Abstract
OBJECTIVE
To assess the impact of continuous glucose monitoring on hypoglycemia in people with type 1 diabetes.
RESEARCH DESIGN AND METHODS
In this randomized, controlled, multicenter study, 120 children and adults on intensive therapy for type 1 diabetes and a screening level of glycated hemoglobin A1c (HbA1c) <7.5% were randomly assigned to a control group performing conventional home monitoring with a blood glucose meter and wearing a masked continuous glucose monitor every second week for five days or to a group with real-time continuous glucose monitoring. The primary outcome was the time spent in hypoglycemia (interstitial glucose concentration <63 mg/dL) over a period of 26 weeks. Analysis was by intention to treat for all randomized patients.
RESULTS
The time per day spent in hypoglycemia was significantly shorter in the continuous monitoring group than in the control group (mean ± SD 0.48 ± 0.57 and 0.97 ± 1.55 h/day, respectively; ratio of means 0.49; 95% CI 0.26–0.76; P = 0.03). HbA1c at 26 weeks was lower in the continuous monitoring group than in the control group (difference −0.27%; 95% CI −0.47 to −0.07; P = 0.008). Time spent in 70 to 180 mg/dL normoglycemia was significantly longer in the continuous glucose monitoring group compared with the control group (mean hours per day, 17.6 vs. 16.0, P = 0.009).
CONCLUSIONS
Continuous glucose monitoring was associated with reduced time spent in hypoglycemia and a concomitant decrease in HbA1c in children and adults with type 1 diabetes.
The benefits of intensive treatment of type 1 diabetes, established almost 20 years ago (1), are difficult to achieve, despite the increased use of insulin analogs and insulin pumps, with only a minority of patients maintaining their glycated hemoglobin A1c (HbA1c) within the target range (2). Intensive insulin treatment and lower HbA1c increase exposure to hypoglycemia (3,4). The risk of hypoglycemia is even higher in children and adolescents (5,6) and increases with the duration of diabetes (7). Frequent hypoglycemia is associated with hypoglycemia unawareness (8,9), which may in turn lead to reduced adherence to therapeutic decisions (10). Finally, hypoglycemia may be associated with permanent damage to the central nervous system (11) and may permanently influence cognitive functions in children (12) but not in adults (13).
Recently, devices for real-time continuous glucose monitoring have been introduced to aid self-management of glycemic control and have been shown to improve HbA1c levels in people with type 1 diabetes (14–17). In clinical practice recommendations, it has also been suggested that continuous glucose monitoring is especially useful in patients with hypoglycemia unawareness and/or frequent episodes of hypoglycemia (18). However, the hypoglycemia preventive effect of continuous glucose monitoring has not been established. Therefore, we designed a randomized, controlled, multicenter clinical trial to evaluate the effect of continuous glucose monitoring on hypoglycemia in children and adults with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Patients
Patients aged between 10 and 65 years with type 1 diabetes diagnosed for more than 1 year, with reasonable metabolic control assessing carbohydrate intake and self-adjusting insulin, and an HbA1c level <7.5%, using intensive insulin treatment with either an insulin pump or multiple daily injections, and not using a real-time continuous glucose monitoring device for at least 4 weeks were eligible for the study. All eligible patients identified from the local diabetes registries were invited to participate and screened consecutively based on the order of their positive reply. The study protocol was designed by the researchers and approved by the institutional or national medical ethics committees from all three centers, and the conduct of the study was consistent with the Good Clinical Practice provisions of the Declaration of Helsinki with all amendments and local regulatory requirements. A written informed consent was obtained from all participants and parents of minors (under 18 years of age, who signed an assent) before enrollment.
At screening patients provided a blood sample for measurement of HbA1c and entered a 4-week run-in period during which self-monitoring of blood glucose (SMBG) was conducted according to patients’ standard glycemic management regimen. A FreeStyle blood glucose meter (Abbott Diabetes Care, Alameda, CA) was provided to familiarize patients with FreeStyle test strips and collect baseline SMBG frequency and glucose levels. Diaries were distributed for recording events of hypoglycemia and associated food intake, insulin doses, and exercise. All patients meeting the eligibility criteria, including HbA1c result from the screening visit, were invited to attend the randomization visit.
