The Joslin Gold Medalists comprise 351 U.S. residents who have survived with type 1 diabetes for >50 years. A high proportion of Medalists remain free from proliferative diabetic retinopathy (PDR) (42.6%), nephropathy (86.9%), neuropathy (39.4%), or cardiovascular disease (51.5%). Current and longitudinal (the past 15 years) glycemic control were unrelated to complications. Advanced glycation end product (AGE) concentrations include carboxyethyl-lysine (CEL), an AGE derived from methylglyoxal; pentosidine, a glycoxidation ascorbate product; and carboxymethyl-lysine (CML), a glycoxidation and advanced lipoxidation product. Fructose-lysine CML and CEL were significantly elevated in the Medalists as compared with nondiabetic, age-matched control subjects (N = 23, mean age 67.7 years). Subjects with both CEL >5.3 μmol/mol lysine and pentosidine (>1.0 pmol/mg protein) were the most likely to have any complication (7.2 times more likely) (P = 0.001), or suffer from nephropathy (3.1 times more likely) (P = 0.007), neuropathy (2.5 times more likely) (P = 0.005), or cardiovascular disease (2.3 times more likely) (P = 0.002). These data are the first to suggest that specific AGEs may decrease risk for diabetes complications because high current CML and fructose-lysine concentrations were negatively correlated with PDR development. Previous reports indicated that AGEs and their precursors may be important in the pathogenesis of diabetes complications; however, it is unexpected that lower current levels of CML and fructose-lysine are inversely related to PDR development in light of reports indicating an association between elevations of these AGEs measured in other tissues and retinopathy. The Medalist population is likely enriched for protective factors against complications. The question is, what are the protective factors?
Metabolic memory in diabetes
The notion of good metabolic memory has been applied to the beneficial effects of an initial period of good glycemic, lipid, and blood pressure control resulting in effects going beyond that of the period of near euglycemia and followed by a rebound hyperglycemia, referred to as the Nike pattern of glycemic control, yet the benefits observed are maintained. This is good metabolic memory and has acquired a number of synonyms including a legacy effect, glycemic memory, hyperglycemic memory, among others (1,2). The Diabetes Control and Complications Trial (DCCT) study showed that the risk of retinopathy remained significantly reduced in the formerly intensely treated subjects in the first 4 years after completion of the trial (3). After 10 years, in the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up in which the A1C levels had converged, the rates of retinopathy and proliferative retinopathy remained better than in the conventional group albeit the risk reduction was somewhat lower at 10 years than the initial 4 years (4). Diabetic nephropathy showed reduction of microalbuminuria, clinical albuminuria, and fewer cases of hypertension and kidney transplantation after 8 years (5). Diabetic neuropathy also fared well with a significant reduction of both somatic and autonomic neuropathy at 8 years of follow-up (6). During the 17 years of follow-up of the EDIC study, intensive therapy reduced the risk of any cardiovascular disease and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease (7). A beneficial effect of early intensive glycemic control was also shown for coronary calcification at 7–9 years (8) and the progression of carotid intima-media thickness (9). This beneficial effect of early glycemic control now stands in stark contrast to the possible bad metabolic memory or legacy of intensive glycemic control later in the course of type 2 diabetes (10).
The sustained benefit from early aggressive treatment is referred to as the legacy effect or metabolic memory, but it is unlikely to account for the Medalist longevity and the paradoxical relationship of freedom from complications and AGEs.
Unfortunately, however, the Gold Medalist trial was initiated before 1993 when glycemic control became important, and no effect was shown on recent or lifetime A1C.
