Abstract
OBJECTIVE
In light of the obesity epidemic, we aimed to characterize novel childhood adiposity trajectories from birth to age 14 years and to determine their relation to adolescent insulin resistance.
RESEARCH DESIGN AND METHODS
A total of 1,197 Australian children with cardiovascular/metabolic profiling at age 14 years were studied serially from birth to age 14 years. Semiparametric mixture modeling was applied to anthropometric data over eight time points to generate adiposity trajectories of z scores (weight-for-height and BMI). Fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) were compared at age 14 years between adiposity trajectories.
RESULTS
Seven adiposity trajectories were identified. Three (two rising and one chronic high adiposity) trajectories comprised 32% of the population and were associated with significantly higher fasting insulin and HOMA-IR compared with a reference trajectory group (with longitudinal adiposity z scores of approximately zero). There was a significant sex by trajectory group interaction (P < 0.001). Girls within a rising trajectory from low to moderate adiposity did not show increased insulin resistance. Maternal obesity, excessive weight gain during pregnancy, and gestational diabetes were more prevalent in the chronic high adiposity trajectory.
CONCLUSIONS
A range of childhood adiposity trajectories exist. The greatest insulin resistance at age 14 years is seen in those with increasing trajectories regardless of birth weight and in high birth weight infants whose adiposity remains high. Public health professionals should urgently target both excessive weight gain in early childhood across all birth weights and maternal obesity and excessive weight gain during pregnancy.
The worldwide increase in overweight and obesity in childhood is associated with increasing rates of early-onset diabetes (1) and is likely to lead to greatly increased incidence of adult cardiovascular disease. There has therefore been considerable interest in patterns of childhood growth and the subsequent rapid increases in obesity as a predictor of adult cardiovascular risk (2). Previous studies have generally assumed that childhood adiposity trajectories are homogenous or limited to few in number according to whether infants were low or normal birth weight (3). Barker et al. (3) showed that adults developing cardiovascular disease had below average early BMI, which subsequently exceeded the average after 11 years. Although a low early birth weight followed by higher BMI by 11 years may have been the dominant pattern in the earlier birth cohort (1934–1944) in the study by Barker et al., we hypothesized that other latent growth trajectories predisposing to cardiovascular disease may exist in contemporary populations because of general increases in birth weight consequent on improved maternal nutrition and increased maternal obesity. In all likelihood, there are multiple pathways to obesity given the many lifestyle (diet and physical activity) and genetic factors contributing to obesity.
Epidemiologic studies suggest an inverse linear relation between birth weight and cardiovascular and metabolic risk (4). Some reports show a U-shaped relationship in which both low and high birth weight infants are at increased risk (5,6). In this context, the issue of critical importance is the effect on insulin resistance of accelerated weight gain over the entire range of birth weight.
Some studies using dichotomized BMI (7) determined from early childhood (8) have confirmed heterogeneity in BMI trajectories (7–9), but they have not been able to interrogate the role of childhood trajectories across the whole spectrum of birth weight.
To allow for the possibility that more than one pattern of infant and childhood growth is associated with greater insulin resistance, we have used a group-based trajectory modeling technique (10), well established for defining developmental and behavioral trajectories.
In a prospective study of the West Australian Pregnancy Cohort (Raine Study), our first aim was to determine whether multiple adiposity trajectories existed from birth to age 14 years and to assess their relation to a cross-sectional measure of insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR] and fasting insulin) at age 14 years. In adolescents, sex differences and pubertal stage have also been shown to strongly affect cardiovascular risk factors (11). The second aim was to determine whether sex differences occurred in the relationship between adiposity trajectories and insulin resistance at age 14 years. We hypothesized that rapid gain in weight/adiposity in early life is causative for insulin resistance regardless of birth weight. Further, we proposed that increased insulin resistance demonstrated for higher birth weight infants (12) would also be influenced by their childhood adiposity trajectories.
RESEARCH DESIGN AND METHODS
Population
The Raine Cohort (5) enrolled pregnant women in the ≤18th week of gestation (1989–1991) into a randomized controlled trial to evaluate the effects of repeated ultrasound in pregnancy (N = 2,900) through the antenatal clinic at King Edward Memorial Hospital (KEMH) and nearby private clinics. A total of 2,868 live births eventuated. Detailed clinical assessments were performed at birth. Birth information (including birth weight) was obtained by midwife records. The children were followed up at 1, 2, 3, 5, 8, 10, and 14 years of age by questionnaire and physical assessments as shown in Supplementary Table 1. Questionnaires included sociodemographic and behavioral data.