Study design
Following the 1-month run-in period, patients were randomized to participate in a 6-month intervention period. Patients were assigned to home monitoring with a FreeStyle blood glucose meter and a masked continuous glucose monitor to be worn for 5 days every second week (control group) or to a group with real-time continuous glucose monitoring, wearing individual sensors for 5 days continuously for 26 weeks (continuous monitoring group). Patients and investigators were masked for the continuous glucose monitoring data in the control group. Patients were allocated to either group by permuted block randomization stratified according to age (10 to 17 years pediatric, 18 to 65 years adult) and study center. The randomization sequence was computer generated, and allocations were concealed using envelopes. Both groups were provided with the FreeStyle Navigator (Abbott Diabetes Care), a continuous glucose monitoring system that measures glucose in interstitial fluid.
All patients were trained to insert and calibrate subcutaneous sensors and to operate the continuous monitoring device. Patients in the continuous monitoring group were instructed in the use of real-time glucose readings; however, no written guidelines were given on adjustment of diabetes management based on the real-time readings. Patients also individually set their glucose alarms. All patients were encouraged to maintain their blood glucose concentration within the preprandial target range of 70 to 130 mg/dL with peak postprandial values below 180 mg/dL.
The first subcutaneous sensor was inserted upon randomization at visit 2 (day 1). The schedule of follow-up visits was identical for both groups. All patients returned for a visit 2–6 days after randomization for upload of all devices to confirm that continuous data were recorded and to replace the subcutaneous sensor under supervision. Further visits were conducted at days 60, 120, and 180 (±7 days). Data were uploaded at each visit, and adverse events including severe hypoglycemia (19), hyperglycemia resulting in ketoacidosis requiring intravenous fluids, device-related or study-related untoward events, and serious adverse events regardless of cause, were reviewed and reported.
Diabetes self-management was adjusted by patients based on the blood glucose measurements in the control group and blood glucose measurements and continuous glucose data in the continuous glucose monitoring group. Samples for HbA1c were collected at days 1, 60, and 180. All samples for HbA1c were sent to a central laboratory (Laboratorium Klinische Forschung, Kiel, Germany; measurement by Bio-Rad Variant II Turbo analyzer, Bio-Rad Laboratories, Hercules, CA).
Statistical analysis
The primary outcome was time spent in hypoglycemia (<63 mg/dL) during the 26-week study period. A sample size of 120 patients was planned to have a power of 80% to detect a difference in the time spent in hypoglycemia between study groups, assuming a population difference of 42%, a SD of 1.12, an α-level of 0.05, and a loss to follow-up of no more than 17%.
All analyses were performed according to the intent-to-treat principle, including all data collected for patients that discontinued prematurely. An excursion was defined as all consecutive recordings outside the boundary covering at least 10 min. The duration of an excursion was defined as the elapsed time from first excursion to the first reading indicating return inside the excursion boundary. Continuous glucose monitoring data in both groups were used to estimate the amount of time per day the glucose level was hypoglycemic (<63 mg/dL, <70 mg/dL, or <55 mg/dL), hyperglycemic (>180 mg/dL or >250 mg/dL), and in the target range (70 to 180 mg/dL or 90 to 180 mg/dL) for each patient. The number of hypoglycemic excursions (<55 and <63 mg/dL) per day and separately during the night period of 0000–0600 h was also calculated. The risk associated with glucose concentration outside the recommended range was assessed by calculating low blood glucose indexes (LBGIs) and high blood glucose indexes (HBGIs) (20). Comparisons between study groups were performed with the use of the Mann-Whitney U test. CIs for the ratio of the study group means (continuous monitoring/control) were calculated using the bias-corrected accelerated (BCa) bootstrap method. A P value of less than 0.05 (two-sided test) was considered to indicate statistical significance.
