Abstract
Cytomegalovirus (CMV) establishes a lifelong persistent infection, and viral immune modulating strategies are important to facilitate this. A particularly diverse CD8 T cell response develops as a result of this host-virus détente, with the CMV-specific memory T cell pool displaying unique functions and phenotypes. To gain insight into the factors that regulate CMV-specific CD8 T cell responses, we examined the influence of the B7-CD28 costimulatory pathway on the magnitude, kinetics and phenotype. Initial expansion of mouse CMV-specific CD8 T cells that establish stable memory pools was severely lower in mice lacking B7-CD28 signaling, and the resulting memory levels also remaining reduced during persistent/latent infection. In contrast, expansion of CD8 T cells that undergo memory inflation during chronic infection was less impacted in the absence of B7-CD28 costimulatory signals, eventually reaching the levels seen in wild-type mice at later times. Regardless of their differential requirements for B7-CD28 signals, both stable and inflationary memory T cell populations showed normal cytotoxic capacity. These results reveal that B7-CD28 costimulation differentially regulates the magnitude and kinetics of the multifaceted CD8 T cell response that develops during CMV infection.
Introduction
CD8 T cells play a critical role in controlling pathogens that establish both acute and persistent infections. However, the resulting nature and quality of the T cell response is highly dependent upon the course of infection, with both host and pathogen factors contributing to its development. The priming of antigen-specific CD8 T cells is initiated by TCR binding to MHC class I molecules presenting pathogen-derived peptide epitopes. TCR signals operate in conjunction with costimulatory signals provided by members of the Ig and TNFR families (e.g. CD28, CD27, CD134/OX40 and CD137/4-1BB), and together with the specific inflammatory milieu induced upon infection, CD8 T cells can become fully activated. The ensuing CD8 T cell expansion and differentiation follows a programmed, yet plastic, path towards effector and memory cell formation (1). Understanding the mechanisms that operate to induce relevant CD8 T cell responses specific for individual pathogens is imperative for the rationale design of T cell based vaccines.
Human cytomegalovirus (HCMV) is a widespread pathogen, which infects the majority of the world’s population. HCMV is usually acquired early in life and establishes a lifelong, largely asymptomatic infection in healthy persons, but can lead to significant disease in neonates and immunocompromised individuals (2,3). An extremely diverse and heterogeneous population of CD8 T cells specific for CMV antigens develops over the course of infection, and these cells suppress viral reactivation from latency and are also able to protect immunocompromised humans and mice from CMV disease when adoptively transferred (4-11). Generally, CMV-specific memory CD8 T cells can be divided into two distinct subsets: 1) “central-memory” cells (IL-2+/CD27+/CD62Lhi/CD127+), which peak early during infection, contract and establish a stable memory pool and 2) “effector-memory” like T cells (IL-2−/CD27−/CD62Llo/CD127−), which increase in numbers during the persistent and/or latent phase of infection (“inflationary” responses). It is these inflationary T cell responses that have been associated with an immune risk profile and immune senescence in the elderly, as they compose an exceptionally large portion of the memory pool (6,9,11-13). Inflationary T cell responses are thought to require chronic or intermittent re-exposure to their cognate viral antigens (14-17), but the collage of factors shaping these unique subsets of CMV-specific T cells are only just beginning to be understood (16-18).
It has often been speculated that the multitude of CMV strategies targeting adaptive immunity have evolved to enable the establishment of persistence. The targeting of the costimulatory B7.1 (CD80) and B7.2 (CD86) molecules by CMV (19-21) implies an important role for the B7-CD28 costimulatory pathway in antiviral immunity. Here we evaluated the role of the B7-mediated costimulatory signals on the kinetics and immunodominance of stable and inflationary CD8 T cell responses during mouse CMV (MCMV) infection. We found that stable memory CD8 T cell responses are highly dependent on the B7-CD28 axis for their development, while the accumulation and function of inflationary CD8 T cells does not strictly require these costimulatory molecules.
Materials and Methods
Mice
C57BL/6 (wild-type (WT)), CD28−/−, B7.1−/−, B7.2−/−, CD28−/− mice (all knockout mice are on a C57BL/6 background) were purchased from The Jackson Laboratory (Bar Harbor, ME). B7.1−/− and B7.2−/− double deficient mice (B7.1/2−/−) on a C57BL/6 background were kindly provided by Dr. A. Sharpe. CD45.1 (SJL) OT-I, CD28−/− CD45.1 OT-I, and B7.1/2−/− CD45.1 OT-I TCR transgenic mice were bred in-house. Mice were maintained under specific pathogen-free conditions in the Department of Laboratory Animal Care at the La Jolla Institute for Allergy and Immunology. All experiments were approved by the La Jolla Institute IACUC in accordance with the guidelines by the Association for assessment and Accreditation of laboratory Animal Care.
MCMV stock preparation, infection and quantification
The MCMV (Smith strain) was obtained from the American Type Culture Collection (VR-194, Manassas, VA), and salivary gland stocks were prepared in BALB/c mice as described (22). MCMV-OVA has been described elsewhere (17), and was obtained from Dr. Ann Hill with the permission of Dr. Geoffrey R. Shellam (School of Biomedical, Biomolecular and Chemical Sciences, Crawley, Western Australia). Age (8-12-week old) and gender-matched mice were infected intraperitoneally (i.p.) with 5 × 104 PFU of MCMV. MCMV PFU was determined by plaque assay in NIH 3T3 cells as described (22). Quantitative PCR analysis of MCMV gene expression was performed as described (22).
In vivo antibody treatment
Hybridomas were cultured in Life Technologies Protein-Free Hybridoma Medium-II (Invitrogen, San Diego, CA), and mAbs were purified by dialysis. For in vivo blockade of B7 costimulatory molecules, 200 μg of anti-mouse B7.1 (clone 16-10A1) and/or anti-mouse B7.2 (clone GL1) was injected i.p. on days −1, 0 and +2 of infection. To deplete CD25+ CD4+ Tregs, mice received an i.p. administration of 500 μg anti-CD25 (clone PC61) 2 days prior and 2 days after infection. The efficiency of Treg depletion was measured by staining of blood lymphocytes for cell surface CD4 and CD25 (clone 7D4, non-competitive epitope) and intracellular FoxP3, and indicated a consistent reduction of >95% of CD25+ CD4+ Tregs 2 days post-antibody treatment. All control mice received in parallel similar amounts of rat IgG. All mAbs were administered in 200 μl PBS.