Participants
To be eligible for enrollment, the women were required to have a pregnancy between 16 and 20 weeks gestation, proficient English language, an expectation to deliver at KEMH, and an intention to reside in Western Australia. Ninety percent of eligible women agreed to participate in the study. Informed consent to participate in the study was obtained from the mother of each child at enrollment and at each subsequent follow-up. The Human Ethics Committees (KEMH or Princess Margaret Hospital) approved all protocols.
Loss to follow-up at 14-year contact
A total of 1,860 of the original 2,868 live births were included in the 14-year follow-up (357 deferred from participating, 412 had withdrawn, 207 were lost to follow-up, and 32 were deceased). Of these, 1,377 consented and underwent phlebotomy. Because multiple births and congenital anomalies (13) are significant potential confounders previously identified to affect both cardiovascular risk and birth size/growth, these exclusions were made. Excluding congenital anomalies resulted in 1,255 participants. Excluding multiple births resulted in 1,197 participants. The original cohort more closely reflected those patients referred to a tertiary center, overrepresenting socially disadvantaged families. Socially disadvantaged participants were less likely to remain in the study beyond 3 years (14). The remaining study participants had sociodemographic characteristics equivalent to those of the general Western Australian population by age 3 years (14) and 14 years (15).
Comparison of participants with nonparticipants in cardiovascular follow-up at age 14 years
Those without cardiovascular data at 14 years had lower maternal education, family income, and maternal age of conception. There was no difference in gestational age, birth weight, or birth length (Supplementary Table 2).
Anthropometry
At each follow-up, height and weight were measured by Holtain Infantometer and Stadiometer (nearest 0.1 cm) and Wedderburn Chair Scales (nearest 100 g). BMI z scores (2–14 years) and weight-for-height z scores (<2 years) customized by age and sex were calculated using U.S. Centers for Disease Control and Prevention growth chart software recommended for Australian children. Because the Centers for Disease Control and Prevention does not provide BMI z scores for those aged ≤24 months, weight-for-height z scores were used as the best adiposity measurement available for this age. Because BMI-for-age and weight-for-length percentiles are highly correlated (16), combining z scores (weight-for-height and BMI) achieved a consistent scale for approximating adiposity.
Assessment of 14-year-old children
Biochemistry.
Overnight fasting venous blood samples obtained at the children’s homes were analyzed (Royal Perth Hospital) for serum insulin and glucose by previously described methods (17). HOMA-IR approximating insulin resistance was calculated by (insulin [μU/mL] × glucose [mmol/L])/22.5 (18).
Other measures.
Physical activity was scored from questionnaires answered by the adolescents as exercise causing breathlessness or sweating (monthly or less, weekly, 2–3 times weekly, 4–6 times weekly, daily). Kilojoules per day were based on adolescent food frequency questionnaires developed by Commonwealth Scientific and Industrial Research Organization, Adelaide, Australia.
Socioeconomic status was assessed by annual family income (Australian dollars <$25,000 and >$25,000) at the 14-year survey in 2004–2007. Adolescents self-rated puberty by Tanner stage diagrams (19). Maternal weight and height were measured at 18 and 34 weeks gestation by a trained midwife. Prepregnancy weight was self-reported by questionnaire. Occurrence of self-reported gestational diabetes was recorded by midwives 2 days after delivery.
Data analysis
SPSS v15.0 (SPSS Inc., Chicago, IL) and SAS v9.1 (SAS Institute Inc., Cary, NC) were used for statistical analyses. Log transformation was undertaken for outcome variables (insulin and HOMA-IR) not normally distributed. Significance was set for P values <0.05.
Stage 1: identification of distinctive adiposity trajectories (birth to 14 years).
To allow for the possibility that more than one pattern of infant and childhood growth is associated with cardiovascular risk, we used a group-based trajectory modeling technique (10), well established for defining developmental and behavioral trajectories.
This technique contrasts with conventional growth modeling (linear mixed effects). This technique allows data grouping and identifies subpopulations. In regard to obesity, in which multiple physiologic, genetic, and environmental factors are implicated, groupings may represent components for approximating unknown (possibly complex) data distribution. The trajectories were identified a priori before relating them to cardiovascular risk. We avoided the assumption that all those with high cardiovascular risk had a homogeneous adiposity growth trajectory.
