Abstract
OBJECTIVE
To determine the prevalence of metabolic syndrome and to define optimal ethnic-specific waist-circumference cutoff points in a rural South African black community.
RESEARCH DESIGN AND METHODS
This was a cross-sectional survey conducted by random-cluster sampling of adults aged >15 years. Participants had demographic, anthropometric, and biochemical measurements taken, including a 75-g oral glucose tolerance test. Metabolic syndrome was defined using the 2009 Joint Interim Statement (JIS) definition.
RESULTS
Of 947 subjects (758 women) studied, the age-adjusted prevalence of metabolic syndrome was 22.1%, with a higher prevalence in women (25.0%) than in men (10.5%). Peak prevalence was in the oldest age-group (≥65 years) in women (44.2%) and in the 45- to 54-year age-group in men (25.0%). The optimal waist circumference cutoff point to predict the presence of at least two other components of the metabolic syndrome was 86 cm for men and 92 cm for women. The crude prevalence of metabolic syndrome was higher with the JIS definition (26.5%) than with the International Diabetes Federation (IDF) (23.3%) or the modified Third Report of the National Cholesterol Education Program Adult Treatment Panel (ATPIII) (18.5%) criteria; there was very good agreement with the IDF definition (κ = 0.90 [95% CI 0.87–0.94]) and good concordance with ATPIII criteria (0.77 [0.72–0.82]).
CONCLUSIONS
There is a high prevalence of metabolic syndrome, especially in women, suggesting that this community, unlike other rural communities in Africa, already has entered the epidemic of metabolic syndrome. Waist circumference cutoff points differ from those currently recommended for Africans.
Metabolic syndrome is a cluster of risk factors for type 2 diabetes and cardiovascular disease (CVD), with insulin resistance proposed as a linking factor (1–8). Metabolic syndrome is common and is increasing in prevalence worldwide, largely attributed to increasing obesity and sedentary lifestyles, and now is both a public health and clinical problem (5).
Since the first formalized definition (6) of metabolic syndrome, there have been several definitions using different criteria (1–4,7,8), leading to widely differing prevalence estimates (5). The two major sets of criteria that have been used are those of the National Cholesterol Education Program Third Adult Treatment Panel (ATPIII) (2–4) and International Diabetes Federation (IDF) (1). The main difference between the two systems is that central obesity, as measured by waist circumference, is a prerequisite in the IDF definition, with cut points of waist circumference being ethnic specific and lower than in the ATPIII definition.
In 2009, an additional definition of metabolic syndrome was proposed as a joint interim statement (JIS) by several organizations in an attempt to harmonize the definition of metabolic syndrome (5). The available information based on ATPIII and IDF criteria suggests that metabolic syndrome is pandemic but that prevalence varies widely depending on the ethnic groups studied and criteria applied (9).
Sub-Saharan Africa currently is experiencing one of the most rapid demographic and epidemiological transitions with one of the fastest rates of urbanization, which is thought to be mainly responsible for the rising burden of diabetes and other noncommunicable diseases (10–13).
The available information on the prevalence of metabolic syndrome in epidemiology studies in sub-Saharan Africa is limited to reports on West Africans in Cameroon (14), Benin (15), and Nigeria (16) and based on ATPIII (2–4) or IDF definitions (1). The crude prevalence in these studies ranged from an absence or low prevalence (0–4.1%) in rural communities in all three countries as well as in an urban community in Cameroon. In Benin, prevalence was higher in semiurban (6.4%) and urban samples (11.0%). To date, there are no reports on the prevalence of metabolic syndrome from epidemiology studies in South Africa and none (urban or rural) using the JIS definition.
A cross-sectional diabetes epidemiology study in rural South Africans of Zulu descent allowed for the determination of the prevalence of metabolic syndrome using the JIS definition and the optimal waist circumference cutoff points to predict the presence of metabolic syndrome in this population.
RESEARCH DESIGN AND METHODS
A detailed description of the survey design and procedures has been previously published (17).