Ex vivo phenotypic analysis, intracellular staining and flow cytometry
Spleens were harvested and single-cell suspensions were prepared by mincing the tissue through a 70 μm cell strainer (BD Biosciences). Lymphocytes from perfused livers and lungs were obtained by mincing the tissue, followed by collagenase treatment for 0.5 h and lympholyte gradient. Erythrocytes were lysed in a hypotonic (0.82%) ammonium chloride buffer. For cell surface staining, cells were resuspended in staining buffer (PBS + 2% FCS + 0.05% sodium azide) and incubated with fluorescent conjugated antibodies and tetramers for 0.5-1 h at 4C°. For BrdU staining, the BD Pharmingen BrdU Flow kit was used. Intracellular staining of granzyme B (Caltag) and Ki-67 (BD Pharmingen) was performed according to the manufacturer’s instructions.
For intracellular cytokine staining, CD8 T cells were in vitro restimulated with medium containing 2 μg/ml peptide and 1 μg/ml brefeldin A for 5 h in 96-well flat-bottom plates (1.5 × 106 splenocytes per well). After stimulation, cells were transferred to U-bottom 96-well plates, and cell surface stained with fluorescent conjugated antibodies at 4C° for 0.5 h in staining buffer (1% FCS). After washing, cells were fixed with 1% paraformaldehyde at 4C° for 1-2 h followed by intracellular staining for cytokines at 4C° for 0.5-1 h in Perm/Wash buffer (BD Biosciences). After washing and resuspending in staining buffer, cells were acquired using a BD LSRII flow cytometer and data was analyzed using FlowJo software (Tree Star). Fluorochrome-conjugated monoclonal antibodies specific for CD8, CD27, CD62L, CD44, CD45.1, CD127, IFN-γ, IL-2, TNF and Vα2 were purchased from BD Biosciences (San Diego, CA) or eBioscience (San Diego, CA).
Peptides and Tetramers
The following MCMV peptide epitopes were used; MHC class I-restricted M45985-993, M38316-323, m139419-426, M57816-824, IE3416-423, m14115-23, M788-15, M861062-1070, M3347-55. Peptides were HPLC purified and synthesized by A&A Systems (San Diego). MHC class I tetramers bound to the M45, M38 or IE3-derived peptides and conjugated to allophycocyanin (Invitrogen) or Extravidin PE (Sigma) were generated as described previously (7).
Adoptive transfer
For adoptive transfer experiments, 1 × 104 WT or CD28−/− OT-I or 1 × 103 WT or B7.1/2−/− OT-I T cells (all CD45.1+) were transferred into naïve (CD45.2) WT mice prior to infection with 5 × 105 PFU MCMV-OVA (i.p.). OT-I T cell expansion was determined in the spleens of recipient mice 8 days post-transfer by flow cytometric analysis of transgenic TCRα chain (Vα2) expression and the CD45.1 (SJL) congenic marker on CD8 T cells.
In vivo cytotoxicity assay
In vivo epitope-specific killing was measured using a published protocol (23), with the following alterations. Target splenocytes from naive CD45.1 mice were differentially costained with CellTrace Far Red DDAO-SE (2 μM; Molecular Probes) and CFSE (0.075 or 1 μM; Sigma-Aldrich) to identify four unique populations. Populations were pulsed with three distinct MCMV peptide epitopes (M45, M38 and IE3) and an unpulsed control population was included. After 1 h peptide pulsing at 37°C, cells were washed, pooled and adoptively transferred i.v. in equal quantities (5 × 106 per population) into WT and B7.1/2−/− recipient mice infected either 8 or 40 days previously. Either 2 hours (for day 8) or 8 hours (for day 40) after adoptive transfer, spleens of recipient mice were harvested and single cell suspensions were analyzed by flow cytometry after incubation with Pacific Blue-conjugated CD45.1 mAbs (Caltag). The relative survival/numbers of each target population was normalized to the same population which was not pulsed with peptide, and the percentage of specific killing was calculated by comparing the survival of that population in naive animals (n = 2), as follows: 100 − ((percentage of peptide pulsed in infected/percentage of unpulsed in infected)/(percentage of peptide pulsed in uninfected/percentage of unpulsed in uninfected)) × 100.
Statistical analysis
Statistical significance of viral titers was determined with the Mann-Whitney test and statistical significance of T cell responses was determined with the two-tailed Student’s t test. Differences between groups were considered significant at p-values <0.05 (* indicates p<0.05 and ** p<0.005).