The shape (rising, falling, stable, or hump-shaped) and the estimated population proportion in each trajectory were defined using SAS Proc Traj (10). The optimal model was selected by maximum Bayesian Information Criterion (BIC). Models were estimated with three to seven groups and linear or quadratic shapes using adiposity z scores at eight time points. Improvement in BIC was judged using the Bayes Factor, which was required to be greater than 10 and was calculated by eBIC i – BIC j. Model adequacy was judged using three diagnostic tests (Supplementary Appendix 1). The posterior probability of membership for each trajectory was estimated, and individuals were assigned to their highest probability trajectory best conforming to their z scores. These trajectories were identified a priori to the cardiovascular disease risk analysis.
Proc Traj accommodates data missing completely at random (10). When Proc Traj was applied to all available data points without restricting to those who had cardiovascular assessment at 14 years or alternatively to a dataset restricted to only participants with complete data for every available time point, comparable shape and proportions were obtained in each trajectory.
Stage 2: relation of adiposity trajectories to cardiovascular risk at age 14 years.
Significant sex with trajectory interactions were present (P = 0.001 for interaction). Insulin resistance with strong sex differences was analyzed separately by sex. One-way ANOVA and χ2 assessed differences between trajectories for HOMA-IR and insulin. Linear regression was performed without (model 1) and with (model 2) adjustment for age, gestational age, ethnicity, diet, exercise, and family income. Additional adjustment was made for puberty (model 3), excluding 170 girls and 164 boys who were incorrectly shown diagrams with ambiguous labeling. Multivariate models were built with inclusion of covariates that play a role in cardiovascular risk.
RESULTS
Adiposity trajectories
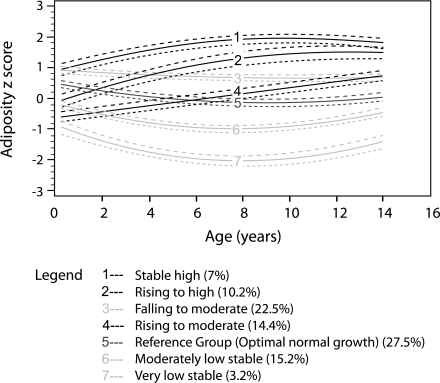
Seven adiposity trajectories were identified (Fig. 1). Two trajectories reached high adiposity, designated “stable high” (trajectory 1) and “rising to high” (trajectory 2). Two trajectories reached moderate adiposity, designated “falling to moderate” (trajectory 3) and “rising to moderate” (trajectory 4). Three trajectories were stable low-moderate adiposity (trajectories 5–7). Group 5, the largest group (27.5%) of individuals, had lifelong average adiposity z scores approximating zero and slightly above average adiposity at birth designated “optimum normal growth.”
Figure 1.
The seven adiposity trajectories identified across the lifecourse of these adolescents from birth to 14 years of age. The trajectories are shown in solid lines, and the 95% CIs are shown in dotted lines. The proportions in each trajectory are shown in brackets. Trajectory 5 (“optimum normal growth”) is the reference group with adiposity z scores approximating zero across the lifetime of these individuals after the period of infancy.
Characteristics of the different adiposity trajectories
All trajectories were similar for sex, age, ethnicity, and maternal education. Trajectories 1, 2, and 7 had a greater proportion (24–29%) of parents in the lowest income group compared with the remaining groups (15–20%) (χ2 P = 0.025). Group 7 (very low stable trajectory) included more preterm births than the other trajectories (P = 0.01). There were no differences in gestational age at delivery for the remaining six trajectory groups (Table 1).
Table 1.
