Summary
Malignantly transformed cells can express aberrant cell surface glycosylation patterns, which serve to distinguish them from normal cells. This phenotype provides an opportunity for the development of carbohydrate-based vaccines for cancer immunotherapy. Synthetic carbohydrate-based vaccines, properly introduced through vaccination of a subject with a suitable construct, should be recognized by the immune system. Antibodies induced against these carbohydrate antigens could then participate in the eradication of carbohydrate-displaying tumor cells. Advances in carbohydrate synthetic capabilities have allowed us to efficiently prepare a range of complex, synthetic anticancer vaccine candidates. We describe herein the progression of our longstanding carbohydrate-based anticancer vaccine program, which is now at the threshold of clinical evaluation in several contexts. Our carbohydrate-based anticancer vaccine program has evolved through a number of stages: (1) monomeric vaccines; (2) monomeric clustered vaccines; (3) unimolecular multi-antigenic vaccines; (4) dual acting vaccines. This account will focus on our recently developed unimolecular multi-antigenic constructs and potential dual-acting constructs, which contain clusters of both carbohydrate and peptide epitopes.
Keywords: carbohydrate antigens, anticancer vaccines, glycopeptides, glycoconjugates, oligosaccharides, mucin, peptide epitope, antibodies
Introduction
Carbohydrates compose one of the major classes of biological molecules, along with DNA, RNA, proteins, and lipids, and are essential for life [1]. Functionally, carbohydrates can serve as intermediates in cellular energy production, as signaling molecules, or as a wide variety of structural components [2]. These structural roles are critical to the construction of complex multicellular organs and organisms, and to the interaction of the individual cells with one another and with the extracellular matrix. Indeed, all cell types and many macromolecules (e.g. proteins, lipids) in Nature carry a dense and often complex array of covalently attached oligosaccharides. When these structures are expressed on the outer surface of the cell, or on secreted macromolecules, they are well positioned to modulate the cell-cell and cell-matrix interactions crucial to the development and function of complex multicellular organisms. Extracellular carbohydrate expression can also mediate the interactions between disparate organisms (e.g. parasite and host). Aside from these extracellular roles, glycosylated macromolecules – typically glycoproteins – are abundant in the cytoplasm as well as the nucleus, and appear to function as regulatory switches.
It has been known for several decades that glycosylation patterns on cell surfaces rapidly change during mammalian embryogenesis and cell activation [3]. These shifting patterns are known to affect a wide variety of biological processes, including: cellular adhesion and differentiation, receptor activation, and tissue morphogenesis. Indeed, the initial event in the life of every sexually reproducing metazoan (i.e. the act of fertilization of a single egg by a single sperm) involves a specific carbohydrate-receptor relationship [4,5]. The very process of fertilization requires the recognition of the glycoprotein ZP3 on the zona pellucida by the lectin-like protein sp56 localized on the head of the sperm, although the exact protein involved is still debated [6,7].
Following fertilization, the resulting one-cell embryo must make its way to the uterus and then implant itself on the uterine epithelial lining. Along the way, the embryo divides several times and ultimately ends up as a roughly 64-cell blastocyst. During this time, a number of stage-specific embryonic antigens (SSEAs) are present [8]. The function of these SSEAs is not fully understood; however, it is known that they are carbohydrate-containing structures that can exist as both glycoproteins and as glycolipids. It is also known that their expression patterns change significantly during early embryogenesis. For example, SSEA-1, also known as the Lewis X (Lex) tetrasaccharide, is transiently expressed on an 8-cell embryo but is no longer found at the 32- to 64-cell stage. Members of the globo series of glycolipids, including SSEA-3 and 4, are also expressed during the early stages of embryogenesis and subsequently lost around the same time as Lex, only to reappear further on in development [9,10].
While the elucidation of the exact role of the oligosaccharides remains an as yet unmet challenge to modern cell biology, it has been postulated that they may regulate the macromolecular interactions that determine how the cells respond to the surrounding microenvironment, including cell migration and/or differentiation. An important example of this occurs in the developing fetal nervous system, where an everchanging array of gangliosides can be found [11]. Studies have shown that there is a specific gradient of the gangliosides GM1, GM2, and 9-O-acetyl GD3 along the retinal-tectum system of vertebrates. The acylation of GD3 appears to be regulated independently from the overall expression of GD3, since GD3 itself does not show any regional specificity along the same system. Moreover, the expression of 9-O-acetyl GD3 is highest in areas of maximal cell migration [12], although it is likely that other glycoconjugates play a role in this as well [13]. Altogether, the disparity in expression patterns implies that the carbohydrates themselves may modulate cellular and axonal guidance during embryonic neurogenesis.
In the developing lung, the variant expression of several different carbohydrate-containing structures has also been observed. For instance, the manifestation of individual Lewis antigens changes dramatically depending on the stage of development [14]. In the 38-day-old embryo, the appearance of the lung buds is accompanied by the expression of only the Lewis Y (Ley) antigen. More specifically, Ley is found on the proliferating cells in the terminal portion of the lung buds. In the 50 to 53-day-old embryo, during the time when the future bronchi are developing, Ley expression is at its peak and Lex slowly begins to appear. At 12 weeks, the cuboidal epithelial cells that line the buds for the future bronchioles are highly expressive of Lex, while Ley expression is minimal. At 18 weeks, the expression of both Lex and Ley begins to disappear and sialylated Lex (sLex) expression begins in cells of the terminal buds of the future alveoli. By 20 weeks, sLex is only weakly expressed in the terminal buds and by 8 months, all three antigens are undetectable in respiratory cells. The Lewis antigens are normally not found in the adult lung, however they are known to reappear on several human cancer cell lines including lung cancer, suggesting that the carcinoma is a result of the cells reverting to the stage of an immature embryonic lung cell.
It is not surprising then, that a universal feature of malignancy and tumor progression in cancer is the marked change in cellular glycosylation patterns and reemergence of fetal antigens [15]. Also, given the fact that different antigens are expressed on specific developing tissues during embryogenesis, it is reasonable to think that the same would be true in cancer. Indeed, it has been found that the specific type of antigen displayed is often associated with a particular cancer type. In fact, the degree of antigen expression can be diagnostic of the disease’s progression, and may presage the ultimate outcome of treatment [16,17].
One of the longstanding objectives of modern cancer research is to harness the power of the immune system to enable the self-recognition and destruction of tumor cells [18,19]. However, many tumor-bearing hosts are unable to recognize malignant cells, indicating that the antigens alone are insufficiently immunogenic. Given the inherent difficulties in developing new vaccines in general, complicated by the challenges associated with cancer immunotherapy (i.e. many of the targets are self-antigens, vide infra), the realization of an effective antitumor vaccine becomes an intimidating endeavor. Fortunately Nature has provided clues suggesting that such an enterprise may ultimately be successful [20].
