Abstract
DPC4 is known to mediate signals initiated by type β transforming growth factor (TGFβ) as well as by other TGFβ superfamily ligands such as activin and BMP (bone morphogenic proteins), but mutational surveys of such non-TGFβ receptors have been negative to date. Here we describe the gene structure and novel somatic mutations of the activin type I receptor, ACVR1B, in pancreatic cancer. ACVR1B has not been described previously as a mutated tumor-suppressor gene.
Smad4 (Dpc4/Madh4) is a mediator of a tumor-suppressive signaling pathway initiated upon binding of the type β transforming growth factor (TGFβ) ligands to its cell surface receptors and the subsequent phosphorylation of pathway-specific Smad proteins (1, 2). The genetic inactivation of the MADH4, MADH2, and TGFβ receptor genes in human tumors has confirmed this pathway to be responsible for tumor suppression (3–6). Smad4 is also known to mediate signals initiated by other TGFβ superfamily ligands such as activin and BMP (bone morphogenic proteins) (1), but mutational surveys of such non-TGFβ receptors had been negative to date (6). Studies of mutational patterns of known suppressor genes in pancreatic cancer and of the biological responsiveness of pancreatic cancer cells suggested the continued attractiveness of seeking additional mutational targets within these related pathways. Here, mutations of the activin type I receptor are described.
Materials and Methods
Tissue Samples and Cell Lines.
Cancers of the pancreas and distal common bile duct resected at The Johns Hopkins Hospital between 1992 and 1997 were xenografted as described (7). In addition, at the time of the surgery, resected normal duodenum was frozen and stored at −80°C. The breast cell line MDA-MB-468 and pancreatic cell lines Su86.86, CFPAC-1, AsPC-1, CaPan-1, CaPan-2, Panc1, MiaPaCa2, BxPC3, and Hs766T were purchased from American Type Culture Collection. Colo357 was obtained from the European Collection of Animal Cell Cultures. Pancreatic cell line PL45 was established in our laboratory (7).
DNA Analysis.
Genomic DNA samples (40 ng per sample) were screened for homozygous deletions by PCR as described (7, 8). Loss of heterozygosity (LOH) was determined by using three polymorphic markers. The criteria for LOH was previously described (8). All samples that had LOH were subject to sequencing. Each exon was amplified by PCR from genomic DNA, treated with exonuclease I and shrimp alkaline phosphatase (United States Biochemical), and subjected to manual cycle-sequencing (ThermoSequenase, Amersham). Sequencing of the homozygous deletion junction and the MADH4 gene were done by an automated DNA sequencer (PE-Biosystems) and analyzed by sequencher software (Gene Codes Corporation).
DNA Constructs and Transfection Assay.
p6SBE-luc was engineered by inserting six copies of the palindromic SBE (Smad-binding element) behind the minimal simian virus 40 promoter in the pGL3-promoter vector (9) (Promega). The MADH4 cDNA was subcloned into pcDNA3.1 (Invitrogen), resulting in pDPC4-WT (9). Expression vectors containing active forms of the TSR-I (ALK1), ActR-I (ALK2), BMPR-1A (ALK3), ACVR1B (ALK4), TGFBR1 (ALK5), and BMPR-1B (ALK6) genes were gifts from Jeff Wrana (Univ. of Toronto). All are hemagglutinin-tagged cDNA sequences driven by the cytomegalovirus promoter. Constitutive activation is provided by acidic substitutions (Q to D in the GS domain of ALK1, -2, -3, and -6, and T to D at codon 206 of ALK4 and at codon 206 of ALK5) (10, 11). Each transient transfection experiment was done in duplicate in six-well plates as described (9). Lipofectamin (Life Technologies) was used as directed by the manufacturer. The DNA-Lipofectamin mixture was removed from cells after 4–5 h of transfection, and culture medium with or without 0.1 ng/ml human recombinant TGFβ1 (Sigma) was then added to the cells. Sixteen to eighteen hours from the start of the transfection, cell lysates were prepared with Reporter Lysis Buffer (Promega) for luciferase and β-galactosidase assays. Luciferase was measured by using the Luciferase Assay System (Promega), and the β-galactosidase assay was performed as described (9). All cultures within an experiment were transfected with the same total amount of plasmid; pcDNA3.1 parental expression vector was added as needed to equalize cotransfection of expression vectors.
