Abstract
Prins-type macrocyclizations have recently emerged as a successful strategy in the synthesis of polyketide-derived natural products. This reaction provides a concise and selective means to form tetrahydropyran-containing macrocyclic rings of varying size. A high degree of functionality within the macrocycle is tolerated and the yields for these transformations are typically good to excellent. Since the initial report of a Prins macrocyclization reaction in 1979, examples of this approach did not re-emerge until 2008. However, the use of this method in natural product synthesis has rapidly gained momentum in the synthetic community, with multiple examples of this macrocyclization tactic reported in the recent literature.
Keywords: cyclization, macrocycles, polyketides, synthesis design, total synthesis
1. Introduction
Macrocyclic natural products are isolated from a diverse collection of sources including bacteria, fungi, plants, and animals. Their prevalence in nature is proposed to be a result of the macrocyclic core providing a beneficial and delicate balance between conformational rigidity and flexibility, which allows for optimal binding to biological targets.[1] In addition, these architectures have good solubility in water and cell permeability, and they are relatively stable to proteolytic and metabolic activities.[2] All of these factors provide a basis for the wide variety of biological activities exhibited by these natural products including antitumor, antibiotic, antifungal, and insecticidal properties. Despite this promising array of activities, the application of macrocyclic natural products in drug development continues to be a challenge owing to the complex nature of this structural class. However, synthesis of these molecules is crucial for structure confirmation, thorough biological evaluation, and the generation of desired analogue structures. In the development and employment of a synthetic route toward a natural product macrocycle, one of the most important and challenging tasks is the key macrocyclization step because of its associated entropic factors and the potential for oligomerization. While a variety of strategies have evolved to access large ring-containing, bioactive molecules, innovative approaches are still needed to drive this area of research forward.
1.1. Common Macrocyclization Strategies
Substrate compatibility, selectivity. and overall convergence of the route all determine which macrocyclization strategy is employed. The approaches that can be taken to form a macrocycle from a linear precursor generally fall into three basic categories: C–X, C=C, and C–C bond formation (Figure 1).[3]
Figure 1.
Common bond formations in macrocyclizations.
1.1.1. C–X Bond Formation
Macrocycles that contain lactones are important target molecules because they often have significant biological or medicinal properties. The prototypical ring closure for these macrolides involves a macrolactonization with C–O bond formation. These are traditionally performed under Mitsunobu (Ph3P, diethylazodicarboxylate (DEAD)), Yamaguchi (2,4,6-trichlorobenzoyl chloride, iPrNEt2, 4-dimethylaminopyridine (DMAP)), and Keck (1,3-dicyclohexylcarbodiimide (DCC), DMAP, DMAP·HCl) conditions.[1] In addition to these standard approaches many other specialized conditions have been employed.[4] A newer strategy involves a C–H oxidative macrolactonization, which Stang and White utilized in their recent synthesis of 6-deoxyerythronolide B.[5] If the macrocycle contains an amide bond, classic Steglich conditions (DCC/DMAP) can be used to form the C–N bond. However, these reagents have been replaced in recent years by more efficient peptide coupling agents such as the uronium salt HATU, phosphonium salt PyBOP, and phosphinate FDPP (HATU = O-(7-azabenzotriazol-1-yl)-N,N,N′,N-tetramethyluronium hexafluorophosphate, PyBOP = benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate, FDPP = pentafluorophenyl diphenyl phosphinate).[1] Unlike C–O bond formation in macrolactonizations, only mild activation of the carboxylic acid is necessary since the amine is much more nucleophilic than the corresponding alcohol.
Macrocyclic natural products containing biaryl ethers (e.g., vancomycin) have provided the impetus to develop new C–X bond-forming approaches such as SNAr reactions.[1] Prior to the late 1990s, the Ullman macrocyclization was the most commonly used technique for the formation of aryl ether bonds. However, the harsh conditions (heat and/or strong base) associated with Ullman reactions are not necessarily compatible with sensitive stereogenic centers and peptide-containing precursors.[6] This liability was typified in synthetic studies of vancomycin by Boger et al.[7] in which epimerization was observed in Ullman macrocyclizations of vancomycin model systems. Zhu et al.[8] and Rao et al.[9] circumvented this epimerization problem by utilizing an SNAr coupling for the same macrocyclization and improved the yields.
1.1.2. C=C Bond Formation
The most broadly employed strategy for C=C macrocyclization is ring-closing metathesis (RCM).[10] In recent years, this powerful approach has become one of the most reliable and efficient methods in the formation of medium- to large-ring systems. The general strategy to form alkene-containing ring systems by RCM was pioneered by Fu and Grubbs,[11] Martin et al.,[12] and Pandit et al.[13] In addition, one of the first successful applications of RCM in total synthesis was accomplished by Hoveyda et al. in their synthesis of fluvirucin B1.[14] This process tolerates a wide variety of functional groups and can fashion rings in various sizes in good to excellent yields. However, RCM is also subject to equilibrium ring-closing metathesis; in other words, there is a competition between intramolecular ring-closure and intermolecular oligomerization. Extensive work by Fogg and others[15] has demonstrated that this can be overcome by utilizing more reactive (first-generation) catalysts, higher temperatures, and lower concentrations. However, these conditions can lead to catalyst decomposition, necessitating higher catalyst loadings. In general, factors such as catalyst structure, concentration, temperature, addition time, and reaction time greatly influence the success of a ring-closing metathesis. Even if the optimal conditions for a particular transformation are determined, the reproducibility of the reaction and the control of the olefin geometry can still be challenging. In particular, the E/Z selectivity of these processes can suffer during the actual metathesis pathway or in subsequent isomerizations catalyzed by the metal complex.
Other alkene-forming strategies in macrocyclic natural product synthesis include the venerable Julia–Kocienski[16] and Horner–Wadsworth–Emmons olefinations.[17] These anion-based approaches have been highly successful but, similar to RCM, the selectivity of the new olefin geometry can be moderate and difficult to control. Another strategy for the installation of a macrocyclic alkene is an aldol condensation.[1] However, this reaction is rarely utilized owing to problems with selective enolization, competing and equilibrating retroaldol reactions, and competition between inter- and intramolecular reactions. Given the popularity and success of RCM to form macrocycles, reactions involving additions to aldehydes to produce macrocyclic alkenes are utilized much less often.