From an immunological point of view, a number of important issues must be considered if carbohydrate antigens are to be used as targets in active immunotherapy. First, the onset of cancer is often accompanied by an apparent immunosuppression, resulting in the inability of the immune system to adequately recognize cancer cells as foreign. This is exacerbated by the fact that cancer cells are known to inhibit the process of complement lysis and that many tumor antigens are auto- or self-antigens. Furthermore, those antigens that could potentially elicit a response are typically surrounded by auto- or self-antigens, resulting in a heterogenous mixture of carbohydrates on the cell surface. Additionally, carbohydrates have been classified as T-cell-independent antigens, usually generating a humoral response resulting in the production of short-lived B-cell-mediated immunoglobulins (IgM antibodies) that have poor memory and lack the support typically provided by T-cells. However, several accounts have detailed experiments showing that carbohydrate-based vaccines are able to elicit a T-cell response in non-carcinoma related immunity [21,22]. Furthermore, multiple recent studies have established that tumor-associated carbohydrate antigen (TACA)-presenting glycopeptides are able to elicit a conventional class I MHC-restricted CD8+ T-cell response [23,24]. Therefore it is not unreasonable to believe that, while the carbohydrates themselves may not be particularly immunogenic, their conjugation to an appropriate carrier may well overcome this problem [25].
In addition to these biological obstacles, one must consider the overall availability of such antigens. At the present time, isolation of carbohydrate antigens from natural sources is difficult at best, often yielding insignificant quantities of material. Furthermore, given the immense obstacles associated with their purification, acquisition of the homogeneous materials necessary to launch a clinical program is virtually impossible. Consequently, the onus (or perhaps opportunity) falls on the synthetic organic chemist to solve both the availability and purity issues if a successful clinical program is to proceed. Along this avenue, many adaptations in the composition of both the antigen and carrier become possible. With this in mind, our laboratory initiated a program ca. 20 years ago, devoted to the development of methodologies for the de novo chemical synthesis of carbohydrates and glycopeptides [26].
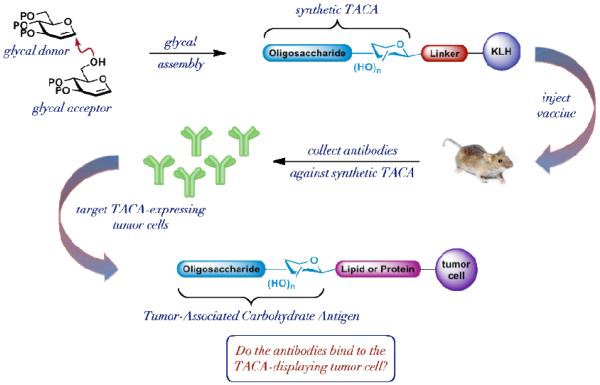
Noting the propensity of malignantly transformed tumor cells to exhibit aberrant glycosylation patterns on their surface (vide supra), and that the tumor-associated carbohydrate antigen (TACA) is covalently bound to cell surface lipids or glycoproteins via N-β or O-α linkages at the carbohydrate’s reducing end, we sought to design a system that would pave the way for reaching this natural array. Our approach to the synthesis and subsequent evaluation of carbohydrate-based antitumor vaccines is broadly outlined in Figure 1. In order to more closely mimic the natural characteristics of the tumor cell’s surface while maximizing the immunogenicity of the vaccine, we envisioned a construct consisting of one or more synthetic TACAs, prepared using the process of glycal assembly [27], presented along a linker molecule, which would ultimately be conjugated to an immunogenic carrier protein such as keyhole-limpet hemocyanin (KLH). The vaccine construct would then be injected into mice with the hope that an antibody response would be elicited. The antibodies would then be harvested and evaluated in vitro to determine their propensity for binding the TACA-displaying tumor cell.
Figure 1.
General strategy for the production of carbohydrate-based antitumor vaccines. (TACA: Tumor-Associated Carbohydrate Antigen)
In a previous account of our work in this journal, we summarized our progress toward the development of carbohydrate-based anticancer vaccines [28]. In this review, we will place particular emphasis on our recently developed unimolecular multi-antigenic constructs and dual acting constructs, containing clusters of both carbohydrate and peptide epitopes.
Monomeric Vaccines
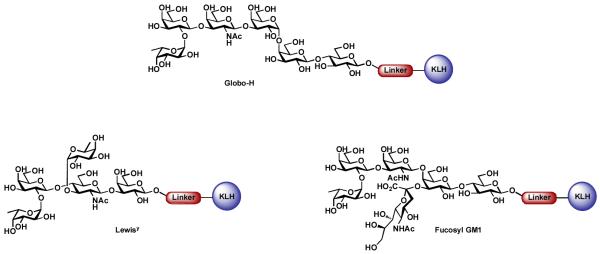
Our first-generation carbohydrate-based anticancer vaccines were monomeric, and consisted of a single carbohydrate antigen conjugated to an immunogenic carrier protein, such as keyhole limpet hemocyanin (KLH). Globo-H ceramide (MBr1 antigen), a hexasaccharide antigen first isolated from the human breast cancer cell line MCF-7 [29,30], was selected as our initial target. Subsequent to our initial interest in this antigen, it was later found to be over-expressed on the surfaces of a number of other cancer cell lines, including colon, lung, ovary, and prostate [31]. Through recourse to the highly convergent glycal assembly methodology developed in our group, we have been able to prepare sufficient quantities of this carbohydrate antigen and its corresponding KLH conjugate (Figure 2) for preliminary immunological evaluations in animal models [32,33,34].
Figure 2.
First generation monomeric carbohydrate-based vaccines.
Immunological evaluations conducted in mice revealed that, when administered in conjunction with a suitable adjuvant (i.e. QS-21), our synthetic Globo-H-KLH conjugate produced high titer IgM and IgG antibody responses to the Globo-H antigen [35]. Importantly, these antibodies were able to distinguish Globo-H positive MCF-7 cells from Globo-H negative B78.2 melanoma cells, and were able to induce complement-mediated lysis in the MCF-7 cells.
On the basis of these and other preclinical studies, we advanced Globo-H into a Phase I clinical trial against prostate cancer, with the results serving to establish a safety profile and dose response curve for the vaccine [36, 37]. The vaccine’s immunogenicity profile was confirmed across a range of cancer stages and tumor burden levels, as each patient exhibited good IgM responses against Globo-H. In fact, the level of antibody response appeared to be somewhat independent of the cancer stage, as antibody titers collected from patients with relatively advanced prostate cancer were nearly as high as those obtained from patients who exhibited only modest elevations in prostate specific antigen (PSA). Importantly, the results from our preclinical studies were confirmed as the IgM antibodies were specific to Globo-H, reacted only with Globo-H positive tumor cells, as evidenced by flow cytometry analysis, and induced complement-mediated lysis in Globo-H expressing cell lines. A subsequent Phase I trial performed in metastatic breast cancer patients yielded similarly promising results. Indeed, at the present time, plans are underway for launching a pivotal Phase II/Phase III trial [38].
While the Globo-H KLH conjugate was our first synthetic vaccine entered into clinical trials, we have since prepared a variety of monomeric vaccine conjugates against various cancer types, including monomeric Fucosyl GM1 KLH [39, 40] and Ley KLH conjugates [41].