General characteristics, anthropometry, and cardiovascular risk factor levels in the seven adiposity trajectory groups
| 1 |
2 |
3 |
4 |
5 |
6 |
7” |
|||
|---|---|---|---|---|---|---|---|---|---|
| Trajectory | All trajectories N = 1,197 | “Stable high” | “Rising to high” | “Falling to moderate” | “Rising to moderate” | “Optimal growth” | “Moderate low stable” | “Very low stable” | P value |
| General | |||||||||
| Sex (proportion of males) | 0.52 | 0.60 | 0.51 | 0.52 | 0.48 | 0.49 | 0.56 | 0.49 | 0.46* |
| Age (years) | 14.1 (14.0–14.1) | 14.1 (14.0–14.1) | 14.1 (14.0–14.1) | 14.1 (14.0–14.1) | 14.0 (14.0–14.1) | 14.1 (14.0–14.1) | 14.1 (14.0–14.1) | 14.1 (14.0–14.1) | 0.27 |
| Gestational age (weeks) | 39.3 (39.2–39.4) | 39.4 (39.1–39.8) | 39.2 (38.9–39.6) | 39.5 (39.3–39.7) | 39.3 (38.9–39.6) | 39.4 (39.2–39.6) | 39.3 (39.0–39.7) | 38.1 (37.1–39.2) | 0.01 |
| Family income (proportion in the lowest income group <$25,000) | 0.19 | 0.25 | 0.29 | 0.19 | 0.20 | 0.15 | 0.17 | 0.24 | 0.025* |
| Ethnicity (proportion with Caucasian mother) | 0.90 | 0.94 | 0.87 | 0.94 | 0.88 | 0.91 | 0.81 | 0.90 | 0.07* |
| Anthropometry | |||||||||
| Birth weight (g) | 3,357 (3,326–3,388) | 3,526 (3,402–3,651) | 3,315 (3,227–3,403) | 3,528 (3,466–3,591) | 3,136 (3,054–3,219) | 3,407 (3,354–3,460) | 3,252 (3,178–3,327) | 2,821 (2,517–3,070) | <0.001 |
| BMI at age 3 years | 16.15 (16.07–16.24) | 18.36 (18.04–18.67) | 16.52 (16.33–16.72) | 17.03 (16.91–17.14) | 15.52 (15.38–15.67) | 15.94 (15.86–16.03) | 14.90 (14.77–15.03) | 14.01 (13.79–14.23) | <0.001 |
| BMI at age 5 years | 15.86 (15.76–15.97) | 19.70 (1,915–20.25) | 17.45 (17.21–17.70) | 16.47 (16.37–16.57) | 15.36 (15.24–15.48) | 15.22 (15.15–15.30) | 14.18 (14.09–14.26) | 13.18 (12.99–13.37) | <0.001 |
| BMI at age 14 years | 21.4 (21.2–21.6) | 29.4 (28.3–30.6) | 26.6 (25.9–27.4) | 21.9 (21.6–22.2) | 22.2 (21.8–22.5) | 19.3 (19.1–19.5) | 18.0 (17.8–18.3) | 16.1 (15.7–16.6) | <0.001 |
| Cardiovascular risk factors measured at age 14 years | |||||||||
| Insulin (U/L), males | 11.9 (11.1–12.7) | 16.4 (13.8–19.0) | 18.1 (14.2–21.9) | 10.8 (9.5–12.1) | 15.7 (12.3–19.1) | 9.6 (8.8–10.4) | 9.5 (8.2–10.8) | 7.4 (5.6–9.3) | <0.001 |
| Insulin (U/L), females | 12.6 (11.9–13.3) | 18.2 (15.2–21.2) | 16.1 (13.3–18.9) | 11.3 (10.5–12.2) | 12.6 (10.2–15.0) | 11.8 (10.6–12.9) | 11.3 (10.2–12.4) | 14.4 (8.0–20.7) | <0.001 |
| HOMA-IR, males | 2.7 (2.5–2.9) | 3.6 (3.0–4.2) | 4.1 (3.1–5.2) | 2.4 (2.1–2.8) | 3.8 (2.7–4.8) | 2.1 (1.9–2.3) | 2.0 (1.7–2.3) | 1.6 (1.2–2.1) | <0.001 |
| HOMA-IR, females | 2.7 (2.5–2.8) | 3.9 (3.2–4.5) | 3.4 (2.8–4.0) | 2.3 (2.2–2.5) | 2.7 (2.1–3.2) | 2.5 (2.2–2.8) | 2.4 (2.1–2.8) | 3.1 (1.7–4.5) | <0.001 |
| Maternal characteristics | |||||||||
| Maternal BMI | 22.40 (22.15–22.65) | 25.17 (23.90–26.44) | 25.59 (23.55–25.64) | 22.68 (22.17–23.18) | 22.34 (21.70–22.99) | 21.60 (21.21–22.0) | 21.19 (20.68–21.69) | 20.61 (19.61–22.65) | <0.001 |
| % Maternal obesity (BMI >30) | 7 | 26 | 16 | 8 | 4 | 3 | 4 | 0 | <0.001 |
| Pregnancy weight gain (g/week) | 403 (393–413) | 447 (393–500) | 396 (361–431) | 413 (393–433) | 392 (366–417) | 404 (388–420) | 391 (369–414) | 341 (295–387) | <0.001 |
| Gestational diabetes | 1.7 | 6.5 | 3.6 | 0.7 | 0.6 | 1.8 | 1.2 | 0.0 | 0.015* |
Adiposity trajectory 5 is the comparison group.