Immunohistochemistry.
Unstained 5-μm sections were cut from the paraffin blocks and deparaffinized by using standard methods. Slides were processed and labeled with monoclonal antibody to Dpc4 (clone B8, Santa Cruz) as described (12). Slides were reviewed by three of the authors (E.M., R.H.H., and S.E.K.) and recorded as positive or negative for both nuclear and cytoplasmic labeling as has been described (12). Focal labeling was interpreted as positive. Stromal cells served as a positive control, and the primary antibody was omitted in negative controls. Pancreatic carcinomas with known Dpc4 genetic status were also included as positive and negative controls (12).
Results
The Effects of Activated Acvr1b on Activation of an SBE Reporter.
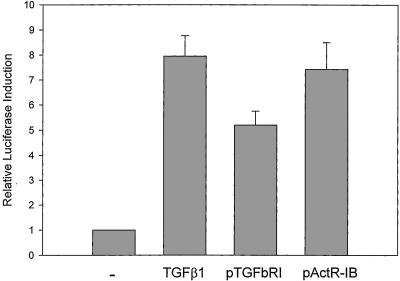
The underexpression of TGFβ receptors is common among pancreatic cancers, but the genetic structure and expression of the receptor-activated MADH genes (Smads 1–3) is intact in these cells (13, 14). Therefore, to bypass any receptor defects, we chose to compare the relative levels of Smad4 nuclear localization achieved by transfection of constitutively active forms of the TSR-I (ALK1), ActR-I (ALK2), BMPR-1A (ALK3), ACVR1B (ALK4), TGFBR1 (ALK5), and BMPR-1B (ALK6) genes (10, 11, 15, 16). We used the SBE reporter, which expresses upon the nuclear localization of Smad4 (17), a common feature of all Smad-mediated signal transduction studied to date (9). The activated Acvr1b and Tgfbr1 receptors caused efficient transcription from the SBE reporter in Panc-1 pancreas cancer cells (Fig. 1). Other activated receptors induced less than 2-fold responses under the same conditions (data not shown). This Acvr1b- and Tgfbr1-induced activation of the SBE reporter was absent in MDA-MB-468 cells that lack Smad4 (ref. 18 and data not shown). We also studied the ability of pancreatic cancer cells to respond to hormones that are present in high amounts within pancreatic parenchyma, at levels approaching 100 times that of peripheral blood, and others, including insulin, VIP, somatostatin, glucagon, epidermal growth factor, estradiol, hydrocortisone, progesterone, pancreatic polypeptide, and secretin. No hormone-induced Smad activation was observed in Panc-1 cells that contained a stably integrated SBE reporter (ref. 19 and data not shown). These cells used with equal efficiency the TGFβ- and activin-receptor signals, but not the other signaling systems tested, to activate the SBE reporter.
Figure 1.
Similarity of Tgfbr1 and Acvr1b effects on activation of the SBE reporter. Panc-1 cells were transfected with the Smad reporter, p6SBE-luc, and with various activated forms of the TGFβ superfamily type I receptors or exposed to TGFβ. The. activated TGFBR1 (ALK5) and ACVR1B (ALK4) genes caused 5-fold or greater induction of the Smad reporter in Panc-1 cells. —, Transfection of parental vector; TGFβ1, transfection of parental vector and addition of TGFβ1; pTGFbRI and pActR-IB, transfection of expression vectors for TGFBR1 and ACVR1B, respectively. Data represent averages of two experiments and SEM.
Somatic Mutations of ACVR1B in Pancreatic Adenocarcinomas.
