Abstract
The luteal phase of the female menstrual cycle is associated with both 1) elevated serum progesterone (P4) and estradiol (E2), and 2) reduced insulin sensitivity. Recently, we demonstrated a link between skeletal muscle mitochondrial H2O2 emission (mEH2O2) and insulin resistance. To determine whether serum levels of P4 and/or E2 are related to mitochondrial function, mEH2O2 and respiratory O2 flux (Jo2) were measured in permeabilized myofibers from insulin-sensitive (IS, n = 24) and -resistant (IR, n = 8) nonmenopausal women (IR = HOMA-IR > 3.6). Succinate-supported mEH2O2 was more than 50% greater in the IR vs. IS women (P < 0.05). Interestingly, serum P4 correlated positively with succinate-supported mEH2O2 (r = 0. 53, P < 0.01). To determine whether P4 or E2 directly affect mitochondrial function, saponin-permeabilized vastus lateralis myofibers biopsied from five nonmenopausal women in the early follicular phase were incubated in P4 (60 nM), E2 (1.4 nM), or both. P4 alone inhibited state 3 Jo2, supported by multisubstrate combination (P < 0.01). However, E2 alone or in combination with P4 had no effect on Jo2. In contrast, during state 4 respiration, supported by succinate and glycerophosphate, mEH2O2 was increased with P4 alone or in combination with E2 (P < 0.01). The results suggest that 1) P4 increases mEH2O2 with or without E2; 2) P4 alone inhibits Jo2 but not when E2 is present; and 3) P4 is related to the mEH2O2 previously linked to skeletal muscle insulin resistance.
Keywords: sex hormones, estradiol, insulin resistance, oxidative stress
the current and increasing epidemic of type 2 diabetes constitutes one of the greatest health concerns in the industrialized world. Reduced mitochondrial content and intramuscular accumulation of lipid are associated with insulin resistance in skeletal muscle (29). Increasingly however, the role of reactive oxygen species (ROS) and oxidative stress has been implicated in the etiology of insulin resistance in multiple tissues, including skeletal muscle (reviewed in Refs. 7, 38). The nonradical ROS, hydrogen peroxide (H2O2), a relatively mild oxidant, has been identified as a biological second messenger (reviewed in Ref. 47). In addition to its role in cellular energy production, mitochondria have long been recognized as a potential source of substantial rates of H2O2 release (10). The physiological/pathophysiological implications of intracellular mitochondrial H2O2 signaling is supported in the literature, with one generally proposed mechanism involving differential alterations in thiol residues on redox-sensitive proteins, a shift in the overall redox tone of the cell, or both (3, 47). Indeed, a recent study by our group demonstrated a link between high dietary fat intake and insulin resistance involving elevated mitochondrial H2O2 emission (mEH2O2) in skeletal muscle (3). More recently, we (25) demonstrated a selective attenuation of succinate-supported mEH2O2 in skeletal muscle of male obese (fa/fa) Zucker rats treated with the popular antidiabetic drug metformin. The reduction in mEH2O2 in that study was accompanied by improved glycemic control.
The volume of published data demonstrating that ovarian sex steroids affect the sensitivity of tissues to insulin in animal models is substantial (reviewed in Ref. 28). While fewer data exist regarding the effects of the ovarian sex hormones in nonmenopausal women, most suggest a negative relationship with insulin sensitivity (28). Indeed, high circulating levels of both estrogens and progesterone accompany normal pregnancy (30), which is also associated with reduced insulin sensitivity (23, 28). Similarly, both serum estrogen and progesterone levels are elevated in healthy, nonmenopausal women during the luteal phase relative to the follicular phase of the menstrual cycle (30), and reduced insulin sensitivity has been reported in healthy, nonmenopausal women in the luteal phase (28). Because skeletal muscle is responsible for the majority of peripheral glucose disposal (1), it would appear that sex steroids may directly affect the insulin sensitivity of skeletal muscle (28). However, despite evidence relating sex steroids and insulin resistance, the exact nature of the link remains unclear.
Nearly 50 years ago, very high concentrations of progesterone were shown to inhibit complex I-linked respiration in the mitochondria isolated from pigeon hearts in vitro (13). Since then, studies examining the effects of estradiol (E2) and progesterone (P4) on mitochondrial function have employed treatment designs that increase the physiological relevancy of data supporting the inhibitory effects of sex steroids on mitochondrial respiration (20, 21). Nevertheless, most studies continue to employ supraphysiological concentrations of sex steroids to investigate the nongenomic effects of female sex steroids on mitochondrial function (16, 45). In a recent study, for example, it was shown that adding P4 to preparations of isolated rat liver mitochondria during the experimental measurements decreased the mitochondrial membrane potential, calcium retention capacity, and the capacity for complex I-linked state 3 respiration (16). However, the P4 concentrations used were 80–150 μM, over 1,000 times greater than the luteal phase serum P4 concentrations in women (41). Hence, the effects of elevated levels of ovarian sex steroids, at concentrations that are relevant to the menstrual cycle in women, on skeletal muscle mitochondrial function remain largely unknown.
In the present study, it was hypothesized that a link between skeletal muscle mEH2O2, insulin sensitivity, and/or the menstrual cycle hormones E2 and P4 would exist in women. To this end, serum E2 and P4 were measured in a group of eight insulin-resistant and 24 insulin-sensitive subjects on the same day they were biopsied for skeletal muscle mitochondrial function analyses. Additionally, nonmenopausal female subjects were biopsied in the menstrual cycle early follicular phase for ex vivo incubation experiments using elevated midluteal phase-relevant concentrations of P4 (11, 41), late follicular phase-relevant (i.e., ovulatory) E2 (11, 41), or both. Our findings reveal that serum levels of P4 influence the mitochondrial O2 flux (Jo2) and mEH2O2 linked to insulin resistance. This effect may be an acute, posttranslational phenomenon whereby an E2 + P4 combination, as occurs in vivo, promotes an increase in mEH2O2 but has little or no effect on Jo2.
MATERIALS AND METHODS
Subjects.
All subjects were nonmenopausal female U.S. citizens between the ages of 22 and 45 yr (subject characteristics presented in Tables 1 and 2). All participants were sedentary nonsmokers with no history of metabolic disease. The first set of subjects (Group A, n = 32; Table 1) consisted of African American (AW) and Caucasian women (CW) of varying body compositions and menstrual cycle status. After confirming that neither race nor obesity exerted an effect on any of the major outcome variables measured in the current study, AW and CW obese and lean women were pooled and divided by insulin resistance as determined by HOMA-IR (see below).
Table 1.
Group A subject characteristics
| Insulin Sensitive | Insulin Resistant | |
|---|---|---|
| N | 24 | 8 |
| Age, yr | 31.6 ± 1.4 | 35.1 ± 2.4 |
| BMI, kg/m2 | 30.2 ± 1.4 | 36.5 ± 2.4* |
| Body fat, % | 44.5 ± 1.5 | 47.3 ± 2.6 |
| HOMA-IR | 1.7 ± 0.2 | 4.6 ± 0.3*** |
| E2, pM | 407.2 ± 61.1 | 313.1 ± 113.1 |
| P4, nM | 8.8 ± 2.8 | 15.2 ± 5.2 |
| P4/E2 | 29.3 ± 6.8 | 42.4 ± 12.6 |
Results are means ± SE. E2, estradiol; P4, progesterone.
