Abstract
Sodium absorption in the mammalian small intestine occurs predominantly by two primary pathways that include Na/H exchange (NHE3) and Na-glucose cotransport (SGLT1) on the brush border membrane (BBM) of villus cells. However, whether NHE3 and SGLT1 function together to regulate intestinal sodium absorption is unknown. Nontransformed small intestinal epithelial cells (IEC-18) were transfected with either NHE3 or SGLT1 small interfering RNAs (siRNAs) and were grown in confluent monolayers on transwell plates to measure the effects on Na absorption. Uptake studies were performed as well as molecular studies to determine the effects on NHE3 and SGLT1 activity. When IEC-18 monolayers were transfected with silencing NHE3 RNA, the cells demonstrated decreased NHE3 activity as well as decreased NHE3 mRNA and protein. However, in NHE3 siRNA-transected cells, SGLT1 activity, mRNA, and protein in the BBM were significantly increased. Thus, inhibition of NHE3 expression regulates the expression and function of SGLT1 in the BBM of intestinal epithelial cells. In addition, IEC-18 cells transected with silencing SGLT1 RNA demonstrated an inhibition of Na-dependent glucose uptake and a decrease in SGLT1 activity, mRNA, and protein levels. However, in these cells, Na/H exchange activity was significantly increased. Furthermore, NHE3 mRNA and protein levels were also increased. Therefore, the inhibition of SGLT1 expression stimulates the transcription and function of NHE3 and vice versa in the BBM of intestinal epithelial cells. Thus this study demonstrates that the major sodium absorptive pathways together function to regulate sodium absorption in epithelial cells.
Keywords: small interfering RNA, Na-glucose cotransport, Na/H exchanger 3, intestinal epithelial transport, regulation of sodium absorption
in the normal intestine, water absorption occurs secondary to electrolyte and nutrient absorption (26). Absorption of electrolytes, nutrients, and fluid by the intestine is essential for the survival of the organism. The primary electrolyte absorbed in the mammalian intestine is sodium (13). Na/H exchange (NHE3, SLC9A3) and Na-glucose cotransport (SGLT1, SLC5A1) are the two predominant pathways of sodium absorption on the brush border membrane (BBM) of villus cells in the mammalian intestine. In addition to the critical role SGLT1 plays in sodium absorption, it is also equally important for the assimilation of glucose, which is one of the most abundant nutrients in the diet (33).
Dysregulated increases in sodium absorption contributes to the development of hypertension (19). In diarrheal diseases such as cholera, the primary cause of infant mortality in developing countries, sodium absorption via NHE3 is known to be diminished (31). The most important treatment modality for these conditions, oral rehydration therapy, is based on the preservation of SGLT1 function (32). So while the importance of these two BBM transporters in health and disease is unparalleled, whether these two proteins may affect the function of each other in physiological or pathophysiological states is unknown.
In the SLC5 family of transporters, SGLT1–6 exist in mammals (32). Of these six Na-glucose cotransporters, SGLT1, SGLT4, and SGLT6 are present in the intestine. SGLT1 is the most highly expressed in the mucosa of the small intestine and transports glucose more efficiently than any of the other Na-glucose cotransporters (32, 34). SGLT1 is a 14 transmembrane protein with a substantial cytoplasmic tail which is known to be essential for proper activity (34). In the SLC9 family of proteins, nine Na/H exchanger isoforms are also known to exist (14, 35). In this family, NHE1, NHE2, and NHE3 are expressed in the epithelial cells of the intestine. They are 10–12 membrane proteins, and NHE3 and NHE2 are on the brush border of villus cells while NHE1 is present on the basolateral membrane of both villus and crypt cells (35). The majority of these Na/H exchangers are specific for sodium and proton exchange although some, such as NHE8, can transport other ions (e.g., K+). NHE3 is largely responsible for whole body sodium absorption while NHE1 is more responsible for cellular pH and volume regulation. In humans, under basal conditions, the majority of sodium absorption is believed to be mediated by NHE3 in the intestine and colon (7). Therefore, NHE3 and SGLT1 are primarily responsible for whole body sodium absorption. How NHE3 and SGLT1 may together regulate sodium absorption in the small intestine is not known.
METHODS
Cell culture.
Rat small intestinal cells (IEC-18, American Type Culture Collection), between passages 5 and 30, were routinely maintained in the laboratory in DMEM [4.5 g/l glucose, 3.7 g/l sodium bicarbonate, 2 mM l-glutamine, 10% (vol/vol) bovine fetal serum, 100 U/l human insulin, 0.25 mM β-hydroxybutyric acid, 100 U/ml penicillin, and 100 μg/ml streptomycin] in a humidified atmosphere of 10% CO2 at 37°C. The cells were fed with fresh medium every 2 to 3 days, and the cells were passaged with 0.1% trypsin-0.04% EDTA in phosphate-buffered saline (PBS).
RNA interference.
Silencer predesigned negative control (catalog no. 4611), SGLT1 (SLC5A1)-specific (ID 197575) and NHE3 (SLC9A3)-specific [small interfering RNA (siRNA) ID 200129] siRNAs (Ambion) were used for siRNA transfections. The siRNAs (1.5 μg of each) were suspended in nucleofector solution (pH 7.4, 7.1 mM ATP, 11.6 mM MgCl2.6H2O, 13.6 mM NaHCO3, 84 mM KH2PO4 and 2.1 mM glucose) and were individually nucleofected into IEC-18 cells using a Nucleofector II device (Amaxa) according to the manufacturer's recommended protocol. Approximately 200,000 cells were plated into each transwell [Millicell Hanging Cell Culture Inserts, 24 mm diameter, 1 μm pore-size polyethylene terephthalate filter supports (Millipore), placed into six-well cell culture plates]. The cells were grown for 7 days for transport studies and Western blotting analyses and 48 h for quantitative RT-PCR (RTQ-PCR) experiments.
Uptake studies in IEC-18 cells.