Monomeric Clustered Vaccines
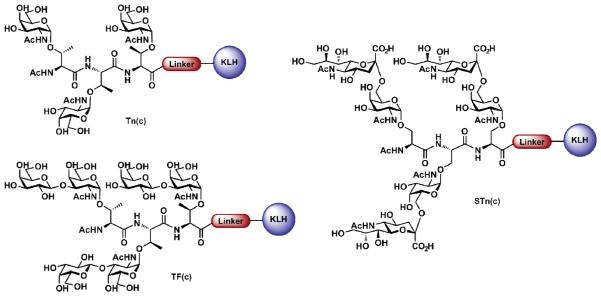
In the process of refining our vaccine structures, we next sought to prepare constructs that would more closely mimic the architecture of the tumor cell surface. Noting that mucins, a family of glycoproteins overexpressed on tumor cell surfaces, often present clusters of two to five adjacent carbohydrates domains [42], we designed a second generation of monomeric anticancer vaccines containing repeats of the relatively smaller mono- and disaccharide antigens typical of the mucins (Figure 3).
Figure 3.
Monomeric clustered carbohydrate-based vaccines.
As part of this program, we developed the methodology necessary to gain access to a building block common to the mucin family of carbohydrate antigens. Termed the cassette method of glycopeptide assembly, an orthogonally protected galactosamine (GalNAc) residue is stereospecifically α-linked to either serine or threonine, resulting in a “cassette” that can be further glycosylated, depending on the antigen required [43]. The cassette method has been implemented in the preparation of several trimeric clustered vaccine constructs, including Tn(c) [44,45,46], Tf(c) [44], STn(c) [47], 2,6-STF(c) [48], and Ley(c) [49]. Biological studies with these constructs determined that the clustered antigens were able to stimulate a more robust immune response in comparison with their non-clustered congeners. Many such constructs are currently being evaluated in preclinical and clinical trials.
Polyvalent monomeric vaccines
While the monovalent vaccines discussed above are promising and are advancing through clinical trials, they do not account for the distinct multiplicity of carbohydrate antigens expressed on many cancer cell types. As such, we speculated that the combination of several carbohydrate antigens closely associated with a particular cancer type might induce a stronger immune response and increase the chance of tumor cells being targeted. Two approaches to this strategy may be envisioned: the polyvalent monomeric approach and the unimolecular multivalent approach (vide infra).
In the polyvalent monomeric approach, a mixture of several monomeric KLH conjugates is utilized in the anticipation that an antibody response to each individual antigen will be induced. In one such study, four monomeric vaccines (GD3 KLH, Ley KLH, and the mucins MUC1 KLH and MUC2 KLH) were co-administered to mice along with QS-21 adjuvant [50]. For comparative purposes, each monomeric KLH conjugate was separately administered to a control group of mice. In each case, IgG and IgM antibody levels for each antigen were determined by ELISA analysis, and there was essentially no difference in antibody production regardless of whether the antigen was administered alone or in a polyvalent context. In another study we employed a heptavalent monomeric KLH conjugate involving seven antigens (GM2, Globo-H, Ley, TF(c), Tn(c), STn(c), and glycosylated MUC1) and found similar results [51]. In this case, the antibodies reacted with both purified synthetic antigens (ELISA analysis) and with naturally expressed antigens on the cancer cell surface (FACS analysis).
With very promising preclinical results in hand, a hexavalent monomeric KLH conjugates was prepared including GM2, Globo-H, Ley, glycosylated MUC-1-32 mer, Tn(c) and TF(c). The conjugate was co-administered with QS-21 to 30 high-risk prostate cancer patients in a Phase II clinical setting [52]. All 30 patients had significant elevations in antibody titers for at least two of the six antigens, and 22 patients had increased reactivity with FACS. This hexavalent vaccine of synthetic “self” antigens broke immunologic tolerance against two or more antigens in all 30 vaccinated patients and was safe, but antibody titers against several of the antigens were lower than those seen in individual monovalent trials. Another trial using a heptavalent monomeric vaccine consisting of GM2, Globo-H, Ley, Tn(c), STn(c), TF(c), Tn-MUC1, with co-administration of QS-21, was performed in eleven patients with epithelial ovarian, fallopian tube, or peritoneal cancer in second or greater complete clinical remission [53]. All patients were included in the safety analysis, and 9 of 11 patients remained on study for at least 2 weeks past the fourth vaccination and were included in the immunologic analysis (two withdrew due to disease progression). The vaccine was well tolerated, no clinically relevant hematologic abnormalities were noted and no clinical, or laboratory evidence of autoimmunity was seen. Eight of nine patients developed responses against at least three antigens. FACS and complement-dependent cytotoxicity analysis showed substantially increased reactivity against MCF7 cells in seven of nine patients, with some increase seen in all patients. This heptavalent-KLH conjugate plus QS-21 vaccine safely induced antibody responses against five of seven antigens.
While the polyvalent monomeric approach appears to be successful, it does carry some potential problems: 1) the approach necessitates the use of substantially increased levels of carrier protein which can lead to a drop in the immunogenicity of the carbohydrate antigen [54,55,56]; 2) the synthesis of multiple monomeric KLH conjugates involves multiple low-yielding and hard-to-reproduce bio-conjugation reactions; 3) the polyvalent monomeric strategy requires the nontrivial regulatory validation of each individual component of the vaccine mixture.
Unimolecular multivalent vaccines
After careful consideration of the drawbacks in the polyvalent monomeric vaccine approach, we designed a perhaps more attractive alternative: a vaccine structure that contains various carbohydrate antigens incorporated into a single peptide backbone. The synthesis of this unimolecular multivalent vaccine structure would only necessitate a single, presumably low-yielding bio-conjugation, rather than one for each component and could simplify regulatory approval. Moreover, this consolidation may also serve as an avenue to minimize the potential for adverse immune suppression caused by the carrier protein [57].
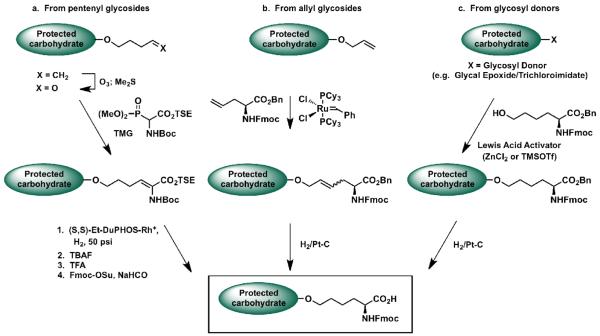
The primary building block for this vaccine type utilizes a non-natural amino acid linker instead of the native serine or threonine linkage at the reducing end of the carbohydrate antigen. This non-natural linker was chosen because: 1) it could avoid problems associated with the instability of O-glycosyl serine/threonine [58]; 2) it appears that suitable glycopeptide mimics can simulate the activity of their native counterparts. In the case of enzyme inhibitors, unnatural linkages can even result in better therapeutic agents [59, 60]. Therefore, it would not be unreasonable to anticipate that synthetic compounds approximating the natural linkage may prove to be more immunogenic, presumably because of the higher likelihood that they would be recognized as “nonself” by the immune system [61]; 3) the anomeric n-pentenyl glycoside linkage has served as an efficient linker for immunoconjugation to the carrier protein KLH and has also provided some advantages in terms of synthetic convergency [62,63].