Survey design
In brief, this was a cross-sectional study of nonpregnant individuals aged >15 years, selected by random-cluster sampling, undertaken over a 3-month period in a rural African (black) community of Zulu descent in the Ubombo district of the province of KwaZulu-Natal in South Africa. Informed consent was obtained from all participants, and the University of KwaZulu-Natal Ethics Committee approved the study.
Survey procedure
The survey methodology was based on the World Health Organization field guide for diabetes and noncommunicable disease risk factor surveys (18). On the test day, all consenting subjects provided information for a questionnaire and underwent an anthropometric examination and a 75-g oral glucose tolerance test (OGTT) with additional fasting blood tests.
The anthropometric examination included measurement of height, weight, waist and hip circumference, and blood pressure (18). BMI was used as a measure of total-body obesity and waist circumference and waist-to-hip ratio (WHR) as measures of central (upper-body or abdominal) obesity (19). Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg.
Venous blood samples were drawn after an overnight fast and 2 h after a 75-g glucose load (OGTT) (6) for measurement of plasma glucose and fasting serum lipids and uric acid. Blood samples were kept on ice and centrifuged within 6 h and were separated and stored at −30°C until determination.
Biochemical methods
Plasma glucose was measured by a glucose oxidase method, and serum total cholesterol, HDL cholesterol, total triglycerides, and uric acid was measured by an enzymatic calorimetric method with kits from Boehringer Mannheim (Mannheim, Germany) and Diagnostica (monotest cholesterol, cholesterol oxidase–phenol+aminophenazone [CHOD-PAP]; peridochrome uric acid). LDL cholesterol was calculated by the Freidewald formula. Interassay coefficients of variation for the biochemical assays used were as follows: glucose 2.7–3.1%, cholesterol 1.6–2.4%, triglycerides 1.5–3.0%, and uric acid 2.4–3.7%.
Metabolic syndrome definitions
Metabolic syndrome was defined based on the 2009 JIS definition (5) (i.e., defined by any three of the following five components [risk factors]: central obesity as elevated waist circumference [≥94 cm for male subjects and 80 cm for female subjects]; elevated serum triglycerides [≥1.7 mmol/L]; reduced serum HDL cholesterol [<1.0 mmol/L for male subjects and 1.3 mmol/L for female subjects]; elevated blood pressure [systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg]; and elevated fasting blood glucose [≥5.6 mmol/L]). IDF Europid cut points (1) were used for elevated waist circumference, as recommended for sub-Saharan Africa populations (5). The JIS definition was used to determine the prevalence of metabolic syndrome and its individual components. For comparison with other reported studies, the IDF (1) and modified ATPIII criteria (3,4) also were applied to the dataset.
Statistical analysis
Statistical analysis was performed using SPSS (version 15; SPSS, Chicago, IL). Numerical variables are expressed as means ± SD. In bivariate analysis, the Student t test was used. The χ2 test was used for categorical variables. A test was considered significant if P < 0.05.
For prevalence estimates, the JIS, IDF, and ATPIII criteria were applied to subjects who had all five components of the metabolic syndrome measured. Additional intergroup analyses were undertaken using the JIS criteria. The age-standardized prevalence rates were calculated with the direct method, using the world population as a standard (20).
The receiver operating characteristic (ROC) curve for waist circumference to predict the presence of at least two other components of the metabolic syndrome, as defined by JIS criteria, was plotted. The optimal cutoff values of waist circumference were calculated by plotting the true-positive rate (sensitivity) against the false-positive rate (1-specificity). To assess the level of concordance (agreement) between the JIS and IDF or ATPIII definitions, the κ statistic was calculated (21).
RESULTS
Response rate and study group
Of 1,300 subjects selected, 1,025 (210 male and 815 female) participated, with an overall response rate of 78.9% (17). The study group for this analysis included 947 subjects (189 male and 758 female) who had data on all components of the metabolic syndrome.