Uptake studies for SGLT1 were performed using [3H]-3-O-methyl-d-glucose (3-OMG; GE Healthcare Biosciences) in confluent nucleofected IEC-18 cells. Cells were incubated with Leibovitz's L-15 medium (Invitrogen) containing supplements (as described for DMEM above) for 1 h at room temperature. The cells were subsequently washed and incubated for 10 min at room temperature with 130 mM trimethylammonium chloride (TMA-Cl) in HEPES buffer (pH 7.4, 4.7 mM KCl, 1 mM MgSO4, 1.2 mM KH2PO4, 20 mM HEPES, and 1.25 mM CaCl2). Glucose uptake studies were performed by incubating the cells with 100 μM 3-OMG in 130 mM NaCl or 130 mM TMA-Cl buffer to measure the uptake in the presence and absence of sodium, respectively. Uptake was arrested by washing the cells with ice-cold HEPES buffer containing 130 mM TMA-Cl as described above. Cells were lysed in 800 μl of 1 N NaOH by incubation for 30 min at 70°C and mixed with 5 ml Ecoscint A (National Diagnostics), and the radioactivity retained by the cells was determined in a Beckman 6500 scintillation counter.
Na/H exchange activity measurements for all conditions were performed in a similar fashion using 1 mM 22Na chloride as substrate added to 130 mM TMA in HEPES buffer as described above. 5-(N-ethyl-N-isopropyl) amiloride (EIPA)-sensitive Na/H exchange was measured by deducting the Na/H exchange activity in the presence of 50 μM EIPA (Sigma-Aldrich) from the total Na/H exchange activity of the IEC-18 cells. EIPA inhibits only NHE3 since this is added only to the BBM (which would not inhibit NHE1 on the basolateral membrane, and not NHE2 either since it is not detectable in these cells).
Na-alanine uptake measurements for all conditions were performed as described above for Na-glucose cotransport, except using 200 μM [3H]-l-alanine in 130 mM NaCl or TMA-Cl in HEPES buffer.
Protein determination.
Total protein was measured by the Lowry method, using the Bio-Rad protein assay kit (Hercules, CA). BSA was used as a standard.
Isolation of total RNA and mRNA expression analysis by RTQ-PCR.
For all conditions, total RNA was isolated form IEC-18 cells using RNeasy Plus total RNA purification mini kits (Qiagen). First-strand cDNA synthesis was performed using SuperScript III (Invitrogen Life Technologies). The cDNAs synthesized were used as templates for RTQ-PCR using the TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturer's protocol. RTQ-PCR experiments for rat β-actin were performed using TaqMan Gene Expression Assay (assay ID 4352931E; Applied Biosystems). RTQ-PCR primers for rat SGLT1 and NHE3 were custom-synthesized using the oligonucleotide synthesis service provided by Applied Biosystems.
The primer and probe sequences used for rat SGLT1 RTQ-PCR were as follows: forward primer, 5′-TTGTGGAGGACAGTGGTGAA-3′; reverse primer, 5′-AAAATAGGCGTGGCAGAAGA-3′; TaqMan probe, 5′-FAM CATCAACGGCATCATCCTCCTGG TAMRA-3′.
The primer and probe sequences used for rat NHE3 RTQ-PCR were as follows: forward primer, 5′-CTCTGGGGCAGGAATTGATA-3′; reverse primer, 5′-CACCCTGGATAGGATGCTTG-3′; TaqMan probe, 5′-FAM TGTGTTCTCCCCTGACGAGGATCTG TAMRA-3′.
The expression of β-actin RTQ-PCR was run along with the SGLT1 and NHE3-specific RTQ-PCR as an endogenous control under similar conditions to normalize the expression levels of SGLT1 and NHE3 between individual samples. RTQ-PCR analyses were performed in triplicate and repeated three times using RNA isolated from three sets of IEC-18 cells.
Western blot analysis.
Western blot experiments were performed according to standard protocols, with slight modifications (3). For all conditions, IEC-18 cells were solubilized in RIPA buffer [50 mM Tris·HCl pH 7.4, 1% Igepal, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 1 mM NaF, and protease inhibitor cocktail (SAFC Biosciences)] and separated on a 4–20% Ready Gel (Bio-Rad). Separated proteins were transferred onto BioTrace PVDF Transfer Membrane (Pall) for SGLT1 Western blotting or Amersham Hybond-C Extra nitrocellulose membrane (GE Healthcare Biosciences) for NHE3 Western blotting. SGLT1 was probed by a primary rabbit polyclonal antibody raised against a synthetic peptide, corresponding to amino acids 603–623 of human SGLT1 (Abcam). NHE3 was probed by a primary chicken polyclonal antibody (Alpha Diagnostics) raised against a synthetic peptide corresponding to COOH-terminal 22 amino acid peptide of rat NHE3. Appropriate horseradish peroxidase-conjugated secondary antibodies were used to monitor the binding of the primary antibodies. ECL Western Blotting Detection Reagent (GE Healthcare) was used to detect the immobilized SGLT1 and NHE3 by chemiluminescence. The intensity of the bands was quantitated using a densitometric scanner (Molecular Dynamics).
Immunocytochemistry studies.
Transfected IEC-18 cells were grown on a coverslip for 7 days and fixed in 4% (vol/vol) paraformaldehyde for 20 min. Antigen retrieval was performed by incubating the cells with 0.5% Triton X-100 (Sigma-Aldrich) in PBS for 2 min at room temperature. Nonspecific binding sites in the tissue sections were blocked by incubation with 2% fetal bovine serum in PBS for 30 min. The cells were then incubated overnight with 1:500 diluted goat anti-rat SGLT1 (Santa Cruz Biotechnology) and 1:250 diluted chicken anti-rat NHE3 (Alpha Diagnostic) primary polyclonal antibodies. Tissue sections were washed with PBS to remove excess antibodies and were incubated with either Alexa Fluor 555 rabbit anti-goat IgG or Alexa Fluor 488 goat anti-chicken IgG (Invitrogen) to detect SGLT1 and NHE3, respectively. Excess secondary antibodies were removed by washing with PBS, and the tissue sections were mounted using ProLong Gold Antifade Reagent (Invitrogen). Finally, the fluorescence signals generated were observed under a Zeiss LSM510 confocal microscope, and image files of the mucosal surface were acquired. In addition, multiple image stacks (4 μm) of the xz plane of the apical side were photographed by Zeiss LSM image software. Densitometric analyses of the xz plane were performed using MacBiophotonics ImageJ software to compare the expression of SGLT1 and NHE3 in different conditions (6).