We have thus far developed three protocols to access these glycosyl amino acids bearing non-natural amino acid linkers (Figure 4). The first method (a) relies on the previously reported allyl glycoside ozonolysis protocol and commences with the more stable n-pentenyl glycoside [64]. Ozonolysis of the terminal olefin followed by Horner-Emmons reaction yields the dehydroamino acid. Enantioselective reduction of the olefin, followed by standard functional group manipulations, affords the requisite glycosylamino acid cassette. A liability of this method lies in the fact that the α-stereogenic center of the amino acid must be installed through an enantioselective reduction subsequent to coupling of the carbohydrate fragment. The second method (b) commences with the appropriately protected allyl glycoside [65] or protected n-pentenyl glycoside [66,67]. This intermediate is subjected to olefin cross-metathesis with an orthogonally protected allyl glycine in the presence of a ruthenium catalyst. Reduction of the resultant olefin with concurrent benzyl ester cleavage provides the glycosylamino acid cassette. Since optically pure allyl glycine is commercially available, this method does not require the installation of a stereogenic center and is suitable for the preparation of glycosyl amino acids bearing large carbohydrate moiety such as Gb3 [66,67], GM2 [68], and Globo-H [69]. Finally, we have developed a protocol (c) that allows for the amino acid functionality to be directly introduced using either a glycal epoxide [70] or trichloroacetimidate as a glycosyl donor [71] and hydroxynorleucine as the glycosyl acceptor in the presence of Lewis acid. Although the efficiency of the protocol is limited by the moderate stereoselectivity of the glycal epoxidation in this particular case and by the need to synthesize hydroxynorleucine, it is suitable for the preparation of glycosyl amino acids bearing relatively small carbohydrate moieties.
Figure 4.
Protocols used for the preparation of the glycosylamino acids.
1. Unimolecular trivalent vaccines
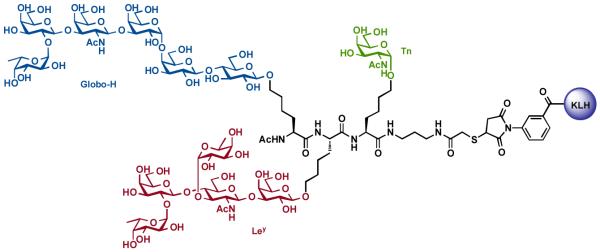
With all of the requisite methods developed, we initially prepared a unimolecular trivalent vaccine structure that contained three known tumor-associated carbohydrate antigens: Globo-H, Ley and Tn (Figure 5) [64]. Subsequent biological evaluation in mice revealed that the unimolecular trivalent vaccine indeed induced antibodies against each of the three component antigens (ELISA analysis). In addition, the antibody titer was shown to react well with the MCF-7 breast cancer cell line [72].
Figure 5.
Protocols used for the preparation of the glycosylamino acids.
2. Unimolecular pentavalent vaccines containing five antigens including Globo-H, Ley, STn, TF and Tn [73]
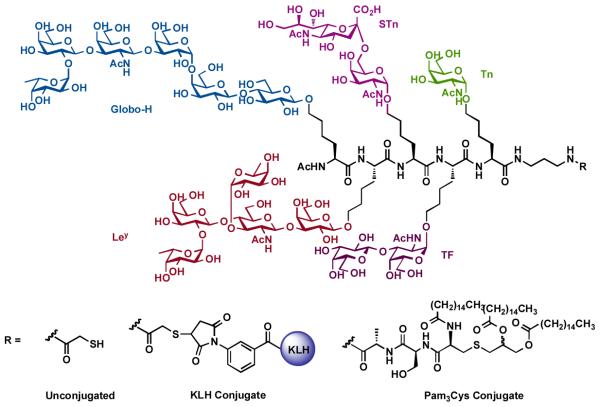
After having demonstrated the potential to stimulate a multifaceted immune response necessary for optimal targeting of the heterogenous population of cells associated with a particular cancer type, we probed further by preparing the rather complex first-generation unimolecular pentavalent construct, which contains five different prostate and breast cancer associated carbohydrate antigens, including Globo-H, Ley, STn, TF and Tn (Figure 6). Synthesis of the unimolecular pentavalent construct was accomplished according to our cassette method of glycopeptide assembly, from relatively small Tn to highly complex Globo-H antigen in order to increase synthetic efficiency. This construct was next subjected to the preparation of its corresponding KLH conjugate and Pam3Cys conjugate as synthetic vaccines.
Figure 6.
First generation unimolecular pentavalent vaccine construct containing five carbohydrate antigens: Globo-H, Ley, STn, TF, and Tn.
Preliminary biological evaluation in mice indicated that KLH conjugate was successful in inducing antibodies against all of the carbohydrate antigens, with the exception of Ley [74]. The disappointing immunogenicity observed with the Ley antigen most likely arises from the fact that it is endogenously expressed at a relatively high level. FACS analysis indicated that the antibodies induced by this first-generation unimolecular pentavalent vaccine reacted significantly with the three cell lines evaluated, which each express high levels of two or more of the corresponding antigens.
3. Second-generation unimolecular pentavalent vaccines and hexavalent vaccines
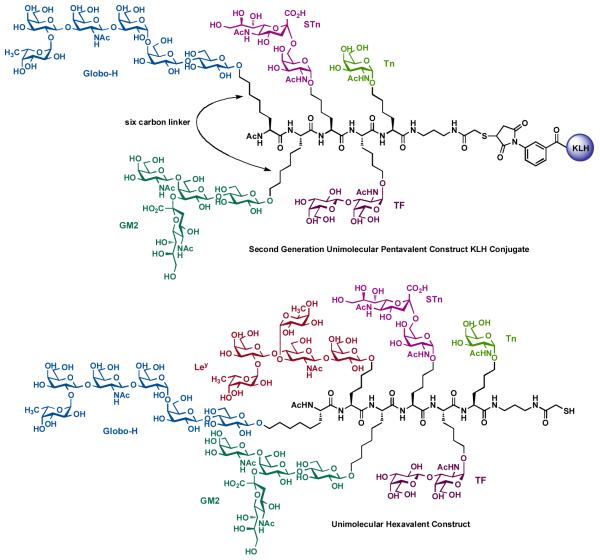
The results of biological studies with this unimolecular pentavalent vaccine led us to pursue the synthesis of a second-generation unimolecular pentavalent construct as well as a unimolecular hexavalent construct against prostate and breast cancer (Figure 7) [74]. In this particular construct, the previously used pentasaccharide Ley antigen was replaced with the prostate and breast cancer-associated tetrasaccharide antigen GM2 [75,76]. GM2 was selected for inclusion on the basis of reports indicating that antibodies induced through this antigen are active against human GM2-positive cells. Moreover, human clinical trials conducted with GM2 alone have established a correlation between elevated GM2 antibody levels and survival. Synthesis of this unimolecular pentavalent construct was accomplished in analogy to the first generation construct. In particular, for synthetic efficiency improvement we elected to extend the distance between the Globo-H and GM2 residues and the peptide backbone through the use of a six-carbon linker. The preparation of the unimolecular hexavalent glycopeptide (Figure 7) followed the general glycopeptide assemble protocol for the synthesis of these type of constructs. Recently, covalent conjugation of the unimolecular pentavalent construct to carrier protein KLH has been carried out to prepare the corresponding UPC-KLH conjugate [77,78]. Bio-conjugation of the unimolecular hexavalent construct to carrier protein is currently under investigation.