Total study group
Table 1 shows the characteristics of the total (n = 947) study group by sex. The mean age was 46.5 ± 18.1 years. Mean BMI, waist and hip circumference, WHR, and the prevalence of total-body and central adiposity were higher in women.
Table 1.
Characteristics of the study group (n = 947) by sex
| Men | Women | P | |
|---|---|---|---|
| n (%) | 189 (20) | 758 (80) | |
| Age (years) | 46.4 ± 20.6 | 46.6 ± 18.2 | 0.9 |
| BMI (kg/m2) | 22.8 ± 6.9 | 26.0 ± 6.5 | <0.001 |
| Waist (cm) | 83.1 ± 12.1 | 86.0 ± 13.4 | 0.004 |
| Hip (cm) | 95.8 ± 11.1 | 102.0 ± 13.5 | <0.001 |
| WHR | 0.87 ± 0.07 | 0.84 ± 0.07 | <0.001 |
| Systolic blood pressure (mmHg) | 128.2 ± 16.7 | 126.5 ± 29.9 | 0.5 |
| Diastolic blood pressure (mmHg) | 80.5 ± 10.6 | 80.3 ± 18.7 | 0.8 |
| BMI (kg/m2) (%) | |||
| ≥25 to <30 | 13.2 | 24.7 | <0.001 |
| ≥30 | 8.5 | 22.6 | <0.001 |
| Waist (cm) (men/women) (%) | |||
| ≥94/80 | 18.5 | 63.3 | <0.001 |
| ≥102/88 | 8.5 | 39.0 | <0.001 |
| WHR >1.0 men/0.85 women (%) | 2.1 | 45.8 | <0.001 |
| Hypertension (%) | 30.7 | 24.7 | 0.1 |
| Plasma glucose (mmol/L) | |||
| 0-min | 4.9 ± 2.1 | 4.9 ± 1.4 | 0.7 |
| 120-min | 6.1 ± 2.3 | 6.2 ± 2.5 | 0.7 |
| Serum lipids (mmol/L) | |||
| Total cholesterol | 4.0 ± 1.0 | 4.1 ± 1.1 | 0.2 |
| Total triglycerides | 1.1 ± 0.7 | 1.0 ± 0.7 | 0.6 |
| HDL cholesterol | 1.24 ± 0.44 | 1.24 ± 0.38 | 0.5 |
| LDL cholesterol | 2.2 ± 1.0 | 2.4 ± 0.9 | 0.006 |
| Serum uric acid (μmol/L) | 0.31 ± 0.08 | 0.26 ± 0.07 | <0.001 |
Data are means ± SD, unless otherwise indicated. P values are for comparison between men and women.
Fewer women than men reported a history of any urban living (20.8 vs. 39.7%; P < 0.001) and in the proportion of life spent in an urban area (4.2 vs. 9.5%; P < 0.001). The majority of men (61.9%) and women (79.5%) either were pensioners (men 21.7% vs. women 25.1%; P < 0.001), engaged in home duties (men 24.9% vs. women 40.0%; P < 0.001), scholars (men 15.9% vs. women 7.4%; P < 0.001), or unemployed (men 15.3% vs. women 14.4%; P < 0.001). Only 22.1% of men and 13.1% of women were employed. The occupational history (men vs. women) was as follows: manual laborer (13.2 vs. 6.3%; P < 0.001); skilled worker (7.9 vs. 6.3%; P < 0.001); and office worker (1.1 vs. 0.5%; P < 0.001). The average monthly income (South African Rand [ZAR]) per household (men vs. women) was low (620.5 ± 757.4 ZAR vs. 543.5 ± 578.9 ZAR; P = 0.1), with many family members (n = 8.9 ± 5.0 vs. 8.0 ± 4.2; P = 0.02) being supported per household.
Prevalence
Metabolic syndrome.