Data presentation.
When data were averaged, means ± SE are shown, except when error bars are inclusive within the symbol. All uptakes and RTQ-PCR were done in triplicate unless otherwise specified. The number (n) for any set of experiments refers to uptake experiments, total RNA, or protein extracts from different sets of cells. Student's t-test was used for statistical analysis.
RESULTS
Effect of silencing NHE3 on SGLT1.
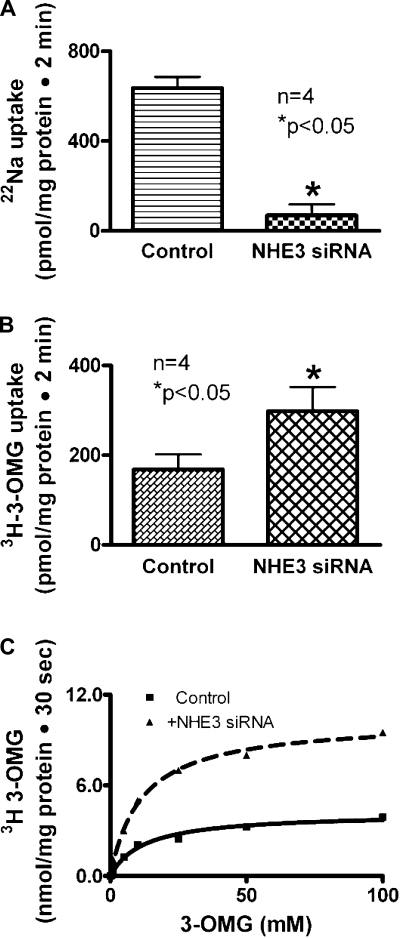
Na-glucose cotransport activity (SGLT1) was measured as Na-dependent glucose uptake. Na/H exchange activity (NHE3) was measured as proton-dependent EIPA-sensitive Na uptake. To determine whether the inhibition of NHE3 expression regulates SGLT1 activity, IEC-18 cells were transfected with NHE3 siRNA. In these cells, NHE3 activity was significantly reduced (Fig. 1A). However, SGLT1 activity was markedly increased (Fig. 1B). Thus these data indicated that inhibition of NHE3 expression stimulates SGLT1 activity in intestinal epithelial cells.
Fig. 1.
Effect of Na/H exchanger 3 (NHE3) silencing on Na-glucose cotransporter 1 (SGLT1) in small intestinal epithelial cells (IEC-18). A: in NHE3-silenced cells, NHE3 activity was significantly reduced compared with IEC-18 cells transfected with negative control small interfering RNA (siRNA). B: However, in NHE3-silenced cells, SGLT1 activity was increased significantly compared with IEC-18 cells transfected with negative control siRNA. Thus, silencing NHE3 stimulates SGLT1 activity. C: kinetics of SGLT1 stimulation of Na-dependent [3H]-3-O-methyl-d-glucose (OMG) uptake is shown as a function of varying concentrations of extracellular glucose in NHE3-silenced cells. Uptake for all concentrations was determined at 30 s. A representative kinetics plot shows that as the concentration of extracellular glucose was increased, uptake of glucose was stimulated and subsequently became saturated in IEC-18 cells in the negative control siRNA and NHE3 siRNA-transfected cells. Analysis of the data provided kinetic parameters. The affinity for glucose was not affected in NHE3 siRNA-treated cells (Km was 12.4 ± 2.3 mM in control and 11.6 ± 1.3 in NHE3 siRNA-transfected cells; n = 5). However, the Vmax or maximal rate of uptake of glucose was increased in cells transfected with NHE3 siRNA (Vmax was 4.2 ± 0.2 nmol/mg protein for 30 s in control and 10.3 ± 0.3 in NHE3 siRNA-transfected cells, P < 0.01, n = 5). Thus, the mechanism of stimulation of SGLT1 when NHE3 is silenced is due to increased transporter numbers.
Effect of silencing NHE3 on alanine, serine, cysteine transporter 1.
To determine whether inhibition of NHE3 selectively regulates SGLT1 rather than just any Na-nutrient cotransport process in the BBM of IEC-18 cells, we investigated Na-alanine cotransport that is mediated by alanine, serine, cysteine transporter 1 (ASCT1; 25). In NHE3 siRNA-transfected IEC-18 cells, alanine transport was unaffected (Fig. 3 of Ref. 25; Na-dependent alanine uptake was 5.9 ± 0.8 nmol·mg protein−1·2 min−1 in control and 6.1 ± 0.3 in NHE3 siRNA-transfected cells, n = 4). These data indicated that stimulation of SGLT1 by NHE3 siRNA may be specific for SGLT1.
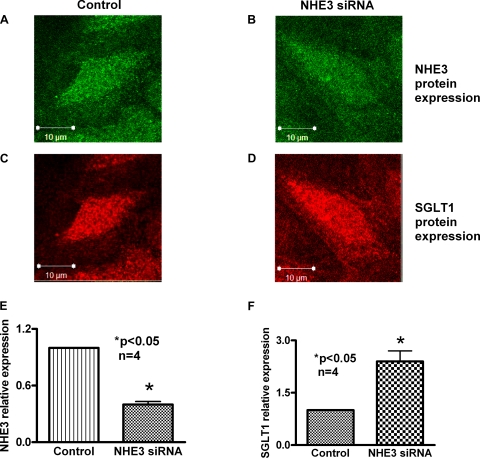
Fig. 3.
