Abstract
Background:
Joint instability has long been empirically recognized as a leading risk factor for osteoarthritis. However, formal mechanistic linkage of instability to osteoarthritis development has not been established. This study aimed to support a clinically accepted, but heretofore scientifically unproven, concept that the severity and rapidity of osteoarthritis development in unstable joints is dependent on the degree of instability. In a survival rabbit knee model of graded joint instability, the relationship between the magnitude of instability and the intensity of cartilage degeneration was studied at the organ level in vivo.
Methods:
Sixty New Zealand White rabbits received either complete or partial (medial half) transection of the anterior cruciate ligament or sham surgery (control) on the left knee. At the time that the animals were killed at eight or sixteen weeks postoperatively (ten animals for each treatment and/or test-period combination), the experimental knees were subjected to sagittal plane stability measurement, followed by whole-joint cartilage histological evaluation with use of the Mankin score.
Results:
Sagittal plane instability created in the partial transection group was intermediate between those in the complete transection and sham surgery groups. The partial and complete transection groups exhibited cartilage degeneration on the medial femoral and/or medial tibial surfaces. The average histological score (and standard deviation) for the medial compartment in the partial transection group (2.9 ± 0.9) was again intermediate, significantly higher than for the sham surgery group (1.9 ± 0.8) and significantly lower than for the complete transection group (4.5 ± 2.3). The average histological scores for the medial compartment in the partial transection group correlated significantly with the magnitude of instability, with no threshold effect being evident. The significance level of alpha was set at 0.05 for all tests.
Conclusions:
The severity of cartilage degeneration increased continuously with the degree of instability in this survival rabbit knee model of graded instability.
Clinical Relevance:
These results are supportive of the current intuitively based concept for orthopaedic treatment of unstable joints, which is that surgical reconstruction to reduce pathological joint instability contributes to prevention of posttraumatic osteoarthritis regardless of the degree of instability initially present.
Joint instability has been closely associated with the pathogenesis of osteoarthritis, especially following trauma. Abnormal joint laxity resulting from ligament injuries has been regarded as a major cause of posttraumatic osteoarthritis in the knee1-3 and ankle4,5. In highly congruous joints such as the ankle (in which stability under compressive loading is primarily provided by articular surface restraint6), residual incongruity decreases joint stability under weight-bearing conditions, causing cartilage loading aberrations that may accumulatively damage cartilage7.
The terms stability and instability commonly are used to describe the pathological mechanisms of posttraumatic joint disorders. However, the definition of these terms remains nebulous. Burstein and Wright8 defined stability as “the ability of a joint to maintain an appropriate functional position throughout its range of motion. Thus, a joint is stable if, when moving through a normal range of motion, it can carry the required functional loads without pain and produce joint contact forces of normal intensity on its articular cartilage surfaces.” They also said that, for a stable joint, “the application of small additional increments of functional load does not produce sudden, large changes in the position of joint contact. Similarly, small changes in the direction of functional load do not produce large, sudden changes in joint contact position.” Unstable joints, which have lost this self-stabilization function, are therefore at a high risk of abrupt repositioning events that presumably involve supraphysiological changes in articular cartilage loading. Given that chondrocytes are particularly sensitive to loading rate9-14, these instability-related dynamic aberrations in cartilage loading plausibly help to drive the pathogenesis of posttraumatic osteoarthritis in unstable joints.
Clinically, it is commonly believed, although never explicitly proven, that higher degrees of instability lead to more severe and more rapid onset of posttraumatic osteoarthritis. Surgical restoration of physiological joint stability, by means of close anatomical ligament reconstruction and/or anatomical reduction of a fractured articular surface, has been presumed to mitigate the risk of posttraumatic osteoarthritis. However, even after functionally successful anterior cruciate ligament (ACL) reconstruction, 50% to >80% of injured knees have radiographic osteoarthritis at the time of follow-up at nine years or more15-17. In the ankle, clinical series of tibial plafond fractures have shown that more than half of patients treated by surgical reconstruction of the joint develop posttraumatic osteoarthritis, often within two years of injury18-21. The pathogenesis of posttraumatic osteoarthritis is multifactorial, with various confounding factors. To support the clinical utility of stability restoration surgeries in osteoarthritis prevention, the empirically based notion that the degree of instability is linked to osteoarthritis development requires more objective evidence.