1.1.3. C–C Bond Formation
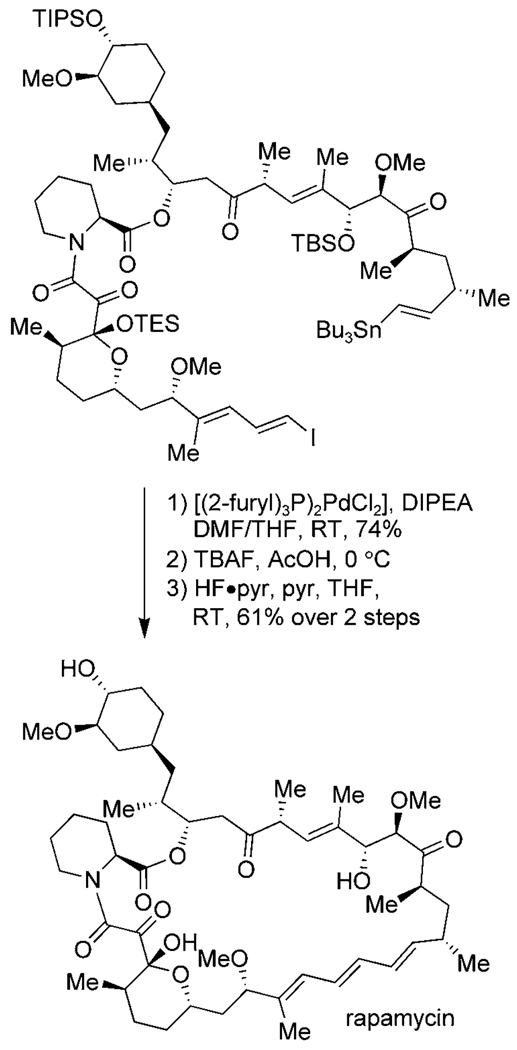
A majority of C–C bond-forming macrocyclizations over the past two decades involve metal-mediated reactions. Palladium-catalyzed cross-coupling reactions, such as the Stille, Suzuki—Miyaura, and Heck reactions, have been employed extensively to form C–C bonds in macrocycles.[1] The conditions are typically mild and the reactions have good functional-group tolerance, as demonstrated by Smith’s synthesis of the 31-membered macrocycle of rapamycin by a Stille coupling (Scheme 1).[18] However, the yields for the macrocyclization variants can be lower than those of standard cross-coupling reactions, and installation of the necessary coupling components, such as vinyl tin or vinyl iodide groups, can be difficult.
Scheme 1.
Stille macrocyclization and completion of the synthesis of rapamycin by Smith et al. pyr = pyridine, TBS = tert-butyldimethylsilyl, TES = triethylsilyl.
1.2. The Prins Reaction: Merged C–C and C–O Formation
1.2.1. Background
In 1919, Prins reported the condensation of formaldehyde with styrene in the presence of an acid catalyst to form a diol product.[19] The generally accepted mechanism of the Prins reaction involves initial activation of an aldehyde with a Brønsted or Lewis acid, followed by addition of an olefin to form a cationic intermediate (Scheme 2).[20] Depending on the reaction conditions, multiple products can be formed. If R2 is a suitable leaving group, the cationic intermediate can then undergo elimination to give an allylic alcohol. Additionally, the intermediate can be trapped with a nucleophile to afford a 3-substituted alcohol. If the nucleophile is water, then a 1,3-diol product results, which can then undergo elimination to form an allylic alcohol if the reaction occurs under forcing conditions. Furthermore, a second aldehyde equivalent could add to the cationic intermediate, leading to a 1,3-dioxane product through subsequent ring closure. Despite the synthetic potential of the Prins reaction, the early development of this process was hampered by the myriad of possible products and harsh conditions necessary to initiate the reaction.
Scheme 2.
The Prins reaction mechanism and possible reaction pathways. Nuc = nucleophile.
1.2.2. Synthesis of Tetrahydropyran Rings
In 1955, Hanschke was the first to report the selective synthesis of tetrahydropyran (THP) rings through a Prins reaction by combining 3-buten-1-ol with a variety of aldehydes or ketones in the presence of acid (Scheme 3).[21] When sulfuric acid was used, 2,2′-disubstituted tetrahydropyran-4-ol products were formed. However, when hydrochloric acid was employed, 4-chlorotetrahydropyran products resulted.[20c] The mechanism involves the condensation of the alcohol onto the activated aldehyde to give an oxocarbenium intermediate. This intermediate is then attacked by the olefin to give a tetrahydropyranal carbocation, which exists in a chair conformation with the hydrogen adjacent to the carbocation in a pseudo-axial position. As a result, this configuration predisposes the empty p orbital to the equatorial position, effecting attack at this site by the nucleophile to afford the THP product. Alder et al. proposed that this chair conformation allows for optimal orbital overlap of the equatorial lone pair on the oxygen with the C2–C3 and C5–C6 σ and σ* orbitals and the vacant p orbital of the carbocation.[22] However, axial attack of a nucleophile can occur under different reaction conditions. Rychnovsky and co-workers have demonstrated that if a small counteranion is employed in the reaction, it will associate with the carbocation of the intermediate pyran. Together, these form a contact ion pair and axial attack of the anion will subsequently occur by the principle of least motion.[23] However, if a large counteranion is utilized in the reaction, it is not only less nucleophillic, but it also forms a solvent-separated ion pair. This consequently effects preferential axial attack by the nucleophile.
Scheme 3.
The first report and mechanism of the synthesis of THP rings by the Prins reaction. [a] When H2SO4 was used. [b] When HCl was used.