P < 0.05 vs. Insulin Sensitive;
P < 0.0001 vs. Insulin Sensitive.
Table 2.
Group B subject characteristics
| N | Age, yr | Weight, kg | BMI, kg/m2 | %Body Fat |
|---|---|---|---|---|
| 5 | 22.4 ± 1.4 | 67.2 ± 3.8 | 22.8 ± 1.2 | 32.6 ± 2.2 |
Data are means ± SE.
The second set of female subjects (Group B, n = 5; Table 2) were lean and healthy with no history of metabolic disease (e.g., HOMA-IR < 3.0) and were not taking medications known to alter carbohydrate or lipid metabolism. All subjects in Group B were scheduled for biopsy, such that the procedure would occur during the early follicular phase of their menstrual cycle (days 1–10), when E2 and P4 levels are lowest (30). Biopsies from subjects in Group B were used in hormone incubation experiments.
Percent body fat (%BF) was determined for each subject by dual-energy X-ray absorptiometry (DEXA). Dietary intake was recorded by subjects 3 days prior to procedure and analyzed for energy, fiber, and macronutrient intake. These protocols were approved by the East Carolina University Policy and Review Committee on Human Research in accordance with the Declaration of Helsinki principles. Informed consent was obtained from each subject after both written and oral information was presented about the procedure.
Procedures.
On the day of the skeletal muscle biopsy, subjects reported between the hours of 0630 and 0900 following an overnight fast (∼12 h). Body mass and height were recorded for body mass index (BMI) determination, and a fasting venous blood sample was obtained prior to the skeletal muscle biopsy for subsequent analysis. With regard to the subjects in Group A, plasma and serum were separated from the blood for subsequent analysis of glucose (YSI 2300 STAT Plus Glucose and Lactate Analyzer; YSI, Yellow Springs, OH), serum insulin, 17β-estradiol, and progesterone (Access Immunoassay System; Beckman-Coulter, Fullerton, CA). A homeostasis model assessment value for insulin resistance was calculated as HOMA-IR = [glucose (mg/dl) × insulin (μU/ml)] ÷ 405 (31). Subjects from Group A were divided by presence of insulin resistance as defined by Stern et al. (46). Group A subjects were therefore described as insulin sensitive (IS, HOMA-IR < 3.60) or insulin resistant (IR, HOMA-IR > 3.60; Table 1).
Skeletal muscle biopsies were obtained from the lateral aspect of the vastus lateralis by the percutaneous needle biopsy technique, with constant suction under local subcutaneous anesthesia (1% Lidocaine). A portion of each biopsy sample was flash-frozen in liquid N2 for subsequent protein analysis. The remaining portion of the biopsy (∼50 mg wet wt) was transferred to ice-cold physiological relaxing buffer (buffer X) for transport, on ice, to the laboratory (< 5 min) for dissection, permeabilization, and mitochondrial function assays for both Groups A and B.
Preparation of permeabilized human myofibers.
This technique is partially adapted from previous methods (26, 48) and has been described previously (3–5, 25). The technique was used for both Groups A and B. After dissection, connective tissue was removed, and fiber bundles were separated with fine forceps under a binocular dissecting microscope in ice-cold buffer X, containing (in mM) 60 K-MES, 35 KCl, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.7 ATP, 15 PCr, 6.56 MgCl2-6H2O (pH 7.4, 295 mOsm). After separation, myofiber bundles were placed in 4°C buffer X containing 30 μg/ml saponin for 30 min and then washed individually in ice-cold buffer Z, containing (in mM) 110 K-MES, 35 KCl, 1 EGTA, 10 K2HPO4, 3 MgCl2-6H2O, 5 mg/ml BSA (pH 7.4, 295 mOsm) until analysis (< 1 h). To determine the acute effects of E2 and P4 on mitochondrial function, washes for the permeabilized myofibers obtained from subjects in Group B contained hormone treatments: two of the Z washes contained 60 nM P4, two contained 1.4 nM E2, two contained 60 + 1.4 nM P4 + E2, and two contained vehicle (DMSO, < 2.0%). Fibers from both Groups A and B used in the H2O2 emission experiments were briefly washed in cold buffer Z containing 10 mM Na-pyrophosphate prior to analysis to prevent Ca+2-independent contraction.
The concentrations of P4 and E2 used in the ex vivo incubation experiments were chosen in consultation with the serum clinical reference values specified in Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (41). These luteal-phase reference values for nonmenopausal women are as follows (in nM): 6.4–79.5 P4 and 1.10–1.65 E2 (41). However, the chosen 1.4 nM E2 concentration exceeds the reference intervals for the luteal phase found in other textbooks (e.g., Ref. 11, clinical reference interval of 0.15–1.25 nM E2), but not the reference intervals for late follicular (i.e., ovulatory) phase E2 values (0.55–2.75 nM in Ref. 41, 0.18–2.83 nM in Ref. 11). Thus, the 1.4 nM E2 is more appropriately referred to in the current study as relevant to the late follicular phase.
Mitochondrial respiration and H2O2 emission measurements in permeabilized human myofibers.
O2 consumption rate was measured by polarographic high-resolution respirometry (Oroboros O2K Oxygraph, Innsbruck, Austria) at 30°C in air-saturated (∼220–150 μM O2) buffer Z + 20 mM creatine hydrate and 50 μM N-benzyl-p-toluene sulfonamide (BTS, an inhibitor of myosin II ATPase) under the following protocol: 25 μM palmitoylcarnitine + 1 mM malate followed by sequential additions of 2 mM ADP, 10 μM cytochrome c, 2 mM glutamate, 3 mM succinate, 10 μg/ml oligomycin (inhibitor of mitochondrial ATP synthase), and finally 2 μM carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP, a protonophoric uncoupler). With regard to the acute E2 and P4 incubation experiments, neither oligomycin nor FCCP were added due to time constraints associated with multiple testing.
H2O2 emission was measured at 30°C in buffer Z during state 4 respiration (10 μg/ml oligomycin) by continuously monitoring oxidation of Amplex red (excitation/emission λ = 563/587 nm) using a Fluorolog-3 (Horiba Jobin Yvon, Edison, NJ) spectrofluorometer under the following protocol: 25 μM palmitoylcarnitine + 1 mM malate followed by sequential additions of 2 mM glutamate, 3 mM succinate, and 10 mM glycerophosphate. At the conclusion of each experiment, permeabilized fiber bundles were washed in distilled H2O to remove salts and then freeze-dried in a lyophilizer (LabConco). Mitochondrial respiration rates (Jo2) are expressed as picomoles per second per milligram dry weight and H2O2 emission rates (mEH2O2) as picomoles per minute per milligram dry weight.
As with the buffer Z washes, respective treatments of P4 and/or E2 conditions were also created by adding the hormones (dissolved in DMSO) to the respective experimental chamber/cuvette (final DMSO concentration <2.0%). A parallel volume of DMSO alone was added to the control chamber/cuvette (i.e., final DMSO concentration <2.0%). Neither O2 consumption nor Amplex red fluorescence (standard curve) was differentially affected by any of the treatment conditions in the absence of biological sample.
Optimization of the saponin-permeabilized myofiber preparation for human female subjects.