A survival animal study was designed to mechanically quantify the relationship between the magnitude of instability and the intensity of osteoarthritis development at the organ level in vivo. Two ranges of joint instability were modeled in the rabbit knee (in which cartilage degeneration reproducibly develops after complete transection of the ACL in eight to twelve weeks22,23, much faster than in the human knee), with use of a novel technique of partial medial transection of the ACL, along with the established technique of complete ACL transection22,23. It was hypothesized that the greater the instability, the more severely and rapidly cartilage degeneration would develop. Implicit in this hypothesis was that there would not be a threshold below which joint instability would not tend to cause cartilage degeneration.
Materials and Methods
Sixty New Zealand White rabbits (twelve to fifteen months old with an average weight of 4.5 kg) were utilized in this study, which was approved by the Institutional Animal Care and Use Committee. These animals received one of three surgical treatments on the left knee: complete or partial medial transection of the ACL or control sham surgery (twenty animals per treatment). Surgery was performed in a surgical suite approved by the Association for Assessment and Accreditation of Laboratory Animal Care with use of inhalation anesthesia and aseptic technique. The rabbits were anesthetized with a preanesthetic intramuscular injection of ketamine HCl (26 mg/kg), acepromazine maleate (0.15 mg/kg), and xylazine HCl (0.78 mg/kg). Gas inhalation (isoflurane, 1.5% to 3.0% with O2) was used for anesthesia. Cephalothin (13 mg/kg) was used prior to surgery and at twelve-hour intervals for two days postoperatively. The ACL was exposed through a medial parapatellar arthrotomy with lateral patellar dislocation. The midsubstance of the ACL was lifted up with a surgical hook to expose the entire ligament. For the complete transection group, the entire ACL was transected with use of a number-11 scalpel. For the partial transection group, the tip of the number-11 blade was inserted into the center of the ACL, and the medial half alone was transected. The sham surgery included arthrotomy and ACL exposure in the identical manner as for the transection groups, without ligament transection. The joint capsule and skin were closed in layers with use of bioabsorbable suture. Butorphanol (0.1 to 0.5 mg/kg, at four-hour intervals) and flunixin (1.1 mg/kg, at twelve-hour intervals) were given for analgesia, by means of intramuscular injection, for forty-eight hours postoperatively. Subsequently, on the basis of investigator observation, analgesics were administered as individually needed to ensure that the animals were comfortable. Postoperatively, the animals were allowed free activity and ad libitum diet in their cages.
For each treatment group, half of the animals were killed eight weeks after surgery (the minimum testing duration in which distinct cartilage degeneration reproducibly developed in a previous study23), and the other half were killed after a twofold longer time period (sixteen weeks). Accordingly, the sample size was ten animals per each combination of treatment and test period. Immediately after the animals were killed, the experimental knees were subjected to sagittal plane stability testing. Finally, all experimental knees were dissected and sampled as a whole-joint construct for histomorphological evaluation of the articular cartilage. For the last thirty-seven animals, the gross conditions of the ACL and menisci were macroscopically inspected and recorded during this dissection.