Mean and 95% CIs are shown.
*χ2 P value.
Maternal BMI, weight gain, and gestational diabetes in the adiposity trajectories.
Maternal BMI, pregnancy weight gain, and prevalence of gestational diabetes differed among the trajectory groups (all P < 0.001). Group 1 had a higher than average pregnancy maternal weight gain of 450 g per week vs. 341–413 g seen in the other trajectories. Mothers giving birth to group 1 trajectory children also had a higher prevalence of gestational diabetes at 6.5%, approximately 4 times the average prevalence of gestational diabetes in the sample, and a prevalence of obesity of 26%, >3.5 times the average prevalence of the sample.
Insulin resistance and trajectories
Group 5 was used as the reference group for comparison because it had average adiposity z scores stabilized at approximately zero up to age 14 years, resembling “normal” growth, and included the largest proportion of children (27.5%). In boys, HOMA-IR and insulin were higher in “stable high,” “rising to high,” and “rising to moderate” trajectories (1,2,4) compared with group 5 (all adjusted models P < 0.001). In girls, HOMA-IR and insulin were higher in the “stable high” trajectory (1) and “rising to high” trajectory (2) compared with reference group 5 (all P ≤ 0.002 in models 1 and 2). Model 3, with additional adjustment for puberty in a restricted number of children, did not reach significance in relation to trajectory 2 (Table 2).
Table 2.
Linear regression models determining whether adiposity trajectories are associated with insulin resistance in boys and girls
| Adiposity trajectory | 1 “Stable high” |
2 “Rising to high” |
3 “Falling to moderate” |
4 “Rising to moderate” |
6 “Moderate low stable” |
7 “Very low stable” |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β coefficient (95% CI) | P value | β coefficient (95% CI) | P value | β coefficient (95% CI) | P value | β coefficient (95% CI) | P value | β coefficient (95% CI) | P value | β coefficient (95% CI) | P value | |
| Cardiovascular risk | ||||||||||||
| Boys | ||||||||||||
| HOMA-IR | ||||||||||||
| Model 1 | 1.6 (1.3–2.0) | <0.001 | 1.8 (1.5–2.1) | <0.001 | 1.1 (0.9–1.2) | 0.27 | 1.4 (1.2–1.7) | <0.001 | 0.9 (0.8–1.1) | 0.22 | 0.7 (0.6–1.0) | 0.04 |
| Model 2 | 1.6 (1.3–1.9) | <0.001 | 1.7 (1.4–2.1) | <0.001 | 1.1 (0.9–1.2) | 0.39 | 1.4 (1.2–1.7) | <0.001 | 0.9 (0.8–1.0) | 0.17 | 0.7 (0.6–1.0) | 0.04 |
| Model 3 | 1.6 (1.3–2.0) | <0.001 | 1.8 (1.1–1.5) | <0.001 | 1.1 (1.0–1.3) | 0.07 | 1.5 (1.2–1.8) | <0.001 | 0.9 (0.8–1.1) | 0.17 | 0.9 (0.6–1.2) | 0.39 |
| Insulin | ||||||||||||
| Model 1 | 1.6 (1.4–2.0) | <0.001 | 1.7 (1.5–2.1) | <0.001 | 1.1 (0.9–1.2) | 0.34 | 1.4 (1.2–1.6) | <0.001 | 0.9 (0.8–1.1) | 0.21 | 0.8 (0.6–1.0) | 0.04 |
| Model 2 | 1.6 (1.3–1.9) | <0.001 | 1.7 (1.4–2.0) | <0.001 | 1.0 (0.9–1.2) | 0.48 | 1.4 (1.2–1.6) | <0.001 | 0.9 (0.8–1.0) | 0.16 | 0.8 (0.6–1.0) | 0.04 |
| Model 3 | 1.6 (1.3–2.0) | <0.001 | 1.8 (1.5–2.1) | <0.001 | 1.1 (1.0–1.3) | 012 | 1.4 (1.2–1.7) | <0.001 | 0.9 (0.8–1.0) | 0.16 | 0.9 (0.6–1.2) | 0.40 |