The activin type I receptor, ACVR1B, is located on human chromosome 12q13. It had not been studied for mutations, despite the strong structural and functional similarities of the activin and TGFβ receptors. We determined the genomic structure of ACVR1B through the sequencing of PAC clones and a BLASTN search of the National Center for Biotechnology Information (NCBI) Database (http://www.ncbi.nlm.nih.gov:80/BLAST/). Two unordered sequences were found to contain ACVR1B sequences (GenBank entries AC019244 and AC025259). ACVR1B comprises 9 exons and spans over 23 kb (Fig. 2). The size of the intron between exons 1 and 2 remains unresolved. We screened for homozygous deletions among 95 pancreatic adenocarcinoma xenografts by using primers specific for exons 2, 5, and 8. A homozygous deletion of 657 bp, including the entire exon 8 of ACVR1B, was detected in xenograft PX226 (also producing a frame-shift if splicing of exons 7 to 9 were to occur) (Fig. 3a and Table 1). The mutation was somatic and was verified by the study of genomic DNA of the normal tissue and primary cancer specimens of the patient (Fig. 3a). The precise structure of the deletion was determined by sequencing across the deletion in the xenograft tumor (Fig. 3 a and b). The deletion appeared to be a result of slippage during DNA replication that occurred at a 4-nt repeated sequence (5′-ggct-3′) (Fig. 3b). The panel of pancreatic cancer xenografts was further analyzed for LOH and intragenic mutations.
Figure 2.
The genomic structure of ACVR1B. Intron/exon boundaries were determined through the sequencing of PAC clones and from GenBank entries AC019244 and AC025259. The first coding exon was assigned as exon 1. The actual size of intron 1 remains undetermined. Exons are shaded.
Figure 3.
Coexistent homozygous deletion of ACVR1B and somatic mutation of MADH4. Pancreatic cancer xenograft, PX226, had a 657-bp deletion and LOH affecting the ACVR1B gene and a nonsense mutation and LOH of the MADH4 gene. The somatic mutation deleted the entire exon 8 of the ACVR1B gene. The deletion was mapped by PCR (a) and verified by sequencing (b). (a) PCR products of the normal DNA of two patients (N; lanes 1, 6, and 11 from a second patient, lanes 2, 7, and 12 from the patient of PX226), the xenograft PX226 (X; lanes 3, 8, and 13), the primary cancer of the patient (C; lanes 4, 9, and 14), and a negative control lacking template (—; lanes 5, 10, and 15) using three primer sets. Gene segments are numbered according to a published ACVR1B sequence (GenBank entry L31848). (b) Genomic PCR products were sequenced with a nested primer. The size of the deletion was determined according to the most current sequences of the ACVR1B gene (GenBank entry AC019244). (c) Genomic PCR products of the MADH4 locus from the xenograft PX226 and its corresponding normal were sequenced with a nested primer. The nonsense mutation was confirmed in the primary cancer of the patient (data not shown).
Table 1.
ACVR1B gene mutations in pancreatic cancers
| Tumor | Allelic status | Mutation site | Gene alteration | Predicted product | Origin of mutation |
|---|---|---|---|---|---|
| PX224 | LOH | Codon 387, exon 7 | CTT GATGAA ACC to CTT AAC | Frame-shift | Somatic |
| PX226 | LOH | Exon 8 | Homozygous deletion | Deletion, frame-shift | Somatic |
LOH involving the ACVR1B locus was determined with the polymorphic markers D12S368, D12S390, and D12S359. LOH was found in 29 of the 85 pancreatic cancer xenografts (34%) and in 5 of the 11 pancreatic cancer cell lines (45%). All coding sequences and splice junctions of the ACVR1B gene (except exon 1, which was incompletely studied because of its high GC content) in the 34 selected cancers exhibiting LOH were then sequenced. A 5-bp deletion that would cause a frame-shift and early termination of protein translation was detected in xenograft PX224 (Table 1). The mutation was somatic and was confirmed in the corresponding primary cancer tissue from the patient. Both mutations eliminated part of the kinase domain of Acvr1b (20). The cytoplasmic kinase domains of Tgfbr1 and Tgfbr2 (the TGFβ receptors types I and II, respectively) are both required in efficient TGFβ signal transduction (21), and Acvr1b is expected to function similarly.