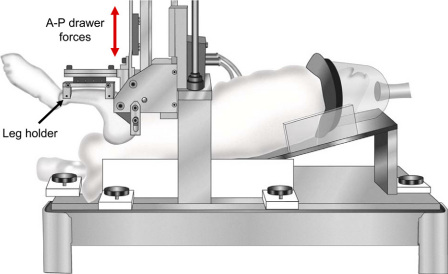
Sagittal plane joint stability was quantified with use of a custom loading device developed for the rabbit knee24 (Fig. 1). Each rabbit was placed in the supine position in a cradle, with the femur secured vertically with use of percutaneous half-pins. The tibia was secured with transverse pins attached to a horizontal leg holder, with the knee held flexed at 90°. The leg holder was in turn attached to a stepper motor-driven actuator. The actuator applied cyclic anterior and posterior knee drawer displacement at a test speed of 1 mm/sec, with limits for direction reversal set as ±75 N or ±3 mm, whichever was reached first. Force-versus-displacement data were continuously recorded during loading. The test sequence consisted of four discrete cycles for adjustment of the so-called zero displacement position, followed by four continuous cycles of preconditioning, and then four continuous loading cycles for data collection.
Fig. 1.
Custom anteroposterior (A-P) loading device for sagittal plane stability measurement, analogous to a clinical so-called drawer test. (Reprinted, with permission, from: Heiner AD, Rudert MJ, McKinley TO, Fredericks DC, Bobst JA, Tochigi Y. In vivo measurement of translational stiffness of rabbit knees. J Biomech. 2007;40:2313-7.)
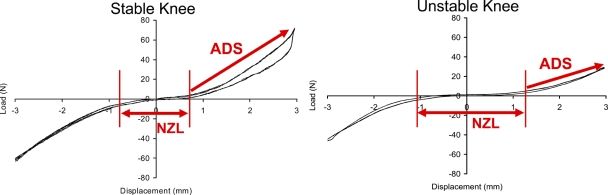
The knee drawer force-versus-displacement relationship was typically characterized by a sigmoid curve (Fig. 2). The length (in millimeters) of the low-resistance central flat portion of the curve, within the range of ±7.5 N of drawer force, was defined as the neutral zone length. The regression slope (N/mm) of the anterior drawer curve during load uptake, from 7.5 N to the attainment of maximum force, was defined as the anterior drawer stiffness. These measurements were averaged across the four continuous data collection loading cycles.
Fig. 2.
Load-displacement relationships in a stable control knee (left) and an unstable knee after partial ACL transection (right). Sagittal plane stability was quantified in terms of anterior drawer stiffness (ADS: regression slope from +7.5 N to the maximum force) and neutral zone length (NZL: displacement length between ±7.5 N data points). Higher instability was characterized by a decrease of anterior drawer stiffness and an increase of neutral zone length.
The histological preparations followed the Osteoarthritis Research Society International guidelines25. The whole-knee specimen from each animal was fixed in neutral buffered 10% formalin after harvesting and was subsequently decalcified in 5% formic acid. Each decalcified joint was separated into four pieces: the medial and lateral femoral condyles and the medial and lateral tibial plateaus. Each of these pieces was then cleaved in a parasagittal plane along the central portion of the weight-bearing area, before embedding in paraffin wax. Five-micrometer-thick sections were stained with safranin O-fast green. High magnification digital images were then captured at 743,028 pixels/mm2 resolution, with use of a QICAM 12-bit camera (QImaging, Surrey, British Columbia, Canada) mounted on a microscope (model BX60; Olympus, Tokyo, Japan) with a 4× objective, coupled with a stepper motor-driven stage (Prior Scientific, Rockland, Maryland). Individual high-resolution image fields were concatenated with use of Image-Pro (Media Cybernetics, Silver Spring, Maryland) to produce (stitched) full-cartilage-thickness osteochondral images.
The histological appearance of the cartilage was evaluated in a blinded fashion, with use of the histological histochemical grading scale described by Mankin et al.26. For each section, a region with representative histological findings from the central one-third of the joint surface (approximately 1 mm wide) was cropped, and a unique identification number was randomly assigned. Inclusion of potential technical artifacts, such as a solitary fissure without proteoglycan depletion, was carefully avoided. Two board-certified orthopaedic surgeons (T.V. and Y.T.) who were trained for cartilage histological analysis rated these blinded images on a computer display. Each observer individually scored each image twice (i.e., a total of four scores per image), and the mean of those values was recorded as the overall score. The femoral and tibial surfaces in both the medial and lateral compartments were rated individually, and the average of the femoral and tibial scores for each compartment was defined as the compartmental average score.