Comparison between the two study groups of HbA1c levels at days 60 and 180 was performed using ANCOVA on baseline HbA1c level and adjusted for clinical center and adult or pediatric patient. Missing HbA1c data were imputed using the last-observation-carried-forward method, including patients that discontinued prematurely.
Analyses were conducted with the use of SAS software, version 8.02 (SAS Institute, Cary, NC). All P values were two-sided.
All researchers had full access to the whole database after it was locked.
RESULTS
Patients
In the period from October 2008 to May 2009, 122 eligible patients were screened. Of these, two patients dropped out before randomization, and 58 were randomized to the control and 62 were randomized to the continuous glucose monitoring group. The study was completed by 48 patients (83%) and 53 patients (85%), respectively. The study flowchart is depicted in Supplementary Fig. 1. Patient baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the patients
| Control group | Continuous monitoring group | |
|---|---|---|
| N | 58 | 62 |
| Female sex, number (%) | 19 (33) | 26 (42) |
| Age (years)* | 26.0 ± 14.6 | 25.7 ± 14.1 |
| Pediatric, number (%) | 26 (45) | 27 (44) |
| BMI (kg/m2)* | 22.0 ± 3.8 | 22.4 ± 3.8 |
| Duration of diabetes (years)* | 11.4 ± 11.4 | 11.6 ± 11.3 |
| Insulin administration, number (%) | ||
| Pump | 34 (59) | 47 (76) |
| MDI | 24 (41) | 15 (24) |
| Glycated hemoglobin at screening (%)* | 6.90 ± 0.47 | 6.83 ± 0.44 |
| Glycated hemoglobin at baseline (%)* | 6.91 ± 0.67 | 6.92 ± 0.56 |
| Record of severe hypoglycemia in last year, number (%) | 7 (12) | 5 (8) |
| Diagnosed with hypoglycemia unawareness, number (%) | 4 (7) | 6 (10) |
| Daily insulin dose (units/kg)* | 0.67 ± 0.32 | 0.66 ± 0.25 |
| Education, number (%) | ||
| Pediatric patient still in education | 26 (45) | 29 (47) |
| Completed education by age 18 | 3 (5) | 6 (10) |
| Completed further education | 29 (50) | 27 (44) |
| Prior use of continuous glucose monitor, number (%) | 18 (31) | 21 (34) |
| Mean blood glucose in 1-month run-in period (mg/dL)* | 148 ± 28 | 147 ± 23 |
| SMBG measurements per day in 1-month run-in period* | 5.1 ± 2.5 | 5.3 ± 2.2 |
Differences between the control and the continuous monitoring group were not statistically significant. MDI, multiple daily injection.
*Means ± SD.
During the one-month run-in period, the mean concentration and frequency of home blood glucose monitoring was similar in the control and continuous monitoring groups (Table 1). Mean HbA1c measured at the end of the run-in period before randomization was 6.9 ± 0.7 and 6.9 ± 0.6%, respectively.
In the six-month randomized study period, median sensor wear was 5.6 (pediatric 5.6, adults 4.9) and 6.1 (pediatric 6.1, adults 6.1) days per week of instructed use in the control and continuous monitoring groups, respectively. Median sensor wear in the continuous monitoring group in month 6 was 5.9 days per week. Detailed data on sensor wear are presented in Supplementary Table 1 and Supplementary Figs. 2 and 3.
Hypoglycemia
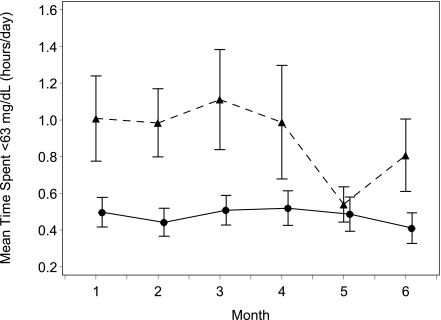
The primary outcome, time spent in hypoglycemia below 63 mg/dL, was significantly shorter in the continuous glucose monitoring group (ratio of means 0.49 [95% CI 0.26–0.76], P = 0.03) (Supplementary Fig. 4). Similarly, time spent in hypoglycemia below 70 mg/dL and below 55 mg/dL was statistically significantly shorter in the continuous glucose monitoring group (P = 0.01 and P = 0.05, respectively; Table 2). The reduction in the primary outcome was evident from the first month and was sustained throughout the 6 months (Fig. 1).