Immunocytochemical analysis of the effect of NHE3 silencing on SGLT1 protein in IEC-18 cells. IEC-18 cells transfected with negative control or NHE3 siRNA were subjected to immunocytochemical analysis using NHE3 and SGLT1-specific primary antibodies. A and C: top views of IEC-18 cells treated with negative control siRNA expressing NHE3 (A) and SGLT1 (C). B and D: top views of IEC-18 cells expressing NHE3 (B) and SGLT1 (D) after transfection with NHE3 siRNA. E and F: quantitative data of the fluorescence intensities of negative control and NHE3 siRNA-transfected cells (n = 4, *P < 0.05). These data demonstrated that siRNA-mediated reduction in NHE3 in IEC-18 cells enhances SGLT1 expression.
SGLT1 kinetic studies in NHE3 siRNA-transfected cells.
To determine the mechanism of regulation of SGLT1 by NHE3 silencing, kinetic studies were performed. In NHE3 siRNA- transfected cells, Na-dependent glucose uptake was stimulated and subsequently became saturated as the extracellular concentration of glucose was increased (Fig. 1C). The affinity for glucose was not significantly altered in NHE3 siRNA-treated cells (Km was 12.4 ± 2.3 mM in control and 11.6 ± 1.3 in NHE3 siRNA-transfected cells; n = 5). However, the Vmax or maximal rate of uptake of glucose was significantly increased in cells transfected with NHE3 siRNA (Vmax was 4.2 ± 0.2 nmol·mg protein−1·30 s−1 in control and 10.3 ± 0.3 in NHE3 siRNA-transfected cells; P < 0.01, n = 5). These studies indicated that the mechanism of stimulation of SGLT1 activity by NHE3 siRNA transfection in IEC-18 cells was due to an increase in the number of cotransporters rather than an alteration in the affinity of the cotransporter for glucose.
NHE3 and SGLT1 mRNA expression in IEC-18 cells transfected with NHE3 siRNA.
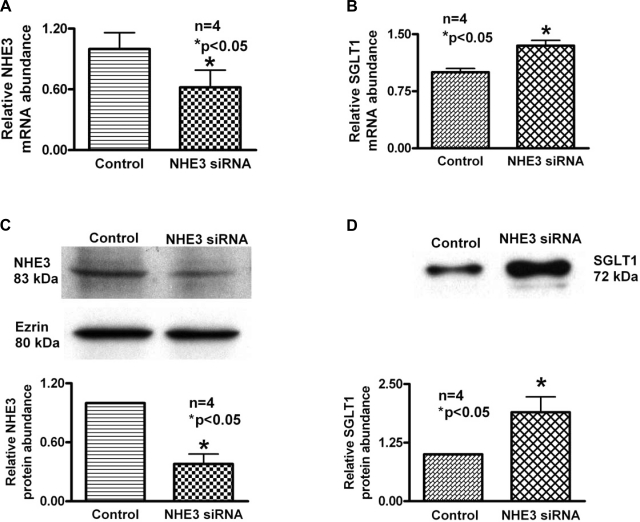
To determine the molecular mechanism of stimulation of SGLT1 by NHE3 siRNA in IEC-18 cells, mRNA levels were determined by RTQ-PCR. Transfection of IEC-18 cells with NHE3 siRNA decreased NHE3 mRNA levels in these cells (Fig. 2A). However, in these cells, SGLT1 mRNA levels increased significantly (Fig. 2B). These data indicated that inhibition of NHE3 with NHE3 siRNA results in the stimulation of SGLT1 activity due to an increase in the message for SGLT1. Thus, inhibition of NHE3 expression appears to regulate the expression of SGLT1 at the transcriptional level in IEC-18 cells.
Fig. 2.
Effect of NHE3 silencing on SGLT1 message and protein expression in IEC-18 cells. Quantitative RT-PCR (RTQ-PCR) analysis of NHE3 and SGLT1 in NHE3 siRNA-transfected IEC-18 cells is shown. A: as expected, in NHE3-silenced cells, NHE3 message was significantly reduced compared with control IEC-18 cells transfected with negative control siRNA. B: however, in NHE3-silenced cells, SGLT1 message was increased significantly compared with control IEC-18 cells. Thus, silencing NHE3 stimulates SGLT1 mRNA levels. C: in NHE3-silenced cells, NHE3 protein was significantly reduced compared with IEC-18 cells transfected with negative control siRNA. D: however, in NHE3-silenced cells, SGLT1 protein was increased significantly compared with IEC-18 cells transfected with negative control siRNA. Ezrin control is for both blots. Thus, silencing NHE3 stimulates SGLT1 at the protein level.
NHE3 and SGLT1 protein expression in IEC-18 cells transfected with NHE3 siRNA.
Since mRNA levels do not necessarily correlate with functional protein levels on the BBM, to further decipher the molecular mechanism of regulation of SGLT1 by NHE3 in IEC-18 cells Western blot studies were performed. In IEC-18 cells transfected with the NHE3 siRNA, NHE3 protein levels were decreased significantly in the BBM (Fig. 2C). However, in these cells SGLT1 protein levels were increased significantly (Fig. 2D). These data in conjunction with the kinetic studies and the mRNA RTQ-PCR studies clearly indicates that when NHE3 expression is inhibited in IEC-18 cells, it stimulates SGLT1 by increasing the synthesis and the number of SGLT1 transporters in the BBM.
Immunocytochemistry of NHE3 silencing on SGLT1 expression.
To demonstrate the effect of NHE3 siRNA at the cellular level, immunocytochemistry experiments were performed using NHE3 and SGLT1-specific primary antibodies. As seen in Fig. 3, after transfection of NHE3 siRNA, NHE3 protein is decreased (compare Fig. 3B with Fig. 3A) while SGLT1 protein levels are increased (compare Fig. 3D with Fig. 3C). To more accurately measure BBM protein expression levels of both NHE3 and SGLT1, side view images (xz projections) were generated (data not shown) and the fluorescence intensity of the proteins of several tissues was measured. The fluorescent intensity in the negative control siRNA-transfected cells was given an arbitrary value of 1, and the intensities obtained after NHE3 siRNA transfection were plotted and compared. NHE3 flourescence decreased significantly (Fig. 3E) while SGLT1 protein fluorescence increased significantly (Fig. 3F). Thus, these data demonstrated that siRNA-mediated reduction in NHE3 in IEC-18 cells enhances SGLT1 expression on the BBM.