For statistical analysis, because the number of animals for each combination of ACL treatment (complete transection, partial transection, or sham surgery) and test period (eight or sixteen weeks) was relatively small (ten animals), a nonparametric test (the Kruskal-Wallis test) analogous to the conventional analysis-of-variance test was used to test for equality of treatment means, with use of the pooled eight-week and sixteen-week data. In primary analyses, this test was applied individually to the two stability measures (neutral zone length and anterior drawer stiffness) and to the four individual surface histological scores. If this global test for testing the null hypothesis of equal treatment means was significant, follow-up pairwise comparisons were performed for testing differences across the three treatment groups (twenty per group, including both eight-week and sixteen-week knees). Exploratory comparisons were performed between test periods (ten per subgroup) within each treatment group and between the compartmental average surface histological scores again with use of the Kruskal-Wallis test. Furthermore, associations of histological score with the joint laxity measures in the knees that had partial transection were assessed by means of linear regression analysis. All tests were performed with use of a significance level of alpha set at 0.05.
Source of Funding
This research was supported by the Centers for Disease Control and Prevention (grant R49 CCR721745) and by the National Institutes of Health (grants 5 P50 AR048939 and 5 P50 AR055533).
Results
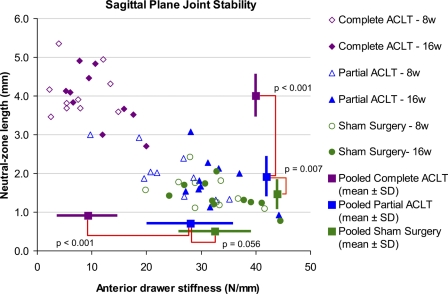
The medial menisci in the complete transection group were frequently torn (thirteen of the fifteen dissected knees), whereas no meniscal tears were identified in the ten knees in the partial transection group or twelve knees in the sham surgery control groups. None of the ten knees that had partial transection had progression to complete ACL rupture. For the joint stability measurement (Fig. 3), data from the partial transection group were widely distributed, in a range from the level of the sham surgery group to a level nearly equivalent to that of the complete transection group. However, in the statistical comparisons across treatments (for which data from eight-week and sixteen-week animals were pooled; Fig. 3), the neutral zone length in the partial transection group was significantly longer (p = 0.007) than in the sham surgery group and significantly shorter (p < 0.001) than in the complete transection group. The anterior drawer stiffness was also nearly significantly lower (p = 0.056) than in the sham surgery group and significantly higher (p < 0.001) than in the complete transection group. These data indicated that the sagittal plane stability of the partial transection group was intermediate between those of the sham surgery and complete transection groups. Regarding test periods, the anterior drawer stiffness for the partial transection knees in the eight-week group (24.1 ± 7.5 N/mm) was significantly lower (p = 0.011) than in the sixteen-week group (32.2 ± 5.1 N/mm). Otherwise, no significant differences in stability parameters between test periods were identified.
Fig. 3.
Individual animal data plots for sagittal plane stability measures: anterior drawer stiffness (horizontal axis) and neutral zone length (vertical axis). The mean values for each treatment group from both eight-week and sixteen-week animals are indicated by the blank square plots at the right (anterior drawer stiffness) or at the bottom (neutral zone length) of the graph. The dispersion bars indicate standard deviations (SD). ACLT = anterior cruciate ligament transection.