Table 2.
Glycemic outcomes
| Variable | Control group | Continuous monitoring group | Ratio of means | 95% CI for ratio of means | P |
|---|---|---|---|---|---|
| N | 54 | 62 | |||
| Hours per day in hypoglycemia <63 mg/dL | 0.97 ± 1.55 | 0.48 ± 0.57 | 0.49 | 0.26–0.76 | 0.03 |
| Median (interquartile range) | 0.54 (0.23–1.31) | 0.26 (0.14–0.54) | |||
| Number of hypoglycemic excursions per day <63 mg/dL | 0.76 ± 0.94 | 0.53 ± 0.60 | 0.70 | 0.43–1.03 | 0.08 |
| Integrated glucose excursion index (area under the curve) <63 mg/dL | 11.1 ± 14.2 | 5.4 ± 7.6 | 0.49 | 0.29–0.79 | 0.02 |
| Hours per day in hypoglycemia <55 mg/dL | 0.41 ± 0.48 | 0.22 ± 0.34 | 0.55 | 0.34–0.91 | 0.05 |
| Number of hypoglycemic excursions per day <55 mg/dL | 0.37 ± 0.40 | 0.28 ± 0.54 | 0.76 | 0.47–1.43 | 0.07 |
| Hours per day in hypoglycemia <70 mg/dL | 1.60 ± 2.02 | 0.91 ± 0.81 | 0.57 | 0.36–0.80 | 0.01 |
| Low blood glucose index | 1.74 ± 1.62 | 1.18 ± 0.82 | 0.68 | 0.49–0.89 | 0.02 |
| Hours per day in hyperglycemia | |||||
| >180 mg/dL | 6.4 ± 3.4 | 5.5 ± 3.2 | 0.86 | 0.71–1.06 | 0.08 |
| >250 mg/dL | 1.66 ± 1.53 | 1.14 ± 1.46 | 0.69 | 0.48–1.07 | 0.06 |
| High blood glucose index | 6.0 ± 3.2 | 5.1 ± 3.1 | 0.85 | 0.70–1.05 | 0.05 |
| Hours per day in normoglycemia | |||||
| 90–180 mg/dL | 13.5 ± 3.1 | 15.1 ± 2.7 | 1.12 | 1.04–1.21 | 0.003 |
| 70–180 mg/dL | 16.0 ± 3.4 | 17.6 ± 3.2 | 1.10 | 1.02–1.18 | 0.009 |
Data are means ± SD. An excursion is defined as all consecutive recordings outside the boundary and covering at least 10 min. The duration of an excursion is defined as the elapsed time from first excursion to the first reading indicating return inside the excursion boundary.
Figure 1.
Time spent below 63 mg/dL by month. Mean values ± SEs for hours per day spent <63 mg/dL over the 6-month study period in all patients. ●, continuous monitoring group; ▲, control group.
The integrated glucose excursion index for <63 mg/dL was reduced in the continuous monitoring group (P = 0.02) as was the low blood glucose index (P = 0.02). Although not statistically significant, the number of hypoglycemic excursions (<55 and <63 mg/dL) per 24 h/day was also lower in the continuous monitoring group (P = 0.07 and P = 0.08, respectively). The number of hypoglycemic excursions <55 and <63 mg/dL during the night, however, was significantly lower in the continuous monitoring group compared with the control (0.13 ± 0.30 vs. 0.19 ± 0.19, P = 0.01; and 0.21 ± 0.32 vs. 0.30 ± 0.31, P = 0.009).
