain human and Drosophila Ste20 kinases function as negative regulators of MAPK signaling cascades (8, 50). Recent studies in C. elegans have shown that GCK-1 is a negative regulator of MPK-1 and that the two kinases physically interact (45). Interestingly, GCK-3 contains putative ERK docking sites conforming to the R/K1–2X2–6I/L/V X I/L/V consensus (9). Clearly, extensive additional biochemical and functional studies are needed to determine if changes in ERK signaling directly modulate GCK-3 phosphorylation state and/or activity.
Numerous studies in mammalian cells have demonstrated that ERK signaling is activated by hypertonic stress. For example, hypertonicity activates ERK1/2 in nucleus pulposus cells, which in turn activates tonicity-responsive enhancer binding protein (TonEBP) (51), a transcription factor that regulates the expression of genes required for organic osmolyte accumulation (26). Hypertoncity-induced expression of monocyte chemoattractant protein-1 in rat kidney cells is regulated by ERK signaling in a TonEBP-dependent manner (28). In tracheal epithelial cells, hypertonic stress activates the Na-K-2Cl cotransporter NKCC1 via activation of ERK (34). Given the well-established role of SPAK/OSR1 in phosphorylating and activating NKCC1 in response to cell shrinkage (12, 27), it is conceivable that ERK kinases function upstream to regulate their activity in response to cell volume changes.
GCK-3 has recently been shown to be an important regulator of development and cell cycle events in C. elegans (22, 29). The Drosophila GCK-3 ortholog Fray also functions in the regulation of cell cycle and developmental events (4, 32, 54). It will be important to determine how these roles are coordinated with the well-established osmosensory and osmoregulatory functions of GCK VI kinases. In addition, it is critical to determine the upstream signaling components that modulate MEK-2/MPK-1 activity in response to cell volume changes in the C. elegans oocyte (Fig. 9) and to determine their role in other GCK-3 signaling pathways. It is interesting to note that WNK kinases have been shown to function upstream of ERKs in mammalian cells (39, 48, 53). While we could find no role for WNK kinases in the cell volume-dependent regulation of CLH-3b (Figs. 1–5), it will be important to determine whether they function together with ERKs in osmosensitive signaling cascades in mammalian cells.
In conclusion, we have demonstrated that the C. elegans STK39/SPAK ortholog GCK-3 regulates the ClC anion channel CLH-3b in a WNK-independent manner and that the kinase, instead, is regulated by ERK signaling. Mammalian WNK kinases have been proposed to be cell volume and Cl− sensors and to function upstream from and to regulate SPAK and OSR1 (12, 27). Consistent with this model, we demonstrated previously that C. elegans WNK functions together with GCK-3 to regulate whole animal volume recovery during hypertonic stress (6). These findings, taken together with the current studies, suggest a remarkable plasticity in the components that participate in GCK-3 signaling cascades. This plasticity undoubtedly allows GCK-3 to regulate physiological processes as diverse as cell cycle control (29), development (22, 29), osmotic homeostasis (6), and CLH-3b activity and ovulation (43). Molecular dissection of these signaling pathways is critical to fully understand how cells detect osmotic stress and activate appropriate response mechanisms and to fully understand the functional interactions of WNK and GCK VI kinases.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-51610 (to K. Strange) and Fellowship 5F32 DK-080576-02 (to R. A. Falin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Experiments described in this study were proposed and designed by R. A. Falin, H. Miyazaki, and K. Strange. R. A. Falin and H. Miyazaki, performed the experimental studies. All of the authors participated in the analysis and interpretation of data, writing of the manuscript, and approval of the final version of the manuscript for publication.
REFERENCES
- 1.Ahlstrom R, Yu AS. Characterization of the kinase activity of a WNK4 protein complex. Am J Physiol Renal Physiol 297: F685–F692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270: 27489–27494, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, Hay A, Golden A, Schedl T. Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc Natl Acad Sci USA 106: 4776–4781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock WG, Balloux F, Zafiropoulos PJ, Yamaguchi S, Winter S, Carthew RW, Cooper M, Jones D, Frenz L, Glover DM. Genome-wide survey of protein kinases required for cell cycle progression. Nature 432: 980–987, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chien LT, Hartzell HC. Drosophila bestrophin-1 chloride current is dually regulated by calcium and cell volume. J Gen Physiol 130: 513–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival following hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol 293: C915–C927, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development 121: 2525–2535, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett SE, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell 137: 949–960, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Costa-Junior HM, Suetsugu MJ, Krieger JE, Schechtman D. Specific modulation of protein kinase activity via small peptides. Regul Pept 153: 11–18, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol 11: 220–230, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Delpire E. The mammalian family of sterile 20p-like protein kinases. Pflügers Arch 458: 953–967, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409: 321–331, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Denton J, Nehrke K, Rutledge E, Morrison R, Strange K. Alternative splicing of N- and C-termini of a C. elegans ClC channel alters gating and sensitivity to external Cl− and H+. J Physiol 555: 97–114, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol 125: 113–125, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 92: 7686–7689, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falin R, Morrison R, Ham A, Strange K. Identification of regulatory phosphorylation sites in a Ste20 kinase regulated cell cycle- and volume-sensitive ClC anion channel. J Gen Physiol 133: 29–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998 [DOI] [PubMed] [Google Scholar]