Figure 7.
Second generation of unimolecular pentavalent vaccine construct containing Globo-H, GM2, STn, TF and Tn.
Vaccination of the unimolecular pentavalent construct KLH conjugate in mice did produce high titers of corresponding IgG and IgM antibodies against all five antigens through ELISA studies. Further flow cytometry analysis (FACS) confirmed that these antibodies could strongly bind to cancer cells, such as MCF-7 breast cancer cell line that expresses all five antigens displayed on the pentavalent construct. This unimolecular pentavalent construct KLH conjugate will enter the phase I trial at Memorial Sloan-Kettering Cancer Center in the near future.
Dual acting vaccines
As previously described, the unimolecular clustered and multiantigenic vaccine constructs typically contain a number of carbohydrate domains presented along a peptide backbone. In the further refinement and progression of our vaccine design, we began to evaluate the incorporation of antigenic peptidyl markers into the peptide backbone with the hope of eliciting a B- and/or T-cell response. Again, following the lead of the mucin family of O-linked glycoproteins, we have designed a new type of antitumor vaccine construct featuring both a carbohydrate-based antigen and a mucin-derived peptide-based marker in an alternating pattern (Figure 8). This type of design seeks to mimic the molecular architecture of the tumor cell’s surface, thus provoking a more realistic and effective immune response. With these clustered carbohydrate–peptide constructs, incorporation of either repeats of the same carbohydrate antigen or a combination of diverse carbohydrate antigens are possible and the array could be modified to suit a particular carcinoma. We envision that this type of vaccine construct has three potential advantages: 1) a mucin derived peptide marker may act not only as a B-cell epitope for the production of antibodies against mucins, but also as a T-helper cell epitope to activate T-cells; 2) the tandem repeats of both carbohydrate and peptide are anticipated to maximally expose these B-cell and T-helper cell epitopes on the surface of the carrier protein. Hopefully, this feature will fall in line with our previous immunogenic studies demonstrating that clustered monomeric antigenic peptides are able to elicit a substantial IgG and IgM antibody response; 3) vaccines composed of numerous carbohydrate antigens associated with a specific cancer type may provide a heightened and more varied antibody response, thereby increasing the efficiency of target cell binding [67]. In this regard, success in the design and synthesis of such constructs would pave the way for the preparation of more complex vaccine structures that are better able to mimic the “bioarchitecture” of natural cell surfaces.
Figure 8.
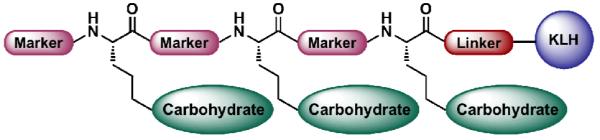
A proposal for a novel carbohydrate–peptide based vaccine.
As a proof of principle, we designed a vaccine structure targeting ovarian cancer which contains alternating repeats of the Gb3 antigen (globotriaosyl ceramide) [79] and MUC5AC peptide marker [80] (Figure 9). Structurally, MUC5AC consist of tandem repeats of an 8-amino acid sequence (TTSTTSAP), which are potentially responsible for the activation of T cells. Since Gb3 naturally exists as a ceramide, we decided to prepare the Gb3 construct using the nonnatural extended hydroxynorleucine linker in the hopes of mimicking the ceramide chain. Synthesis of this dual acting construct was accomplished by standard peptide ligation methods. In particular, a Gb3-MUC5AC thioester was utilized as a key building block for efficient preparation of this glycopeptide construct. Final covalent conjugation of this construct to KLH provided the synthetic vaccine [67]. Immunological evaluation of this KLH conjugate in mice is currently in progress and its results will be disclosed in due course.
Figure 9.
A clustered Gb3-MUC5AC construct KLH conjugate targeting ovarian cancer.
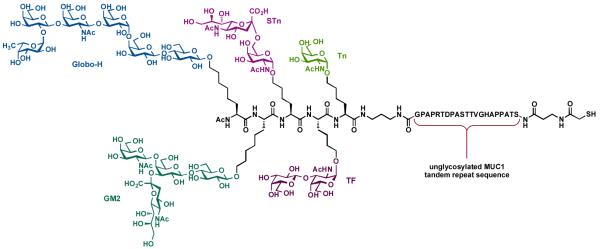
In addition, it was noted that the over-expression of MUC1, a human tumor-associated epithelial mucin, is correlated with the progression of breast [81], ovarian [82], and colon [83] cancer. Structurally, MUC1 contains repeating units of a 20-amino acid sequence (HGVTSAPDTRPAPGSTAPPA) in the extracellular portion of this glycoprotein [84]. Both animal studies and clinical trials have shown MUC1 to be capable of inducing a T-helper type I response [85]. Based on these observations and the promising biological properties of the second-generation unimolecular pentavalent vaccine, we prepared a hybrid vaccine construct containing a unimolecular pentavalent glycopeptide domain covalently linked to the MUC1 peptide (Figure 10) [86]. The preparation of the KLH conjugate of this construct is currently underway.
Figure 10.
A complex unimolecular pentavalent MUC1 glycopeptide construct for prostate and breast cancer.
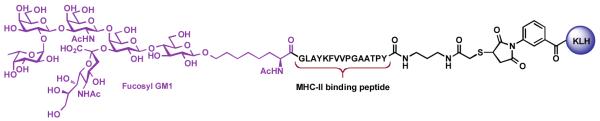
Along the same lines, we considered the possibility of using the HLA-DR binding peptide sequence GLAYKFVVPGAATPY, derived from Plasmodium falciparum, as a T-cell epitope [87]. Given the success of our recent fucosyl GM1-based vaccine in the treatment of small cell lung cancer (SCLC) [88], we designed a new vaccine that combines the specificity of GM1 for SCLC [89] with the potentially T-cell activating HLA-DR peptide sequence (Figure 11). This construct has been conjugated to KLH and we have recently begun immunological studies.
Figure 11.
A bidomainal fucosyl GM1-based vaccine for the treatment of small cell lung cancer.
Conclusions
When we first began our synthetic carbohydrate-based antitumor vaccine program over two decades ago, our major efforts were focused on the development of doable synthetic methods in the synthesis of carbohydrate antigens. During the past years, we have discovered efficient glycal assembly methods for synthesis of complex oligosaccharide antigens as well as corresponding glycosyl amino acids. These substantial achievements have allowed us to provide a number of highly complex carbohydrate-based anticancer vaccine constructs in very convergent manner. From the first-generation monomeric version to the most recent developed dual acting constructs, all these fully synthesized vaccines have been or are being evaluated in preclinical and clinical immunological settings in close collaboration with immunologists and oncology based clinicians. The resulting promising biological data have provided very valuable information in our continuous refinement of the design of antitumor vaccine constructs.
Expert commentary
A number of carbohydrate-based antitumor vaccines have been efficiently prepared using the power of chemical synthesis and, upon immunological evaluation, have demonstrated promising biological activities. In particular, we have recently developed several prominent unimolecular multivalent constructs. The promising preclinical data of unimolecular multivalent vaccines have resulted in the advancement into Phase I clinical trials and also brought us hope in the development of more robust and clinically useful antitumor vaccines.