Time spent in hypoglycemia below 63 mg/dL was reduced in the continuous monitoring group by 41% (mean 0.48 vs. 0.81 h/day) in pump users and by 59% (0.49 vs. 1.20) in subjects on multiple daily injections. This end point was reduced by 48% (0.34 vs. 0.65) in pediatric subjects (10–17 years of age) and by 54% (0.59 vs. 1.27) in adults (18–65 years of age). In the post hoc per protocol analysis, where only patients that wore the sensor for >20 days (corresponding to one third of the required time in the control group) were included (44 of 53 pediatric and 53 of 63 adult patients), the primary outcome was reduced by 64% (P < 0.001) and 50% (P = 0.02) in pediatric and adult patients, respectively.
Glycated hemoglobin and glycemic control
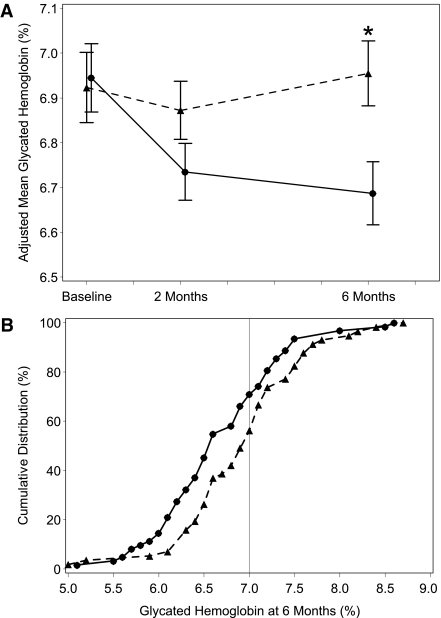
HbA1c at 6 months, adjusted for baseline HbA1c (visit 2), center and age-group was significantly lower in the continuous glucose monitoring group (mean 6.69% compared with 6.95%, difference in means −0.27, 95% CI −0.47 to −0.07, P = 0.008) (Fig. 2). Mean HbA1c at 6 months, adjusted for baseline HbA1c, center and age-group, was reduced by 0.39 in pump users (6.72 vs. 7.11). For subjects using multiple daily injections, the difference was −0.06 (6.70 vs. 6.65). Adjusted mean HbA1c was reduced by 0.23 (6.92 vs. 7.15) in pediatric subjects (10–17 years of age) and by 0.31 (6.51 vs. 6.83) in adults (18–65 years of age).
Figure 2.
Glycated HbA1c at 2, 4, and 6 months and cumulative distribution of HbA1c levels at 6 months. Mean values are ± SEs for HbA1c (in %) over the 6-month study period in all patients. The means were adjusted for clinical center and adult or pediatric patient. An asterisk denotes statistical significance for comparison between the continuous monitoring and control groups with P < 0.05 (A); cumulative distribution of HbA1c levels at 6 months among all patients is shown. The vertical line represents the American Diabetes Association target of 7.0% (B). ●, continuous monitoring group; ▲, control group.
Time spent in normoglycemia (70 to 180 mg/dL and 90 to 180 mg/dL) was significantly longer in the continuous glucose monitoring group (mean hours per day, 17.6 vs. 16.0, P = 0.009; and 15.1 vs. 13.5, P = 0.003). Concurrently, the time spent per day in hyperglycemia >250 mg/dL was shorter in the continuous monitoring group compared with control group (mean hours per day, 1.14 and 1.66), although this was not statistically significant.
Adverse events
Four serious adverse events were reported although none were related to the study or device (Supplementary Table 2). There was an incident of mild diabetic ketoacidosis (DKA) in a patient in the continuous monitoring group, because of the patient disconnecting his or her insulin pump. However, this patient had stopped wearing the continuous glucose monitoring system 2 weeks before the incident. No incidents of severe hypoglycemia were reported.
CONCLUSIONS
This randomized controlled trial, designed to evaluate the effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes, demonstrated a significant reduction by half in time spent in hypoglycemia in relatively well-controlled children and adults with type 1 diabetes. Notably, this finding was paralleled by a significant decrease in HbA1c, contrary to the results in the Diabetes Control and Complication Trial (DCCT) study where the rate of hypoglycemia increased considerably with lower HbA1c levels (21).