- 17a.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol 26: 689–698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Han SM, Cottee PA, Miller MA. Sperm and oocyte communication mechanisms controlling C. elegans fertility. Dev Dyn 239: 1265–1281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, Denton J, Nehrke K, Strange K. Carboxy terminus splice variation alters ClC channel gating and extracellular cysteine reactivity. Biophys J 90: 3570–3581, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep 9: 70–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang P, Stern MJ. FGF signaling functions in the hypodermis to regulate fluid balance in C. elegans. Development 131: 2595–2604, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn 218: 2–22, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki K, McCarter J, Francis R, Schedl T. emo-1, a Caenorhabditis elegans Sec61p γ-homologue, is required for oocyte development and ovulation. J Cell Biol 134: 699–714, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon US, Kim JA, Sheen MR, Kwon HM. How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol (Oxf) 187: 241–247, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Kojima R, Taniguchi H, Tsuzuki A, Nakamura K, Sakakura Y, Ito M. Hypertonicity-induced expression of monocyte chemoattractant protein-1 through a novel cis-acting element and MAPK signaling pathways. J Immunol 184: 5253–5262, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Kupinski AP, Muller-Reichert T, Eckmann CR. The Caenorhabditis elegans Ste20 kinase, GCK-3, is essential for postembryonic developmental timing and regulates meiotic chromosome segregation. Dev Biol 344: 758–771, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol 59: 201–220, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 177: 2039–2062, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron 28: 793–806, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Hu J, Vita R, Sun B, Tabata H, Altman A. SPAK kinase is a substrate and target of PKCθ in T-cell receptor-induced AP-1 activation pathway. EMBO J 23: 1112–1122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liedtke CM, Cole TS. Activation of NKCC1 by hyperosmotic stress in human tracheal epithelial cells involves PKC-δ and ERK. Biochim Biophys Acta 1589: 77–88, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20-related kinases. Cell Signal 20: 1237–1247, 2008 [DOI] [PubMed] [Google Scholar]
- 36.McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol 205: 111–128, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 291: 2144–2147, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Moghal N, Sternberg PW. The epidermal growth factor system in Caenorhabditis elegans. Exp Cell Res 284: 150–159, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Moniz S, Verissimo F, Matos P, Brazao R, Silva E, Kotelevets L, Chastre E, Gespach C, Jordan P. Protein kinase WNK2 inhibits cell proliferation by negatively modulating the activation of MEK1/ERK1/2. Oncogene 26: 6071–6081, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Nicholas HR, Hodgkin J. The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C. elegans. Curr Biol 14: 1256–1261, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Okuyama T, Inoue H, Ookuma S, Satoh T, Kano K, Honjoh S, Hisamoto N, Matsumoto K, Nishida E. The ERK MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in C. elegans. J Biol Chem 285: 30274–30281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pombo CM, Force T, Kyriakis J, Nogueira E, Fidalgo M, Zalvide J. The GCK II and III subfamilies of the STE20 group kinases. Front Biosci 12: 850–859, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Rutledge E, Bianchi L, Christensen M, Boehmer C, Morrison R, Broslat A, Beld AM, George A, Greenstein D, Strange K. CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C. elegans oocytes. Curr Biol 11: 161–170, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Rutledge E, Denton J, Strange K. Cell cycle- and swelling-induced activation of a C. elegans ClC channel is mediated by CeGLC-7α/β phosphatases. J Cell Biol 158: 435–444, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schouest KR, Kurasawa Y, Furuta T, Hisamoto N, Matsumoto K, Schumacher JM. The germinal center kinase GCK-1 is a negative regulator of MAP kinase activation and apoptosis in the C. elegans germline. PLoS One 4: e7450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith L, Smallwood N, Altman A, Liedtke CM. PKCδ acts upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells. J Biol Chem 283: 22147–22156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strange K, Denton J, Nehrke K. Ste20-type kinases: evolutionarily conserved regulators of ion transport and cell volume. Physiology 21: 61–68, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Gao L, Yu RK, Zeng G. Down-regulation of WNK1 protein kinase in neural progenitor cells suppresses cell proliferation and migration. J Neurochem 99: 1114–1121, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Sundaram MV. RTK/Ras/MAPK signaling. WormBook 11: 1–19, 2006. 18050474 [Google Scholar]
- 50.Tassi E, Biesova Z, Di Fiore PP, Gutkind JS, Wong WT. Human JIK, a novel member of the STE20 kinase family that inhibits JNK and is negatively regulated by epidermal growth factor. J Biol Chem 274: 33287–33295, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res 22: 965–974, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Xu BE, Stippec S, Lenertz L, Lee BH, Zhang W, Lee YK, Cobb MH. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J Biol Chem 279: 7826–7831, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto Y, Izumi Y, Matsuzaki F. The GC kinase Fray and Mo25 regulate Drosophila asymmetric divisions. Biochem Biophys Res Commun 366: 212–218, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Han SM, Miller MA. MSP hormonal control of the oocyte MAP kinase cascade and reactive oxygen species signaling. Dev Biol 342: 96–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]