| Girls | ||||||||||||
| HOMA-IR | ||||||||||||
| Model 1 | 1.6 (1.3–2.0) | <0.001 | 1.3 (1.1–1.5) | <0.001 | 1.0 (0.9–1.1) | 1.0 | 1.1 (0.9–1.2) | 0.25 | 1.0 (0.9–1.2) | 0.75 | 1.1 (0.9–1.5) | 0.26 |
| Model 2 | 1.6 (1.3–2.0) | <0.001 | 1.3 (1.1–1.5) | 0.002 | 1.0 (0.9–1.1) | 0.76 | 1.1 (1.0–1.2) | 0.23 | 1.0 (0.9–1.2) | 0.85 | 1.1 (0.8–1.3) | 0.65 |
| Model 3 | 1.5 (1.2–1.8) | 0.001 | 1.2 (1.0–1.4) | 0.056 | 1.0 (1.0) | 0.84 | 1.1 (0.9–1.2) | 0.31 | 1.0 (0.9–1.2) | 0.73 | 1.0 (0.8–1.4) | 0.75 |
| Insulin | ||||||||||||
| Model 1 | 1.6 (1.3–1.9) | <0.001 | 0.3 (1.1–1.5) | 0.001 | 1.0 (0.9–1.1) | 0.72 | 1.1 (1.0–1.2) | 0.19 | 1.0 (0.9–1.2) | 0.78 | 1.1 (0.9–1.4) | 0.26 |
| Model 2 | 1.6 (1.3–1.9) | <0.001 | 1.3 (1.1–1.5) | 0.001 | 1.0 (0.9–1.2) | 0.50 | 1.1 (1.0–1.2) | 0.16 | 1.0 (0.9–1.1) | 0.91 | 1.1 (0.8–1.3) | 0.61 |
| Model 3 | 1.5 (1.2–1.8) | <0.001 | 1.2 (1.0–1.4) | 0.065 | 1.0 (0.9–1.2) | 0.60 | 1.1 (0.9–1.3) | 0.22 | 1.0 (0.9–1.2) | 0.66 | 1.1 (0.8–1.4) | 0.64 |
Model 1 unadjusted for any covariates.
Model 2 adjusted for age, gestational age, ethnicity, diet, exercise, and family income (annual income in Australian dollars ≤$25,000 or >$25,000).
Model 3 also adjusted for puberty, excluding 170 girls and 164 boys accidently shown diagrams with ambiguous labeling.
Adiposity trajectory 5 is the comparison group. The bold text indicates results associated with a P value < 0.05.
Because insulin resistance is highly correlated with current BMI, we specifically compared risk factors within pairs of trajectories with similar 14-year-old BMI (1 vs. 2, and 3 vs. 4) but different gradients (Table 3). Boys in a “rising to moderate” trajectory 4 had higher insulin resistance compared with boys in a “falling to moderate” trajectory 3, despite both trajectories having similar current BMI (all P = 0.001).
Table 3.
Comparison of trajectories reaching similar final BMI to ascertain importance of accelerated adiposity gain
| Cardiovascular risk factor | Comparison groups 1 and 2 |
P value | Comparison groups 3 and 4 |
P value |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | |||
| Boys | ||||
| HOMA-IR | ||||
| Model 1 | 1.2 (1.0–1.5) | 0.063 | 1.3 (1.1–1.5) | 0.001 |
| Model 2 | 1.2 (1.0–1.5) | 0.072 | 1.3 (1.1–1.5) | 0.001 |
| Insulin | ||||
| Model 1 | 1.2 (1.0–1.5) | 0.090 | 1.3 (1.1–1.5) | 0.001 |
| Model 2 | 1.2 (1.0–1.5) | 0.096 | 1.3 (1.1–1.5) | 0.001 |
| Girls | ||||
| HOMA-IR | ||||
| Model 1 | 1.0 (0.8–1.3) | 0.84 | 1.1 (0.9–1.2) | 0.34 |
| Model 2 | 1.0 (0.8–1.3) | 0.83 | 1.0 (0.9–1.2) | 0.54 |
| Insulin | ||||
| Model 1 | 1.1 (0.8–1.3) | 0.76 | 1.1 (0.9–1.2) | 0.41 |
| Model 2 | 1.0 (0.8–1.3) | 0.75 | 1.1 (0.9–1.2) | 0.64 |
Model 1 adjusted for age, gestational age, ethnicity, diet, exercise, low family income, and current BMI. Model 2 also adjusted for birth weight.
Groups 1 (“stable high”) and 3 “falling to moderate” are referent groups. These referent groups are compared with rising trajectories that reach similar current BMI. The bold text indicates results associated with a P value < 0.05.