What plagues scientists is the inability to predict good versus bad and understanding the vast differences between individuals and even ethnic groups at risk for complications. The notion that a period of glycemic control improves vascular, mitochondrial, or other functions providing long-lasting effects seems somewhat naïve in light of observations such as those in Pima Indians who, despite glycemic control, die of renal insufficiency because their parents did so thereby implicating a strong genetic predisposition to complications including ApoE gene, Toll receptor, aldose reductase, angiotensin (1), and choline transporter polymorphism (11), which increase susceptibility to the negative effects of hyperglycemia if present. The clear advantages accorded to the Joslin Gold Medalists may reside in such protection but have yet to be studied. In terms of identifying protection, one needs to examine the risk factors—not the absence of risk. The secret may lie in their absence, which is a much more daunting task to uncover. The clue is in the failure of the dog to bark, my dear Watson!!! As beautifully illustrated in his book The Music Lesson, Victor L. Wooten points out the need to listen not only to the notes but to the silences! However, we first need to address the notes, i.e., the traditional risk factors and those that may contribute to the reduced risk by their absence or their overexpression.
The major mechanism thought to contribute to bad metabolic memory includes glucotoxicity, lipotoxicity, hexosamine, increased polyol pathway activity, and the accumulation of AGEs.
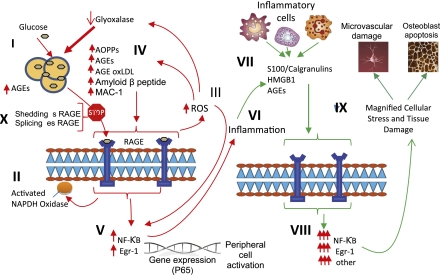
The accumulation of AGEs may be one of the important factors in metabolic memory. AGEs are a heterogeneous group of compounds occurring as a result of nonenzymatic glycation of proteins, lipids, and nucleic acids. Their role in the complication of diabetes has recently been reviewed (12–15) (Fig. 1).
Figure 1.
The relationship between binding of ligands to the pattern recognition AGE receptor, RAGE and inflammation, gene expression, oxidative and nitrosative stress, and damage to the macro- and microvasculature. Elevated levels of glucose bind to proteins and form AGEs (I), which bind to RAGEs. RAGE signaling activates NADPH oxidase (II) and production of reactive oxygen species (ROS) (III). Increased ROS increases advance oxidation protein products (AOPPs), more AGEs, and AGE-modification of oxidized LDLs (oxLDLs). Furthermore, increased ROS may deplete glutathione, thereby suppressing glyoxalase I activity, a mechanism favoring further AGE accumulation (IV). AGEs, AOPPs, macrophage glycoprotein (MAC-1), and AGE-oxLDL ligands of RAGE sustain stimulation of RAGE, and these processes, together with increased ROS, activate key transcription factors such as nuclear factor-κB (NF-κB) and Egr-1 (V), which increase gene transcription factors and activate inflammatory mechanisms (VI). Consequences include increased migration and activation of RAGE-expressing neutrophils, monocytes/macrophages, T-cells, and dendritic cells (VII). This results in the release of the proinflammatory RAGE ligands S100/calgranulins and high-mobility group protein box-1 (HMGB1). In this inflammatory environment, further AGEs may be formed as well. Via interaction with RAGE, these ligands magnify activation of NF-κB, Agr-1, and other factors (VIII), thereby amplifying cellular stress and tissue damage leading to neurovascular and bone dysfunction. Soluble RAGE (sRAGE) formed from cleavage of RAGE by disintegrins such as ADAM 10, a metalloproteinase, and β- and γ-secretases. sRAGE or a spliced variant (esRAGE) compete for binding of ligands to RAGE, and a deficiency could theoretically initiate the sequence of events activating an inflammatory cascade with an increase in the expression of proinflammatory cytokines (E-selectin, endothelin-1 tissue factor, vascular endothelial growth factor, and other proinflammatory cytokines [interleukin-6 and tumor necrosis factor-α]) and damage to neurons, kidney, eye, the vasculature, and even bone. Increasing sRAGE or its administration could competitively reduce activation of the AGE/RAGE pathway and it consequences. Modified with permission from Yan et al. (14). (A high-quality digital representation of this figure is available in the online issue.)