1.2.3. Competitive Reaction Pathways in THP Cyclizations
Forming tetrahydropyran rings through a Prins cyclization is typically a diastereoselective process that occurs with excellent transfer of chirality from the starting material. However, unusual and racemic products have been observed that result from competing 2-oxonia-Cope rearrangements (Scheme 4). As was just discussed in Section 1.2.2, the Prins reaction proceeds through a chairlike transition state with the C2 substituent located equatorially and the oxocarbenium in an E configuration. Upon ring closure, a new stereocenter is formed at the C6 position with chirality transfer from the C2 stereocenter. The transient C4 carbocation is then trapped by a nucleophile, resulting in an equatorial orientation for the substituent (pathway A). In the competing 2-oxonia-Cope rearrangement, however, the oxocarbenium intermediate undergoes a [3,3] sigmatropic rearrangement, destroying the integrity of the stereocenter at C2. Therefore, upon 6-endo ring closure, a tetrahydropyran product results with scrambling of the stereochemistry to afford a mixture of diastereomers (pathway B). It is also possible for the achiral intermediate to undergo a 5-endo ring closure to afford a tetrahydrofuran product, as was first observed by Speckamp et al. (pathway C).[24] A third pathway, which was elucidated by Roush and Dilley in studies toward scytophycin C,[25] involves hydrolysis of the achiral oxocarbenium ion to form an epimeric allylic alcohol. This alcohol can then reversibly react with aldehydes in solution and form exchange products upon eventual 6-endo ring closure (pathway D). Elegant studies from the Rychnovsky lab demonstrated that the oxonia-Cope rearrangement occurs during Prins cyclizations when the two possible oxocarbenium intermediates are similar in energy, thus promoting thermodynamic control of the process.[26] Fortunately, the Prins reaction can be kinetically controlled by destabilizing the oxocarbenium intermediate or by stabilizing the resulting tetrahydropyranal carbocation through substrate design.
Scheme 4.
Competing reaction mechanisms during Prins cyclizations to form tetrahydropyran rings.
The loss of optical activity through other mechanisms has also been reported. Willis et al. observed decreased enantiomeric excess during Prins cyclizations of benzylic alcohols through a direct ionization pathway (pathway E).[27] Another mechanism of racemization was recently discovered by Jasti and Rychnovsky while investigating a Grob fragmentation/Prins cyclization sequence.[28] Extensive mechanistic studies demonstrated that the complete loss of chirality in the tetrahydropyran product was the result of successive 2-oxonia-Cope rearrangements of the (Z)-oxocarbenium ion, which was proposed to have isomerized from the (E)-oxocarbenium through an addition–elimination pathway (pathway F).
Thorough mechanistic studies have resulted in approaches to suppress pathways that might lead to possible loss of enantiomeric purity or potential competitive side reactions during a Prins cyclization. Thus, the Prins reaction is not only an efficient strategy to construct tetrahydropyran rings, but it has also emerged as a powerful C–C and C–O bond-forming macrocyclization technique in the synthesis of polyketide natural products.
1.3. Early Prins Macrocyclizations
The clear first example of a Prins reaction being utilized in a macrocyclization was the formal synthesis of (R,S)-muscone (6) outlined by Schulte-Elte et al. (Scheme 5).[29] The ozonolysis of (Z,E,E)-1,5,9-cyclododecatriene (1), monoprotection of the resulting dialdehyde, and subsequent addition of a Grignard reagent generated from methallylmagnesium chloride afforded the cyclization precursor 2 (Scheme 5). Macrocyclization of the 15-membered ring was facilitated with p-toluenesulfonic acid (1 mol%) in refluxing toluene to afford the bicyclic dihydropyran product 3 in a 75% yield. Interestingly, lower yields were observed when the saturated macrocyclization precursor or the pure E,E diene were used. This early report underscored the strong impact of conformational effects on the formation of macrocylic oxocarbenium ions. Conversion of bicycle 3 to muscone (6) was accomplished by heating to 135–270°C in the presence of activated Pd/C in xylenes under H2. The proposed mechanism for this process is the initial reduction of the diene mixture in the macrocycle, followed by a dehydration to give pyran 4. A 6π electrocyclic ring opening of pyran 4, followed by hydrogenation of the resulting diene provided muscone (6). Thus, this five-step synthetic route afforded (R,S)-muscone in an overall 40% yield, effectively demonstrating the power and efficiency of the Prins macrocyclization. However, despite the obvious synthetic utility of this strategy, it would not be thoroughly capitalized on for another three decades.
Scheme 5.
Formal synthesis of (R,S)-muscone by Schulte-Elte et al. TsOH = p-toluenesulfonic acid.
2. Applications in Natural Product Synthesis
2.1. The Syntheses of Neopeltolide
Neopeltolide is a 14-membered macrolide[30] that was isolated from a Daedalopelta Sollas related sponge off the Jamaican coast (Scheme 6).[31] Contained within the 14-membered macrolactone are six stereogenic centers, a 2,6-cis-tetrahydropyran unit, and an appended oxazole and carbamate-containing side chain also found in the natural product leucascandrolide A. In 2007, Wright and co-workers reported neopeltolide to inhibit tumor cell growth with promising IC50 values against a variety of tumor cell lines. In 2008, Kozmin, Kron, and co-workers established that neopeltolide targets the cytochrome bc1 complex, causing inhibition of mitochondrial ATP synthesis.[32] In addition to these biological studies, our laboratory, in collaboration with the Crews group at Yale, demonstrated that both the C11/C13 and C3/C7 diastereomeric analogues retain most of the biological activity of neopeltolide against human breast adenocarcinoma (MCF-7) and murine leukemia (P-388) cell lines.[33] Further analogue testing by us and by Maier and Vintonyak independently demonstrated that both the entire oxazole side chain and the macrocycle are necessary for full anticancer activity.[34]
Scheme 6.
The proposed and corrected structures of neopeltolide.
One of the first total syntheses and the structural revision of neopeltolide, which came from our laboratory, employed an aggressive Prins macrocyclization strategy to construct the macrocycle and embedded pyran ring simultaneously.[35, 36] Shortly thereafter, Lee et al. also reported a total synthesis,[37] followed by Yadav and Kumar’s formal synthesis,[38] both of which utilized a Prins macrocyclization. The strong interest in this molecule’s biological activity has called for efficient methods to access this macrolide, and three of the 13 reported formal and total syntheses utilized a Prins macrocyclization as a key bond-forming step.
2.1.1. Total Synthesis of Neopeltolide by the Scheidt Group
In December 2007, our laboratory reported the total synthesis and structural revision of neopeltolide.[35] Our strategy relied on a Sc(OTf)3-catalyzed key step with concomitant formation of the tetrahydropyran ring and the macrocycle (Scheme 7). The macrocyclization precursor 11 was formed by Yamaguchi esterification of the alcohol fragment 12 with the β-hydroxy dioxinone acid fragment 13. For alcohol 12, the stereochemistry of C13 was installed by a Noyori reduction and a pseudoephedrine-controlled alkylation established the R configuration at C9.[39] An Evans–Tishchenko reduction effectively set the stereochemistry at C11. Acid fragment 13 was constructed utilizing a titanium(IV)/(R)-binol-catalyzed vinylogous aldol reaction developed by Scettri et al.[40]
Scheme 7.