CONCLUSIONS
This study identified seven different trajectories of adiposity in a contemporary childhood population from a developed country studied from in utero to 14 years of age. Three adiposity trajectories (two rising and one “chronic high” adiposity) were related to significantly higher insulin resistance than the reference group. The three highest risk trajectories comprised approximately one third of the sample. Higher insulin resistance at age 14 years was not restricted to those with low birth weight and occurred with both moderate adiposity at age 14 years and moderate birth weight, providing there was accelerated adiposity gain (rising trajectory). Higher HOMA-IR and insulin levels were also seen in the subpopulation whose trajectory was associated with sustained high adiposity. This group also showed higher maternal BMI, greater maternal weight gain during pregnancy, and increased rates of gestational diabetes.
If more than one subpopulation of growth leading to cardiovascular disease were to exist, a priori group-based trajectory modeling would be more likely to detect this. We identified two distinct high-risk trajectories (1 and 2) not identified in the 1934–1944 cohort (3). Arguably, these trajectories, containing individuals achieving high adolescent adiposity, may have only reached prominence in contemporary cohorts in which both mothers (12) and children (20) are exposed to obesogenic lifestyles.
Li et al. (21) demonstrated an “early-onset overweight” trajectory corresponding to our trajectory 1 (“chronic high” adiposity) and a “late-onset overweight” trajectory corresponding to trajectory 2 (“moderate to high rising adiposity”). Ventura et al. (9) identified an upward percentile group associated with more metabolic risk factors. This corresponds to our “rising to high” adiposity trajectory 2. We describe a further trajectory 4 (rising to moderate adiposity) containing individuals not ultimately obese and therefore undetectable with dichotomized BMI, but nevertheless associated with high metabolic risk.
The importance of accelerated adiposity gain in relation to insulin resistance at age 14 years was attenuated in females compared with males. In females, the “rising to moderate adiposity” trajectory 4, most closely akin to the low birth weight (3,4) groups underlying Barker’s hypothesis, showed no increase in insulin resistance. Fetal programming is more often observed in males than females in animal and human studies (22). During puberty, girls are more insulin resistant at all pubertal stages. This needs to be further studied to see whether sex differences in fetal programming persist when the subjects become adults.
An increasing proportion of women of childbearing age are becoming obese and developing gestational diabetes (20,23). These women give birth to large for gestational age babies and infants who subsequently are at risk for diabetes and obesity themselves (12). Group 1 (the “stable high” adiposity) is likely to become more common and the descending trajectory 3 (“falling to moderate” adiposity) less common. Current contemporary trends toward early onset of childhood obesity (20) are also likely to be reflected in a greater proportion of children in the “rising to high” trajectory 2 and “stable high” trajectory 1.
A limitation of the study is its moderate size and selective attrition. A total of 1,197 subjects who had a complete metabolic dataset at 14 years (42% of original cohort) defined the trajectories. Nevertheless, the number of subjects is larger than in previous obesity studies using latent class or semiparametric mixture models. Some degree of selective attrition occurred with loss of families from lower socioeconomic status (14). Because the population initially recruited was at relatively high risk, this pattern of attrition is likely to result in those remaining being more characteristic of the general community (15,16). Furthermore, subjects with and without metabolic data at age 14 years did not differ with regard to critical birth anthropometry.
“Proc Traj” accommodates random missing data (10). Missing data in the Raine study is not entirely random. However, using “Proc Traj” on subjects with no missing data revealed similar trajectories albeit with wider CIs (data not shown).
BMI may not be the optimal measure of adiposity, but it is a good approximation for a large population study in which measures have been repeated in a cohort of 1,197 at eight time points. Further, we have shown that BMI correlates strongly with all the other traditional cardiovascular risk factors at age 14 years in this population (24). The outcome measurement of insulin resistance at the final time end point is cross-sectional and reflects risk at age 14 years. Nevertheless, insulin resistance is known to track from adolescence into adult life (25); thus, a cross-sectional measure of insulin resistance at age 14 years can approximate risk of adult cardiovascular disease and diabetes.
In conclusion, by using the statistical approach of semiparametric mixture modeling, we show the existence of multiple adiposity trajectories. We also demonstrated increased insulin resistance at age 14 years with rising trajectories across the full spectrum of birth weights. This is more obvious in boys than girls. In addition, a constantly high trajectory is associated with increased insulin resistance at age 14 years, maternal gestational diabetes, and obesity and weight gain during pregnancy. The three highest risk trajectories comprise 32% of the population. These data provide refined definitions of growth trajectories in childhood and may give further impetus for identifying and targeting appropriate individuals for prevention of childhood and maternal obesity and managing excessive weight gain during pregnancy.