Table 2 shows the prevalence of metabolic syndrome by sex. Using JIS criteria, the crude overall prevalence of metabolic syndrome was 26.5%, with a higher prevalence in women (30.2%) than in men (11.6%). Using age-specific rates, the prevalence of metabolic syndrome increased with age in both men and women; peak prevalence was in the 45- to 54-year age-group in men (25.0%) and in the oldest age-group (≥65 years) in women (44.2%). The age-adjusted prevalence was lower at 22.1% for the total study group, 10.5% for men, and 25.0% for women. In the total study group, the prevalence (crude/adjusted) was lower with both IDF (23.3/19.2%) and ATP III (18.5/15.0%) criteria. This also was true when the sexes were examined separately (Table 2).
Table 2.
Age- and sex-specific and age-adjusted prevalence of metabolic syndrome based on definitions of the JIS, the IDF, and the ATPIII (n = 947)
| Age-group (years) | n | Metabolic syndrome |
||
|---|---|---|---|---|
| JIS | IDF | ATPIII | ||
| Men (n = 189) | ||||
| 15–24 | 44 | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| 25–34 | 20 | 5.0 (1) | 10.0 (2) | 5.0 (1) |
| 35–44 | 22 | 13.6 (3) | 13.6 (3) | 4.5 (1) |
| 45–54 | 28 | 25.0 (7) | 25.0 (7) | 21.4 (6) |
| 55–64 | 31 | 12.9 (4) | 12.9 (4) | 12.9 (4) |
| ≥65 | 44 | 15.9 (7) | 13.6 (6) | 11.4 (5) |
| Total crude | 189 | 11.6 (22) | 11.6 (22) | 9.0 (17) |
| Age adjusted | — | 10.5 | 11.2 | 7.9 |
| Women (n = 758) | ||||
| 15–24 | 101 | 5.9 (6) | 4.0 (4) | 4.0 (4) |
| 25–34 | 123 | 16.3 (20) | 12.2 (15) | 6.5 (8) |
| 35–44 | 149 | 26.2 (39) | 22.8 (34) | 17.4 (26) |
| 45–54 | 106 | 39.6 (42) | 33.0 (35) | 28.3 (30) |
| 55–64 | 124 | 42.7 (53) | 39.5 (49) | 29.8 (37) |
| ≥65 | 154 | 44.2 (68) | 39.6 (61) | 34.4 (53) |
| Missing | 1 | 0 (1) | 0 (1) | 0 (1) |
| Total crude | 758 | 30.2 (229) | 26.3 (199) | 20.9 (158) |
| Age adjusted | — | 25.0 | 21.2 | 16.8 |
Data are % (n).
Individual components.
The prevalence of the individual components of metabolic syndrome is shown in Table 3. The most frequent individual component was low serum HDL cholesterol in women (65.2%) and elevated blood pressure in men (47.1%); the least frequent component in women was elevated fasting plasma glucose (FPG) (10.6%) and in men was both elevated FPG (13.8%) and elevated serum total triglycerides (13.8%).
Table 3.
Prevalence (%) of the individual components of metabolic syndrome based on 2009 JIS criteria (n = 947)
| Metabolic syndrome component* | Total | No metabolic syndrome | Metabolic syndrome | P |
|---|---|---|---|---|
| Men | ||||
| n | 189 | 167 | 22 | |
| High waist circumference | 16.4 | 6.6 | 90.9 | <0.001 |
| High triglycerides | 13.8 | 7.8 | 59.1 | <0.001 |
| Low HDL cholesterol | 29.1 | 26.3 | 50.0 | 0.02 |
| High blood pressure | 47.1 | 42.5 | 81.8 | 0.001 |
| High FPG | 13.8 | 9.0 | 50.0 | <0.001 |
| Women | ||||
| n | 758 | 529 | 229 | |
| High waist circumference | 62.4 | 47.4 | 96.9 | <0.001 |
| High triglycerides | 11.7 | 2.1 | 34.1 | <0.001 |
| Low HDL cholesterol | 65.2 | 54.3 | 90.4 | <0.001 |
| High blood pressure | 48.4 | 30.6 | 89.5 | <0.001 |
| High FPG | 10.6 | 3.4 | 27.1 | <0.001 |
Data are percentages, unless otherwise indicated.