Effect of silencing SGLT1 on NHE3.
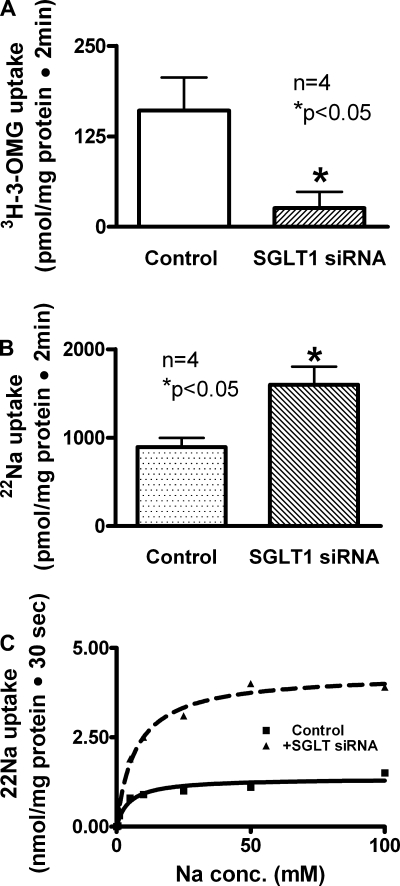
To determine whether the inhibition of SGLT1 expression regulates NHE3, Na/H exchange activity was measured as proton-dependent EIPA-sensitive Na uptake. In SGLT1 siRNA-transfected IEC-18 cells, SGLT1 was significantly reduced (Fig. 4A). On transwell plates, only NHE3 is present on the BBM while NHE2 expression is undetectable (data not shown). In cells transfected with SGLT1 siRNA, NHE3 activity was markedly increased (Fig. 4B). These data indicated that inhibition of SGLT1 stimulates NHE3 activity in intestinal epithelial cells.
Fig. 4.
Effect of SGLT1 silencing on NHE3 in IEC-18 cells. A: in SGLT1-silenced cells, SGLT1 activity was significantly reduced compared with IEC-18 cells transfected with negative control siRNA. B: however, in SGLT1-silenced cells, NHE3 activity was increased significantly compared with IEC-18 cells transfected with negative control siRNA. Thus, silencing SGLT1 stimulates NHE3 activity. C: a representative kinetics plot of NHE3 stimulation of EIPA-sensitive proton-dependent 22Na uptake is shown as a function of varying concentrations of extracellular sodium in SGLT1-silenced cells. Uptake for all concentrations was determined at 30 s. As the concentration of extracellular sodium was increased, uptake of sodium was stimulated and subsequently became saturated in IEC-18 cells in the negative control siRNA and SGLT1 siRNA-transfected cells. Analysis of the data provided kinetic parameters. The affinity for sodium was not affected statistically by SGLT1 siRNA transfection (Km was 4.70 ± 0.3 mM in control and 7.20 ± 0.1 in SGLT1 siRNA-transfected cells;, n = 4). However, the Vmax or maximal rate of uptake of sodium was significantly increased in cells transfected with SGLT1 siRNA (Vmax was 1.4 ± 0.1 nmol·mg protein−1·30 s−1 in control and 4.2 ± 0.1 in SGLT1 siRNA-transfected cells; P < 0.01, n = 4). Thus, the mechanism of stimulation of NHE3 when SGLT1 is silenced is due to increased transporter numbers.
NHE3 kinetic studies in SGLT1 siRNA-transfected cells.
To determine the mechanism of regulation of NHE3 by the inhibition of SGLT1 expression, kinetic studies were performed. In SGLT1 siRNA-transfected IEC-18 cells, the uptake of proton-dependent EIPA-sensitive Na uptake was stimulated and subsequently became saturated as the extracellular concentration of Na was increased (Fig. 4C). The affinity for sodium was not altered significantly by SGLT1 siRNA transfection (Km was 4.70 ± 0.3 mM in control and 7.20 ± 0.1 in SGLT1 siRNA-transfected cells; n = 4). However, the Vmax or maximal rate of uptake of sodium was significantly increased in cells transfected with SGLT1 siRNA (Vmax was 1.4 ± 0.1 nmol·mg protein−1·30 s−1 in control and 4.2 ± 0.1 in SGLT1 siRNA-transfected cells; P < 0.01, n = 4). These studies indicated that the mechanism of NHE3 stimulation by SGLT1 siRNA transfection in IEC-18 cells was the result of an increase in NHE3 exchanger expression in the BBM rather than an alteration in the affinity of the exchangers for sodium.
NHE3 and SGLT1 mRNA expression in IEC-18 cells transfected with NHE3 siRNA.
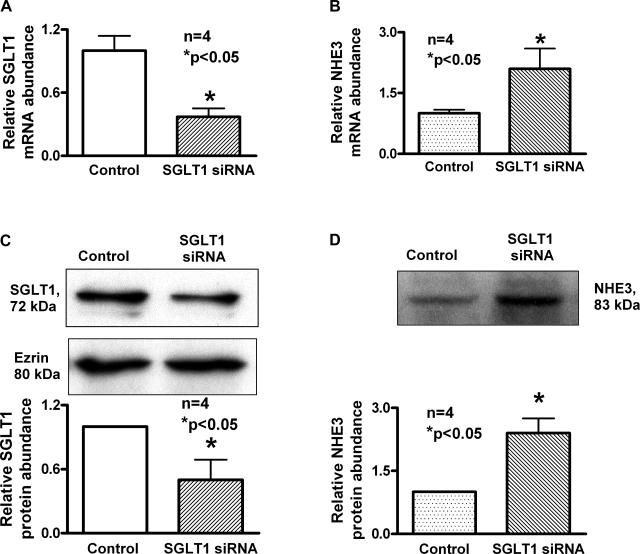
To determine the molecular mechanism of stimulation of NHE3 activity by SGLT1 siRNA transfection in IEC-18 cells, mRNA levels were determined by RTQ-PCR. Transfection of IEC-18 cells with SGLT1 siRNA decreased SGLT1 mRNA levels (Fig. 5A). However, in these cells, NHE3 mRNA increased significantly (Fig. 5B). These data indicated that inhibition of SGLT1 with siRNA results in the stimulation of NHE3 activity due to an increase in the message for NHE3. Thus, the inhibition of SGLT1 expression stimulates NHE3 at the transcriptional level in IEC-18 cells.