The formation of AGEs, driven by hyperglycemia and oxidative stress, is an important biochemical abnormality that accompanies diabetes and inflammation in general. AGEs act to induce the cross-linking of long-lived proteins such as collagen and to promote vascular stiffness altering structure and function. AGEs also interact with cellular receptors to induce cellular signaling leading to enhanced oxidative stress thereby irreversibly modifying mitochondrial function. The accumulation of AGEs in skin with an increase in CML and glycated collagen has recently been obtained from skin biopsies in the DCCT/EDIC study (16) with the prediction of progression of retinopathy and nephropathy even controlling for A1C. In the Gold Medalist study, current and longitudinal (the past 15 years) glycemic control were unrelated to complications. Subjects with high plasma CEL and pentosidine were 7.2-fold more likely to have any complication. The AGE results confirm a robust association with complications and suggest that AGE formation may be independent of glycemia (Fig. 1). The association of complications with CEL implicates increased methyglyoxal production, which itself is associated with various cellular and matrix dysfunctions (17), while increased pentosidine likely reflects increased ascorbic acid degradation because of oxidant production. Of the Medalists without PDR, 96% with no retinopathy progression over the first 17 years of follow-up experienced worsening retinopathy thereafter. The great paradox in the Medalist study is the observation that the unique combination of AGEs were predictive of the development of microvascular complications of diabetes but at follow-up the relation between AGEs and complications were inverse suggesting that 1) other pathways may be involved, 2) there were compensatory mechanisms operating with time, or 3) the body had a self-contained defense mechanism for AGE interaction with its receptor(s) with the elaboration of subsequent events. Thus, an adaptive mechanism, which can alter the processing of CML and fructose-lysine, may provide protection against development of PDR and possibly other complications of diabetes. If so, then what are these defense mechanisms?
AGEs have also been implicated in the development of neuropathy and most recently in Charcot neuroarthropathy (18). The highest AGE levels are found in tissues with the slowest turnover such as skin, tendon, bone, amyloid plagues, and cartilage (19). The AGE/receptor for AGE (RAGE) axis is a likely mechanism linking microangiopathy and neuropathy supported by the finding of colocalization of CML, RAGE, nuclear factor-κB, and interleukin-6 to epineural vessels, perineurium, and endoneurial blood vessels. AGEs have been demonstrated in murine models to worsen nerve conduction velocity and blood flow to nerves (20). Administration of AGEs duplicate the effects of hyperglycemia and can be neutralized by anti-AGE antibodies (21,22). However, even in RAGE null mice, the activation of the inflammatory process is incompletely inhibited suggesting that AGE/RAGE may be an incomplete part of the mechanism for the development of neuropathy. In the kidney and possibly in the eye, there are significant increases in the accumulation of AGEs including basement membrane thickening, mesangial expansion, glomerulosclerosis and tubulointerstitial fibrosis, and increased collagen 1 V and transforming growth factor-β. The AGE blocker aminoguanidine, a cross-link breaker known as alagebrium, and treatment with soluble RAGE (sRAGE) have been shown to reduce the renal damage of diabetes (12). AGEs may negatively influence bone by interfering with osteoblast differentiation and the production of important matrix proteins such as collagen and osteocalcin. AGEs have also been shown to stimulate apoptosis of human mesenchymal stem cells (23) and osteoblast apoptosis through a nuclear factor-κB–independent mechanism that further limits bone formation (24). A proposed mechanism through which diabetes induces apoptosis by stimulating AGEs is via CML-collagen, mediated through RAGE, the pattern recognition receptor for AGE. However, a decoy mechanism is found in which AGEs and other ligands (14) bind to RAGE a circulating form of RAGE and thereby preclude access of the RAGE ligand to sRAGE mitigating the activation of the pathways of oxidative and nitrosative stress. Although not reported in the Gold Medalist study, measurements of circulating sRAGE may well have contributed further to the understanding of the protection afforded these individuals. While it appears that RAGE knockouts experience protection from complications of diabetes, gene polymorphisms of the multitude of regulatory sites for control of AGE/RAGE interactions have been suggestive but none can be held accountable for the protection or susceptibility to complications of diabetes (13).