Pilot data collected with permeabilzed myofibers from female subjects by use of standard protocols (27) exhibited abnormally low rates of Jo2. This was accompanied by a more than 40% increase in complex I-linked Jo2 (i.e., glutamate + malate substrates) after addition of 10 μM cytochrome c (Supplemental Fig. 1), indicating disruption of the outer mitochondrial membranes, likely due to excessive permeabilization. We therefore tested lower concentrations of saponin and found that 30 μg/ml saponin (vs. 50 μg/ml) resulted in optimal mitochondrial function in permeabilized fibers from these human subjects (Supplemental Fig. 1; supplementary material is found with the online version of this paper at the Journal's website). Moreover, it was determined that the percent coefficient of variation (%CV) for repeated measurements of respirometric O2 flux (Jo2) during state 3 respiration in four myofibers permeabilized with 30 μg/ml saponin was less than one-half the %CV of the Jo2 measured under the same conditions but permeabilzed with the standard 50 μg/ml.
Statistics.
Data are presented as means ± SE. Statistical analyses were performed with GraphPad Prism (GraphPad Software,) using two-way ANOVA (as appropriate) with Bonferroni's post hoc method for analysis of significance among groups. Pearson bivariate correlations and variable adjustments for %BF were performed using ANCOVA with SPSS 17 software. Dietary record data were processed using Nutritionist Pro software (Axxya Systems). The α-level of statistical significance was set a priori at P < 0.05.
RESULTS
Subject data are presented in Tables 1 and 2. Dietary record assessments indicated no difference between groups with regard to body mass (kg) proportional daily energy (kcal/kg), fiber (g/kg), or macronutrient intake (i.e., g carbohydrate, fat, or protein; P = 0.85, data not shown).
Mitochondrial H2O2 emission and respiratory O2 flux in permeabilized myofibers from insulin-sensitive and insulin-resistant subjects.
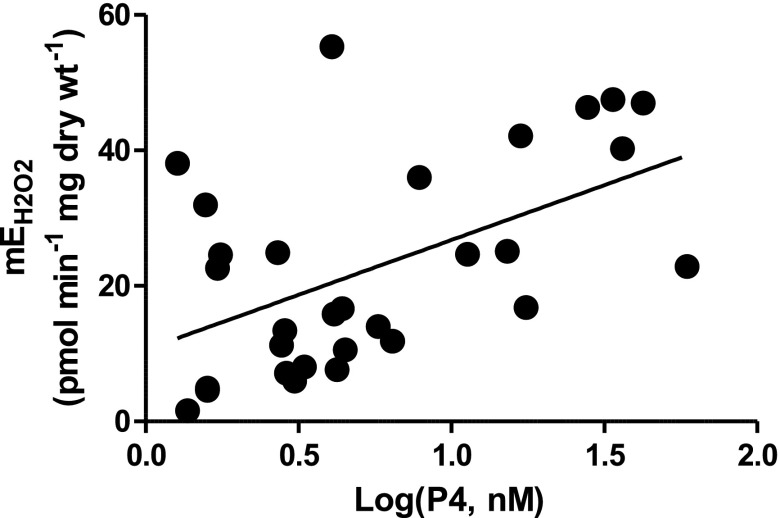
We hypothesized that serum levels of E2 and/or P4 would associate with measured mEH2O2 and/or HOMA-IR in the Group A subjects. Interestingly, only serum P4 concentration (nM, log transformed) correlated with P-C/MG+S-supported mEH2O2 (r = 0.53; P < 0.01; Fig. 1). This supports the possibility for P4 as the sex steroid responsible for increasing mEH2O2 and not E2.
Fig. 1.
Relationship between serum progesterone (P4) and mitochondrial H2O2 emission (mEH2O2) in women. Rates of mEH2O2 plotted against the log-transformed serum concentrations of P4 (nM) from 32 women in Group A. A significant correlation was present with respect to the substrate conditions: 10 μM oligomycin + 25 μM palmitoylcarnitine + 1 mM malate + 2 mM glutamate + 3 mM succinate (P-C/MGS;
r = 0.53, P < 0.01).
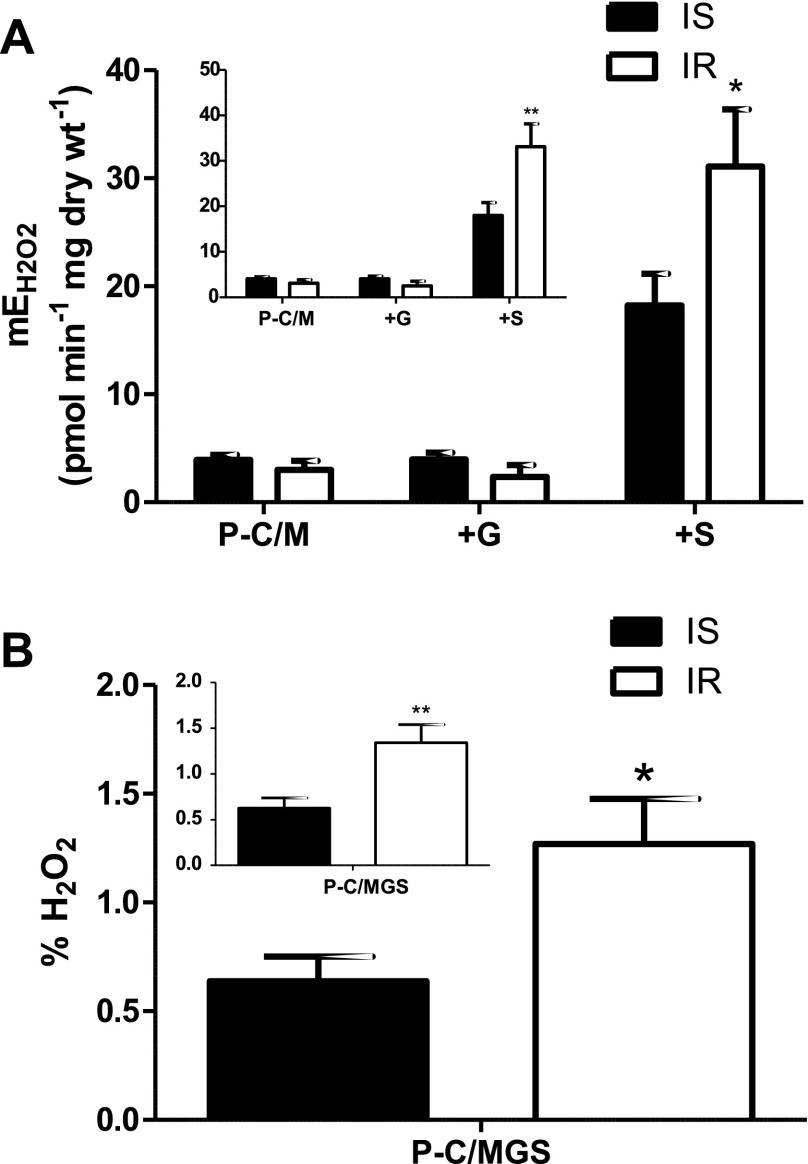
The rates of P-C/MG+S-supported mEH2O2 in permeabilized myofibers from the IR women were more than 50% greater than that of the IS women (P < 0.05; Fig. 2A). This supports a link between mEH2O2 and insulin resistance. Initially, we did not observe an effect of obesity (%BF) on rates of mEH2O2 (data not shown). However, it has been suggested that cytokines originating from adipose tissue may influence insulin sensitivity (reviewed in Ref. 35). Interestingly, statistically controlling for the potential secondary effect of adiposity (%BF; ANCOVA) on skeletal muscle mEH2O2 and HOMA-IR revealed a difference in the mEH2O2 between IS and IR women (P < 0.01; Fig. 2A, inset). This supports the hypothesis that skeletal muscle mEH2O2 influences insulin resistance and further suggests that adipose modulates insulin sensitivity.