Coexistent Mutations of ACVR1B and MADH4 in Pancreatic Adenocarcinomas.
The primary tumors of PX224 and PX226, which harbored an inactivated ACVR1B gene, were examined immunohistochemically for their Madh4 status. The samples were immunohistochemically negative for Smad4/Dpc4/Mahd4 expression in the neoplastic cells of the tumors (Fig. 4 b and c). Sequencing analysis of the MADH4 gene revealed a nonsense mutation in the exon 5 (codon 245) of the MADH4 gene in PX226 (Fig. 3c). The somatic mutation was confirmed in the primary tumor of the patient (data not shown). Neither homozygous deletions nor LOH were detected at the TGFBR1 and TGFBR2 genes of these two samples (6), suggesting that the MADH4 inactivation of these two tumors may have served as the means to ablate the TGFβ pathway.
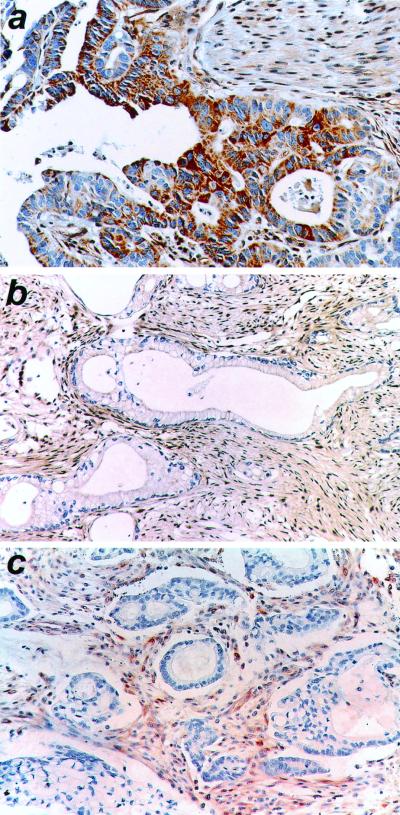
Figure 4.
The absence of Smad4 expression in ACVR1B-mutant tumors. Smad4 expression was present in the ductal epithelium of a normal pancreas (a) but absent from the two tumors with mutations in the ACVR1B gene (b and c). There was no immunodetectable Smad4 in the nucleus or cytoplasm of tumor cells in the primary carcinomas for xenograft PX224 (b) and xenograft PX226 (c). The desmoplastic stroma, which expressed Smad4, served as an internal control. Immunohistochemistry using anti-Smad4 antibody, counterstained with hematoxylin.
Discussion
Here we provide the first genetic evidence from human tumors to support ACVR1B as a tumor-suppressor gene. The identification of signaling pathways that link known oncogenes and tumor-suppressor genes has been a major accomplishment of cancer research. Because the mutations observed are determined by selective pressures, tumor mutations in regulatory pathways are often seen to be reciprocal, i.e., human neoplasms as a rule have activation or inactivation of one, but not more than one, member of certain regulatory pathways. Such examples include the MDM2/p53, the APC/β-catenin, and the p16INK4/CDK4/RB1 pathway relationships (22–25). Multiple considerations, however, had supported a combined input (rather than linear) model for Smad4 tumor-suppression in pancreatic cancer. First, pancreatic cancer arises from an intraductal precursor, PanIN (pancreatic intraepithelial neoplasia), and loss of Smad4 expression is restricted to PanIN-3, the most advanced stage before invasion (26). Second, reports suggest that the inactivation of a TGFβ receptor is not reciprocal with that of MADH4, because some tumors are known to have genetically inactivated both members (6, 27). These findings would fit with the expectations of a combined input model, which could rationalize the observed coexistence of genetic inactivations of these genes. We therefore re-examined the ability of these branches to contribute signals that would be mediated by the MADH4 gene in pancreatic cancers. We searched for such mutations in tumors having intact and those with disrupted MADH4 genes. The coexistence of ALK4 and MADH4 inactivation in two pancreatic adenocarcinomas thus fits well with prior evidence from human tumors regarding mutations in the TGFβ superfamily/Smad system.