Development of vaccines that closely mimic the nature of tumor cell surface architecture and efficiently activate T cells, as we believe, would be critical for this program to be ultimately successful, because these two key elements would help elicit substantial antibodies that strongly react with natural cancer cells. Towards this direction, dual acting vaccine constructs and their KLH conjugate bearing tumor associated mucin-derived peptidyl factors have been prepared and are currently being evaluated in a preclinical setting. On the other hand, optimization of the structure of adjuvants, such as QS-21, is in progress at MSKCC. If successful, it may greatly help the production of substantial antibodies for elimination of micrometastatic cancer cells.
Five-year view
Through chemical synthesis, preclinical and clinical evaluation, our efforts so far have demonstrated that synthetic carbohydrate-based anticancer vaccines, in the presence of appropriate adjuvant, were able to activate immune system and elicit corresponding antibodies against these carbohydrate antigens when administered into either animals or humans. Currently, representative monomeric vaccines, monomeric clustered vaccines, and polyvalent monomeric vaccines are being evaluated in clinical trials, and will hopefully advance into Phase II/III trials in the near future. In addition, based on the promising preclinical data, a second-generation unimolecular pentavalent vaccine is scheduled to enter a Phase I clinical trial at Memorial Sloan-Kettering Cancer Center. In the next few years, the immunological results from such Phase I studies will provide valuable information in the structure and reactivity correlation, and hopefully this superior vaccine will enter the Phase II trials. Moreover, preclinical evaluation of the latest dual acting vaccines will be accomplished and hopefully will bring us more insight into the development of more promising cancer vaccines. The authors would expect some of them to be further studied in Phase I clinical trials. It is anticipated that biologically experimental outcome will further direct us to the ultimate goal – development of clinically efficient vaccines to help patients fight against cancers.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Seyrek K. The Third Alphabet of Life: Carbohydrate-Protein Interactions. Turk. J. Vet. Anim. Sci. 2004;28:784–792. [Google Scholar]
- 2.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. * Provides an overview of the many biological roles of oligosaccharides.
- 3.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu. Rev. Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 4.Easton RL, Patankar MS, Lattanzio FA, et al. Structural analysis of murine zona pellucida glycans. J. Biol. Chem. 2000;275:7731–7742. doi: 10.1074/jbc.275.11.7731. [DOI] [PubMed] [Google Scholar]
- 5.Sutton-Smith M, Wong NK, Khoo K-H, et al. Analysis of protein-linked glycosylation in a sperm-somatic cell adhesion system. Glycobiology. 2007;17:553–567. doi: 10.1093/glycob/cwm025. [DOI] [PubMed] [Google Scholar]
- 6.Aitken RJ. The complexities of conception. Science. 1995;269:39–40. doi: 10.1126/science.7604276. [DOI] [PubMed] [Google Scholar]
- 7.Florman HM, Wassarman PM. O-Linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985;41:313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 9.Pennington JE, Rastan S, Roelcke D, Feizi T. Saccharide structures of the mouse embryo during the first eight days of development. J. Embryol. Exp. Morphol. 1985;87:115–128. [PubMed] [Google Scholar]
- 10.Fenderson BA, Eddy EM, Hakomori S-I. Glycoconjugate expression during embryogenesis and its biological significance. BioEssays. 1990;12:173–179. doi: 10.1002/bies.950120406. [DOI] [PubMed] [Google Scholar]
- 11.Schwarting GA, Yamamoto M. Expression of glycoconjugates during development of the vertebrate nervous system. BioEssays. 1988;9:19–23. doi: 10.1002/bies.950090106. [DOI] [PubMed] [Google Scholar]
- 12.Blum AS, Barnstable CJ. O-Acetylation of a cell-surface carbohydrate creates discrete molecular patterns during neural development. Proc. Natl. Acad. Sci. USA. 1987;84:8716–8720. doi: 10.1073/pnas.84.23.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang CR, Liour SS, Dasgupta S, Yu RK. Inhibition of neuronal migration by JONES antibody is independent of 9-O-acetyl GD3 in GD3-synthase knockout mice. J. Neurosci. Res. 2007;85:1381–1390. doi: 10.1002/jnr.21264. [DOI] [PubMed] [Google Scholar]
- 14.Miyaki M, Zenita K, Tanaka O, Okada Y, Kannagi R. Stage-specific expression of SSEA-1-related antigens in the developing lung of human embryos and its relation to the distribution of these antigens in lung cancers. Cancer Res. 1988;48:7150–7158. [PubMed] [Google Scholar]
- 15.Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Nat. Acad. Sci. USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 17.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim. Biophys. Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 18.Livingston PO, Zhang S, Lloyd KO. Carbohydrate vaccines that induce antibodies against cancer. 1. Rationale. Cancer Immunol. Immunother. 1997;45:1–9. doi: 10.1007/s002620050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol. Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. ** Provides insight into the synthesis, conjugation, clinical administration and immunological potential of several carbohydrate-based cancer vaccines.
- 20.Le Poole IC, Gerberi MAT, Kast WM. Emerging strategies in tumor vaccines. Curr. Opin. Oncol. 2002;14:641–648. doi: 10.1097/00001622-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Dzhambazov B, Holmdahl M, Yamada H, et al. The major T cell epitope on type II collagen is glycosylated in normal cartilage but modified by arthritis in both rats and humans. Eur. J. Immunol. 2005;35:357–366. doi: 10.1002/eji.200425637. [DOI] [PubMed] [Google Scholar]
- 22.Purcell AW, van Driel IR, Gleeson PA. Impact of glycans on T-cell tolerance to glycosylated self-antigens. Immunol. Cell Biol. 2008;86:574–579. doi: 10.1038/icb.2008.48. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Sette A, Sidney J, Gendler SJ, Franco A. Tumor-associated carbohydrate antigens: A possible avenue for cancer prevention. Immunol. Cell Biol. 2005;83:440–448. doi: 10.1111/j.1440-1711.2005.01347.x. [DOI] [PubMed] [Google Scholar]
- 24.Bettahi I, Dasgupta G, Renaudet O, et al. Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B-cell, CD4+ and CD8+ T cell epitopes. Cancer Immunol. Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingale S, Wolfert MA, Buskas T, Boons GJ. Increasing the antigenicity of synthetic tumor-associated carbohydrate antigens by targeting toll-like receptors. Chembiochem. 2009;10:455–463. doi: 10.1002/cbic.200800596. * Disclosure demonstrating that the optimization of synthetic carbohydrate-based cancer vaccines is amenable to structure-activity relationship studies.