Witzke et al. (18) reported on a 50% reduction in circulating sRAGE in diabetes and an 85% reduction in patients with Charcot neuroarthropathy. Geroldi et al. (25) studied centenarians and found sRAGE levels that were inordinately high, suggesting that sRAGE is a marker of health and/or an innate protective mechanism for complications of diabetes. Can sRAGE levels be changed? It has been shown that certain drugs can raise sRAGE levels (e.g., α-lipoic acid, benfotiamine, vitamin C, ACE inhibitors, statins, and glitazones) (14), suggesting that these medications may have a role in the prevention of complications of diabetes. Alternatively the availability of sRAGE suggests a potential means of treating or preventing complications of diabetes. Pyridoxamine is a vitamin B6 derivative that prevents degradation of protein Amadori products to their AGE equivalents and has been shown to decrease microalbuminuria and other elements of renal dysfunction. Putative cross-link breakers such as N-phenacylthiaxolium bromide and a stable derivative alagebrium, ALT 711, have shown salutary effects on RAGE accumulation in several tissues with improvement in albuminuria and cardiac collagen 111 expression and have improved ejection fractions and arterial stiffness in clinical trials (12). Benfotiamine, a lipid-soluble thiamine derivative (B1), prevents activation of three main pathways of hyperglycemia damage—hexosamine, intracellular AGE formation, and the diacylglycerol kinase pathway—by inhibiting the action of transketolase in renal glomeruli–reduced microalbuminuria (26,27). Benfotiamine improves nerve function in the peroneal nerve (28) and alleviates neuropathic pain (29) in diabetic humans. Benfotiamine reduces the microvascular and macrovascular endothelial dysfunction and oxidative stress after a high AGE meal (30). Indeed angiotensin 11 inhibitors lower AGE levels and increase the expression of sRAGE as a potential decoy. Drugs such as thiazolidinediones, metformin, and angiotensin receptor blockers have been shown to decrease AGE formation and promote AGE clearance and degradation possibly by inducing the formation of sRAGE. Even dietary restriction of AGEs may have significant effects upon AGE accumulation in clinical diabetes and animal models of diabetes complications (12). This leads us to wonder if in the early years of the DCCT, before it became fashionable, the Joslin Gold Medalists reduced their intake of AGEs and enhanced their capacity to clear AGEs by one of the mechanisms outlined and, with time, the inverse correlation reflected only an incomplete compensation of the AGE/RAGE mechanism and not that they had any less damage potential. We eagerly await studies on the haloed who remain free of complications of diabetes because this will offer new therapeutic potential in an area hungry for novel forms of intervention.
Other theories on the mechanism for metabolic memory include the possibility that there is a long prodrome that precedes the advent of hyperglycemia in which there are increases in inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α, in oxidative and nitrosative stress, and in the selectins such as E-selectin and P-selectin (1,31). Good metabolic memory may derive from suppression of these proinflammatory cytokines despite failure of glycemic control by e.g., insulin (1) and episodic spikes in glucose (32). The role of anti-inflammatory agents, particularly those involving the cyclooxygenase pathways with reduction in neuropathy, retinopathy, and nephropathy in mice with 5- and/or 12-lipoxygenase knockouts (33–35), further suggest that novel mechanisms may contribute to metabolic memory and confer advantages in individuals protected from the ravages of diabetes.