Supplementary Material
Acknowledgments
This study was supported by the Raine Medical Foundation, Healthway, Western Australia; The Telethon Institute for Child Health Research (University of Western Australia); and the Australian National Health and Medical Research Council.
No potential conflicts of interest relevant to this article were reported.
R.-C.H. conceptualized, analyzed, and wrote the article. N.H.d.K., A.S., and G.E.K. provided statistical advice. L.I.L. initiated the cohort. T.A.M. supplied laboratory data. J.P.N. and F.J.S. initiated the cohort. W.H.O. supplied dietary data. B.H. supplied exercise data. L.J.B. extensively reviewed the article. All authors reviewed the article.
The authors thank all the families who took part in this study and the Raine Study team, which includes data collectors, cohort managers, clerical staff, research scientists, and volunteers. The authors thank Tammy Gibbs (The Telethon Institute for Child Health Research) for help with graphics.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1809/-/DC1.
References
- 1.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346:802–810 [DOI] [PubMed] [Google Scholar]
- 2.Wilkin TJ, Metcalf BS, Murphy MJ, Kirkby J, Jeffery AN, Voss LD. The relative contributions of birth weight, weight change, and current weight to insulin resistance in contemporary 5-year-olds: the EarlyBird Study. Diabetes 2002;51:3468–3472 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med 2005;353:1802–1809 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–417 [DOI] [PubMed] [Google Scholar]
- 5.Huang RC, Burke V, Newnham JP, et al. Perinatal and childhood origins of cardiovascular disease. Int J Obes (Lond) 2007;31:236–244 [DOI] [PubMed] [Google Scholar]
- 6.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 2003;41:451–456 [DOI] [PubMed] [Google Scholar]
- 7.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring) 2007;15:760–771 [DOI] [PubMed] [Google Scholar]
- 8.Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. Obesity and psychiatric disorder: developmental trajectories. Pediatrics 2003;111:851–859 [DOI] [PubMed] [Google Scholar]
- 9.Ventura AK, Loken E, Birch LL. Developmental trajectories of girls’ BMI across childhood and adolescence. Obesity (Silver Spring) 2009;17:2067–2074 [DOI] [PubMed] [Google Scholar]
- 10.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods 2001;6:18–34 [DOI] [PubMed] [Google Scholar]
- 11.Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab 1995;80:172–178 [DOI] [PubMed] [Google Scholar]
- 12.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296 [DOI] [PubMed] [Google Scholar]
- 13.Aberg A, Westbom L, Källén B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev 2001;61:85–95 [DOI] [PubMed] [Google Scholar]
- 14.Kendall GE. Children in Families in Communities: A Modified Conceptual Framework and an Analytic Strategy for Identifying Patterns of Factors Associated with Developmental Health Outcomes in Childhood. PhD thesis. Perth, University of Western Australia, 2003 [Google Scholar]
- 15.Robinson M, Oddy WH, McLean NJ, et al. Low-moderate prenatal alcohol exposure and risk to child behavioural development: a prospective cohort study. BJOG 2010;117:1139–1150 [DOI] [PubMed] [Google Scholar]
- 16.Nash A, Secker D, Corey M, Dunn M, O’Connor DL. Field testing of the 2006 World Health Organization growth charts from birth to 2 years: assessment of hospital undernutrition and overnutrition rates and the usefulness of BMI. JPEN J Parenter Enteral Nutr 2008;32:145–153 [DOI] [PubMed] [Google Scholar]
- 17.Huang RC, Mori TA, Burke V, et al. Synergy between adiposity, insulin resistance, metabolic risk factors, and inflammation in adolescents. Diabetes Care 2009;32:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 1976;51:170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JM, Pilli S, Gebremariam A, et al. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) 2010;34:614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Hardy R, Kuh D, Lo Conte R, Power C. Child-to-adult body mass index and height trajectories: a comparison of 2 British birth cohorts. Am J Epidemiol 2008;168:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol 2002;175:757–767 [DOI] [PubMed] [Google Scholar]
- 23.Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Am J Obstet Gynecol 2001;185:845–849 [DOI] [PubMed] [Google Scholar]
- 24.Burke V, Beilin LJ, Simmer K, et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int J Obes (Lond) 2005;29:15–23 [DOI] [PubMed] [Google Scholar]
- 25.Bao WH, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med 1994;154:1842–1847 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.