- 26.Halcomb RL, Danishefsky SJ. On the direct epoxidation of glycals: Application of a reiterative strategy for the synthesis of b-linked oligosaccharides. J. Am. Chem. Soc. 1989;111:6661–6666. [Google Scholar]
- 27.Danishefsky DJ, Bilodeau MT. Glycals in organic synthesis: the evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew. Chem. Int. Ed. 1996;35:1381–1419. [Google Scholar]
- 28.Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Synthetic carbohydrate-based antitumor vaccines: Challenges and opportunities. Expert Rev. Vaccines. 2005;4:677. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- 29.Kannagi R, Levery SB, Ishigami F, et al. New globo series glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen. J. Biol. Chem. 1983;258:8934. [PubMed] [Google Scholar]
- 30.Bremer EG, Levery SB, Sonnino S, et al. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J. Biol. Chem. 1984;259:14773. [PubMed] [Google Scholar]
- 31.Livingston PO. Augmenting the immunogenicity of carbohydrate tumor antigens. Semin. Cancer Biol. 1995;6:357. doi: 10.1016/1044-579x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 32.Bilodeau MT, Park TK, Danishefsky SJ, et al. Total Synthesis of a Human Breast Tumor Associated Antigen. J. Am. Chem. Soc. 1995;117:7840. [Google Scholar]
- 33.Park TK, Kim IJ, Hu SH, et al. Total synthesis and proof of structure of a human breast tumor (Globo-H) antigen. J. Am. Chem. Soc. 1996;118:11488. [Google Scholar]
- 34.Allen JR, Allen JG, Zhang XF, et al. A second generation synthesis of the MBr1 (Globo-H) breast tumor antigen: new application of the n-pentenyl glycoside method for achieving complex carbohydrate protein linkages. Chem. Eur. J. 2000;6:1366. doi: 10.1002/(sici)1521-3765(20000417)6:8<1366::aid-chem1366>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Ragupathi G, Park TK, Zhang SL, et al. Immunization of mice with a fully synthetic globo-H antigen results in antibodies against human cancer cells: a combined chemical - immunological approach to the fashioning of an anticancer vaccine. Angew. Chem., Int. Ed. 1997;36:125. [Google Scholar]
- 36.Ragupathi G, Slovin SF, Adluri S, et al. A fully synthetic globo-H carbohydrate vaccine induces a focused humoral response in prostate cancer patients: a proof of principle. Angew. Chem., Int. Ed. 1999;38:563. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Slovin SF, Ragupathi G, Adluri S, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo-H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. USA. 1999;96:5710. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilewski T, Ragupathi G, Danishefsky SJ, et al. Immunization of metastatic breast cancer patients with a fully synthetic globo-H conjugate: a Phase I trial. Proc. Natl. Acad. Sci. USA. 2001;98:3270. doi: 10.1073/pnas.051626298. Erratum in: Proc. Natl. Acad. Sci. USA 98, 14186 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickler MN, Ragupathi G, Liu NX, et al. Immunogenicity of a fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin. Cancer Res. 1999;5(10):2773. [PubMed] [Google Scholar]
- 40.Krug LM, Ragupathi G, Danishefsky SJ, et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM1 conjugated to keyhole limpet hemocyanin. Clin. Cancer Res. 2004;10(18 Pt 1):6094. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 41.Sabbatini PJ, Kudryashov V, Danishefsky SJ, et al. Immunization of ovarian cancer patients with a synthetic Lewis(y)-protein conjugate vaccine: a Phase 1 trial. Int. J. Cancer. 2000;87(1):79–85. [PubMed] [Google Scholar]
- 42.Carlstedt I, Davies JR. Glycoconjugates facing the outside world. Biochem. Soc. Trans. 1997;25:214. doi: 10.1042/bst0250214. [DOI] [PubMed] [Google Scholar]
- 43.Chen XT, Sames D, Danishefsky SJ. Exploration of modalities in building O-linked systems through glycal assembly: a total synthesis of the mucin-related F1 antigen. J. Am. Chem. Soc. 1998;120(31):7760. [Google Scholar]
- 44.Kuduk SD, Schwarz JB, Danishefsky SJ, et al. Synthetic and immunological studies on clustered modes of mucin-related TN and TF O-linked antigens: the preparation of a glycopeptide-based vaccine for clinical trials against prostate cancer. J. Am. Chem. Soc. 1998;120:12474. [Google Scholar]
- 45.Kagan E, Ragupathi G, Danishefsky SJ, et al. Comparison of antigen constructs and carrier molecules for augmenting the immunogenicity of the monosaccharide epithelial cancer antigen Tn. Cancer Immunol. Immunother. 2005;54:424. doi: 10.1007/s00262-004-0584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slovin SF, Ragupathi G, Danishefsky SJ, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with α-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J. Clin. Oncol. 2003;11:4292. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz JB, Kuduk SD, Danishefsky SJ, et al. A broadly applicable method for the efficient synthesis of O-linked glycopeptides and clustered sialic acid residues. J. Am. Chem. Soc. 1999;121:2662. [Google Scholar]
- 48.Sames D, Chen XT, Danishefsky SJ. Convergent total synthesis of a tumor-associated mucin motif. Nature. 1997;389:587. doi: 10.1038/39292. [DOI] [PubMed] [Google Scholar]
- 49.Glunz PW, Hintermann S, Williams LJ, et al. Design and synthesis of Ley-bearing glycopeptides that mimic cell surface Ley mucin glycoprotein architecture. J. Am. Chem. Soc. 2000;122:7273. [Google Scholar]
- 50.Ragupathi G, Cappello S, Danishefsky SJ, et al. Comparison of antibody titers after immunization with monovalent or tetravalent KLH conjugate vaccines. Vaccine. 2002;20:1030. doi: 10.1016/s0264-410x(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 51.Ragupathi G, Koide F, Sathyan N, et al. A preclinical study comparing approaches for augmenting the immunogenicity of a heptavalent KLH-conjugate vaccine against epithelial cancers. Cancer Immunol. Immunother. 2003;52:608. doi: 10.1007/s00262-003-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slovin SF, Ragupathi G, Fernandez C, et al. A polyvalent vaccine for high-risk prostate patients: “are more antigens better?”. Cancer Immunol. Immunother. 2007;56:1921. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabbatini PJ, Ragupathi G, Hood C, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS-21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin. Cancer Res. 2007;13:4170. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 54.Barington T, Gyhrs A, Kristensen K, Heilmann C. Opposite effect of actively and passively acquired immunity to the carrier on response of human infants to Haemophilus influenzae type b conjugate vaccine. Infect. Immun. 1994;62:9. doi: 10.1128/iai.62.1.9-14.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters CC, Tenbergen-Meeks AM, Poolman JT, et al. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect. Immun. 1974;59:3504. doi: 10.1128/iai.59.10.3504-3510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarvas H, Makela O, Toivanen P, Toivanen A. Effect of carrier preimmunization on the anti-hapten response in chicken. Scand. J. Immunol. 1974;3:455. doi: 10.1111/j.1365-3083.1974.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 57.Herzenberg LA, Tokuhisa T. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 58.Kihlberg J, Elofsson M. Solid-phase synthesis of glycopeptides: immunological studies with T cell stimulating glycopeptides. Curr. Med. Chem. 1997;4:85. [Google Scholar]
- 59.Sears P, Wong CH. Carbohydrate mimetics: a new strategy for tackling the problem of carbohydrate-mediated biological recognition. Angew. Chem., Int. Ed. Engl. 1999;28:2301. [PubMed] [Google Scholar]
- 60.Muller B, Schaub C, Schmidt RR. Efficient sialyltransferase inhibitors based on transition-state analogs of the sialyl donor. Angew. Chem., Int. Ed. 1998;37:2893. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2893::AID-ANIE2893>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 61.Glunz PW, Hintermann S, Williams LJ, et al. Design and Synthesis of Ley-Bearing Glycopeptides that Mimic Cell Surface Ley Mucin Glycoprotein Architecture. J. Am. Chem. Soc. 2000;122:7273. [Google Scholar]
- 62.Allen JR, Allen JG, Zhang XF, et al. A second generation synthesis of the MBr1 (globo-H) breast tumor antigen: new application of the n-pentenyl glycoside method for achieving complex carbohydrate protein linkages. Chem. Eur. J. 2000;6:1366. doi: 10.1002/(sici)1521-3765(20000417)6:8<1366::aid-chem1366>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 63.Allen JR, Ragupathi G, Livingston PO, Danishefsky SJ. New applications of the n-pentenyl glycoside method in the synthesis and immunoconjugation of fucosyl GM1: a highly tumor-specific antigen associated with small cell lung carcinoma. J. Am. Chem. Soc. 1999;121:10875. [Google Scholar]
- 64.Allen JR, Harris CR, Danishefsky SJ. Pursuit of optimal carbohydrate-based anticancer vaccines: preparation of a multiantigenic unimolecular glycopeptide containing the Tn, MBr1, and Ley antigens. J. Am. Chem. Soc. 2001;123:1890. doi: 10.1021/ja002779i. [DOI] [PubMed] [Google Scholar]
- 65.Biswas K, Coltart DM, Danishefsky SJ. Construction of carbohydrate-based antitumor vaccines: synthesis of glycosyl amino acids by olefin cross-metathesis. Tetrahedron Lett. 2002;43:6107. [Google Scholar]
- 66.Wan Q, Cho YS, Lambert TH, Danishefsky SJ. Olefin cross-metathesis: a powerful tool for constructing vaccines composed of multimeric antigens. J. Carbohydr. Chem. 2005;24:425. [Google Scholar]
- 67.Zhu J, Wan Q, Ragupathi G, George CM, Livingston PO, Danishefsky SJ. Biologics through chemistry: total synthesis of a proposed dual acting vaccine targeting ovarian cancer by orchestration of oligosaccharide and polypeptide domains. J. Am. Chem. Soc. 2009;131:4151. doi: 10.1021/ja810147j. * Describes the first synthesis of a highly complex dual-acting glycopeptide construct featuring clustered Gb3-MUC5AC targeting ovarian cancer.