Fig. 5.
Effect of SGLT1 silencing on NHE3 message and protein expression in IEC-18 cells. RTQ-PCR analysis of NHE3 and SGLT1 in SGLT1 siRNA-transfected IEC-18 cells is shown. A: as expected, in SGLT1-silenced cells, SGLT1 message was significantly reduced compared with control IEC-18 cells transfected with negative control siRNA. B: however, in SGLT1-silenced cells, NHE3 message was increased significantly compared with control IEC-18 cells. Thus, silencing SGLT1 stimulates NHE3 mRNA levels. C: in SGLT1-silenced cells, SGLT1 protein was significantly reduced compared with IEC-18 cells transfected with negative control siRNA. D: however, in SGLT1-silenced cells, NHE3 protein was increased significantly compared with IEC-18 cells transfected with negative control siRNA. A representive blot for each condition and densitometric analysis of four blots are given. Ezrin control is for both blots. Thus, silencing NHE3 stimulates SGLT1 at the protein level.
SGLT1 on NHE3 protein expression in IEC-18 cells transfected with SGLT1 siRNA.
Since mRNA levels do not necessarily correlate with functional protein levels in epithelial cells, Western blot analysis was performed. In IEC-18 cells transfected with the SGLT1 siRNA, SGLT1 protein levels were decreased (Fig. 5C). However, in these cells, NHE3 protein levels were increased significantly (Fig. 5D). These data in conjunction with the kinetic and RTQ-PCR studies clearly indicate that when SGLT1 expression is inhibited in IEC-18 cells it stimulates NHE3 activity by increasing the expression of NHE3 transporters in epithelial cells.
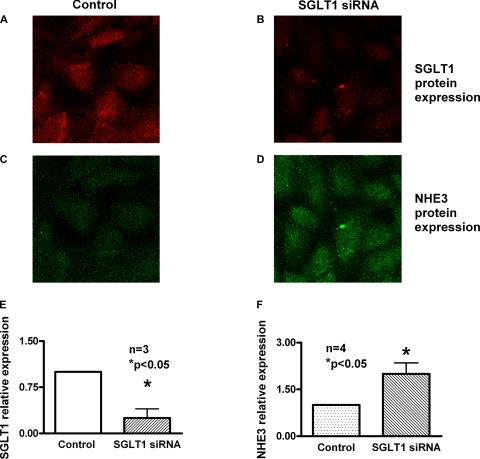
Immunocytochemistry of SGLT1 silencing on NHE3 expression.
To determine whether there are changes in the brush border expression of NHE3 protein, immunocytochemistry experiments of IEC-18 cells were performed (Fig. 6). With the transfection of SGLT1 siRNA, the levels of SGLT1 expression on the BBM decreased (Fig. 6B) compared with control (Fig. 6A). However, in these cells, BBM NHE3 expression is increased (Fig. 6D) compared with control cells (Fig. 6C). To accurately quantitate BBM expression of SGLT1 and NHE3, an xz projection of the cells was generated (data not shown). The fluorescence intensity in negative control siRNA-treated cells was given an arbitrary value of 1. The intensities measured after transfection of SGLT1 siRNA into IEC18 cells show that SGLT1-specific fluorescence decreased (Fig. 6E) while NHE3-specific fluorescence increased (Fig. 6F). These results indicated a significant reduction in BBM SGLT1 expression after transfection with SGLT1 siRNA while it enhances BBM NHE3 expression.
Fig. 6.
Immunocytochemical analysis of the effect of SGLT1 silencing on NHE3 protein in IEC-18 cells. IEC-18 cells transfected with negative control or SGLT1 siRNA were subjected to immunocytochemical analysis using NHE3 and SGLT1-specific primary antibodies. A and C: top views of IEC-18 cells treated with negative control siRNA expressing SGLT1 (A) and NHE3 (C). B and D: top views of IEC-18 cells expressing SGLT1 (B) and NHE3 (D) after transfection with SGLT1 siRNA. E and F: quantitative data of the fluorescence intensities of negative control and SGLT1 siRNA-transfected cells (n = 4, *P < 0.05). These data demonstrated that siRNA-mediated reduction in NHE3 in IEC-18 cells enhances SGLT1 expression.
DISCUSSION
Because sodium is one of the primary electrolytes absorbed, its assimilation is critical for the maintenance of health. When sodium homeostasis is disrupted, water balance is also affected, contributing to hypertension and diseases that promote diarrhea. In the mammalian intestine the predominant sodium absorptive pathways are Na/H exchange and Na-glucose cotransport found on the BBM of villus cells (13, 31, 32). Na/H exchange in conjunction with Cl/HCO3 exchange is the means by which coupled NaCl absorption occurs in the mammalian small intestine (23).
In view of the significance of these two transport proteins in mammalian intestinal physiology, many studies have been conducted involving their regulation in health and in disease (21, 32). However, no study has been undertaken to determine whether the expression and activity of these two transporters found on the BBM of the villus cells function together to regulate sodium absorption. This study for the first time demonstrates that sodium absorption is regulated by the primary absorptive pathways in epithelial cells.