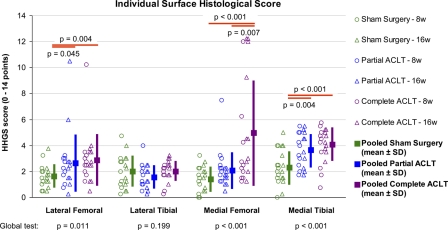
Cartilage degeneration identified in the complete and partial transection groups (Fig. 4) was typically modest, except in some of the knees that had complete transection (at the medial femoral surface) and in a few outliers (at the lateral and medial femoral surfaces). In the statistical comparisons across treatments (for which data from eight-week and sixteen-week animals were again pooled), the average histological scores for the medial tibial surface in the complete and partial transection groups were significantly higher (p < 0.001 and p = 0.004, respectively) than those in the sham surgery group (Fig. 4). On the medial femoral surface, the histological score for the complete transection group was significantly higher than the scores for the sham surgery and partial transection groups (p < 0.001 and p = 0.007, respectively). The lateral femoral surface also had slightly elevated average histological scores for the complete and partial transection groups (p = 0.004 and 0.045, respectively) than for the sham surgery group. No significant differences between test periods were identified in any treatment groups for any joint surface.
Fig. 4.
Results of histological evaluation for each individual articular surface. The scores on the histological histochemical grading scale (HHGS) for each individual animal are plotted, along with the mean values (from both eight-week and sixteen-week animals) and the standard deviation (SD) for each treatment group. ACLT = anterior cruciate ligament transection.
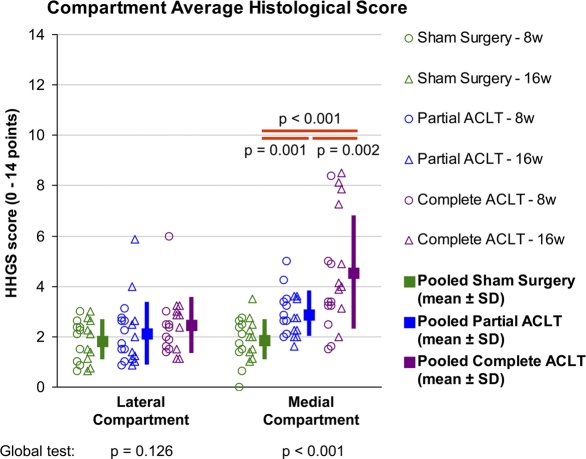
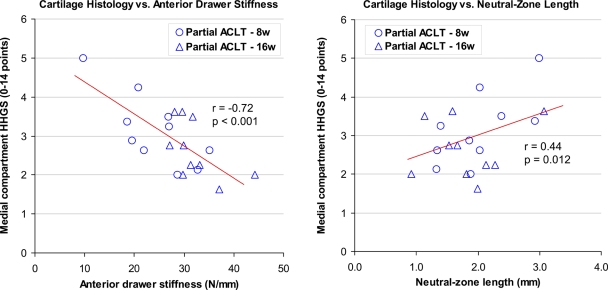
In statistical analysis of the average histological scores for the compartments (Fig. 5), the medial compartment score in the partial transection group was significantly higher (p = 0.001) than in the sham surgery group and significantly lower (p = 0.002) than in the complete transection group. This indicated that global cartilage degeneration in the medial compartment in the partial transection group (mean and standard deviation, 2.9 ± 0.9 points) was intermediate between those in the sham surgery (1.9 ± 0.8 points) and complete transection groups (4.5 ± 2.3 points). In linear regression analysis of histological scores versus stability measures in the partial transection group, the average histological scores for the medial compartment correlated significantly with both anterior drawer stiffness (r = −0.72, p < 0.001) and with neutral zone length (r = 0.44, p = 0.012), indicating that the severity of medial compartment global degeneration increased in an approximately linear fashion with the degree of instability. No particular threshold effects (characterized by discontinuity in the cause-and-effect relationship) were evident.
Fig. 5.