Fig. 2.
mEH2O2 and insulin resistance in women. mEH2O2 was measured in permeabilzed myofibers and fractional mEH2O2 [mEH2O2/mitochondrial respiratory O2 flux (Jo2), %] in insulin-sensitive (IS, n = 22) and insulin-resistant (IR, n = 7) women. Inset: graphs represent data adjusted for %body fat (ANCOVA). A: substrate conditions were, in the presence of 10 μM oligomycin: 25 μM palmitoylcarnitine + 1 mM malate (P-C/M); P-C/M + 2 mM glutamate (+G); and P-C/MG + 3 mM succinate (+S). B: fractional mEH2O2 is expressed as a percentage of the Jo2 (pmol·min−1·mg dry wt−1) measured in parallel substrate conditions (i.e., P-C/MGS + 10 μM oligomycin). *P < 0.05, **P < 0.01 vs. IS.
To examine whether the link between mEH2O2 and insulin resistance might be mirrored in mitochondrial respiration and/or coupling, we measured Jo2 in premeabilized fibers from IS and IR women and subsequently calculated ratios of respiratory control (Table 3). While no differences in Jo2 were detected with insulin resistance, after adjusting for %BF, a difference in the uncoupling control ratio (UCR, ratio of uncoupled Jo2 to oligomycin-inhibited Jo2) was detected (P < 0.05; Table 3). This suggests that skeletal muscle mitochondrial coupling may be greater in IR than in IS women and that this relationship may be confounded by adiposity. Furthermore, when expressed relative to Jo2, the rate of mEH2O2 was still significantly greater in the IR than in the IS women (P < 0.05; Fig. 2B), suggesting that the increase mEH2O2 with IR was independent of differences in Jo2.
Table 3.
Jo2 and control in insulin-sensitive and insulin-resistant women
| Insulin Sensitive (n = 23) | Insulin Resistant (n = 8) | |
|---|---|---|
| P-C/M4 | 8.8 ± 1.2 | 8.4 ± 2.2 |
| P-C/M3 | 49.7 ± 2.7 | 42.2 ± 4.9 |
| P-C/MG3 | 145.5 ± 7.8 | 147.8 ± 14.1 |
| P-C/MGS3 | 207.8 ± 9.7 | 198.0 ± 17.7 |
| P-C/MGSO | 49.7 ± 2.8 | 40.3 ± 5.0 |
| P-C/MGSU | 253.6 ± 12.0 | 245.9 ± 21.8 |
| RCR | 7.2 ± 0.8 | 6.3 ± 1.5 |
| ACR | 1.2 ± 0.0 | 1.3 ± 0.1 |
| UCR | 5.2 ± 0.2 | 6.4 ± 0.4* |
Data are means ± SE. Jo2, respiratory O2 flux (pmol·s−1·mg dry wt−1); Substrates: P-C/M4, 25 μM palmitoylcarnitine + 1 mM malate; P-C/M3, P-C/M +2 mM ADP; P-C/M, P-C/MG, State 3 + 2 mM glutamate; P-C/MGS, P-C/M, G, State 3 + 3 mM succinate; P-C/MGSO, P-C/MGS + 10 μM oligomycin; P-C/MGSOU, P-C/MGSO + 2 μM FCCP. RCR, respiratory control ratio = (Jo2, P-C/M3)·(Jo2, P-C/M4)−1; ACR, adenylate control ratio = (Jo2, P-C/MGSOU)·(Jo2, P-C/MG+S)−1; UCR, uncoupling control ratio = (Jo2, P-C/MGSOU)·(Jo2, P-C/MGSO)−1.
P < 0.05 vs. Insulin Sensitive.
Acute ex vivio effects of P4 and E2 on mitochondrial function in permeabilized myofibers.
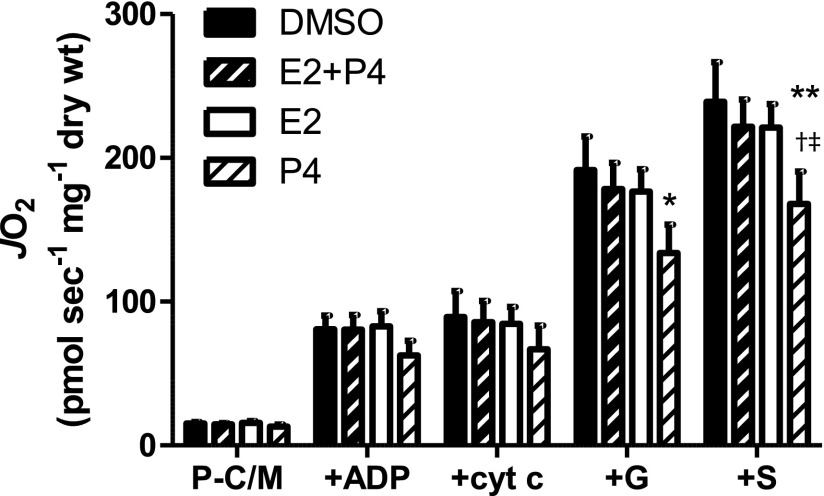
To test the acute, ex vivo effects of E2 and P4 on mitochondrial function, skeletal muscle fibers were incubated in either 1.4 nM E2, 60 nM P4, or both for 1–2 h after permeabilization but before and during the experimental measurements. As Fig. 3 illustrates, a trend, manifested in significant main effects for steroid hormones on Jo2 was present in the respirometric experiments. Compared with vehicle (DMSO), the results indicate that P4 alone significantly inhibited Jo2 during state 3 respiration supported by palmitoylcarnitine/malate + glutamate (P-C/M+G; P < 0.05) and P-C/MG + succinate (P-C/MG+S, P < 0.01; Fig. 3). This suggests that P4 exerts an inhibitory affect on complex I (+G) and possibly also complex II (+S). Interestingly, when combined with E2, P4 (i.e., E2 + P4) did not significantly inhibit Jo2 (Fig. 3). When combined with the results of E2 treatment alone (i.e., no effect on Jo2), the respirometric data with E2 + P4 suggest that E2 may prevent the inhibitory effects of P4 on Jo2.
Fig. 3.
Acute ex vivo effects of P4 and/or 17β-estradiol (E2) on Jo2 in permeabilized human female myofibers. Rates of Jo2 (pmol·s−1·mg dry wt−1) and H2O2 emission (mEH2O2) measured in saponin-permeabilized myofibers from lean, healthy women (n = 5) in menstrual cycle follicular phase (days 1–10). Permeabilized fibers were incubated in either DMSO (<1.5%, vehicle), 1.4 nM E2, 60 nM P4, or both (E2 + P4). Substrate conditions were: 25 μM palmitoylcarnitine + 1 mM malate (P-C/M), state 4; P-C/M + 2 mM ADP (+ADP); P-C/M, state 3 + 10 μM cytochrome c (+cyt c); P-C/M, state 3 + 2 mM glutamate (+G); P-C/M, G, state 3 +3 mM succinate (+S). Results are means ± SE (n = 4). *Less than control (DMSO), P < 0.05; **P < 0.01; †less than E2, P < 0.05; ‡less than E2 + P4, P < 0.05.