Our initial retrosynthesis of neopeltolide. OTf = trifluoromethanesulfonate.
The macrocyclization strategy did in fact work but after we had completed the synthesis to give macrolide 7, the 1H and 13C NMR spectra did not agree with the reported spectra of neopeltolide. As discussed in Section 1.2.3, a 2-oxonia-Cope rearrangement is possible during Prins-type cyclizations, which can lead to unexpected products. We postulated that this might have occurred in our synthesis, causing inversion at the C3, C5, and C7 stereocenters to give diastereomer 15 (Scheme 8). To test this hypothesis, we constructed this diastereomer directly by utilizing acid fragment 14 (with the opposite configuration to that of the previously used acid 13) but the spectra of macrolide 15 also did not match the reported spectra of neopeltolide. After a careful analysis of the spectral data, we proposed that the stereochemistry was inverted at the C11 and C13 positions, which corresponded to two of the stereocenters in the alcohol fragment. To test this hypothesis, new diastereomeric alcohol 16 was synthesized and used to complete the synthesis of macrolide 8, whose spectral data did finally match those of neopeltolide.
Scheme 8.
Our revised synthetic approaches to neopeltolide.
This synthesis was the first example of a Prins-type macrocyclization strategy in natural product synthesis in nearly three decades. The convergency of this route facilitated by the macrocyclization approach was particularly useful when the stereochemistry of the reported structure of neopeltolide came into question.
2.1.2. Total Synthesis of Neopeltolide by the Lee Group
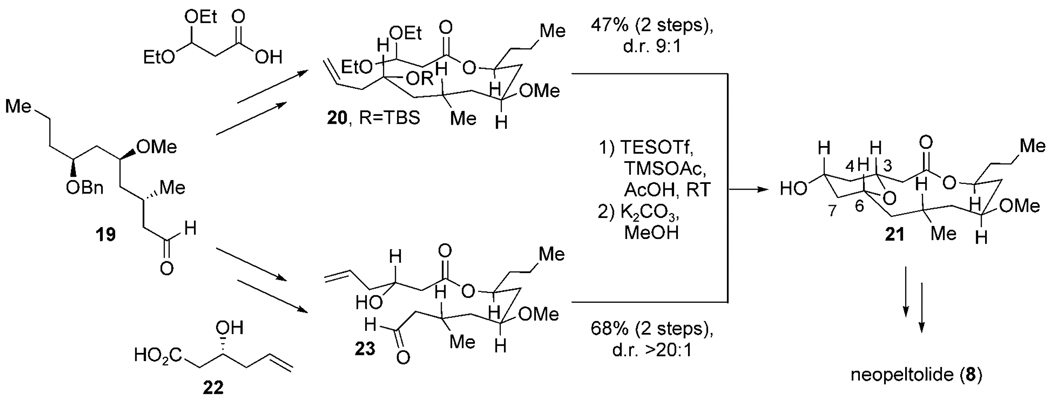
Lee and co-workers reported the total synthesis of neopeltolide utilizing a Prins macrocyclization in March 2008.[37] While we closed the C6–C7 bond during the macrocyclization step in our synthesis, Lee developed two complimentary Prins macrocyclization strategies for the formation of the C3–C4 and C6–C7 bonds following C–O bond formation in the THP ring (Scheme 9). The absolute stereochemistry of common aldehyde fragments 19 was installed by an asymmetric crotyl-transfer reaction, titanium(IV) chloride mediated methallylation and substrate-directed hydroformylation. The macrocyclization precursor 20 was accessed by an asymmetric Brown allylation and Yamaguchi esterification with 3,3-diethoxypropanoic acid to form the C3–C4 bond of macrocycle 21 in 47%yield with diastereomeric ratio (d.r.) of 9:1. Macrocyclization through formation of the C6–C7 bond was achieved in 68% yield and > 20:1 d.r. from precursor 23, which was formed by a Yamaguchi esterification with alcohol 22.
Scheme 9.
Synthetic strategies toward neopeltolide by Lee et al. Bn = benzyl, TMS = trimethylsilyl.
Lee et al. realized an innovative strategy of generating two different macrocyclization precursors (20 and 23) from a common advanced intermediate (19). Each of these related compounds were then processed separately to arrive at the desired natural product, thereby providing an excellent example of the powerful and versatile nature of the Prins macrocyclization strategy.
2.1.3. Formal Synthesis of Neopeltolide by Yadav and Kumar
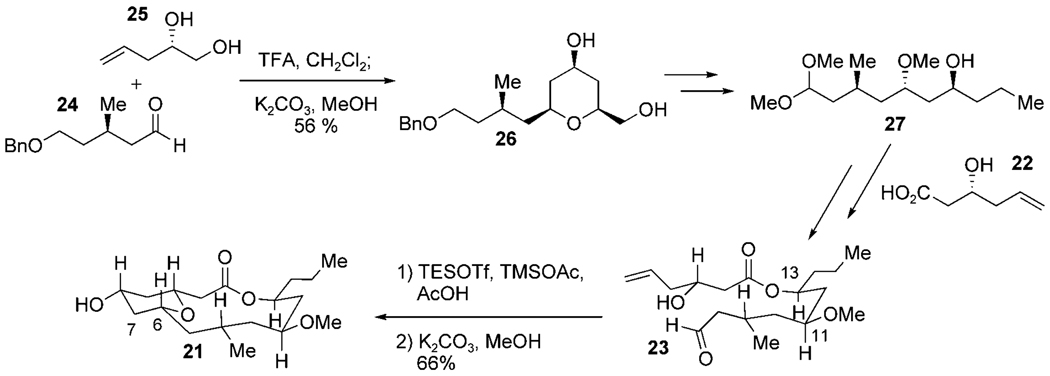
In November 2009, Yadav and Kumar reported a formal synthesis of neopeltolide, in which the C6–C7 bond was formed by a Prins macrocyclization in a similar manner to Lee’s strategy.[38] The major difference was the manner in which the stereochemistry of the macrocyclization precursor 23 was established (Scheme 10).
Scheme 10.
Formal synthetic strategy toward neopeltolide by Yadav and Kumar. TFA = trifluoroacetic acid.