Results
Expansion of MCMV-specific CD8 T cells that eventually undergo memory inflation is less dependent upon B7-CD28 costimulation during acute infection than those that establish stable memory pools
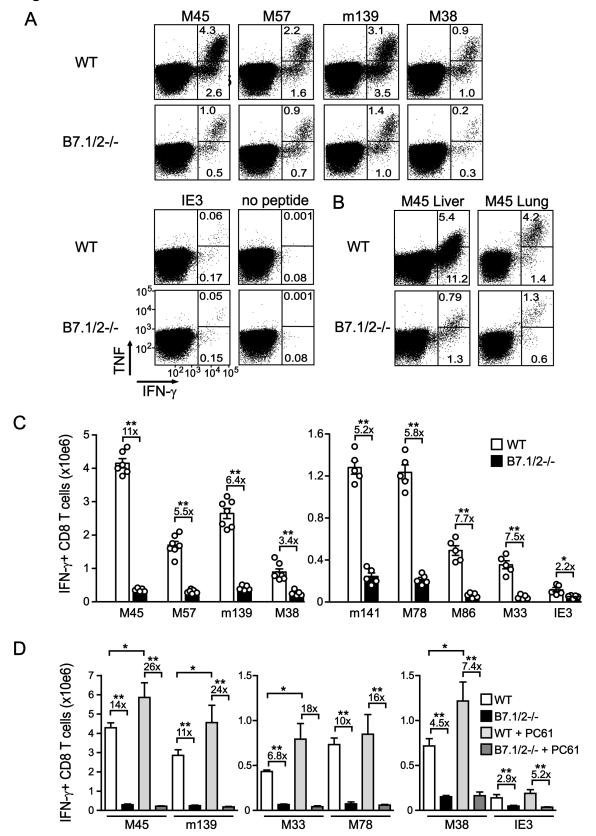
To determine the role of B7-mediated cosignals in regulating MCMV-specific CD8 T cell responses, several epitope-specific responses that either remain stable (e.g. M45 and M57) or inflate in numbers during persistent infection (e.g. m139, M38 and IE3) were analyzed in WT and B7.1/B7.2-deficient (B7.1/2−/−) mice. At day 8 after infection, ~7% of the splenic CD8 T cells reacted specifically to the immunodominant M45-derived peptide epitope in WT mice and produced IFN-γ (of which 4.5% co-produced TNF) upon restimulation, and this was ~6 fold reduced in B7.1/2−/− mice (Fig. 1A). The percentages of M45-specific CD8 T cells were also reduced in both the liver and lungs of B7.1/2−/− mice (Fig.1B). Calculating the absolute numbers of M45-specific CD8 T cells revealed a ~10-fold reduction of these cells in B7.1/2−/− mice, and the magnitude of other MCMV-specific CD8 T cell responses that establish stable memory pools (M57-, m141-, M78-, M86-, M33-specific) were ~6-fold reduced in absolute numbers at day 8 post-infection (Fig. 1C). Also the response to m139, which undergoes intermediate levels of contraction followed by a phase of memory inflation in WT mice, was ~6-fold reduced. However, CD8 T cell responses to the inflationary M38 and IE3 epitopes, which show virtually no contraction, were only ~2 to 4-fold decreased in B7.1/2−/− mice at day 8 post-infection (Fig. 1C).
Figure 1. Expansion of MCMV-specific CD8 T cell responses that undergo inflation is less dependent upon B7-CD28 costimulation.
WT and B7.1/2−/− mice were infected with MCMV and 8 days later the virus-specific CD8 T cell response was analyzed by intracellular cytokine staining after restimulation with the indicated class I epitopes. (A and B) Representative flow plots showing the percentage of CD8+ T cells producing TNF and IFN-γ in (A) spleen, (B) liver or lung. (C) Graphs depict the total number of IFN-γ+ CD8 T cells in the spleen. Numbers are shown as means with standard errors (n=5-7 per group) and each symbol (○) represents an individual mouse. (D) Groups of WT and B7.1/2−/− MCMV-infected mice received anti-CD25 mAb (clone PC61) to deplete Tregs. Graphs show the total number of IFN-γ+ CD8 T cells in the spleen at day 8 post-infection. All bar graphs are shown as means with standard errors (n=5 per group). Statistical significance was determined by Student’s t test (* indicates p<0.05 and ** p<0.005). Data shown are representative of two independent experiments.
To examine whether the reduced expansion of MCMV epitope-specific CD8 T cells in B7.1/2−/− mice might be due to altered control by regulatory T cells (Tregs) (24), we depleted Tregs during acute infection and analyzed the magnitude of MCMV-specific CD8 T cell response 8 days later (Fig. 1D). In WT mice, the CD8 T cell responses to both stable and inflationary epitopes were slightly increased when Tregs were depleted (~1.5-2 fold) (Fig. 1D). However, no effect of Treg depletion was observed in B7.1/2−/− mice, and the preferential impact of B7-CD28 costimulation on the expansion of responses that establish stable memory pools was still observed under these conditions. Altogether, these data indicate that at early times after infection the expansion of MCMV-specific CD8 T cell responses that establish stable memory pools are preferentially dependent upon B7-CD28 signaling.
Both B7.1 and B7.2 promote expansion of MCMV-specific CD8 T cells
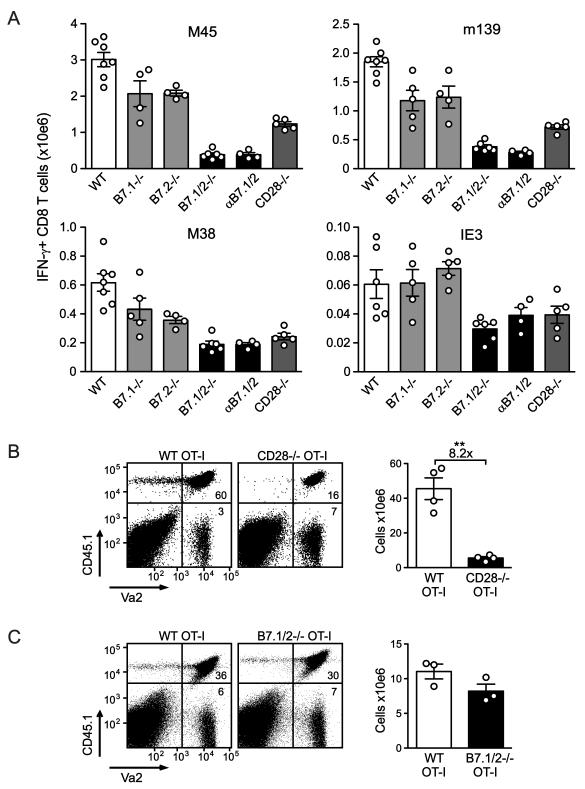
To examine the respective roles of B7.1 and B7.2 in regulating the expansion of MCMV-specific CD8 T cell responses, single knockout mice (B7.1−/− and B7.2−/−) and blocking anti-B7.1/2 antibodies were utilized (Fig. 2A). M45-, m139-, M38- and IE3-specific CD8 T cell numbers were essentially normal in singly-deficient mice at day 8 after infection, demonstrating that redundancy in B7.1 and B7.2-mediated cosignals is operable during MCMV infection (Fig. 2A). CD28−/− mice basically mirrored B7.1/2−/− mice, although the numbers of M45- and m139-specific CD8 T cells were slightly less reduced. Mice treated with anti-B7.1 and/or anti-B7.2 antibodies recapitulated the results obtained in genetically deficient mice, with blockade of both B7 ligands being required for marked reductions in CD8 T cells (Fig. 2A and data not shown). Taken together, these data indicate that both B7.1 and B7.2 can promote the expansion of MCMV-specific effector CD8 T cells during acute infection.