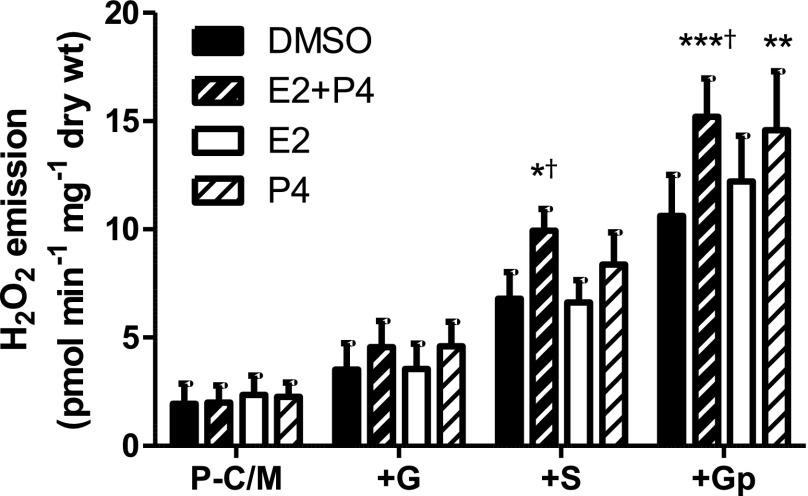
When rates of mEH2O2 were measured in the Group B muscle fibers treated with P4 and/or E2 acutely postpermeabilization, significant differences were observed after the addition of succinate and also glycerophosphate (Fig. 4). Compared with control (DMSO), E2 + P4 treatment resulted in significantly greater rates of mEH2O2 during state 4 respiration supported by either P-C/MG + succinate (+S; P < 0.05) and P-C/MGS + glycerophosphate (+Gp, P < 0.01; Fig. 4). Moreover, P4 alone significantly increased mEH2O2 compared with DMSO during P-C/MGS+Gp (P < 0.01; Fig. 4). Interestingly, however, E2 alone did not increase mEH2O2 (Fig. 4). Because additions of succinate and glycerophosphate are known to elicit reverse electron flow-mediated superoxide production at complex I (34), these data suggest that P4 increases the potential for complex I-linked mitochondrial H2O2 production. Furthermore, the protection conferred by E2 with regard to the inhibitory effects of P4 on Jo2 (Fig. 3) was not paralleled in the mEH2O2 measurements (Fig. 4). Taken together, these data support a model whereby E2 prevents P4-inhibited complex I-linked, and possibly complex II-linked Jo2, and conversely a model whereby E2 does not attenuate a P4-mediated increase in complex I-linked mEH2O2 within the context of elevated menstrual cycle levels of E2 and P4 (Fig. 5).
Fig. 4.
Acute ex vivo effects of P4 and/or E2 on mEH2O2 in permeabilized human female myofibers. Rates of mEH2O2 measured in saponin-permeabilized myofibers from lean, healthy women in menstrual cycle follicular phase (days 1–10). Permeabilized fibers were incubated in either DMSO (<1.5%, vehicle), 1.4 nM E2, 60 nM P4, or both (E2 + P4). Substrate conditions were, in the presence of 10 μM oligomycin: 25 μM palmitoylcarnitine + 1 mM malate (P-C/M); P-C/M + 2 mM glutamate (+G); P-C/MG + 3 mM succinate (+S); and P-C/MGS + 10 mM glycerophosphate (+Gp). Results are means ± SE (n = 5). *Less than control (DMSO), P < 0.05; **P < 0.01; ***P < 0.001; †less than E2, P < 0.05.
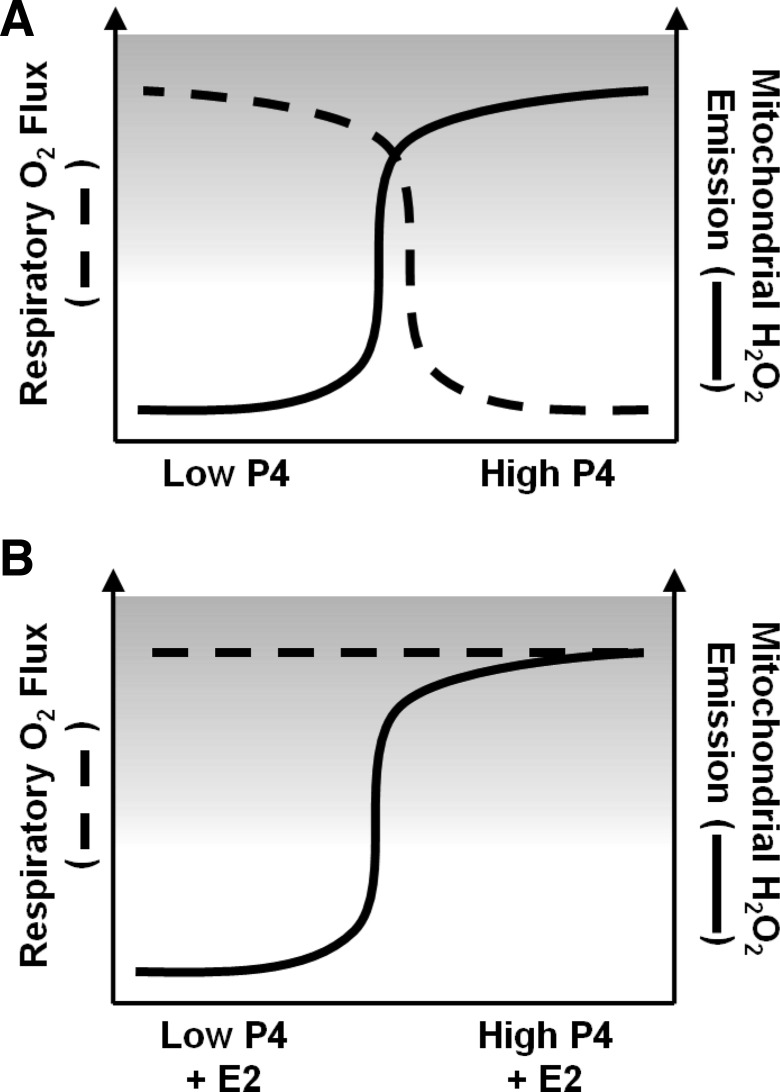
Fig. 5.
Diagrammatic summation of results and proposed effects of E2 and P4 on skeletal muscle mitochondrial function and insulin sensitivity. Both E2 and P4 are elevated during the luteal phase relative to the early follicular phase of the female menstrual cycle. Results of the current study support a model whereby elevated P4, whether alone (A) or in the presence of high E2 (B), increases the propensity for mEH2O2 in skeletal muscle of women. P4-associated inhibition of skeletal muscle Jo2 flux (A) is not observed when high E2 is present (B). Given that a reduction in insulin sensitivity has been reported for women in the luteal phase, and considered in the context of the observed increase in mEH2O2 with insulin resistance, results of the current study also suggest that the rise in P4 that accompanies the luteal phase may lead to insulin resistance via an increase in mEH2O2.