There is no doubt that the absence of evidence is not evidence of absence but, in this instance, there should be a great return from the study of why the dog did not bark among the Joslin Gold Medalist survivors, and what the compensatory mechanisms are for defense against the storm of AGE/RAGE interaction.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
References
- 1.LeRoith D, Fonseca V, Vinik A. Metabolic memory in diabetes—focus on insulin. Diabetes Metab Res Rev 2005;21:85–90 [DOI] [PubMed] [Google Scholar]
- 2.Wright AD. Metabolic memory in type 1 diabetes. Brit J Diabetes & Vascular Disease 2009;9:254 [Google Scholar]
- 3.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NH, Sun W, Cleary PA, et al. ; DCCT EDIC Research Group Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol 2008;126:1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin CL, Albers J, Herman WH, et al. ; DCCT/EDIC Research Group Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Cleary PA, Backlund JY, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary PA, Orchard TJ, Genuth S, et al. ; DCCT/EDIC Research Group The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan DM, Lachin J, Cleary P, et al. ; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Prato S. Megatrials in type 2 diabetes: from excitement to frustration? Diabetologia 2009;52:1219–1226 [DOI] [PubMed] [Google Scholar]
- 11.Neumann SA, Lawrence BA, Jennings JR, Ferrell RE, Manuck SB. Heart rate variability is associated with polymorhic variation in the choline transporter gene. Psychosom Med 2005;67:168–171 [DOI] [PubMed] [Google Scholar]
- 12.Goh SY, Cooper ME. Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 2008;93:1143–1152 [DOI] [PubMed] [Google Scholar]
- 13.Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009;52:2251–2263 [DOI] [PubMed] [Google Scholar]
- 14.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res 2010;106:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med 2009;87:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genuth S, Sun W, Cleary P, et al. ; DCCT Skin Collagen Ancillary Study Group Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009;58:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzke K, Vinik A, Grant L, Parson H, Pittenger G. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke S, Siggelkow H, Wolf G, Hein G. Advanced glycation endproducts influence the mRNA expression of RAGE, RANKL and various osteoblastic genes in human osteoblasts. Arch Physiol Biochem 2007;113:154–161 [DOI] [PubMed] [Google Scholar]
- 20.Chen AS, Taguchi T, Sugiura M, et al. Pyridoxal-aminoguanidine adduct is more effective than aminoguanidine in preventing neuropathy and cataract in diabetic rats. Horm Metab Res 2004;36:183–187 [DOI] [PubMed] [Google Scholar]
- 21.Vlassara H, Brownlee M, Cerami A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc Natl Acad Sci USA 1981;78:5190–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol 2001;169:386–391 [DOI] [PubMed] [Google Scholar]
- 23.Kume S, Kato S, Yamagishi S, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res 2005;20:1647–1658 [DOI] [PubMed] [Google Scholar]
- 24.Alikhani M, Alikhani Z, Boyd C, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 2007;40:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from disease marker to potential therapeutic target. Curr Med Chem 2006;13:1971–1978 [DOI] [PubMed] [Google Scholar]
- 26.Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 2003;9:294–299 [DOI] [PubMed] [Google Scholar]
- 27.Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 2003;52:2110–2120 [DOI] [PubMed] [Google Scholar]
- 28.Stracke H, Lindemann A, Federlin K. A benfotiamine-vitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes 1996;104:311–316 [DOI] [PubMed] [Google Scholar]
- 29.Haupt E, Ledermann H, Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy—a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther 2005;43:71–77 [DOI] [PubMed] [Google Scholar]
- 30.Stirban A, Negrean M, Stratmann B, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care 2006;29:2064–2071 [DOI] [PubMed] [Google Scholar]
- 31.Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am 2004;88:947–999, xi [DOI] [PubMed] [Google Scholar]
- 32.Ceriello A, Esposito K, Ihnat M, Thorpe J, Giugliano D. Long-term glycemic control influences the long-lasting effect of hyperglycemia on endothelial function in type 1 diabetes. J Clin Endocrinol Metab 2009;94:2751–2756 [DOI] [PubMed] [Google Scholar]
- 33.Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 2008;57:1387–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov 2009;8:417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stavniichuk R, Drel VR, Shevalye H, et al. Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radic Biol Med; 2010;49:1036–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]