Comparisons of global histological findings for individual compartments across treatment groups. Average scores on the histological histochemical grading scale (HHGS) for the compartments for each individual animal are plotted, along with the mean values (from both eight-week and sixteen-week animals) and the standard deviation for each treatment group. ACLT = anterior cruciate ligament transection.
Discussion
In this study, two ranges of joint instability were modeled in rabbit knees in vivo, either by completely or partially transecting the ACL, with the organ-level short to mid-term responses of these joints being quantified in terms of whole-joint cartilage histological findings. The sagittal plane stability data demonstrated that partial ACL transection reliably created moderate instability. The relatively wide distribution of stability data in the knees with partial transection (Fig. 3) was presumably due to imprecise control in the amount of ligament fiber transaction of the small ACL as well as to physiological individual variability of initial joint stability. However, this continuous range of instability severity after transection was actually beneficial for testing the hypothesis, since it allowed for regression analysis to correlate the magnitude of instability with the degree of cartilage degeneration (Fig. 6). The partial transections adopted for instability purposes in this rabbit knee model were not intended to mimic partial ACL injuries in humans, in which so-called unruptured ligament fibers also are usually damaged. In the present study, the subpopulation of ligament fibers left intact in these rabbit knees in vivo was presumably fully functional, at least immediately after the partial transection. A human clinical situation perhaps more closely mimicking the partial transection knees in the present study would be knees with moderately successful single-bundle ACL reconstruction.
Fig. 6.
Correlations of global cartilage degeneration in the medial compartment with instability in the twenty knees that had partial transection of the ACL (including both eight-week and sixteen-week animals), as assessed by means of anterior drawer test stiffness (left) and of neutral zone length (right). Note that both the negative slope in the left graph and the positive slope in the right graph indicate positive correlation of cartilage degeneration with instability. No threshold effect is evident. HHGS = histological histochemical grading scale, and ACLT = anterior cruciate ligament transection.
The severity of cartilage degeneration in the rabbit knees after partial ACL transection was intermediate between those after complete transection and those after sham surgery (Fig. 5), as was the magnitude of instability in these joints. More importantly, in these partial transection knees, the intensity of cartilage degeneration correlated with the magnitude of instability (Fig. 6). These data clearly support the postulate that the degree of osteoarthritis development in unstable joints in rabbits is dependent on the degree of instability. Given that the medial meniscus was frequently found to be torn in the knees that received complete ACL transection, the greater degeneration in those knees relative to the partial transection knees might have been confounded by the meniscal tears. However, this would not explain the instability dependency of osteoarthritis development within the partial transection knees, in which no meniscal tears were identified. Presumably, in accordance with the above-described concept of joint instability by Burstein and Wright8, the knees with greater laxity were at a higher risk of repetitive instability events. It is possible that cage confinement may have mitigated degenerative changes in these experimental animals, by reducing exposure to the more injurious instability events that might have occurred during free-ranging activity. However, as suggested by Tochigi et al.27, even small instability events can cause supraphysiological high-rate cartilage loading (i.e., the so-called microinstability concept), and the cumulative effects of such microinstability events may lead to gradual cartilage degeneration. In addition, as demonstrated by Andriacchi et al.28, joint laxity shifts the contact location from the joint-specific primary central weight-bearing region to a more peripheral nonprimary weight-bearing region. Changes in habitual contact location could adversely influence cartilage nutritional support and metabolism. Either or both of these pathological mechanisms would presumably be accentuated by increased instability.
A clear instability threshold beyond which osteoarthritis developed was not evident in the present study (Fig. 6). In the surface-specific histological results (Fig. 4), cartilage degeneration for the medial tibial surface was significant in both the partial and total transection groups, while degeneration in the medial femoral surface was significant only in the total transection group. This inconsistency implies that sensitivity to instability is different across different surfaces, presumably associated with differences in mechanical environments for each surface. Such surface-specific differences, along with individual variability in biological responses, may have made the whole-joint effect of instability more stochastic.