DISCUSSION
In this study, it was hypothesized that in women the ovarian steroid hormones E2 and P4 influence insulin sensitivity via alterations in the production of mitochondrial H2O2 in skeletal muscle. The novel findings presented here demonstrate that menstrual cycle-relevant concentrations of E2 and P4 (11, 41) can directly affect mitochondrial function in skeletal muscle. Furthermore, a clear relationship emerged between P4 measured in serum and mEH2O2 measured in biopsy samples. Finally, these results provide further evidence for a link between skeletal muscle mEH2O2 and insulin resistance in humans.
As early as 1963, Chance et al. (13) reported that high concentrations of P4 (i.e., mM) exhibited an inhibitory, “rotenone-like” effect on complex I-linked respiration and pyridine nucleotide reduction in mitochondria isolated from pigeon heart. A review (45) of this, and subsequent publications regarding posttranslational effects of experimental steroid hormone concentrations on the function of isolated mitochondria questioned the physiological relevance of these findings based on the supraphysiological concentrations of steroid hormones employed. To clarify discrepancies in the earlier literature, the current study utilized permeabilized myofibers from women in the early follicular phase of the menstrual cycle incubated with high late-follicular phase-relevant concentrations of E2 (1.4 nM) and midluteal phase-relevant P4 (60 nM) (11, 41). In agreement with the results of reports demonstrating an inhibitory effect of P4 on mitochondrial respiration in animals (13, 16, 20, 21), we observed a significantly lower Jo2 in fibers incubated with P4 alone but not when combined with E2. In light of the results of our recent study linking mEH2O2 to skeletal muscle insulin resistance (3), the observed increase in mEH2O2 after acute ex vivo treatment with P4 and E2 + P4 (but not E2) provides a potential link between the ovarian sex steroids and the reduced insulin sensitivity reported during the luteal phase of the menstrual cycle (see summary Fig. 5) and perhaps also pregnancy, when P4 levels are at their greatest naturally (28).
The results of the current study support the notion of an inhibitory effect of P4 alone on skeletal muscle mitochondrial respiration but not in combination with E2. In support of the present findings, although in slight contrast, a study of the effects of 150 μM P4 and 36 μM E2 on mitochondria isolated from mouse liver reported that state 3 Jo2 supported by succinate (complex II substrate) was reduced with P4 alone, although the reduction occurred in combination with E2 (21). When the mitochondria were supplied exclusively with glutamate + malate (complex I substrate), both state 3 and state 4 Jo2 were significantly lowered by treatment with either P4 alone or in combination with E2 compared with controls (21). These effects of P4 or E2 + P4 on mitochondrial Jo2 were not observed during TMPD + ascorbate respiration, which supplies electrons exclusively to complex IV (21), suggesting complex I, or possibly complex II, as one of the sites of action by P4. In another study, when mitochondria isolated from male rat livers were incubated briefly (1 min) with 30 μM E2, both state 3 and FCCP-uncoupled Jo2 supported by the complex I substrates glutamate + malate were significantly reduced from controls (33). However, E2 treatment had no effect on the mitochondrial membrane potential (ΔΨ) (33). Furthermore, no effect of E2 on mitochondrial H2O2 production was observed with or without rotenone present (33). This is in contrast to findings from another group demonstrating increased mitochondrial ROS in cultured cells treated for 15 min with greater than 360 nM E2 (17). Even more recently, it was shown that adding P4 to preparations of isolated rat liver mitochondria during experimental measurements decreased the ΔΨ, the calcium retention capacity, and the capacity for complex I-linked state 3 Jo2 (16). However, as with most of the investigations into the nongenomic effects of female sex steroids on mitochondrial function, the P4 concentrations used were unphysiological relative to levels found in circulation; in this case, anywhere from 80 to 150 μM, or over 1,000 times greater than the luteal-phase serum P4 concentration in women (41). Some insight into the rapid, nongenomic nature of late pregnancy-relevant P4 concentrations (1–50 μM) on respiration and glucose metabolism was recently provided (20). In their study, Gras et al. (20) demonstrated inhibition of respiration in isolated muscle strips from male rats that was not matched by the expected compensatory increase in insulin-stimulated glucose transport into the tissue. Importantly, this was a rapid phenomenon, which persisted even when inhibitors of transcription, protein synthesis, and the nuclear progesterone receptor were included in the preparation, strongly supporting the nongenomic nature of P4 on skeletal muscle metabolism (20). Moreover, it was determined that the progesterone receptor membrane component 1, the putative cell-surface mediator of progesterone's nongenomic effects, is clearly present in rat skeletal muscle (20). Whether the membrane progesterone receptor may mediate or is necessary for the observed increase in mEH2O2 in the current study will require further research. It should be noted, however, that the acute incubation experiments in the current study were conducted on permeabilized myofibers, such that sarcolemmal steroid receptors should not necessarily be required for the observed effects of E2 or P4 on mitochondrial function.
In the current study, we chose to address the inconsistencies raised by supraphysiological concentrations of the female sex steroids on isolated mitochondria by introducing concentrations of E2 and P4 relevant to the female menstrual cycle (11, 41) to test for an inhibitory effect of P4 alone on mitochondrial Jo2 in permeabilized myofibers. P4 is one of the female reproductive hormones most associated with pregnancy, as even its namesake implies (2). While extending the findings of the current study to the increase in P4 during the luteal phase of the menstrual cycle and/or pregnancy (30) might predict a decrease in the basal metabolic rate accompanying either the luteal phase or pregnancy, the results of the current study also demonstrate that E2 can prevent the inhibitory effects of P4 on mitochondrial Jo2. Because E2 also increases during the luteal phase [relative to the early follicular phase (11, 30, 41)] and also during pregnancy (30), this may explain why basal metabolic rate does not decrease in the face of increasing P4 during the luteal phase (8, 43, 51) or pregnancy (18), even when maternal and fetal mass are adjusted for (22). The novel finding of the potential ability for E2 to counteract the inhibitory effects of P4 on respiration may also explain why in the current study we found no relationship between serum P4, E2, or the P4/E2 ratio and Jo2 in the Group A subjects (i.e., the IS vs. IR subjects; data not shown).
Many reports describe a reduction in insulin sensitivity with elevated ovarian sex steroids in both humans and animal models (reviewed in Ref. 28), although it increasingly appears that estrogens are involved in the maintenance of insulin sensitivity, at least in mice (6, 39, 40). Indeed, the reported benefits of hormone replacement therapy on insulin sensitivity in women suggests a role for estrogen in maintaining or minimally modulating insulin sensitivity within a narrow physiological range of estrogen levels (28). In the current study, concentrations of serum P4 and E2 were measured in subjects for whom menstrual cycle did not dictate the day on which skeletal muscle biopsies were performed for the subsequent mitochondrial function assays. A significant correlation between succinate-supported mEH2O2 and serum P4 was observed (Fig. 1). Because both succinate and glycerophosphate are known to stimulate reverse electron flow-mediated superoxide production at complex I (34), these results suggest a relationship between P4 and complex I-mediated H2O2 in skeletal muscle mitochondria. Considering our recent report demonstrating that the antidiabetic drug metformin selectively attenuates succinate-supported mEH2O2 in rodent skeletal muscle (25), it would seem likely that P4 may exert its effects on insulin sensitivity through a similar mechanism in skeletal muscle, namely, complex I-linked mEH2O2. Metformin has been suggested to reduce reverse electron flow and superoxide production at complex I while allowing forward electron flow and supported respiration (25). Unfortunately, instrumental and tissue availability constraints in the current study prevented parallel experiments aimed at pinpointing the site of ROS generation at complex I (e.g., substrates supplying forward vs. reverse electron flow at complex I with and without rotenone). Nevertheless, rotenone was added after the P-C/MGS condition in the mEH2O2 experiments involving the acute E2 and P4 incubations. The addition of rotenone elicited a relatively small increase (∼25%) in mEH2O2 that was similar across treatments (data not shown), which would seem to suggest no qualitative difference in the nature of mEH2O2 influenced by E2 and/or P4. However, these data are difficult to interpret, as both forward and reverse electron flow substrates were present with rotenone. Future studies may benefit from additional protocols in which singular substrates supporting either forward or reverse electron flow through complex I are tested with and without rotenone.