- 68.Cho YS, Wan Q, Danishefsky SJ. Organic synthesis in pursuit of immunology: large-scale synthesis of peracetylated GM2 glycosylamino acid for preparation of a multiantigenic prostate cancer vaccine. Bioorg. Med. Chem. 2005;13:5259. doi: 10.1016/j.bmc.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 69.Zhu J, Wan Q, Yang G, Ouerfelli O, Danishefsky SJ. Synthesis of human cancer associated globo-H (MBr1 antigen) glycosylamino acid: some mechanistic and conformational reinvestigations. Heterocycles. 2009 doi: 10.3987/COM-08-S(D)82. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keding SJ, Endo A, Biswas K, et al. Hydroxynorleucine as a glycosyl acceptor is an efficient means for introducing amino acid functionality into complex carbohydrates. Tetrahedron Lett. 2003;44:3413. [Google Scholar]
- 71.Keding SJ, Endo A, Danishefsky SJ. Synthesis of non-natural glycosylamino acids containing tumor-associated carbohydrate antigens. Tetrahedron. 2003;59:7023. [Google Scholar]
- 72.Ragupathi G, Coltart DM, Williams LJ, et al. On the power of chemical synthesis: immunological evaluation of models for multiantigenic carbohydrate-based cancer vaccines. Proc. Natl. Acad. Sci. USA. 2002;99:13699. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keding SJ, Danishefsky SJ. Prospects for total synthesis: a vision for a totally synthetic vaccine targeting epithelial tumors. Proc. Natl. Acad. Sci. USA. 2004;101:11937. doi: 10.1073/pnas.0401894101. * Describes the design rationale and chemical synthesis of the first-generation unimolecular pentavalent glycopeptide construct.
- 74.Ragupathi G, Koide F, Livingston PO, et al. Preparation and evaluation of unimolecular pentavalent and hexavalent antigenic constructs targeting prostate and breast cancer: a synthetic route to anticancer vaccine candidates. J. Am. Chem. Soc. 2006;128:2715. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 75.Livingston PO, Natoli EJ, Calves MJ, et al. Vaccines containing purified GM2 ganglioside elicit GM2 antibodies in melanoma patients. Proc. Natl. Acad. Sci. USA. 1987;84:2911. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Livingston PO, Wong GY, Adluri S, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. Clin. Oncol. 1994;12:1036. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 77.Zhu J, Danishefsky SJ. A unimolecular pentavalent carbohydrate-based antigen KLH conjugate as vaccine targeting prostate and breast cancer. Chinese Science Bulletin (Chinese Version) 2008;53:2126. [Google Scholar]
- 78.Zhu J, Wan Q, Lee D, et al. From synthesis to biologics: preclinical data on the chemistry derived anticancer vaccines. J. Am. Chem. Soc. doi: 10.1021/ja901415s. Submitted. * Provides a better bio-conjugation protocol for the synthesis of unimolecular pentavalent glycopeptide KLH conjugate and the preclinical data of this vaccine in mice.
- 79.Kiguchi K, Iwamori Y, Suzuki N, et al. Characteristic expression of globotriaosyl ceramide in human ovarian carcinoma-derived cells with anticancer drug resistance. Cancer Sci. 2006;97:1321. doi: 10.1111/j.1349-7006.2006.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giuntoli RL, Rodriguez GC, Whitaker RS, et al. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546. [PubMed] [Google Scholar]
- 81.Tampellini M, Berruti A, Gerbino A, et al. Relationship between CA 15-3 serum levels and disease extent in predicting overall survival of breast cancer patients with newly diagnosed metastatic disease. Br. J. Cancer. 1997;75:698. doi: 10.1038/bjc.1997.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bon GG, Verheijen RHM, Zuetenhorst JM, et al. Mucin-like carcinoma-associated antigen serum levels in patients with adenocarcinomas originating from ovary, breast and colon. Gynecol. Obstet. Inv. 1996;42:58. doi: 10.1159/000291890. [DOI] [PubMed] [Google Scholar]
- 83.Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gasteroenterology. 1994;106:353. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 84.Gendler SJ, Lancaster CA, Taylor-Papadimitriou T, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 1990;265:15286. [PubMed] [Google Scholar]
- 85.Butts C, Murray N, Maksymiuk A, et al. Randomized Phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J. Clin. Oncol. 2005;23:6674. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 86.Lee D, Danishefsky SJ. “Biologic” level structures through chemistry: a total synthesis of a unimolecular pentavalent MUCI glycopeptide construct. Tetrahedron Lett. 2009;50:2167. doi: 10.1016/j.tetlet.2009.02.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Southwood S, Sidney J, Kondo A, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immun. 1998;160:3363. [PubMed] [Google Scholar]
- 88.Nagorny P, Kim WH, Wan Q, et al. On the emerging role of chemistry in the fashioning of biologics: synthesis of a bidomainal fucosyl GM1-based vaccine, for the treatment of small cell lung cancer. J. Org. Chem. 2009 doi: 10.1021/jo900918m. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang S, Cordon-Cardo C, Zhang HS, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int. J. Cancer. 1997;73:42. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]