Figure 2. Both B7.1 and B7.2 promote initial expansion of MCMV-specific CD8 T cells.
(A) WT, B7.1−/−, B7.2−/−, B7.1/2−/−, CD28−/− and WT mice treated with blocking B7.1 and B7.2 antibodies (αB7.1/2) were infected with MCMV and 8 days later the virus-specific CD8 T cell response was analyzed by intracellular cytokine staining after restimulation with the indicated class I epitopes. Graphs show the total number of IFN-γ+ CD8 T cells in the spleen. (B) 1 × 104 congenic (CD45.1) WT OVA-specific TCR transgenic CD8 T cells (OT-I) or CD28−/− OT-I cells or (C) 1 × 103 CD45.1 WT OT-I or B7.1/2−/− OT-I cells were adoptively transferred into WT (CD45.2) mice that were subsequently infected with MCMV-OVA. The number of transgenic T cells was determined in the spleen 8 days following transfer. Statistical significance was determined by Student’s t test (** indicates p<0.005). Fold difference is indicated. All bar graphs in this figure are shown as means with standard errors and each symbol (○) represents an individual mouse. Data shown are representative of two independent experiments.
T cells constitutively express CD28 on their cell surface, as well as low levels of B7.1 and B7.2 (25). To determine whether B7 interactions regulate expansion of MCMV-specific CD8 T cells primarily through binding of T cell-expressed CD28, ovalbumin (OVA)-specific CD8 T cells (OT-I) from WT, CD28−/− or B7.1/2−/− background strains mice were transferred into WT mice that were subsequently infected with a recombinant MCMV expressing OVA (MCMV-OVA). A massive expansion of WT OT-I cells was observed after 8 days of infection (>500 fold), while this expansion was substantially decreased when OT-I cells lacked CD28 (~8 fold Fig. 2B). In turn, expansion of B7.1/2−/− OT-I cells were similar to that of WT OT-I cells, establishing that CD28, but not B7.1/2, must be expressed by the T cells. Overall, these data indicate that both B7.1 and B7.2 regulate the expansion of MCMV-specific CD8 T cells through binding of CD28 on T cells.
B7-mediated costimulation contributes, but is not strictly required, for memory CD8 T cell inflation
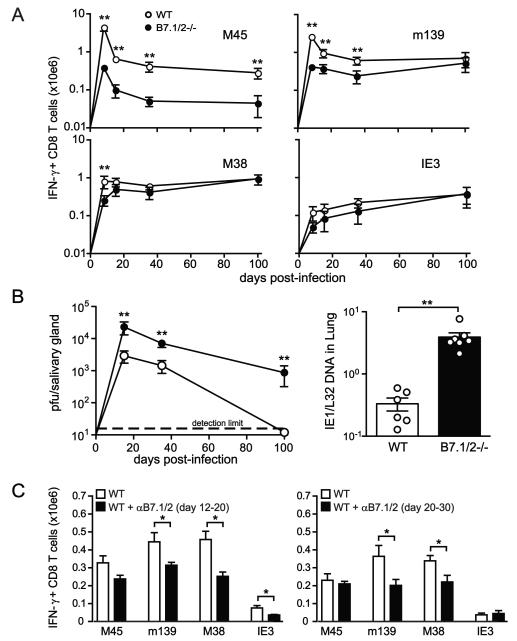
As CMV infection moves into the persistent/latent phase, the immunodominance hierarchy of the MCMV-specific CD8 T cell response changes as inflationary responses increase in magnitude and start to comprise the vast majority of the CMV-specific memory pool. To assess the role of B7-mediated costimulation during this phase of infection, MCMV-specific CD8 T cell responses were measured at several later times of infection (day 15, day 35 and day 100) in B7.1/2−/− and WT mice. Stable memory pools of CD8 T cells (exemplified by M45) remained strongly reduced at all times in B7.1/2−/− mice compared to WT mice (Fig. 3A). In contrast, inflationary responses (m139, M38 and IE3-specific) were only ~2-fold lower at days 15 and 35 post infection, and reached levels roughly equivalent to WT mice by day 100 in B7.1/2−/− mice (Fig. 3A).
Figure 3. Accumulation of inflationary MCMV-specific CD8 T cells in the absence of CD28-B7 costimulation.
(A) WT and B7.1/2−/− mice were infected with MCMV. Graphs depict the time course analysis of the total numbers of IFN-γ producing MCMV-specific CD8 T cells in the spleen at day 8, 15, 35 and 100 after infection. IFN-γ production was determined by intracellular cytokine staining after restimulation with the MHC class I-restricted peptides M45, M38, m139 and IE3. Data are shown as means with standard errors (5-8 mice per time point). (B) Time course analysis of viral titers in the salivary glands at day 15, 35 and 100 post-infection. Data are shown as means with standard errors (5-8 mice per time point). Bar graph shows MCMV DNA levels in the lung at day 15 post-infection as means with standard errors and each symbol (○) represents an individual mouse. (C) MCMV-infected WT mice were treated with blocking B7.1 and B7.2 antibodies (αB7.1/2) or with control IgG from day 12 to day 20 or from day 20 to 30 post-infection. Graphs show the total number of IFN-γ+ CD8 T cells in the spleen at day 20 (αB7.1/2 blockade day 12-20) or day 30 (αB7.1/2 blockade day 20-30) post-infection after restimulation with the class I peptide epitopes M45, m139, M38 and IE3. Bar graphs are shown as means with standard errors (n=5 mice). Statistical significance of viral titers was determined with the Mann-Whitney test and statistical significance of T cell responses was determined with the two-tailed Student’s t test. All data are the average for 4-8 mice per time point. Differences between groups were considered significant at p-values <0.05 (* indicates p<0.05 and ** p<0.005). Data shown are representative of two independent experiments.