Purely positivist application of the present findings to all scenarios is unlikely, however, even with regard to women's health. For example, reduced insulin sensitivity and predisposition toward metabolic syndrome are well documented in menopausal women, a condition in which ovarian sex steroid levels are markedly reduced (12). Mitigating factors during menopause, such as age, increased abdominal adiposity, and physical activity levels highlight the complex relationship between systemic hormonal glycemic control and the role of ovarian sex steroids at different stages throughout life (12). Indeed, the results of the current study also suggest that %BF may affect the relationship between skeletal muscle mEH2O2 and insulin resistance in nonmenopausal women (Fig. 2), supporting the reported influence of adipose on insulin sensitivity (35). Moreover, changes in the serum concentrations of E2 and P4 in women may be dictated by factors such as smoking (52), dietary fiber (42), and fat (19), but typical changes in E2 and P4 are primarily associated with the menstrual cycle in nonpregnant women (30). It is also important to note that peak levels of E2 and P4 do not normally occur during the same phases of the female menstrual cycle, E2 peaking at or about ovulation midcycle and P4 peaking during midluteal phase. Thus, the high levels of both E2 and P4 employed simultaneously in the acute incubation experiments of the present study represent a potential limitation to extrapolating the results to the menstrual cycle in anything more than a general sense. Further research may clarify this issue with larger, longitudinal studies monitoring women throughout the menstrual cycle. Nevertheless, we have demonstrated an association between high P4 and the mEH2O2 linked to skeletal muscle insulin resistance. Indeed, insulin sensitivity is shown to decrease during both the luteal phase and during pregnancy (28), two conditions in which P4 and E2 levels are elevated relative to the follicular phase of the menstrual cycle. If, in fact, high physiological levels of E2 and P4 link conditions such as pregnancy and the luteal phase of the menstrual cycle to skeletal muscle insulin resistance, the next relevant question is which are responsible: E2, P4, or both? In a study involving stable isotope dilution and indirect calorimetry, d'Eon et al. (14) were able to measure glucose uptake and estimate skeletal muscle glucose oxidation during exercise while manipulating the blood levels of E2 and P4 in healthy women. They discovered opposing actions of E2 and P4, the former reducing estimated muscle glycogen utilization and the rate of glucose disappearance from the blood. In contrast, increasing blood levels of P4, in addition to E2, increased the estimated muscle glycogen utilization, but not the rate of glucose disappearance from the blood. These results are supportive of the present findings in that the presence of ovarian sex steroids in combination may alter the singular effect of each alone on cellular metabolism. Indeed, our results demonstrate that, with regard to skeletal muscle mitochondrial Jo2, P4 alone significantly reduced respiration supported by the multisubstrate combination P-C/MGS (Fig. 3). However, even when P4 was present, E2 preserved Jo2 (Fig. 3). This is again in agreement with previous reports indicating that E2 preserves mitochondrial function in neuronal cells challenged with proapoptotic factors (32), inhibitors of succinate dehydrogenase (50), high calcium (36), and oxidative stress (32, 50). Although further research will be necessary to reveal the exact mechanism by which E2 exerts its effects on mitochondrial maintenance of function, studies indicate that E2 exerts a direct antioxidant effect on isolated mitochondria (9), and there is additional evidence from other laboratories involving isolated mitochondria that E2 can directly enhance the activity of the manganese-containing superoxide dismutase (37).
The results of the current study, however, do not support the notion of E2 as a direct antioxidant when in combination with progesterone (Fig. 4), although E2 alone did not increase the rate of mEH2O2 (Fig. 4). Therefore, the present findings suggest that P4 may be related to the insulin resistance observed during conditions of elevated sex steroids. If mEH2O2 is linked to insulin resistance in skeletal muscle (3), the finding that acute exposure of permeabilized myofibers to P4 or P4 + E2 increased mEH2O2 (Fig. 4) permits speculation about potential mechanisms whereby P4 influences skeletal muscle insulin sensitivity via mEH2O2. Because the women in the present study were fasted, their serum P4 levels may actually reflect how their skeletal muscle will respond to metabolic challenges known to reduce insulin sensitivity, such as consuming a large meal (24), prolonged fasting [e.g., 1–2 days (15)], sleep restriction (44), or marathon running (49). During the protocols used in the current investigation, rates of mEH2O2 were in fact stimulated progressively through the addition of various substrates used in mitochondrial oxidative phosphorylation, and not in the absence or progressive lowering of substrates. If the measurements of mEH2O2 are viewed not as a resting level enzymatic activity assay but rather as how the mitochondria in skeletal muscle will respond to an influx of substrate, the implications may be more physiologically relevant. Perhaps the effects of P4 on skeletal muscle are pleiotropic, conditionally specific, and evident experimentally only under extreme conditions, such as a high rate of mitochondrial substrate flux. From an evolutionary perspective, P4 might serve as a regulator of substrate provision associated with pregnancy. Indeed, when a woman becomes pregnant, the rise in P4 that accompanies the luteal phase continues and increases during gestation, as often does insulin resistance (23). Teleologically, the rise in P4 during pregnancy and an increase in skeletal muscle mEH2O2, and, perhaps in turn, muted insulin sensitivity, may have more to do with satisfying the energetic needs of the developing fetus than any pathological condition in carbohydrate metabolism. The rise in P4 may therefore set the stage for a means to divert substrate away from the mother's skeletal muscle following a meal. While changes in glycemic control during situations of elevated ovarian sex steroids are most certainly the combined result of multiple hormones acting on multiple tissues, the results of the present study support the notion that the direct effects of P4 on skeletal muscle could play an important role (20, 28). Establishing the exact degree to which progesterone mitigates insulin sensitivity in skeletal muscle, and perhaps additional tissues, will require further research.
To conclude, the results of the current study support a model in which high luteal phase-relevant levels of P4 increase mEH2O2 and decrease Jo2 in skeletal muscle and in which late-follicular-phase levels of E2 remove the inhibitory effects of P4 on Jo2 but not mEH2O2 (Fig. 5). Furthermore, the results of this study clearly suggest a link between mEH2O2 and insulin resistance in women. Whether the model can explain a causative role for ovarian sex steroids in the etiology of insulin resistance and type 2 diabetes will require further research. Last, these results highlight the need to take into account female menstrual cycle status and the influence of ovarian sex steroids when studying mitochondrial function, even with ex vivo experimental designs.
GRANTS
This study was supported by National Institutes of Health Grants R01 DK-075880 (R. N. Cortright) and DK-074825 and DK-073488 (P. D. Neufer).