*Waist circumference ≥94/80 cm (for men/women), serum triglycerides ≥1.7 mmol/L, serum HDL cholesterol <1.0/1.3 mmol/L (for men/women), systolic ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg, FPG ≥5.6 mmol/L.
In subjects with metabolic syndrome, the most frequent individual component was a high waist circumference in both men (90.9%) and women (96.9%); the least frequent component was elevated FPG in men (50.0%) and women (27.1%) and low serum HDL cholesterol in men (50.0%). As expected, there was a significant difference between those with metabolic syndrome and those without for each of the individual components.
Optimal waist circumference cutoff points
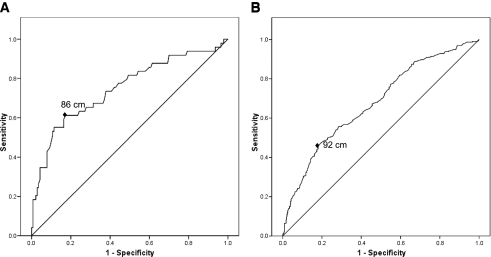
The optimal cut points of waist circumference for predicting at least two other components of the metabolic syndrome, as defined by the JIS criteria, was 86 cm for men (sensitivity 61.2%, specificity 82.9%) and 92 cm for women (sensitivity 45.9%, specificity 81.9%) (Fig. 1).
Figure 1.
ROC curves for waist circumference to predict the presence of at least two other components of the metabolic syndrome, as defined by the 2009 JIS, in men (A) and women (B). Area under the ROC curve: 0.75 in men (A) and 0.68 in women (B). Waist cut point: 86 cm in men (sensitivity 61.2%, specificity 82.9%) (A) and 92 cm in women (sensitivity 45.9%, specificity 81.9%) (B).
Comparison and agreement between the JIS and IDF or ATPIII definitions
Comparison between the JIS and IDF definitions showed that the crude overall prevalence was higher with the JIS criteria (26.5%; n = 251) than with the IDF criteria (23.3%; n = 221); 23.0% (n = 218) had metabolic syndrome using both sets of definitions. The agreement between the two systems was very good in the total study group (κ = 0.90 [95% CI 0.87–0.93]) as well as in men (κ = 0.95 [0.88–1.02]) and women (κ = 0.89 [0.85–0.93]) separately.
The crude overall prevalence also was higher when compared with the ATPIII criteria (18.5%; n = 175); the current system picked up all 175 subjects defined by the ATPIII criteria. The concordance between the two systems was good in the total study group (κ = 0.77 [95% CI 0.72–0.82]) and in women (κ = 0.76 [0.70–0.81]) and very good in men (κ = 0.86 [0.73–0.98]).
CONCLUSIONS
This study in a rural South African community of Zulu descent and using the 2009 JIS definition has highlighted a high prevalence of metabolic syndrome, especially in women. The most frequent individual component in subjects with metabolic syndrome was a high waist circumference. Optimal waist circumference cut points also have been identified.
To our knowledge, this is the first report on the prevalence of metabolic syndrome in sub-Saharan Africa using the JIS definition. Available evidence on the impact of metabolic syndrome from epidemiology studies in sub-Saharan Africa is limited to those using IDF (1) or ATPIII (2–4) criteria in West Africans in Cameroon (14), Benin (15), and Nigeria (16) and with only crude prevalence rates reported.
Using ATPIII criteria, the prevalence of metabolic syndrome in this study (crude 18.5%) is higher than in rural communities in Cameroon (0.0%) or Nigeria (all 3.0%; men 2.1% and women 2.7%). Based on the IDF criteria, the prevalence is higher in this study (crude 23.3%) than in rural Cameroon (men 0.0% and women 0.3%) or Benin (all 4.1%; men 0.0% and women 8.2%) and even when compared with urban communities in Cameroon (men 1.2% and women 1.5%) and Benin (all 11.0%; men 5.0% and women 17.0%).