Aldehyde 24 was derived from (S)-citronellol and diol 25 was built by a Jacobsen hydrolytic kinetic resolution of (±)-epichlorohydrin followed by a copper(I)-catalyzed regioselective epoxide opening with vinyl magnesium bromide. A Prins cyclization of these two fragments afforded the tetrahydropyran 26 in moderate yield, setting the C11 and C13 stereocenters in a novel manner. Further manipulation of this fragment gave alcohol 27, which was coupled to acid 22 (made in a similar manner to diol 25) under Steglich conditions. Submission of macrocyclization precursor 23 to Lee’s conditions afforded macrocycle 21 in an expected 66% yield.
2.2. Synthesis of Bryostatin Analogues by the Wender Group
The bryostatins are a large class of complex natural products isolated starting in the late 1960s from the marine bryozoan Bulgula neritina.[41] The bryostatins share a 26-membered macrolide core, three highly substituted tetrahydropyran rings with at least one appended exocyclic enoate, and at least 11 stereogenic centers. The most extensively studied member of the bryostatin family is bryostatin 1, (35; see Scheme 12) which exhibits multiple potent biological activities arising from its ability to modulate protein kinase C (PKC).[42] It exhibits potent anticancer activity owing to its ability to reverse drug resistance, stimulate the immune system, and restore apoptotic function. It has also been shown to enhance cognition and restore memory in mammals,[43] suggesting its potential use to treat neurological disorders such as depression and Alzheimer’s disease.[44] Despite the remarkable biological activity of the bryostatins, only a few clinical leads of these compounds are available because of their natural scarcity and due to the paucity of facile and scalable routes to these molecules.[45] In an effort to address this problem, Wender and co-workers reported a Prins macrocyclization strategy in May 2008 as an alternative to the reportedly problematic Julia olefination/lactonization sequence most commonly used in syntheses of the bryostatins and their analogues.
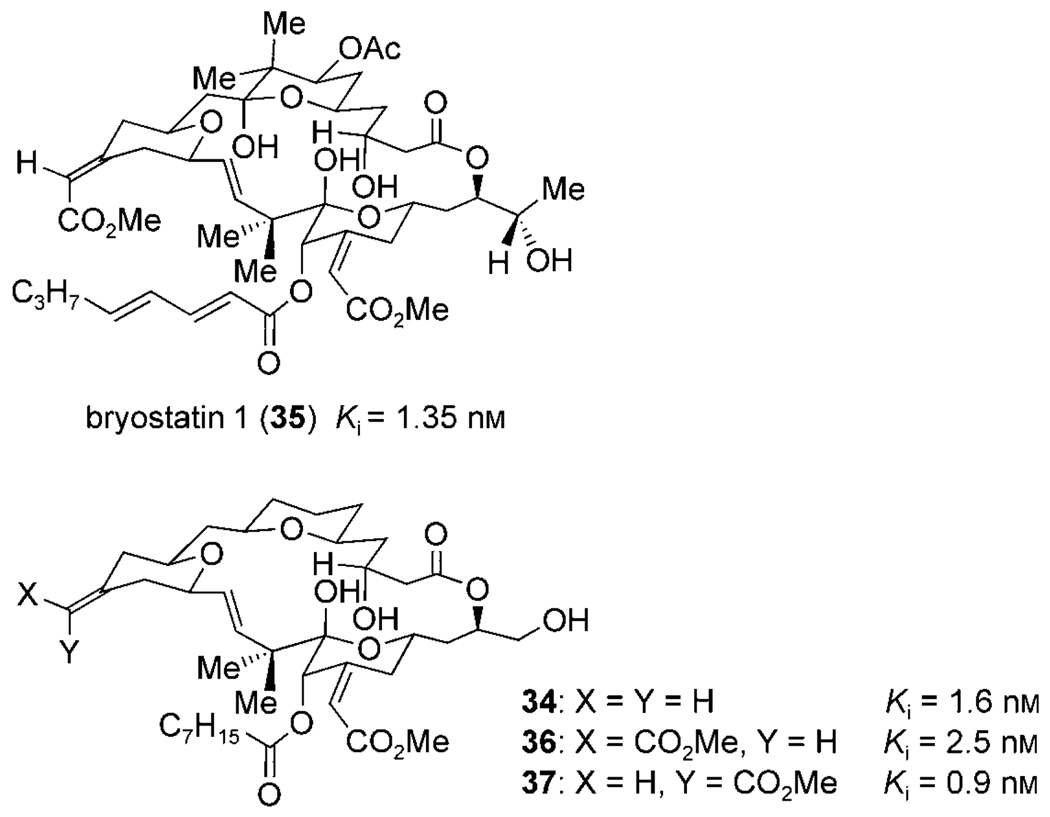
Scheme 12.
Comparison of PKC Ki values for bryostatin 1 and bryostatin analogues.
Wender et al. utilized the previously synthesized intermediates pyran acid 31 and pyran alcohol 32, with just a few modifications of the acid to install the allyl silane moiety (Scheme 11). They established the stereochemistry about pyran acid 31 through a series of a Noyori asymmetric diketone hydrogenations after the assembly of commercially available acyl chloride 28, butanone 29, and ethyl acetoacetate.[46] Pyran alcohol 32 was accessed from commercially available neopentylglycol 30 through a series of transformations including an asymmetric Keck allylation, a diastereoselective reduction, and a Sharpless asymmetric dihydroxylation.[47] The combination of the two pieces by a Yamaguchi esterification, deprotection of the TES-protected alcohol, and treatment with TMSOTf for global desilylation afforded the bryostatin analogue 34.
Scheme 11.
Synthetic strategy toward bryostatin analogues by Wender et al. TBDPS = tert-butyldiphenylsilyl.
Chemoselective, oxidative cleavage of the exocylic olefin of macrocycle 34 and olefination afforded E and Z enoate analogues, 36 and 37. All three bryostatin analogues were shown to have potent binding affinity for protein kinase C (Scheme 12) and, when tested further for antileukemia activity, exhibited nanomolar and subnanomolar EC50 values against K562 human erythroleukemia and MV411 B-myelomonocytic leukemia cell lines. Wender et al. noted in their conclusions that the remarkable functional-group tolerance and efficiency of their Prins-driven macrocyclization approach allowed them to produce bryostatin analogues that were the most active compounds at that time; their biological activity exceeded that of their previously synthesized, most potent analogues by two orders of magnitude.