Intestinal mucosal electrolyte transport proteins have been shown to affect one another. It has been suggested that HCO3 secretion by intestinal crypt cells may occur as a result of stimulation of the Na/H exchange on the basolateral membrane which then alkalinizes the cell, resulting in the stimulation of BBM Cl/HCO3 exchange (22). It has also been demonstrated that coupled NaCl absorption occurs in the BBM of intestinal villus cells via the dual operation of Na/H and Cl/HCO3 exchange and that this coupling is mediated by intracellular pH (23). In support of this hypothesis, in the NHE3 knockout mouse the expression of a Cl/HCO3 exchanger (downregulated in adenoma, DRA) is upregulated (18), whereas in the DRA knockout mouse, NHE3 is upregulated in the colon (20). Others have speculated that inhibition of BBM Na/H exchange may increase bicarbonate secretion by activating anion exchangers in the BBM (7). The cystic fibrosis transmembrane regulator (CFTR) and Cl/HCO3 exchangers have also been shown to regulate one another (10, 15–17, 30) as well as indirectly increasing Na/H exchange activity by altering cell volume (8).
Na-nutrient cotransporters are also affected by other transporter proteins. For example, Na-K-ATPase on the basolateral membrane of villus cells, by maintaining low steady-state intracellular sodium levels, provides the favorable electrochemical gradient necessary for the functioning of BBM Na-solute cotransport processes including SGLT1 (24). Studies using diabetic patients have also shown a link of SGLT1 to glucose transporter 2 (GLUT2) in intestinal villus cells and that SGLT1 function stimulates the expression of GLUT2 in the BBM of intestinal epithelia. Indirect linkage between NHE3 and SGLT1 mediated by intracellular sodium was also postulated as a result of the in vivo studies to inhibit constitutive nitric oxide production in the rabbit small intestine (4, 5).
There have been some reports of nutrient transporters affecting electrolyte transport. It has been reported that PEPT1, a proton-dependent dipeptide transporter, is linked to Na/H exchange in mammalian intestinal epithelial cells to regulate cellular pH (1, 2, 11, 12, 28) as well as the activation of other proton-solute cotransporter processes to stimulate Na/H exchange in intestinal epithelial cells (27). Interestingly, colon cancer cells (Caco-2) transfected with SGLT1 immediately upon activation have been suggested to stimulate Na/H exchange. Such activation alkalinized these cells and would have been expected to inhibit, not stimulate, Na/H exchange (29). This type of regulation is also a very short-term interaction between the two transporters unlike the more stable clinical condition of this study.
Analogous to the NHE3 siRNA-transfected IEC-18 cells, mouse models of NHE3 knockout exist and have been shown to have impaired Na absorption. However, unlike in IEC-18 cells where the compensation appears to be mediated by SGLT1, in mice the compensation appears to be mediated by an amiloride-sensitive mechanism, likely NHE2 (9). This isoform of Na/H exchange is not present in IEC-18 cells. Nevertheless, with this compensation it would have been interesting to see whether the compensatory change in SGLT1 was present. However, in that article, the mucosal incubation media specifically had no glucose, thus removing its effects on Na transport. Therefore, the effect of NHE3 knockout on SGLT1 compensation if any was not known. Since there are no SGLT1 knockouts, likely owing to the fact that this may be a lethal knockout, our findings in vitro with SGLT1 siRNA-transfected IEC-18 cells cannot be correlated with in vivo studies.
However, in none of these studies has it been shown that sodium absorption is regulated by the two primary BBM sodium absorptive pathways in what may be a compensatory fashion in intestinal epithelial cells. Thus, while existing evidence suggests that some transporters do affect one another, there are currently no reports of major villus BBM sodium-absorbing transporters affecting one another to presumably maintain sodium homeostasis. In this present study, inhibition of NHE3 expression with NHE3 siRNA stimulated SGLT1 activity. Kinetic studies demonstrated that the mechanism of stimulation of SGLT1 was secondary to an increase in Vmax without a change in the affinity of the transporter for sodium (Fig. 1C). Molecular studies using RTQ-PCR showed that the mechanism of stimulation was secondary to an increase in SGLT1 BBM expression as reflected by an increase in the message for SGLT1 (Fig. 2B). Finally, Western blot and immunocytochemistry studies indicated that the mechanism of stimulation of SGLT1 when NHE3 expression is inhibited in intestinal epithelial cells is due to an increase in the expression of SGLT1 on the BBM (Fig. 2, C and D>, and Fig. 3). This clearly indicates that inhibition of NHE3 expression regulates SGLT1 in intestinal epithelial cells.
In addition, inhibition of SGLT1 expression with SGLT1 siRNA also stimulated NHE3 activity. Kinetic studies established that, in SGLT1 siRNA-transfected cells, the mechanism of stimulation of Na/H exchange was the result of an increase in the NHE3 expression on the BBM (Fig. 4B). RTQ-PCR studies showed that the stimulation of NHE3 is due to an increase in message (Figs. 5B). Western blot studies showed that the mechanism of stimulation of the increase in NHE3 expression when SGLT1 expression is inhibited in intestinal epithelial cells is due to an increase in the number of exchangers in the cells (Fig. 5, C and D). Finally, results of immunocytochemistry experiments showed that when SGLT1 BBM expression is inhibited, NHE3 expression on the BBM of IEC-18 cells is stimulated (Fig. 6). These studies together show that sodium absorption is regulated and that when SGLT1 expression is inhibited, NHE3 is stimulated. Therefore all of this data taken together shows that when the expression of either of these two transporters is inhibited the expression of the other increases on the BBM of intestinal villus cells, presumably to maintain the proper absorption of sodium. This likely occurs by transcriptional regulation of these transporters by transcription factors and second messenger pathways.
In conclusion, this unique regulation of the two primary sodium absorptive pathways could provide valuable insights into how electrolyte and water balance is regulated. It is important to note that sodium dysregulation leads to changes in water balance as well, which leads to diseases such as hypertension and diarrhea. Alterations in glucose absorption may also contribute to diseases such as diabetes and obesity. Therefore a better understanding of how these two major sodium pathways regulate sodium and glucose absorption may lead to better efficacious treatments for these diseases. For example, oral rehydration therapy itself is a treatment that supplies sodium and glucose in order for SGLT1 to compensate in diarrheal diseases such as cholera where NHE3 is significantly inhibited (32). Thus this unique regulation of the two primary sodium absorptive pathways could form the basis for developing strategies to promote electrolyte and nutrient absorption when necessary and inhibit the same in diseases where it would be advantageous.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK45062 and DK58034 (to U. Sundaram).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge Palanikumar Manoharan, Jamil Talukder, and Subha Arthur for involvement in a few aspects of this project as part of postdoctoral education and training in the lab, albeit no data generated by them are included in this article.