The “catch up” of MCMV-specific inflationary CD8 T cells in B7.1/2−/− mice could be due to a variety of reasons, one potentially being increased exposure to viral antigen in the persistent phase of infection. Accordingly, MCMV replication in the salivary gland was assessed in WT and B7.1/2−/− mice from day 15 up to day 100 post-infection. At all these times, increased MCMV replication was observed in the salivary glands of B7.1/2−/− mice, continuing at high levels at times when it is normally controlled in WT mice (Fig. 3B). This increase in salivary gland replication is almost certainly due to the decreased MCMV-specific CD4 T cell response in these mice (26), as this cellular subset is largely responsible for controlling replication in this organ (27). In addition, at day 15 post-infection the levels of viral DNA in total organ homogenates of lung and liver were also increased (Fig. 3B and data not shown).
The differential requirements of B7-CD28 cosignals for the stable and inflationary MCMV-specific memory T cell pools suggested that this costimulatory pathway was operable during the persistent phase for only select epitope-specific responses. To test this, antibodies blocking B7.1 and B7.2 were injected from day 12-20 post MCMV infection, and the stable and inflationary CD8 T cell responses were subsequently analyzed. The response to M45 (stable) epitope trended lower in mice treated with this B7 blocking regiment, although these reductions did not reach statistical significance (Fig. 3C). However, CD8 T cells specific for all three inflationary responses (m139, M38 and IE3) were significantly decreased at day 20 when B7 was blocked at this later time (Fig. 3C). Blocking of B7-mediated costimulation from day 20-30 also significantly decreased the numbers of CD8 T cells specific for m139 and M38, but not IE3. Taken together, these data indicate that B7-mediated signals are implicated in the regulation of the expansion of inflationary MCMV-specific CD8 T cells during early phases of infection and at later times of infection but still allow the gradual accumulation of these cells.
Effects of B7-CD28 signaling on the phenotype of MCMV-specific CD8 T cells
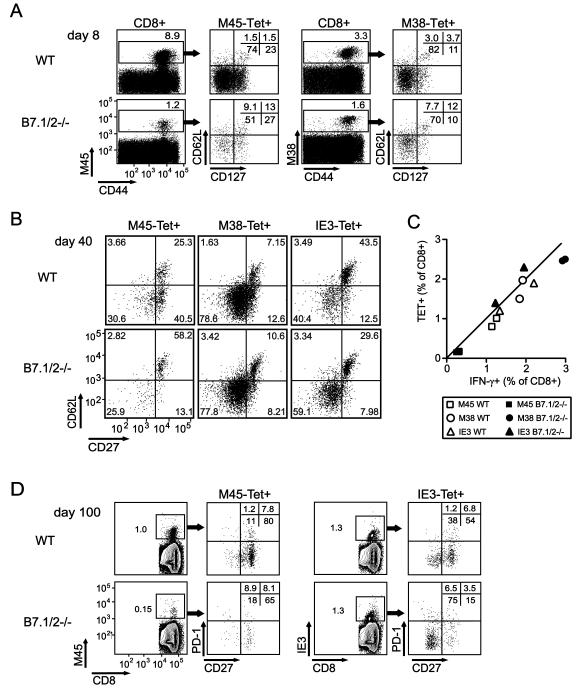
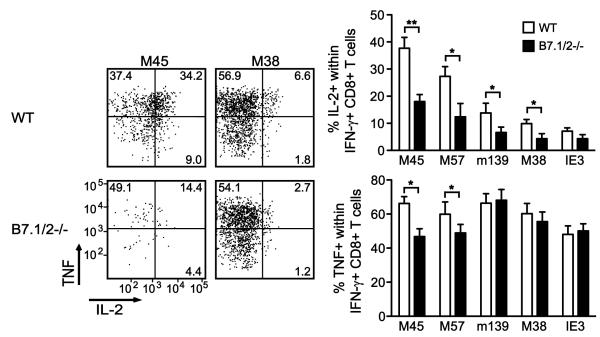
The phenotype of inflationary MCMV-specific CD8 T cells is largely “effector-memory”-like (IL-2−CD27−CD62LloCD127−), whereas the stable memory populations display a “central-memory”-like phenotype (IL-2+CD27+CD62LhiCD127+)(1,7,8,11). To assess whether B7-CD28 impacts the differentiation and/or activation status of these MCMV-specific CD8 T cell populations, the phenotype of class I tetramer-binding (Tet+) CD8 T cells was analyzed at various times following MCMV infection (Fig. 4A). As expected, the number of M45Tet+ CD8 T cells was considerably decreased in B7.1/2−/− mice at day 8 post-infection, but the M45Tet+ CD8 T cells that did develop showed similar levels of CD44 expression as seen in WT mice (Fig. 4A). However, downregulation of CD62L and CD127 on M45Tet+ CD8 T cells was less pronounced in B7.1/2−/− mice (Fig. 4A). Analysis of M38Tet+ CD8 T cells revealed that the difference in downmodulation of CD62L and CD127 in WT and B7.1/2−/− was also observed, albeit not as remarkable as that seen for M45Tet+ CD8 T cells (Fig. 4A). By day 40 after infection, CD62L expression was still higher on M45Tet+ CD8 T cells from B7.1/2−/− mice, but had returned to similar levels on M38Tet+ CD8 T cells (Fig. 4B). In contrast to M45Tet+ CD8 T cells, IE3Tet+ CD8 T cells from B7.1/2−/− displayed a more pronounced downmodulation of CD62L at day 40 post-infection. At early (day 8, day 15) and later (day 40, day 100) times post-infection in both WT and B7.1/2−/− mice, the percentages of Tet+ CD8 T cells correlated closely with IFN-γ producing cells, indicating that functional “exhaustion” does not occur in either case at these times (shown for day 100, Fig. 4C). In addition, at day 40 and day 100 post-infection no difference in PD-1 or CTLA-4 levels was observed on stable or inflationary CD8 T cells when comparing WT and B7.1/2−/− mice (Fig. 4D and data not shown). To test whether B7-CD28 regulation of stable and inflationary CD8 T cell populations correlated with the ability of these cells to produce IL-2, intracellular cytokine staining combined with multi-color flow cytometry was performed. In WT mice at day 15, 40 