2.3. Formal Synthesis of Kendomycin by Rychnovsky and Bahnck
In 1996, kendomycin (43; Scheme 13) was isolated from various Streptomyces bacteria and was shown to exhibit broad-spectrum antibacterial activity again MRSA and VRSA (MRSA: methicillin-resistant Staphylcoccus aureus, VRSA: vancomycin-resistant Staphylcoccus aureus) strains and had potent cytotoxicity against carcinoma cells lines with GI50 values < 100 nm.[48] The all-carbon 18-membered polyketide macrocycle, p-quinone methide moiety, and fully substituted tetrahydropyran ring proved particularly challenging. Prior syntheses by the Mulzer,[49] Smith,[50] and Arimoto groups[51] had utilized a late-stage ring-closing metathesis macrocyclization strategy that proved problematic in both closing the macrocycle and generating the trisubstituted olefin of the macrocycle with good control of the resulting alkene geometry. To avoid this issue, Bahnck and Rychnovsky took a Prins macrocyclization approach in their formal synthesis of kendomycin, which was reported in September 2008.[52]
Scheme 13.
Formal synthetic strategy toward kendomycin by Rychnovsky and Bahnck.
A Suzuki–Miyaura coupling of homoallylic alcohol 38 and the boronic acid generated from alkyl iodide 39 assembled the main core of the macrocyclization precursor, which was further manipulated to install the C5 aldehyde function. A selective sulfonylation of the C4 phenol group then gave precursor 40 (Scheme 13). They found that the benzenesulfonyl group was essential for the Prins cyclization to proceed efficiently, which afforded both the acetate and fluorine products, 41 and 42, in yields of 33% and 48%, respectively. The addition of this unusual phenol protecting group presumably enhances the electron-deficient nature of the aldehyde and promotes formation of the macrocyclic oxocarbenium ion. Without the sulfonyl unit, various exchanged-type products were observed (see Scheme 4). Macrocycles 41 and 42 were then transformed by ethanolysis into the same advanced intermediate utilized in Lee’s synthesis,[53] which could then be easily made into (−)-kendomycin (43) in just three steps. The strength in Bahnck and Rychnovsky’s Prins macrocyclization strategy is that it greatly simplified the construction of kendomycin by simultaneously forming the THP ring, three stereocenters, and the macrocycle in high yield, thereby avoiding intermediates prone to C5–C4a atropisomerism.
2.4. Studies towards Clavosolide A by the Rychnovsky Group
Clavosolide A (44; Scheme 14) was isolated in 2002 from the marine sponge Myriastra clavosa off the coast of the Philippines and was originally shown to be noncytotoxic. However, the limited natural availability of the compound prevented further biological studies.[54] This 20-membered diolide contains two disubstituted cyclopropane rings, two substituted tetrahydropyran rings, and two permethylated d-xylose moieties. In general, common synthetic approaches to macrodiolides involve tandem dimerization/macrocyclization reactions, including double esterification or Suzuki coupling followed by olefin metathesis. Rychnovsky and co-workers set out to develop an elegant strategy based on a Prins cyclization/macrocyclization sequence to enable facile access to natural products such as clavosolide A.[55] In October 2009, they reported a successful Prins dimerization and macrocyclization of model system 46 by utilizing the reliable macrocyclization conditions of TESOTf in acetic acid to afford the desired dimer 27 in a 43%yield (Scheme 14). They found that the dimethyl acetal was essential in this process because of the instability of the aldehyde precursor. Thus, Rychnovsky et al. successfully developed a powerful Prins macrocyclization strategy and applied it toward a novel dimerization/macrocyclization route for the synthesis of macrodiolide natural products.
Scheme 14.
Synthetic studies toward clavosolide A by Rychnovsky et al. Cy = cyclohexyl.
2.5. Total Synthesis of Polycavernoside A by Lee and Woo
The most recent application of a Prins-driven macrocyclization is Woo and Lee’s total synthesis of polycavernoside A (54; Scheme 15), reported in March 2010.[56] In Guam during 1991, thirteen people fell ill and three died after experiencing symptoms of intoxication subsequent to ingesting the red alga Polycavernosa tsudai.[57] In 1993, polycavernoside A was extracted from the red alga and confirmed as the toxin that caused the illness. A combination of the limited natural availability, an incomplete structural assignment, and the puzzling but potent bioactivity of the marine macrolide called for efficient total syntheses so that it could be further studied.
Scheme 15.
Synthetic strategy toward polycavernoside A by Lee and Woo. PMB = para-methoxybenzyl.
This 16-membered macrolide contains a tetrahydropyran ring with an attached fucosyl–xylosyl disaccharide moiety, as well as a triene side chain appended to the macrolide. Prior syntheses by the Murai,[58] Paquette[59] and White groups[60] had utilized a macrolactonization strategy, while Woo and Lee sought to take a Prins macrocyclization approach. The macrocyclization precursor 52 was assembled swiftly in 12 steps from four basic fragments and submitted to the already proven macrocyclization conditions to afford macrolide 53 in 69% yield and 5.5:1 d.r. (Scheme 15). This was actually a secondary strategy, as the original approach had an acetate protecting group on the alcohol at C13. Under the same macrocyclization conditions with this protected substrate, they achieved an 85% yield and a 6:1 d.r. of the desired macrocyclic product. This serves as another example of how the convergency of this Prins macrocyclization strategy is particularly useful in polyketide natural product synthesis when the synthetic route needs to be altered.
3. Summary and Outlook
The Prins cyclization has emerged as a powerful merged C–O and C–C bond-forming macrocyclization technique in the synthesis of polyketide natural products. This process has been successful in closing 12- to 20-membered macrocycles with the simultaneous formation of a tetrahydropyran ring with various substitution patterns. Even though unexpected side products and loss of stereochemical information have been observed during the Prins cyclization, extensive studies have resulted in an arsenal of conditions and approaches to suppress such pathways. This strategy overcomes inherent reactivity issues along with the challenges associated with creating macrocyclic oxocarbenium ions, and has been successfully applied to multiple natural products with more undoubtedly to come. The examples reported herein document the expanding versatility and breadth of functional-group tolerance for the overall process. Additionally, the various ring sizes that have formed thuso far clearly indicate that smaller and larger macrocycles are possible. Overall, the resurgence of the Prins macrocyclization in the past two years reflects the capacity of this approach to construct a tetrahydropyran ring simultaneously with a macrocycle in a convergent, selective, and high-yielding manner. The application of this strategy toward the synthesis of bioactive macrocycles will undoubtedly continue, thereby firmly establishing it as a powerful method to form these important small molecules.