Differences in the histological characteristics of cartilage between test periods (eight versus sixteen weeks) were not evident in either the partial or complete transection groups, suggesting that the cartilage degeneration in this model did not dramatically progress from eight weeks to sixteen weeks after transection. Presumably, the majority of instability-associated cartilage degeneration occurred in the first eight weeks. Capsular incision and ACL transection at the experimental surgery necessarily involved bleeding and inflammatory responses. Both blood29-31 and inflammatory mediators32,33 are known to be deleterious to cartilage. In a previous study, Fahlgren et al.34 reported similarly early osteoarthritis development in a rabbit knee meniscectomy model, and concluded that early-stage disease progression was likely linked to such biological stimuli. Cartilage in the unstable joints in the present study was also presumably more vulnerable to mechanical stresses during the first eight weeks than during the subsequent eight weeks. Besides these biological factors, potential load protection of the experimental joint may have affected chronic disease progression and so may potential compensation associated with neuromuscular adaptation. To quantify purely the chronic effects of instability magnitude on cartilage degeneration, a far longer testing period along with joint usage monitoring would be needed.
The etiology of posttraumatic osteoarthritis is complex and multifactorial35, affected not only by chronic biomechanical residuals but also by acute to subacute cartilage damage at the time of injury and its subsequent propagation by biological stimuli. While it is unknown under what circumstances any of these factors dominate the progression from injury to posttraumatic osteoarthritis, the results of the present investigation support current treatment paradigms that prioritize restoring stability to acutely injured joints. To our knowledge, this is the first study to have quantified the instability-dependence of cartilage degeneration at the organ level in vivo. Cartilage degeneration in this survival rabbit model of graded knee instability was directly correlated to the degree of instability, with no threshold effect being evident.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the Centers for Disease Control (grant R49 CCR721745) and the National Institutes of Health (grants 5 P50 AR048939 and 5 P50 AR055533). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Sommerlath K, Lysholm J, Gillquist J. The long-term course after treatment of acute anterior cruciate ligament ruptures. A 9 to 16 year followup. Am J Sports Med. 1991;19:156-62 [DOI] [PubMed] [Google Scholar]
- 2.Sommerlath K, Odensten M, Lysholm J. The late course of acute partial anterior cruciate ligament tears. A nine to 15-year follow-up evaluation. Clin Orthop Relat Res. 1992;281:152-8 [PubMed] [Google Scholar]
- 3.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756-69 [DOI] [PubMed] [Google Scholar]
- 4.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34:612-20 [DOI] [PubMed] [Google Scholar]
- 5.Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, Amendola A. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-6 [PMC free article] [PubMed] [Google Scholar]
- 6.Tochigi Y, Rudert MJ, Saltzman CL, Amendola A, Brown TD. Contribution of articular surface geometry to ankle stabilization. J Bone Joint Surg Am. 2006;88:2704-13 [DOI] [PubMed] [Google Scholar]
- 7.McKinley TO, Rudert MJ, Tochigi Y, Pedersen DR, Koos DC, Baer TE, Brown TD. Incongruity-dependent changes of contact stress rates in human cadaveric ankles. J Orthop Trauma. 2006;20:732-8 [DOI] [PubMed] [Google Scholar]
- 8.Burstein AH, Wright TM. Fundamentals of orthopaedic biomechanics. Baltimore: Williams & Wilkins; 1994 [Google Scholar]
- 9.Chen CT, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J Orthop Res. 1999;17:870-9 [DOI] [PubMed] [Google Scholar]
- 10.Guilak F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology. 2000;37:27-44 [PubMed] [Google Scholar]