or 100 after infection, the M45-specific CD8 T cells produced the highest amounts of IL-2 within the IFN-γ-producing population (~38%), followed by the subdominant M57-specific response (~28%) that also establishes a stable, central-memory population (shown for day 100, Fig. 5). However, fewer IL-2 producers were found within the inflationary CD8 T cell populations specific for m139 (~14%), M38 (~11%) and IE3 (~8%). In B7.1/2−/− mice, the percentage of IL-2/IFN-γ producing CD8 T cells was ~2-fold reduced for all MCMV-specific responses examined as compared to WT mice, excepting IE3 which was unaltered (Fig. 5). The decreased autocrine IL-2 production in MCMV-specific CD8 T cells in B7.1/2−/− mice is likely due to early “programming” effects since blocking of B7-CD28 interactions by anti-B7.1/2 mAbs from day 20-30 had no significant effect on the percentage of IL-2 producers among the IFN-γ producing CD8 T cells (data not shown). In contrast to the IL-2/IFN-γ double-producing CD8 T cells, TNF production was differentially affected by the lack of B7-mediated cosignals, with an ~30% reduction seen in M45 and M57 specific responses and none seen in the inflationary populations (Fig. 5). Thus, autocrine TNF production is preferentially regulated by B7-CD28 signals in MCMV-specific stable memory CD8 T cells.
Figure 4. Differential contribution of B7-mediated costimulation on the phenotype of stable and inflationary MCMV-specific CD8 T cells.
WT and B7.1/2−/− mice were infected with MCMV. (A) Flow plots show the cell surface phenotype of M45- and M38-tetramer positive (Tet+) CD8 T cells at day 8 post-infection. CD8 T cells were analyzed for tetramer reactivity, CD44, CD62L and CD127 expression. (B) The cell surface phenotype of M45-, M38- and IE3-Tet+ positive CD8 T cells was analyzed at day 40 post-infection. Plots were gated on tetramer positive CD8 T cells and analyzed for CD27 and CD62L expression. (C) At day 100 post-infection, MCMV-specific CD8 T cells were analyzed for tetramer binding or intracellular expression of IFN-γ after restimulation with the indicated class I epitopes. Graph shows the percentage of Tet+ versus IFN-γ+ CD8 T cell population at day 100 post-infection. (D) At day 100 post-infection, M45- and IE3-Tet+ CD8 T cells in the spleen were analyzed for expression of PD-1 and CD27. Data shown are representative of two independent experiments.
Figure 5. Contribution of B7-mediated costimulation to IL-2 production by stable and inflationary memory populations of MCMV-specific CD8 T cells.
WT and B7.1/2−/− mice were infected with MCMV. At day 100 post-infection, MCMV-specific CD8 T cells were analyzed for intracellular expression of IFN-γ, TNF and IL-2 after restimulation with the indicated class I epitopes. Flow cytometry plots are gated on IFN-γ+ CD8 T cells and show the percentage of TNF and/or IL-2 production within this population. Graphs show the percentage of IL-2+ or TNF+ cells within the IFN-γ+ CD8 T cell population. Similar results were found at day 15 and day 40 post-infection. Statistical significance was determined by Student’s t test (* indicates p<0.05 and ** p<0.005). Bar graphs are shown as means with standard errors (n=5 per group). Data shown are representative of two independent experiments.
MCMV-specific CD8 T cells divide more rapidly in mice lacking B7-CD28 signaling during persistent MCMV infection
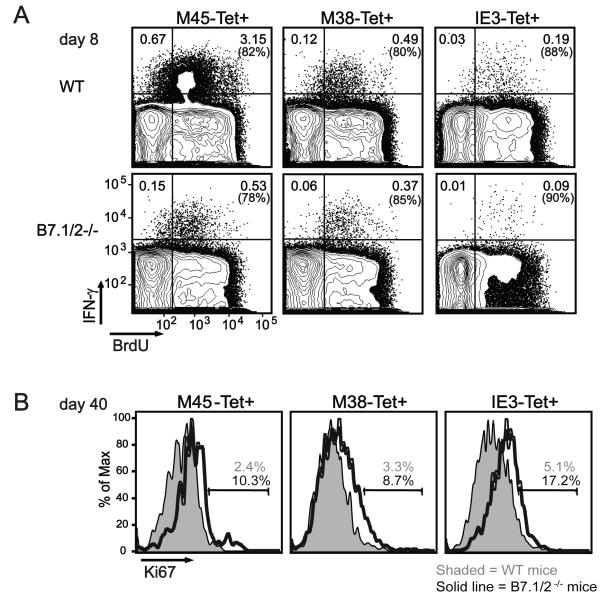
One possible explanation for why inflationary MCMV-specific CD8 T cells in B7.1/2−/− mice reach eventually the levels seen in WT mice could be an increased proliferation rate of these cells at later times of infection. The uptake of the thymidine analog BrdU and the expression of the cell-cycle marker Ki-67 was comparable in MCMV-specific CD8 T cells analyzed from WT and B7.1/2−/− mice at day 8 post-infection (Fig. 6A and data not shown). In contrast, the inflationary M38Tet+ and IE3Tet+ CD8 T cells showed higher Ki-67 levels compared to WT mice at day 40 of infection (Fig. 6B). Also M45Tet+ CD8 T cells showed increased Ki-67 expression in B7.1/2−/− mice, even though these cells remain reduced during the memory phase. These results indicate that the catch-up of inflationary CD8 T cells in B7.1/2−/− mice involves increased proliferation, potentially driven by increased interaction of these cells with their cognate antigen during the persistent phase of MCMV infection.