REFERENCES
- 1. Anderson CM, Mendoza ME, Kennedy DJ, Raldua D, Thwaites DT. Inhibition of intestinal dipeptide transport by the neuropeptide VIP is an anti-absorptive effect via the VPAC1 receptor in a human enterocyte-like cell line (Caco-2). Br J Pharmacol 138: 564–573, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson CM, Thwaites DT. Regulation of intestinal hPepT1 (SLC15A1) activity by phosphodiesterase inhibitors is via inhibition of NHE3 (SLC9A3). Biochim Biophys Acta 1768: 1822–1829, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Stuhl K. Current Protocols in Molecular Biology. New York: Wiley, 1995 [Google Scholar]
- 4. Coon S, Kim J, Shao G, Sundaram U. Na-glucose and Na-neutral amino acid cotransport are uniquely regulated by constitutive nitric oxide in rabbit small intestinal villus cells. Am J Physiol Gastrointest Liver Physiol 289: G1030–G1035, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Coon S, Shao G, Wisel S, Vulaupalli R, Sundaram U. Mechanism of regulation of rabbit intestinal villus cell brush border membrane Na/H exchange by nitric oxide. Am J Physiol Gastrointest Liver Physiol 292: G475–G481, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Di Iorio E, Barbaro V, Ferrari S, Ortolani C, De Luca M, Pellegrini G. Q-FIHC: quantification of fluorescence immunohistochemistry to analyse p63 isoforms and cell cycle phases in human limbal stem cells. Microsc Res Tech 69: 983–991, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Furukawa O, Luke CB, Guth PH, Engel E, Hirokawa M, Kaunitz JD. NHE3 inhibition activates duodenal bicarbonate secretion in the rat. Am J Physiol Gastrointest Liver Physiol 286: G102–G109, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clark LL. cAMP inhibition of murine intestinal Na+/H+ exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125: 1148–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281: G1301–G1308, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflügers Arch 445: 139–146, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Kennedy DJ, Raldua D, Thwaites DT. Dual modes of 5-(N-ethyl-N-isopropyl)amiloride modulation of apical dipeptide uptake in the human small intestinal epithelial cell line Caco-2. Cell Mol Life Sci 62: 1621–1631, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiela PR, Ghishan FK. Na+-H+ exchange in the mammalian digestive tract. In: Physiology of the Gastrointestinal Tract (4th ed.) New York: Elseiver, 2006, p. 1847–1880 [Google Scholar]
- 14. Kiela PR, Xu H, Ghishan FK. Apical Na+/H+ exchangers in the mammalian gastrointestinal tract. J Physiol Pharmacol 57, Suppl 7: 51–79, 2006 [PubMed] [Google Scholar]
- 15. Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO(3)(-) transport in cystic fibrosis. EMBO J 21: 5662–5672, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamprecht G, Heil A, Baisch S, Lin-Wu E, Yun CC, Kalbacher H, Gregor M, Seidler U. The down regulated in adenoma (dra) gene product binds to the second PDZ domain of the NHE3 kinase A regulatory protein (E3KARP), potentially linking intestinal Cl−/HCO3− exchange to Na+/H+ exchange. Biochemistry 41: 12336–12342, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(−)/HCO(3)(−) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J Biol Chem 274: 22855–22861, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Orlov SN, Mongin AA. Salt-sensing mechanisms in blood pressure regulation and hypertention. Am J Physiol Heart Circ Physiol 293: H2039–H2053, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Seidler U, Lenzen H, Cinar A, Tessema T, Bleich A, Riederer B. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann NY Acad Sci 1072: 262–275, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Sundaram U, Knickelbein RG, Dobbins JW. Mechanism of intestinal secretion. Effect of serotonin on rabbit ileal crypt and villus cells. J Clin Invest 87: 743–746, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sundaram U, Knickelbein RG, Dobbins JW. pH regulation in ileum: Na(+)-H+ and Cl(−)-HCO3− exchange in isolated crypt and villus cells. Am J Physiol Gastrointest Liver Physiol 260: G440–G449, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Sundaram U, Wisel S, Rajendren VM, West AB. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am J Physiol Gastrointest Liver Physiol 273: G913–G919, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Talukder JR, Kekuda R, Saha P, Sundaram U. Mechanism of leukotriene D4 inhibition of Na-alanine cotransport in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G1–G6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thiagarajah JR, Verkman AS. Water transport in the gastrointestinal tract. In: Physiology of the Gastrointestinal Tract (4th ed.). New York: Elsevier, 2006, p. 877–894 [Google Scholar]
- 27. Thwaites DT, Ford D, Glanville M, Simmons NL. H+/solute induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest 104: 629–635, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thwaites DT, Kennedy DJ, Raldua D, Anderson CM, Mendoza ME, Bladen CL, Simmons NL. H/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na/H exchanger. Gastroenterology 122: 1322–1333, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na+-glucose cotransport in intestinal epithelia. Am J Physiol Cell Physiol 281: C1533–C1541, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Wheat VJ, Shumaker H, Burnham C, Shull GE, Yankaskas JR, Soleimani M. CFTR induces the expression of DRA along with Cl−/HCO3− exchange activity in tracheal epithelial cells. Am J Physiol Cell Physiol 279: C62–C71, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Wright EM, Hirayama B, Loo D, Turk E, Hager K. Intestinal sugar transport. In: Physiology of the Gastrointestinal Tract. New York: Raven, 1994, p. 1751–1772 [Google Scholar]
- 32. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 261: 32–43, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Wright EM, Loo D, Hirayama B, Turk E. Sugar absorption. In: Physiology of the Gastrointestinal Tract (4th ed.). New York: Elsevier, 2006, p. 1653–1666 [Google Scholar]
- 34. Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology (Bethesda) 19: 370–376, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995 [DOI] [PubMed] [Google Scholar]