DISCLOSURES
No conflicts of interest are reported by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank the subjects who participated in this study. D. A. Kane also thanks C. L. Tweedie for tissue processing and C. G. R. Perry for helpful comments.
Present address for D. A. Kane: Dept. of Human Kinetics, St. Francis Xavier University, Antigonish, NS, Canada B2G 2W5 (e-mail: dkane@stfx.ca).
REFERENCES
- 1. Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010: 476279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen WM. Progesterone: how did the name originate? South Med J 63: 1151–1155, 1970. [DOI] [PubMed] [Google Scholar]
- 3. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Barros RP, Machado UF, Warner M, Gustafsson JA. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc Natl Acad Sci USA 103: 1605–1608, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 89: 27–71, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Bisdee JT, James WP, Shaw MA. Changes in energy expenditure during the menstrual cycle. Br J Nutr 61: 187–199, 1989. [DOI] [PubMed] [Google Scholar]
- 9. Borras C, Gambini J, Lopez-Grueso R, Pallardo FV, Vina J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim Biophys Acta 1802: 205–211, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrne CJ, Saxton DF, Pelikan PK, Nugent PM. Laboratory Tests: Implications for Nursing Care (2nd ed.). Menlo Park, CA: Addison-Wesley Health Sciences Division, 1986, p. 332–342. [Google Scholar]
- 12. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem 238: 418–431, 1963. [PubMed] [Google Scholar]
- 14. D'Eon TM, Sharoff C, Chipkin SR, Grow D, Ruby BC, Braun B. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am J Physiol Endocrinol Metab 283: E1046–E1055, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Dobbins RL, Chester MW, Daniels MB, McGarry JD, Stein DT. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes 47: 1613–1618, 1998. [DOI] [PubMed] [Google Scholar]
- 16. Fedotcheva NI, Teplova VV, Fedotcheva TA, Rzheznikov VM, Shimanovskii NL. Effect of progesterone and its synthetic analogues on the activity of mitochondrial permeability transition pore in isolated rat liver mitochondria. Biochem Pharmacol 78: 1060–1068, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry 44: 6900–6909, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr 47: 942–947, 1988. [DOI] [PubMed] [Google Scholar]
- 19. Goldin BR, Adlercreutz H, Gorbach SL, Woods MN, Dwyer JT, Conlon T, Bohn E, Gershoff SN. The relationship between estrogen levels and diets of Caucasian American and Oriental immigrant women. Am J Clin Nutr 44: 945–953, 1986. [DOI] [PubMed] [Google Scholar]
- 20. Gras F, Brunmair B, Quarre L, Szocs Z, Waldhausl W, Furnsinn C. Progesterone impairs cell respiration and suppresses a compensatory increase in glucose transport in isolated rat skeletal muscle: a non-genomic mechanism contributing to metabolic adaptation to late pregnancy? Diabetologia 50: 2544–2552, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Grimbert S, Fisch C, Deschamps D, Berson A, Fromenty B, Feldmann G, Pessayre D. Effects of female sex hormones on mitochondria: possible role in acute fatty liver of pregnancy. Am J Physiol Gastrointest Liver Physiol 268: G107–G115, 1995. [DOI] [PubMed] [Google Scholar]
- 22. Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am J Obstet Gynecol 178: 1010–1015, 1998. [DOI] [PubMed] [Google Scholar]
- 23. Hollingsworth DR. Alterations of maternal metabolism in normal and diabetic pregnancies: differences in insulin-dependent, non-insulin-dependent, and gestational diabetes. Am J Obstet Gynecol 146: 417–429, 1983. [DOI] [PubMed] [Google Scholar]
- 24. Jenkins DJ, Ocana A, Jenkins AL, Wolever TM, Vuksan V, Katzman L, Hollands M, Greenberg G, Corey P, Patten R, Wong G, Josse RG. Metabolic advantages of spreading the nutrient load: effects of increased meal frequency in non-insulin-dependent diabetes. Am J Clin Nutr 55: 461–467, 1992. [DOI] [PubMed] [Google Scholar]
- 25. Kane DA, Anderson EJ, Price JW, 3rd, Woodlief TL, Lin CT, Bikman BT, Cortright RN, Neufer PD. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med 49: 1082–1087, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem 241: 909–915, 1996. [DOI] [PubMed] [Google Scholar]
- 27. Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond) 102: 151–166, 2002. [DOI] [PubMed] [Google Scholar]
- 29. Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis—part 2. Headache 46: 365–386, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 32. Mattson MP, Robinson N, Guo Q. Estrogens stabilize mitochondrial function and protect neural cells against the pro-apoptotic action of mutant presenilin-1. Neuroreport 8: 3817–3821, 1997. [DOI] [PubMed] [Google Scholar]
- 33. Moreira PI, Custodio J, Moreno A, Oliveira CR, Santos MS. Tamoxifen and estradiol interact with the flavin mononucleotide site of complex I leading to mitochondrial failure. J Biol Chem 281: 10143–10152, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 114: 183–194, 2008. [DOI] [PubMed] [Google Scholar]
- 36. Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA 100: 2842–2847, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell 17: 2125–2137, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perez-Matute P, Zulet MA, Martinez JA. Reactive species and diabetes: counteracting oxidative stress to improve health. Curr Opin Pharmacol 9: 771–779, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150: 2109–2117, 2009. [DOI] [PubMed] [Google Scholar]
- 40. Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab 298: E304–E319, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roberts WL, McMillin GA, Burtis CA, Bruns DE. Reference information for the clinical laboratory. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (4th ed.), edited by Burtis CA, Ashwood ER, Bruns DE. Philadelphia, PA: Elsevier Saunders, 2006, p. 2252–2302. [Google Scholar]
- 42. Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr 54: 520–525, 1991. [DOI] [PubMed] [Google Scholar]
- 43. Solomon SJ, Kurzer MS, Calloway DH. Menstrual cycle and basal metabolic rate in women. Am J Clin Nutr 36: 611–616, 1982. [DOI] [PubMed] [Google Scholar]
- 44. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439, 1999. [DOI] [PubMed] [Google Scholar]
- 45. Starkov AA. “Mild” uncoupling of mitochondria. Biosci Rep 17: 273–279, 1997. [DOI] [PubMed] [Google Scholar]
- 46. Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 54: 333–339, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8: 243–270, 2006. [DOI] [PubMed] [Google Scholar]
- 48. Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflügers Arch 446: 261–269, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Tuominen JA, Ebeling P, Bourey R, Koranyi L, Lamminen A, Rapola J, Sane T, Vuorinen-Markkola H, Koivisto VA. Postmarathon paradox: insulin resistance in the face of glycogen depletion. Am J Physiol Endocrinol Metab 270: E336–E343, 1996. [DOI] [PubMed] [Google Scholar]
- 50. Wang J, Green PS, Simpkins JW. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J Neurochem 77: 804–811, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Webb P. 24-Hour energy expenditure and the menstrual cycle. Am J Clin Nutr 44: 614–619, 1986. [DOI] [PubMed] [Google Scholar]
- 52. Zumoff B, Miller L, Levit CD, Miller EH, Heinz U, Kalin M, Denman H, Jandorek R, Rosenfeld RS. The effect of smoking on serum progesterone, estradiol, and luteinizing hormone levels over a menstrual cycle in normal women. Steroids 55: 507–511, 1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.