When compared with other regions of the world, and using IDF and ATPIII criteria, the prevalence in women in this study is higher than in Asian groups from Japan, China, Mauritius, and India and some European communities but lower than in ethnic groups in the United States, including in African Americans (9).
The higher prevalence of metabolic syndrome in women in this study compared with other rural sub-Saharan Africa communities is likely related to the higher prevalence of obesity and the role of other factors, namely that physical activity and dietary factors needs to be established. Previous reports (10,12,22) have confirmed the high prevalence of obesity in South Africans. In this study, 47.3% of women were overweight or obese. Although this rate is lower than the national prevalence (56.6%) (22), it is higher than in rural communities in Cameroon (9.5%) and Nigeria (4.2%) and similar to rates in urban female subjects in Cameroon (47.0%) (16,23). The proportion of those who were obese in this study (22.6%) also is lower than the national estimate (31.8%) or in semiurban (40.2%) and urban (43.9%) South African women but higher than in rural Nigerians (2.4%) and rural (0.7%) or urban (10.0%) Cameroonians (16,22,23).
The most frequent individual component was low serum HDL cholesterol in women and elevated blood pressure in men. Similar findings have been reported in rural Nigerians (16), whereas elevated blood pressure alone was the most frequent component in men in rural and urban Cameroon and semiurban Benin (14,15). Both these variables are known risk factors for CVD (5); these findings may suggest that if the current trend continues, this population is at increased risk for coronary artery disease. To date, the major type of CVD in Africans is cerebrovascular disease, with coronary artery disease being uncommon (12). On the other hand, it may suggest an ethnic predisposition to this type of dyslipidemia, as has been suggested for Iranians (24). The least common individual component was a high FPG (in men and women) and elevated serum triglycerides (in men). A consistent finding in all the African studies, except in South African women, was that the least frequent component was elevated serum triglycerides. Whether this contributes to the low risk of coronary artery disease in this population is unclear and would require additional studies to establish its significance.
In subjects with metabolic syndrome, the most frequent component was a high waist circumference. In a previous report (17), waist circumference was found to be a risk factor associated with diabetes in this population; the impact of this variable on future CVD in Africans needs to be established.
There was very good agreement between the JIS and IDF definitions. This is not surprising given that the components are the same in both sets; elevated waist circumference was found in >90% of subjects with metabolic syndrome in this study, and the cut points for waist circumference are the same.
To our knowledge, this is the first report in Africans that attempts to define waist circumference cut points to predict metabolic syndrome. The cut point for women (92 cm) is higher than currently recommended for Africans (i.e., IDF Europid cut points of 80 cm) (1,5) but similar to those recently reported in Iranians (24). On the other hand, the cut point in men (86.3 cm) is lower than currently recommended but similar to that reported in Japanese subjects (25). Additional studies are required in other African communities to confirm this finding.
The major limitations of this study are the cross-sectional design and the overrepresentation of women; the latter is accounted for by the migrant labor system in our country, with men in the economically productive age-group moving to urban areas, whereas women, children, and older individuals remain in the rural areas (17).
In summary, this study shows a high prevalence of metabolic syndrome, especially in women, and suggests that this community, unlike other rural communities in Africa, already has entered the global pandemic. It confirms the need for implementing national policies for its prevention and control in this and other sub-Saharan African countries that face a rising burden of diabetes and CVD over the next 2 decades. Waist circumference cutoff points for both men and women differ from those currently recommended for Africans.
Acknowledgments
This study was partially sponsored by the South African Medical Research Council, the South African Sugar Association, Novo Nordisk (South Africa), and Servier Laboratories South Africa. No other potential conflicts of interest relevant to this article were reported.