These results lead us to propose that during the early stages of pancreatic tumorigenesis, in neoplastic clones still harboring a functional MADH4 gene, mutations or expression defects that impair or obviate the function of either the activin or TGFβ receptors can occur. Inactivation of the activin or TGFβ signaling input offers a selective advantage for a clone that carries this new defect. In this clone, signals of the remaining MADH4-mediated pathway (TGFβ or activin), or perhaps other tumor-suppressive receptor inputs yet to be conclusively demonstrated by the identification of tumor mutations, remain active in their partial suppression of the clone. During these early stages, for reasons that are currently unknown, the loss of Smad4/Dpc4/Madh4 function is detrimental and that MADH4-null clones cannot emerge. The loss of Smad4/Dpc4/Madh4 expression is thus not observed earlier than the late PanIN-3 stage (26). At a very late stage in the intraductal evolution of PanIN, within cells that now harbor multiple genetic defects in cell cycle checkpoints and other regulatory systems, the functional loss of Smad4 ceases to be detrimental but becomes advantageous. All remaining Smad4-mediated signals can then be inactivated by the loss of MADH4. This removes the remaining tumor-suppressive signals provided by the surviving pathway(s). A nonreciprocal pattern of inactivation of pathway members can thereby be demonstrated in some carcinomas, and is produced by the stepwise nature of a process that inactivates a branched pathway tumor-suppressive system.
The low frequency of mutations observed in the TGFBR1, TGFBR2, and ACVR1B genes might be a reciprocal manifestation of the high frequency of expression defects that affect the TGFβ receptors (14). In contrast, the incompleteness of the expression defects and the multiplicity of tumor-suppressive inputs to MADH4 would leave a considerable role for MADH4 in continuing to mediate tumor suppression. Such a role would account for the eventual loss of MADH4 in late-stage PanIN-3, and subsequently a rapid evolution to the invasive and extremely lethal stage, that of pancreatic carcinoma.
The parallel contributions of ACVR1B and TGFBR1 signaling pathway in tumor suppression also raise interest in the activin pathway as a legitimate target for cancer therapy. Early pancreatic neoplasia or other tumor types that share the high frequency of TGFβ-unresponsiveness but low frequency of MADH4 mutations may benefit from stimulation of activin-induced tumor suppression. Activin administration to certain cancer cell lines is known to cause apoptotic and other suppressive effects (28, 29).
Acknowledgments
This work was supported by the National Institutes of Health SPORE (Specialized Program of Research Excellence) in Gastrointestinal Cancer Grant CA 62924 and National Institutes of Health Grant CA 68228.