Figure 6. Increased proliferation of MCMV-specific CD8 T cells in mice lacking B7-CD28 signaling during persistent MCMV infection.
WT and B7.1/2−/− mice were infected with MCMV. (A) At day 8 post-infection BrdU staining was performed after in vivo labeling with BrdU (1 mg/ml in drinking water) for 7 days in combination with intracellular cytokine staining after restimulating splenocytes with the indicated class I epitopes. Plots are gated on CD8 T cells and show BrdU expression versus IFN-γ reactivity. (B) At day 40 post-infection the intracellular expression of the cell cycle marker Ki67 was analyzed on M45-, M38- or IE3-tetramer positive (Tet+) CD8 T cells by flow cytometry. Histograms are gated on splenic CD8 Tet+ cells. Data shown are representative of two independent experiments.
MCMV-specific CD8 T cells that develop in the absence of B7 signaling are functional
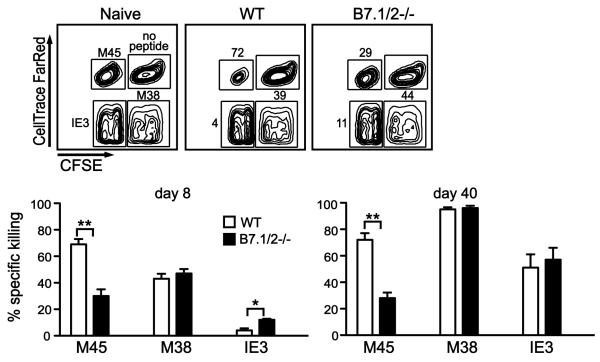
To determine whether B7-CD28 signaling differentially regulates the functional capacity of stable and inflationary MCMV-specific CD8 T cells, an in vivo cytotoxicity assay was performed (Fig. 7). At both day 8 and day 40 post-infection, efficient killing of M45 peptide-pulsed cells was observed in WT mice, while reduced killing was observed in B7.1/2−/− mice (Fig. 7), consistent with the reduction in numbers of M45-specific CD8 T cells in these mice at later times. In contrast, killing of M38 peptide-labeled targets was comparable in WT and B7.1/2−/− mice at both these times, while IE3-specific killing was even increased at day 8. To corroborate these findings, the cytotoxic capacity of MCMV-specific CD8 T cells was analyzed on a per cell basis by determining their expression of granzyme B ex vivo. In WT mice 8% of M45-specific and 11% of M38-specific CD8 T cells express granzyme B at day 8 day after MCMV infection, while in B7.1/2−/− mice these percentages were increased to 11% and 23%, respectively. Taken together, these results reveal that B7-CD28 signals are not required for the cytotoxic function of MCMV-specific CD8 T cells.
Figure 7. B7 costimulation is not required for cytolytic function of MCMV-specific CD8 T cells.
Peptide-pulsed target cells were differentially labeled with two separate fluorescent dyes in order to track 4 separate populations (no peptide, M45-, M38- and IE3-peptide pulsed), and were transferred into uninfected mice (naïve control) or into WT and B7.1/2−/− mice infected with MCMV for 8 or 40 days. The top panels represent a typical analysis of target cells recovered from the spleen after transfer (shown is day 8). Numbers indicate the percent killing of labeled targets after normalization (see methods). Graphs summarize the killing efficiency and show the averaged values. 4-7 mice were used per group and each experiment was repeated twice. Statistical significance was determined by Student’s t test (* indicates p<0.05 and ** p<0.005). Data shown are representative of two independent experiments.
Discussion
The dynamic interactions between CMV and the host immune system are reflected in the diverse populations of CMV-specific T cells that develop over a lifetime of infection. Here we show that B7-CD28 signaling is required to establish normal memory levels of MCMV-specific CD8 T cells that remain stable over time, while CD8 T cells that undergo memory inflation are able to accumulate in the absence of this costimulatory pathway.
The characteristic effector-memory phenotype of CMV-specific CD8 memory T cells that undergo inflation is attributed to repeated antigenic stimulation, presumably from either persistently replicating or reactivating CMV reservoirs (14-17,28), however this remains to be formally proven. Virtually identical numbers of inflationary CD8 T cells were observed in B7.1/2−/− and WT mice at ~100 days post infection, contrasting the defect in initial expansion of these cells in the absence of B7-CD28. The augmented levels of persistent viral antigens, as evidenced by increased salivary gland replication and increased viral DNA levels in B7.1/2−/− mice, may contribute to inflationary cells achieving the levels observed in WT mice. The increased expression of Ki-67 and granzyme B in MCMV-specific CD8 T cells in B7.1/2−/− mice might also be explained by this reasoning. These results substantially extend previous findings (29), which reported that expansion of the polyclonal T cell pool (CD3+/NK1.1−) was decreased in mice lacking B7-CD28 cosignals during early MCMV infection. In addition to potential increased persistent antigen recognition in B7.1/2−/− mice, other costimulatory pathways that regulate memory inflation may contribute differently in the absence of CD28-B7 interactions. For example, the TNFR family members OX40 (CD134) (17) and 4-1BB (CD137) (30) preferentially promote inflationary, but not stable, MCMV-specific memory CD8 T cell numbers, and this contribution is not evident during T cell expansion. Interestingly however, although the impact of these other costimulatory pathways is not revealed until later times, they are operable during the first week of infection, and require CD4 T cells for their effects. This indicates that both “programming” during acute MCMV infection and additional factors during persistence/latency contribute to the regulation of inflation. 4-1BB also plays a unique role in regulating the proliferation of HCMV-specific memory CD8 T cells (31).