In the Juvenile Diabetes Research Foundation (JDRF) trial in children and adults with HbA1c <7% at randomization (15), hypoglycemia below 60 mg/dL was less pronounced in the continuous monitoring group than in the control group at the end of 6 months (median 18 vs. 35 min/day, P = 0.05). Moreover, combined outcomes of hypoglycemia and HbA1c were significantly better in the continuous glucose monitoring patients than in the control patients, which corroborate our findings.
A recent randomized controlled trial named Sensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) comparing sensor-augmented pump therapy with multiple-injection therapy demonstrated a significant reduction of HbA1c in adults and children without an increase in hypoglycemia (22). However, the area under the curve <70 mg/dL and <50 mg/dL did not differ between the two treatment modalities. It is possible that the relatively small amount of continuous sensor data in the multiple-injection therapy group lacked sufficient power to show a difference. Additionally, the baseline HbA1c was considerably higher in the STAR 3 trial as compared with the current study.
None of the participants or parents in the current study reported an event of severe hypoglycemia. Similarly, severe hypoglycemia episodes were infrequent in the JDRF trial (less than 10% of the patients) (15) and the STAR 3 trial (around 13 per 100 patient-years) (22) and did not differ between the study groups. Neither the JDRF trial nor ours was powered to detect differences in the rate of severe hypoglycemic episodes. In the open extension phase of the JDRF trial, episodes of severe hypoglycemia decreased by almost 50% in the control patients after they were switched to continuous glucose monitoring, but this was not statistically significant (P = 0.08) (16).
Although the number of hypoglycemic excursions below 55 mg/dL was not significantly different between the two groups per 24 h, it was significantly lower in the continuous monitoring group during the night. In the JDRF trial, a higher incidence of nocturnal hypoglycemia is associated with lower HbA1c in the continuous glucose monitoring group; however, no comparison is made with the control group (23). Taken together, previous and present data may suggest that the risk of hypoglycemia is alleviated by continuous glucose monitoring. Clinically more meaningful reduction of hypoglycemic events remains to be demonstrated.
Several studies have demonstrated that the use of continuous monitoring above 70% of the time is associated with significantly increased benefit and with a significant lowering of HbA1c in all age groups (17,22,24). Compliance with the sensor wear in the current study in the continuous monitoring group was more than 6 days per week (86%) and did not decrease significantly with time, with an average of around 5.8 days/week (84%) at the end of the 26-week period (Supplementary Fig. 2B). This is roughly similar to the adult group and considerably more than the adolescent or children group of the JDRF trial (16).
This study protocol stated that the control group should wear a masked sensor for 5 days every second week, amounting to 65 days or around 9 weeks during the 26-week study period. In fact, compliance with the sensor wear in the control group was lower than intended (around 80%). However, when compared with previous studies, the amount of masked continuous monitoring data from the control group was substantial. Combined with the high compliance of sensor use in the continuous monitoring group, this contributed to the power of the statistical analysis.
Our results must be interpreted with caution since the patients and their families were highly motivated, demonstrating good metabolic control with an average of more than five blood glucose measurements per day before randomization, and all three participating centers were academic with high penetration of diabetes-related technology. Additionally, because of its nature, the intervention could not be blinded, rendering the results less compelling. The results may therefore not be simply generalized, and further studies are needed to evaluate the hypoglycemia-preventive effects of continuous glucose monitoring in less well-controlled and less motivated patient populations.
In conclusion, the results of the current study demonstrated significantly shorter time spent in hypoglycemia in children and adults with type 1 diabetes who used continuous glucose monitoring compared with standard SMBG, with a concomitant significant decrease of HbA1c.
Supplementary Material
Acknowledgments
This study was supported by Abbott Diabetes Care. T.B. was supported in part by the Slovenian National Research Agency Grants J3-9663, J3-2412, and P3-0343.