A.A.M. wrote the manuscript, researched data, contributed to discussion, and reviewed and edited the manuscript. T.E. and F.J.P. researched data, contributed to discussion, and reviewed and edited the manuscript. M.A.K.O. contributed to discussion and reviewed and edited the manuscript.
The authors thank the community for their cooperation; Serena van Hacht (University of KwaZulu-Natal), the field coordinator; the field workers; the Bethesda Hospital staff; and Rajnee Billy (University of KwaZulu-Natal) for typing the manuscript.
References
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–480 [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA, American Heart Association. National Heart, Lung, and Blood Institute. American Diabetes Association Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 2004;109:551–556 [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 6.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 7.Balkau B, Charles MA, European Group for the Study of Insulin Resistance (EGIR) Comment on the provisional report from the WHO consultation. Diabet Med 1999;16:442–443 [DOI] [PubMed] [Google Scholar]
- 8.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract 2003;9:237–252 [PubMed] [Google Scholar]
- 9.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008;28:629–636 [DOI] [PubMed] [Google Scholar]
- 10.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet 2010;375:2254–2266 [DOI] [PubMed] [Google Scholar]
- 11.International Diabetes Federation Diabetes Atlas. 4th ed. Brussels, International Diabetes Federation, 2009 [Google Scholar]
- 12.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet 2009;374:934–947 [DOI] [PubMed] [Google Scholar]
- 13.Unwin N, McLarty D, Machibya H, et al. Changes in blood pressure and lipids associated with rural to urban migration in Tanzania. J Hum Hypertens 2006;20:704–706 [DOI] [PubMed] [Google Scholar]
- 14.Fezeu L, Balkau B, Kengne AP, Sobngwi E, Mbanya JC. Metabolic syndrome in a sub-Saharan African setting: central obesity may be the key determinant. Atherosclerosis 2007;193:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ntandou G, Delisle H, Agueh V, Fayomi B. Abdominal obesity explains the positive rural-urban gradient in the prevalence of the metabolic syndrome in Benin, West Africa. Nutr Res 2009;29:180–189 [DOI] [PubMed] [Google Scholar]
- 16.Oladapo OO, Salako L, Sodiq O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south-western Nigerian population: a population-based survey. Cardiovasc J Afr 2010;21:26–31 [PMC free article] [PubMed] [Google Scholar]
- 17.Motala AA, Esterhuizen T, Gouws E, Pirie FJ, Omar MA. Diabetes and other disorders of glycemia in a rural South African community: prevalence and associated risk factors. Diabetes Care 2008;31:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Diabetes and Non-Communicable Disease Risk Factor Surveys: A Field Guide. Geneva, World Health Org., 1999. (WHO/NCD/NCS/99-1) [Google Scholar]
- 19.World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation Geneva, World Health Org., 2000 (Tech. rep. ser. no. 894) [PubMed] [Google Scholar]
- 20.Waterhouse J, Correa P, Muir C, Powell J. Cancer Incidence in Five Continents. Vol. III Lyon, France, IARC, 1976, p. 456 [Google Scholar]
- 21.Graphpad Software. QuickCalcs online calculators for scientists: quantify agreement with kappa [Internet]. Available from http://www.graphpad.com/quickcalcs/Kappa2.cfm./. Accessed 19 May 2010
- 22.Puoane T, Steyn K, Bradshaw D, et al. Obesity in South Africa: the South African demographic and health survey. Obes Res 2002;10:1038–1048 [DOI] [PubMed] [Google Scholar]
- 23.Jackson M, Walker S, Cruickshank JK, et al. Diet and overweight and obesity in populations of African origin: Cameroon, Jamaica and the UK. Public Health Nutr 2007;10:122–130 [DOI] [PubMed] [Google Scholar]
- 24.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First nationwide study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care 2009;32:1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara K, Matsushita Y, Horikoshi M, et al. A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care 2006;29:1123–1124 [DOI] [PubMed] [Google Scholar]