The reason for inflation of select MCMV epitope-specific CD8 T cells is poorly understood, and the fact that distinct epitopes derived from the same MCMV gene can either inflate or not (e.g. M38 and M102) highlights this point (8). Latency-specific expression programs have been proposed to potentially account for inflation, and although this may be true in the case of CD8 T cells specific for immediate early antigens (e.g. IE1 or IE3) (28), there is no evidence this is operable for other inflationary responses. In fact, no further increase of inflationary MCMV-specific CD8 T cells occurs between days 100 and 600 post-infection in the vast majority of WT C57/BL6 mice (our unpublished observations), indicating that the window of inflation is largely restricted to the period of persistent replication. This would be consistent with what is observed in humans, where the CMV-specific memory CD8 T cells that become immunodominant after initial systemic infection is controlled remain largely stable for many decades, with inflation potentially occurring again in very old individuals (32).
In summary, we have discovered that B7-CD28 signals are critical for establishing stable memory CD8 T cell pools, but that inflationary memory populations can still develop in their absence during MCMV infection, albeit with delayed kinetics. The severe consequences of HCMV infection in newborns and immunocompromised individuals has spawned a high priority vaccine-development initiative (33). Additionally, recent data indicates that CMV-based vaccine vectors expressing SIV proteins elicit dramatic protection by promoting stable numbers of highly functional T cells displaying an effector-memory phenotype (34). Furthering the understanding of how various costimulatory pathways regulate CMV-elicited T cell responses in these and other contexts will help to provide key insight into these clinically relevant issues.
Acknowledgements
We would like to thank Dr. Ann Hill for providing initial tetramers and Dr. Michael Croft for excellent discussions.
This work was supported by a Veni grant (916.155) from The Netherlands Organization for Research and a Marie Curie Fellowship from the European Commission to R.A., a Deutsche Forschungsgemeinschaft fellowship to A.L. (DFG# 1421/1-1), NIH grants CA80261 and AI76972 to S.P.S. and AI076864 to C.A.B.
Reference List
- 1.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol. Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocarski ES., Jr. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 3.Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–174. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Riddell SR, Greenberg PD. T cell therapy of human CMV and EBV infection in immunocompromised hosts. Rev. Med. Virol. 1997;7:181–192. doi: 10.1002/(sici)1099-1654(199709)7:3<181::aid-rmv200>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat. Rev. Immunol. 2003;3:931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 6.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 8.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 9.Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J. Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- 10.Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 2008;325:315–331. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- 11.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 13.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 2004;173:7481–7489. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 14.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 15.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J. Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamadia LE, van Leeuwen EM, Remmerswaal EB, Yong SL, Surachno S, Wertheim-van Dillen PM, ten Berge IJ, van Lier RA. The size and phenotype of virus-specific T cell populations is determined by repetitive antigenic stimulation and environmental cytokines. J. Immunol. 2004;172:6107–6114. doi: 10.4049/jimmunol.172.10.6107. [DOI] [PubMed] [Google Scholar]
- 17.Snyder CM, Cho KS, Bonnett EL, van DS, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphreys IR, Loewendorf A, De TC, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J. Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 19.Moutaftsi M, Mehl AM, Borysiewicz LK, Tabi Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood. 2002;99:2913–2921. doi: 10.1182/blood.v99.8.2913. [DOI] [PubMed] [Google Scholar]
- 20.Loewendorf A, Kruger C, Borst EM, Wagner M, Just U, Messerle M. Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J. Virol. 2004;78:13062–13071. doi: 10.1128/JVI.78.23.13062-13071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mintern JD, Klemm EJ, Wagner M, Paquet ME, Napier MD, Kim YM, Koszinowski UH, Ploegh HL. Viral interference with B7-1 costimulation: a new role for murine cytomegalovirus fc receptor-1. J. Immunol. 2006;177:8422–8431. doi: 10.4049/jimmunol.177.12.8422. [DOI] [PubMed] [Google Scholar]
- 22.Schneider K, Loewendorf A, De TC, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host. Microbe. 2008;3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Ruckwardt TJ, Chen M, Nicewonger JD, Johnson TR, Graham BS. Epitope-specific regulatory CD4 T cells reduce virus-induced illness while preserving CD8 T-cell effector function at the site of infection. J. Virol. 2010;84:10501–10509. doi: 10.1128/JVI.00963-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 26.Arens R, Loewendorf A, Her MJ, Schneider-Ohrum K, Shellam GR, Janssen E, Ware CF, Schoenberger SP, Benedict CA. B7-mediated costimulation of CD4 T cells constrains cytomegalovirus persistence. J. Virol. 2011;85:390–396. doi: 10.1128/JVI.01839-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 1989;169:1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon CO, Holtappels R, Tervo HM, Bohm V, Daubner T, Oehrlein-Karpi SA, Kuhnapfel B, Renzaho A, Strand D, Podlech J, Reddehase MJ, Grzimek NK. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J. Virol. 2006;80:10436–10456. doi: 10.1128/JVI.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook CH, Chen L, Wen J, Zimmerman P, Zhang Y, Trgovcich J, Liu Y, Gao JX. CD28/B7-mediated co-stimulation is critical for early control of murine cytomegalovirus infection. Viral Immunol. 2009;22:91–103. doi: 10.1089/vim.2008.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, Benedict CA, Ware CF, Croft M. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur. J. Immunol. 2010 doi: 10.1002/eji.200940256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific “effector memory” (CD28(−) CD45RA(HI)) CD8(+) T cells. Blood. 2007;110:4360–4366. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 32.Komatsu H, Inui A, Sogo T, Fujisawa T, Nagasaka H, Nonoyama S, Sierro S, Northfield J, Lucas M, Vargas A, Klenerman P. Large scale analysis of pediatric antiviral CD8+ T cell populations reveals sustained, functional and mature responses. Immun. Ageing. 2006;3:11. doi: 10.1186/1742-4933-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong J, Khanna R. Vaccine strategies against human cytomegalovirus infection. Expert. Rev. Anti. Infect. Ther. 2007;5:449–459. doi: 10.1586/14787210.5.3.449. [DOI] [PubMed] [Google Scholar]
- 34.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]