T.B.’s institution received research grant support, with receipt of travel and accommodation expenses in some cases, from Abbott, Medtronic, Novo Nordisk, and Diamyd. T.B. is on the speaker’s bureaux of Eli Lilly, Novo Nordisk, Bayer, and Medtronic and is a member of scientific advisory boards for Bayer, LifeScan, and Medtronic. M.P.’s institution received research grant support, with receipt of travel and accommodation expenses in some cases, from Medtronic and Dexcom. M.P. is a consultant for Animas, Medtronic, and Bayer and is a member of scientific advisory boards for CGM3, D-Medical, and Physical Logic. N.B. is on the speaker’s bureau of Medtronic. R.N.’s institution received research grant support, with receipt of travel and accommodation expenses in some cases, from Medtronic and Dexcom. J.B. is on the speaker’s bureau of Abbott, Medtronic, and sanofi-aventis and is a member of scientific advisory boards for AstraZeneca, Medtronic, and Merck Sharp & Dohme. Abbott Diabetes Care provided funding, device-related training, and analytical support. Abbott Diabetes Care was permitted to review the manuscript and suggest changes, but the final decision on content and submission of the manuscript was exclusively retained by the authors, who take responsibility for the accuracy and integrity of the data and analyses. This study was an investigator-initiated trial. The study and protocol were designed by the investigators. The manuscript was prepared by the investigators. No other potential conflicts of interest relevant to this article were reported.
T.B. contributed to the study concept and design, collected data, supervised the study, participated in data analysis and interpretation, and drafted, reviewed, and edited the manuscript. M.P. contributed to the study concept and design, collected data, supervised the study, participated in data analysis and interpretation, and reviewed and edited the manuscript. N.B., R.N., and P.O. collected data, participated in data analysis and interpretation, and reviewed and edited the manuscript. J.B. contributed to the study concept and design, collected data, supervised the study, participated in data analysis and interpretation, and reviewed and edited the manuscript.
The authors thank the following colleagues for assistance in conducting the study: Dr. Nina Bratanic and Ivica Zupancic, diabetes nurse, from the University Children’s Hospital Ljubljana, Slovenia; and Ingela Bredenberg and Hilkka Lahnalampi, diabetes nurses from the Karolinska University Hospital Huddinge, Sweden. Statisticians of Abbott Diabetes Care and an independent statistician (James Gallagher, Director, Statistical Services Centre, University of Reading) worked with the investigators to prepare the statistical analysis plan and performed the analyses. Jude Douglass, a medical writer of Healthcom Partners, edited English language and style and formatted the manuscript for publication.
Parts of this study were presented at the 4th International Conference on Advanced Technologies and Treatments for Diabetes, London, U.K., 16–19 February 2011.
Footnotes
Clinical trial reg. no. NCT00843609, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1989/-/DC1.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Hanberger L, Samuelsson U, Lindblad B, Ludvigsson J, Swedish Childhood Diabetes Registry SWEDIABKIDS A1C in children and adolescents with diabetes in relation to certain clinical parameters: the Swedish Childhood Diabetes Registry SWEDIABKIDS. Diabetes Care 2008;31:927–929 [DOI] [PubMed] [Google Scholar]
- 3.Shalitin S, Phillip M. Hypoglycemia in type 1 diabetes: a still unresolved problem in the era of insulin analogs and pump therapy. Diabetes Care 2008;31(Suppl. 2):S121–S124 [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 6.Bulsara MK, Holman CDJ, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care 2004;27:2293–2298 [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 8.Daneman D. Type 1 diabetes. Lancet 2006;367:847–858 [DOI] [PubMed] [Google Scholar]
- 9.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2004;350:2272–2279 [DOI] [PubMed] [Google Scholar]
- 10.Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care 2009;32:1196–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes 2010;59:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care 2008;31:1892–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson AM, Musen G, Ryan CM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deiss D, Bolinder J, Riveline J-P, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 15.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 17.O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The DCCT Research Group Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 1991;90:450–459 [PubMed] [Google Scholar]
- 20.Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther 2005;7:849–862 [DOI] [PubMed] [Google Scholar]
- 21.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 22.Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 23.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care 2010;33:1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck RW, Buckingham B, Miller K, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care 2009;32:1947–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.