- 11.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410-21 [DOI] [PubMed] [Google Scholar]
- 12.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181-8 [DOI] [PubMed] [Google Scholar]
- 13.Morel V, Quinn TM. Cartilage injury by ramp compression near the gel diffusion rate. J Orthop Res. 2004;22:145-51 [DOI] [PubMed] [Google Scholar]
- 14.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619-36 [DOI] [PubMed] [Google Scholar]
- 15.Salmon LJ, Russell VJ, Refshauge K, Kader D, Connolly C, Linklater J, Pinczewski LA. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am J Sports Med. 2006;34:721-32 [DOI] [PubMed] [Google Scholar]
- 16.Sutherland AG, Cooper K, Alexander LA, Nicol M, Smith FW, Scotland TR. The long-term functional and radiological outcome after open reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2010;92:1096-9 [DOI] [PubMed] [Google Scholar]
- 17.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145-52 [DOI] [PubMed] [Google Scholar]
- 18.Etter C, Ganz R. Long-term results of tibial plafond fractures treated with open reduction and internal fixation. Arch Orthop Trauma Surg. 1991;110:277-83 [DOI] [PubMed] [Google Scholar]
- 19.Marsh JL, Weigel DP, Dirschl DR. Tibial plafond fractures. How do these ankles function over time? J Bone Joint Surg Am. 2003;85:287-95 [PubMed] [Google Scholar]
- 20.Ovadia DN, Beals RK. Fractures of the tibial plafond. J Bone Joint Surg Am. 1986;68:543-51 [PubMed] [Google Scholar]
- 21.Teeny SM, Wiss DA. Open reduction and internal fixation of tibial plafond fractures. Variables contributing to poor results and complications. Clin Orthop Relat Res. 1993;292:108-17 [PubMed] [Google Scholar]
- 22.Vignon E, Bejui J, Mathieu P, Hartmann JD, Ville G, Evreux JC, Descotes J. Histological cartilage changes in a rabbit model of osteoarthritis. J Rheumatol. 1987;14 Spec No:104-6 [PubMed] [Google Scholar]
- 23.Yoshioka M, Coutts RD, Amiel D, Hacker SA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1996;4:87-98 [DOI] [PubMed] [Google Scholar]
- 24.Heiner AD, Rudert MJ, McKinley TO, Fredericks DC, Bobst JA, Tochigi Y. In vivo measurement of translational stiffness of rabbit knees. J Biomech. 2007;40:2313-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13-29 [DOI] [PubMed] [Google Scholar]
- 26.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523-37 [PubMed] [Google Scholar]
- 27.Tochigi Y, Rudert MJ, McKinley TO, Pedersen DR, Brown TD. Correlation of dynamic cartilage contact stress aberrations with severity of instability in ankle incongruity. J Orthop Res. 2008;26:1186-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:95-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roosendaal G, Tekoppele JM, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Articular cartilage is more susceptible to blood induced damage at young than at old age. J Rheumatol. 2000;27:1740-4 [PubMed] [Google Scholar]
- 30.Roosendaal G, TeKoppele JM, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a canine in vivo study. Arthritis Rheum. 1999;42:1033-9 [DOI] [PubMed] [Google Scholar]
- 31.Hooiveld M, Roosendaal G, Wenting M, van den Berg M, Bijlsma J, Lafeber F. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am J Pathol. 2003;162:943-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427 Suppl:S27-36 [DOI] [PubMed] [Google Scholar]
- 33.Myers SL, Brandt KD, O'Connor BL, Visco DM, Albrecht ME. Synovitis and osteoarthritic changes in canine articular cartilage after anterior cruciate ligament transection. Effect of surgical hemostasis. Arthritis Rheum. 1990;33:1406-15 [DOI] [PubMed] [Google Scholar]
- 34.Fahlgren A, Chubinskaya S, Messner K, Aspenberg P. A capsular incision leads to a fast osteoarthritic response, but also elevated levels of activated osteogenic protein-1 in rabbit knee joint cartilage. Scand J Med Sci Sports. 2006;16:456-62 [DOI] [PubMed] [Google Scholar]
- 35.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7-16 [PubMed] [Google Scholar]