Acknowledgments
We thank all current and former Scheidt group members for their suggestions and dedication. Financial support for this work was provided in part by NIGMS (CMLD p50 GM086145) and the NCI (R01 CA126827), Amgen, the Sloan Foundation, GlaxoSmithKline, and AstraZeneca. E.A.C. thanks the Malkin Scholars Program for support administered through the Robert H. Lurie Comprehensive Cancer Center at Northwestern University.
Biographies

Karl Scheidt earned his PhD from Indiana University in 1999 under the supervision of William Roush. After an NIH postdoctoral fellowship at Harvard University in David Evans’s group, he joined the faculty of Northwestern University (Evanston, IL, USA) in 2002. His research focuses on the development of new organic methodology and the synthesis of bioactive molecules. He is a Sloan Foundation Fellow and currently holds the Irving Klotz Research Chair in Chemistry as well as the Alumnae of Northwestern Teaching Professorship.

Erika Crane, born in Kettering, Ohio (USA) in 1985, did undergraduate research with Professor Robert Coleman at The Ohio State University and received her BSc from Ohio Northern University in 2007. Since the fall of 2007, her research in the Scheidt group has focused on the synthesis of polyketide natural products and the development of methodology to access biologically relevant heterocyclic cores in order to construct small-molecule libraries of novel structures.
Footnotes
Dedicated to Professor David A. Evans
References
- 1.Wessjohann LA, Ruijter E. Top. Curr. Chem. 2005;234:137–184. [Google Scholar]
- 2.Driggers EM, Hale SP, Lee J, Terrett NK. Nat. Rev. Drug Discovery. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 3.The following is not a comprehensive review of all macrocyclization strategies. Please refer to the reviews cited herein for a comprehensive account of various macrocyclization techniques.
- 4.Parenty A, Moreau X, Campagne JM. Chem. Rev. 2006;106:911–939. doi: 10.1021/cr0301402. [DOI] [PubMed] [Google Scholar]
- 5.Stang EM, White MC. Nat. Chem. 2009;1:547–551. doi: 10.1038/nchem.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao AVR, Gurjar MK, Reddy KL, Rao AS. Chem. Rev. 1995;95:2135–2167. [Google Scholar]
- 7.Boger DL, Nomoto Y, Teegarden BR. J. Org. Chem. 1993;58:1425–1433. [Google Scholar]
- 8.Beugelmans R, Singh GP, Bois-Choussy M, Chastanet J, Zhu JP. J. Org. Chem. 1994;59:5535–5542. [Google Scholar]
- 9.Rao AVR, Reddy KL, Rao AS. Tetrahedron Lett. 1994;35:8465–8468. [Google Scholar]
- 10.a) Gradillas A, Perez-Castells J. Angew. Chem. 2006;118:6232–6247. doi: 10.1002/anie.200600641. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:6086–6101. doi: 10.1002/anie.200600641. [DOI] [PubMed] [Google Scholar]; b) Majumdar KC, Rahaman H, Roy B. Curr. Org. Chem. 2007;11:1339–1365. [Google Scholar]
- 11.a) Fu GC, Grubbs RH. J. Am. Chem. Soc. 1992;114:5426–5427. [Google Scholar]; b) Fu GC, Grubbs RH. J. Am. Chem. Soc. 1992;114:7324–7325. [Google Scholar]
- 12.a) Martin SF, Liao YS, Wong Y, Rein T. Tetrahedron Lett. 1994;35:691–694. [Google Scholar]; b) Martin SF, Liao YS, Chen HJ, Patzel M, Ramser MN. Tetrahedron Lett. 1994;35:6005–6008. [Google Scholar]
- 13.Borer BC, Deerenberg S, Bieraugel H, Pandit UK. Tetrahedron Lett. 1994;35:3191–3194. [Google Scholar]
- 14.Xu Z, Johannes CW, Houri AF, La DS, Cogan DA, Hofilena GE, Hoveyda AH. J. Am. Chem. Soc. 1997;119:10302–10316. [Google Scholar]
- 15.Monfette S, Fogg DE. Chem. Rev. 2009;109:3783–3816. doi: 10.1021/cr800541y. [DOI] [PubMed] [Google Scholar]
- 16.Giesbrecht HE, Knight BJ, Tanguileg NR, Emerson CR, Blakemore PR. Synlett. 2010:374–378. [Google Scholar]
- 17.Morin-Fox ML, Lipton MA. Tetrahedron Lett. 1993;34:7899–7902. [Google Scholar]
- 18.Smith AB, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1995;117:5407–5408. [Google Scholar]
- 19.a) Bloys van Treslong Prins PC. Chem. Weekbl. 1919:1510. [Google Scholar]; b) Bloys van Treslong Prins PC. Chem. Weekbl. 1919:1072. [Google Scholar]
- 20.a) Olier C, Kaafarani M, Gastaldi S, Bertrand MP. Tetrahedron. 2010;66:413–445. [Google Scholar]; b) Pastor IM, Yus M. Curr. Org. Chem. 2007;11:925–957. [Google Scholar]; c) Adams DR, Bhatnagar SP. Synthesis. 1977:661–672. [Google Scholar]
- 21.Hanschke E. Chem. Ber. 1955;88:1053–1061. [Google Scholar]
- 22.Alder RW, Harvey JN, Oakley MT. J. Am. Chem. Soc. 2002;124:4960–4961. doi: 10.1021/ja025902+. [DOI] [PubMed] [Google Scholar]
- 23.Jasti R, Vitale J, Rychnovsky SD. J. Am. Chem. Soc. 2004;126:9904–9905. doi: 10.1021/ja046972e. [DOI] [PubMed] [Google Scholar]
- 24.Lolkema LDM, Hiemstra H, Semeyn C, Speckamp WN. Tetrahedron. 1994;50:7115–7128. [Google Scholar]
- 25.Roush WR, Dilley GJ. Synlett. 2001:955–959. [Google Scholar]
- 26.a) Rychnovsky SD, Marumoto S, Jaber JJ. Org. Lett. 2001;3:3815–3818. doi: 10.1021/ol0168465. [DOI] [PubMed] [Google Scholar]; b) Jasti R, Anderson CD, Rychnovsky SD. J. Am. Chem. Soc. 2005;127:9939–9945. doi: 10.1021/ja0518326. [DOI] [PubMed] [Google Scholar]
- 27.Crosby SR, Harding JR, King CD, Parker GD, Willis CL. Org. Lett. 2002;4:577–580. doi: 10.1021/ol0102850. [DOI] [PubMed] [Google Scholar]
- 28.Jasti R, Rychnovsky SD. J. Am. Chem. Soc. 2006;128:13640–13648. doi: 10.1021/ja064783l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulte-Elte KH, Hauser A, Ohloff G. Helv. Chim. Acta. 1979;62:2673–2680. [Google Scholar]
- 30.Macrocycle size as determined by the all-carbon backbone plus the acylated oxygen atom. Macrocycle sizes for the remainder of the article are defined as such.