Abbreviations
- LOH
loss of heterozygosity
- PanIN
pancreatic intraepithelial neoplasia
- SBE
Smad-binding element
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Nature (London) 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Feng X, We R, Derynck R. Nature (London) 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 3.Thiagalingam S, Lengauer C, Leach F S, Schutte M, Hahn S A, Overhauser J, Willson J K V, Markowitz S D, Hamilton S R, Kern S E, et al. Nat Genet. 1996;13:343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan R S, Zborowska E, Kinzler K W, Vogelstein B, et al. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 5.Hahn S A, Schutte M, Hoque A T M S, Moskaluk C A, da Costa L T, Rozenblum E, Weinstein C L, Fischer A, Yeo C J, Hruban R H, Kern S E. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 6.Goggins M, Shekher M, Turnacioglu K, Yeo C J, Hruban R H, Kern S E. Cancer Res. 1998;58:5329–5332. [PubMed] [Google Scholar]
- 7.Caldas C, Hahn S A, da Costa L T, Redston M S, Schutte M, Seymour A B, Weinstein C L, Hruban R H, Yeo C J, Kern S E. Nat Genet. 1994;8:27–31. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- 8.Su G H, Hruban R H, Bova G S, Goggins M, Bansal R K, Tang D T, Shekher M C, Entius M M, Yeo C J, Kern S E. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai J L, Turnacioglu K, Schutte M, Sugar A Y, Kern S E. Cancer Res. 1998;58:4592–4597. [PubMed] [Google Scholar]
- 10.Attisano L, Wrana J L, Montalvo E, Massague J. Mol Cell Biol. 1996;16:1066–1073. doi: 10.1128/mcb.16.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieser R, Wrana J L, Massague J. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilentz R E, Su G H, Dai J L, Sparks A B, Argani P, Sohn T A, Yeo C J, Kern S E, Hruban R H. Am J Pathol. 2000;156:37–43. doi: 10.1016/S0002-9440(10)64703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riggins G J, Kinzler K W, Vogelstein B, Thiagalinbam S. Cancer Res. 1997;57:2578–2581. [PubMed] [Google Scholar]
- 14.Baldwin R L, Friess H, Yokoyama M, Lopez M E, Kobrin M S, Buchler M W, Korc M. Int J Cancer. 1996;67:283–288. doi: 10.1002/(SICI)1097-0215(19960717)67:2<283::AID-IJC21>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Piek E, Afrakhte M, Sampath K, van Zoelen E J, Heldin C H, ten Dijke P. J Cell Physiol. 1999;180:141–149. doi: 10.1002/(SICI)1097-4652(199908)180:2<141::AID-JCP1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama S, Katagiri T, Namiki M, Yamaji N, Yamamoto N, Miyama K, Shibuya H, Ueno N, Wozney J M, Suda T. Exp Cell Res. 1997;235:362–369. doi: 10.1006/excr.1997.3680. [DOI] [PubMed] [Google Scholar]
- 17.Dai J L, Bansal R K, Kern S E. Proc Natl Acad Sci USA. 1999;96:1427–1432. doi: 10.1073/pnas.96.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schutte M, Hruban R H, Hedrick L, Molnar'Nadasdy G, Weinstein C L, Bova G S, Isaacs W B, Cairns P, Nawro H, Sidransky D, et al. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
- 19.Su G H, Sohn T A, Ryu B, Kern S E. Cancer Res. 2000;60:3137–3142. [PubMed] [Google Scholar]
- 20.Carcamo J, Weis F M, Ventura F, Wieser R, Wrana J L, Attisano L, Massague J. Mol Cell Biol. 1994;14:3810–3821. doi: 10.1128/mcb.14.6.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okadome T, Yamashita H, Franzen P, Moren A, Heldin C H, Miyazono K. J Biol Chem. 1994;269:30753–30756. [PubMed] [Google Scholar]
- 22.Leach F S, Tokino T, Meltzer P, Burrell M, Oliner J D, Smith S, Hill D E, Sidransky D, Kinzler K W, Vogelstein B. Cancer Res. 1993;53:2231–2234. [PubMed] [Google Scholar]
- 23.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 24.Hall M, Peters G. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 25.Sherr C J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 26.Wilentz R E, Iacobuzio-Donahue C A, Argani P, McCarthy D M, Parsons J L, Yeo C J, Kern S E, Hruban R H. Cancer Res. 2000;60:2002–2005. [PubMed] [Google Scholar]
- 27.Grady W M, Myeroff L L, Swinler S E, Rajput A, Thiagalingam S, Lutterbaugh J D, Neumann A, Brattain M G, Chang J, Kim S J, et al. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 28.Wang Q F, Tilly K I, Tilly J L, Preffer F, Schneyer A L, Crowley W F, Jr, Sluss P M. Endocrinology. 1996;137:5476–5483. doi: 10.1210/endo.137.12.8940374. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Woodruff T K, Mayo K E. Endocrinology. 2000;141:1263–1272. doi: 10.1210/endo.141.3.7361. [DOI] [PubMed] [Google Scholar]