- 31.Wright AE, Botelho JC, Guzman E, Harmody D, Linley P, McCarthy PJ, Pitts TP, Pomponi SA, Reed JK. J. Nat. Prod. 2007;70:412–416. doi: 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- 32.Ulanovskaya OA, Janjic J, Suzuki M, Sabharwal SS, Schumacker PT, Kron SJ, Kozmin SA. Nat. Chem. Biol. 2008;4:418–424. doi: 10.1038/nchembio.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Custar DW, Zabawa TP, Hines J, Crews CM, Scheidt KA. J. Am. Chem. Soc. 2009;131:12406–12414. doi: 10.1021/ja904604x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vintonyak VV, Maier ME. Org. Lett. 2008;10:1239–1242. doi: 10.1021/ol8001255. [DOI] [PubMed] [Google Scholar]
- 35.Custar DW, Zabawa TP, Scheidt KA. J. Am. Chem. Soc. 2008;130:804–805. doi: 10.1021/ja710080q. [DOI] [PubMed] [Google Scholar]
- 36.Panek et al. reported the first total synthesis and structural revision of neopeltolide: See: Youngsaye W, Lowe JT, Pohlki F, Ralifo P, Panek JS. Angew. Chem. 2007;119:9371–9374. doi: 10.1002/anie.200704122. Angew. Chem. Int. Ed. 2007;46:9211–9214. doi: 10.1002/anie.200704122..
- 37.Woo SK, Kwon MS, Lee E. Angew. Chem. 2008;120:3286–3288. [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:3242–3244. doi: 10.1002/anie.200800386. [DOI] [PubMed] [Google Scholar]
- 38.Yadav JS, Kumar GGKSN. Tetrahedron. 2010;66:480–487. [Google Scholar]
- 39.a) Larcheveque M, Ignatova E, Cuvigny T. Tetrahedron Lett. 1978;19:3961–3964. [Google Scholar]; b) Myers AG, Yang BH, Chen H, McKinstry L, Kopecky DJ, Gleason JL. J. Am. Chem. Soc. 1997;119:6496–6511. [Google Scholar]
- 40.Villano R, Acocella MR, De Rosa M, Soriente A, Scettri A. Tetrahedron: Asymmetry. 2004;15:2421–2424. [Google Scholar]
- 41.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. J. Am. Chem. Soc. 1982;104:6846–6848. [Google Scholar]
- 42.Szallasi Z, Smith CB, Pettit GR, Blumberg PM. J. Biol. Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- 43.Hongpaisan J, Alkon DL. Proc. Natl. Acad. Sci. USA. 2007;104:19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Proc. Natl. Acad. Sci. USA. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wender PA, DeChristopher BA, Schrier AJ. J. Am. Chem. Soc. 2008;130:6658–6659. doi: 10.1021/ja8015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wender PA, Mayweg AVW, VanDeusen CL. Org. Lett. 2003;5:277–279. doi: 10.1021/ol0272390. [DOI] [PubMed] [Google Scholar]
- 47.Wender PA, Baryza JL, Bennett CE, Bi FC, Brenner SE, Clarke MO, Horan JC, Kan C, Lacote E, Lippa B, Nell PG, Turner TM. J. Am. Chem. Soc. 2002;124:13648–13649. doi: 10.1021/ja027509+. [DOI] [PubMed] [Google Scholar]
- 48.Bode HB, Zeeck A. J. Chem. Soc. Perkin Trans. 2000;1:323–328. [Google Scholar]
- 49.Mulzer J, Pichlmair S, Green MP, Marques MMB, Martin HJ. Proc. Natl. Acad. Sci. USA. 2004;101:11980–11985. doi: 10.1073/pnas.0401503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith AB, Mesaros EF, Meyer EA. J. Am. Chem. Soc. 2006;128:5292–5299. doi: 10.1021/ja060369+. [DOI] [PubMed] [Google Scholar]
- 51.Sengoku T, Uemura D, Arimoto H. Chem. Lett. 2007;36:726–727. [Google Scholar]
- 52.Bahnck KB, Rychnovsky SD. J. Am. Chem. Soc. 2008;130:13177–13181. doi: 10.1021/ja805187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan Y, Men HB, Lee CB. J. Am. Chem. Soc. 2004;126:14720–14721. doi: 10.1021/ja0447154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson KL, Gustafson KR, Pannell LK, Beutler JA, Boyd MR. J. Nat. Prod. 2002;65:1303–1306. doi: 10.1021/np020193z. [DOI] [PubMed] [Google Scholar]
- 55.Gesinski MR, Tadpetch K, Rychnovsky SD. Org. Lett. 2009;11:5342–5345. doi: 10.1021/ol9022062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woo SK, Lee E. J. Am. Chem. Soc. 2010;132:4564–4565. doi: 10.1021/ja1009579. [DOI] [PubMed] [Google Scholar]
- 57.Yotsu-Yamashita M, Haddock RL, Yasumoto T. J. Am. Chem. Soc. 1993;115:1147–1148. [Google Scholar]
- 58.Fujiwara K, Murai A, Yotsu-Yamashita M, Yasumoto T. J. Am. Chem. Soc. 1998;120:10770–10771. [Google Scholar]
- 59.Paquette LA, Barriault L, Pissarnitski D. J. Am. Chem. Soc. 1999;121:4542–4543. [Google Scholar]
- 60.White JD, Blakemore PR, Browder CC, Hong J, Lincoln CM, Nagornyy PA, Robarge LA, Wardrop DJ. J. Am. Chem. Soc. 2001;123:8593–8595. doi: 10.1021/ja011256n. [DOI] [PubMed] [Google Scholar]